Abstract
Several observational studies on the association between Cd exposure and risk of prostate cancer have yielded inconsistent results. To address this issue, we conducted a meta-analysis to evaluate the correlation between Cd exposure and risk of prostate cancer.
Relevant studies in PubMed and Embase databases were retrieved until October 2015. We compared the highest and lowest meta-analyses to quantitatively evaluate the relationship between Cd exposure and risk of prostate cancer. Summary estimates were obtained using a random-effects model.
In the general population, high Cd exposure was not associated with increased prostate cancer (OR 1.21; 95% CI 0.91–1.64), whereas the combined standardized mortality ratio of the association between Cd exposure and risk of prostate cancer was 1.66 (95% CI 1.10–2.50) in populations exposed to occupational Cd. In addition, high D-Cd intake (OR 1.07; 95% CI 0.96–1.20) and U-Cd concentration (OR 0.86; 95% CI 0.48–1.55) among the general population was not related to the increased risk of prostate cancer. In the dose analysis, the summary relative risk was 1.07 (95% CI 0.73–1.57) for each 0.5 μg/g creatinine increase in U-Cd and 1.02 (95% CI 0.99–1.06) for each 10 μg/day increase of dietary Cd intake. However, compared with nonoccupational exposure, high occupational Cd exposure may be associated with the increased risk of prostate cancer.
This meta-analysis suggests high Cd exposure as a risk factor for prostate cancer in occupational rather than nonoccupational populations. However, these results should be carefully interpreted because of the significant heterogeneity among studies. Additional large-scale and high-quality prospective studies are needed to confirm the association between Cd exposure and risk of prostate cancer.
INTRODUCTION
Prostate cancer is one of the most common malignancies in developed countries and is the second most common cancer in men, following lung cancer, worldwide.1,2 The incidence and mortality rates of prostate cancer vary markedly among different ethnic groups, with the lowest rates found in China and other parts of Asia and the highest rates detected in Western populations.3,4 These differences are caused by genetic susceptibility, exposure to unknown external risk factors, differences in health care and cancer registration, or a combination of these factors. In 2015, up to 220,800 men were diagnosed with prostate cancer, and 27,540 men will die of it in the United States.2 In recent decades, a rapid increase in prostate cancer incidence has been observed in fast-developing countries, in which lifestyles have significantly changed. The etiology of prostate cancer comprises multiple factors. Some causative risk factors for prostate cancer have been implicated, including obesity, androgen, and exposure to selenium, lycopene, vitamins D and E, dietary fat, and Cd.5–7
Cd is a minor metal found naturally in the earth's crust and has been widely distributed in the environment as a result of anthropogenic activity. Cd presents an elimination half-life of 10 to 30 years and may exert a wide range of negative effects on human health.8 Besides being a carcinogen,9 Cd exposure is associated with osteoporosis and bone fracture,10,11 type 2 diabetes,12 kidney disease,13 and cardiovascular disease.14,15 Cd is currently one of the most extensive occupational and environmental pollutants. Occupational Cd exposure is used in various industries, such as Cd-emitting industries and metal mines. Occupational Cd exposure occurs when dust and fumes are inhaled. In particular, major sources of natural and anthropogenic Cd in the general population include cigarette smoking and diet choices: tobacco, grains, potatoes, and vegetables taking up Cd from the soil.16 Several epidemiologic studies investigating the association between Cd exposure and susceptibility to prostate cancer have yielded inconsistent findings. Some studies have demonstrated a significant correlation17–22 or little association23–25 between Cd exposure and risk of prostate cancer, but others failed to show any significant association.26–39 Therefore, we systematically performed a meta-analysis by combining all available data from observational studies to evaluate the association between Cd exposure and risk of fracture. Our meta-analysis followed the Preferred Reporting Items for Systematic Reviews.40
METHODS
This article presents a systematic review and meta-analysis of previously published studies; therefore, ethical approval and written informed consent from patients are not required. This research was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.40
Data Source and Search Strategy
We searched the PubMed and Embase databases until October 2015 to identify relevant studies that evaluated the association between Cd exposure and prostate cancer risk. We used the following search terminologies: “prostate carcinoma” OR “prostatic cancer” OR “prostate cancer” OR “prostatic carcinoma” combined with “Cadmium.” The search was limited to human subjects. Moreover, we manually searched the reference lists of previous reviews and related article references to identify other potentially eligible studies.
Eligibility Criteria and Study Selection
The inclusion criteria were as follows: (1) Cd was the heavy metal used for exposure to humans; (2) the outcome of interest was prostate cancer incidence, prevalence, and mortality; (3) the report was a cohort, case-control study; and (4) the relative risk (RR), odds ratio (OR), hazard risk, or standardized mortality ratio (SMR) with corresponding 95% confidence interval (CI) was reported or calculated from available data. If the study was reported more than once, we included the study with the most comprehensive data.
Data Extraction and Quality Assessment
Two authors (JKS and DBY) separately extracted data from selected studies, and discrepancies were resolved through discussion and consensus. For each study, we extracted the first author's name, year of publication, study design, country, total number of cases and subjects, sex, exposure type of Cd, and adjusted variables. When more than 1 adjusted OR was reported, the OR with the most fully adjusted model was selected. For dose analysis, the number of cases and participants of person-years for each category of Cd exposure must also be provided (or data available for calculations).
We evaluated the methodological quality of the included studies by using the Newcastle–Ottawa scale (NOS).41 The checklist contained 9 items for case-control studies and cohort studies, with every item accounting for 1 point. We considered high-quality studies as those with a score of >5.
Statistical Analysis
Differences were expressed as OR with 95% CI for nonoccupational exposure studies and SMR with 95% CI for occupational exposure studies. Prostate cancer caused by Cd was considered a rare event, and the RR in the cohort study was considered as approximations of OR. Three studies reported stratified risk estimates by age23 and region.19,38 We combined these estimates by using a random-effects model and used the pooled estimates for the meta-analysis. The OR in 1 study34 was not extracted; thus, we computed the crude risk estimates and their corresponding CI. A random-effects model of the DerSimonian and Laird method was used to calculate the summary risk estimates, irrespective of heterogeneity, which incorporated both within-study and between-study variability.42 Subgroup analysis was stratified by geographic region, study design, quality of NOS scale, type of outcome, and type of exposure. We conducted sensitivity analyses by omitting 1 study in each turn to investigate whether the results were attributed to 1 large study or a study with extreme results. Furthermore, we explored the heterogeneity of the different variables mentioned above through a single-variable meta-regression analysis. We conducted a 2-stage random-effects dose-response meta-analysis by using the method proposed by Greenland and Longnecker.43 This method required that the distribution of cases, person-years, non-cases, and risk estimates within the variance are known for at least 3 quantitative exposure categories. We assigned the median values or middle point of Cd exposure for each category to the corresponding RR. If the highest category of the studies was open-ended, we assumed the range to be the same as the adjacent interval. First, we estimated a restricted cubic spline model via generalized least-square regression with 4 knots at 5%, 35%, 65%, and 95% distribution. Second, we pooled the study-specific risk estimates by using the restricted maximum likelihood method in a random-effects meta-analysis. Nonlinear relation was estimated by testing the null hypothesis, which indicated that the coefficient of the second spline is equal to 0.
We also evaluated the potential publication bias by using funnel plot and Egger tests, with a priori P < 0.1 indicating a significant publication.44 If asymmetry evidence was detected, the trim-and-fill method was employed to correct the publication bias.45 All statistical analyses were conducted using Stata version 13.1 (Stata Corp, College Station, TX).
RESULTS
Literature Search
Figure 1 shows a flow chart of the inclusion criteria. Following the development of our search strategy, we identified 478 records from PubMed and Embase databases. After excluding the duplicates and articles that did not meet the inclusion criteria, we obtained 36 articles with full-texts read for further evaluation. Five articles were duplicate publications,17,46–49 4 articles presented no data useful for the meta-analysis,50–53 2 articles were excluded because the Cd contents in prostate tissues were measured,54,55 2 articles were reviews,10,56 and 1 article reported an association between Cd and prostate-specific antigen.57 Finally, 22 studies that met the meta-analysis criteria were included.
FIGURE 1.
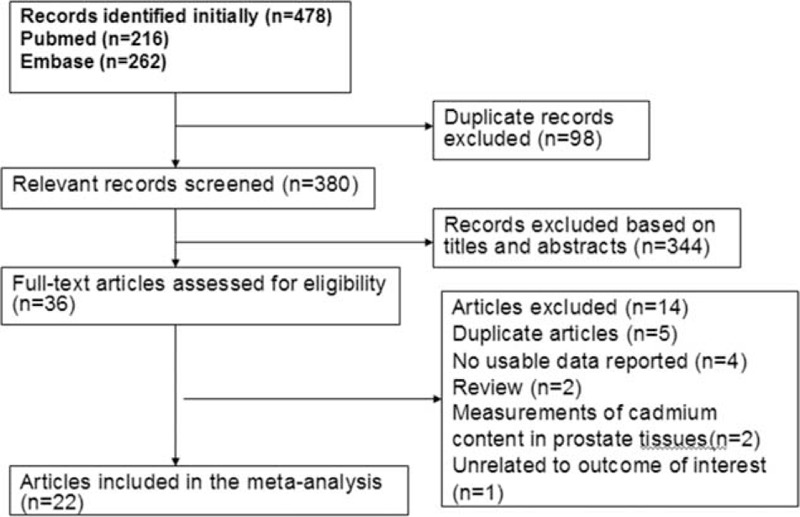
Flow diagram of the literature search and study selection.
Study Characteristics
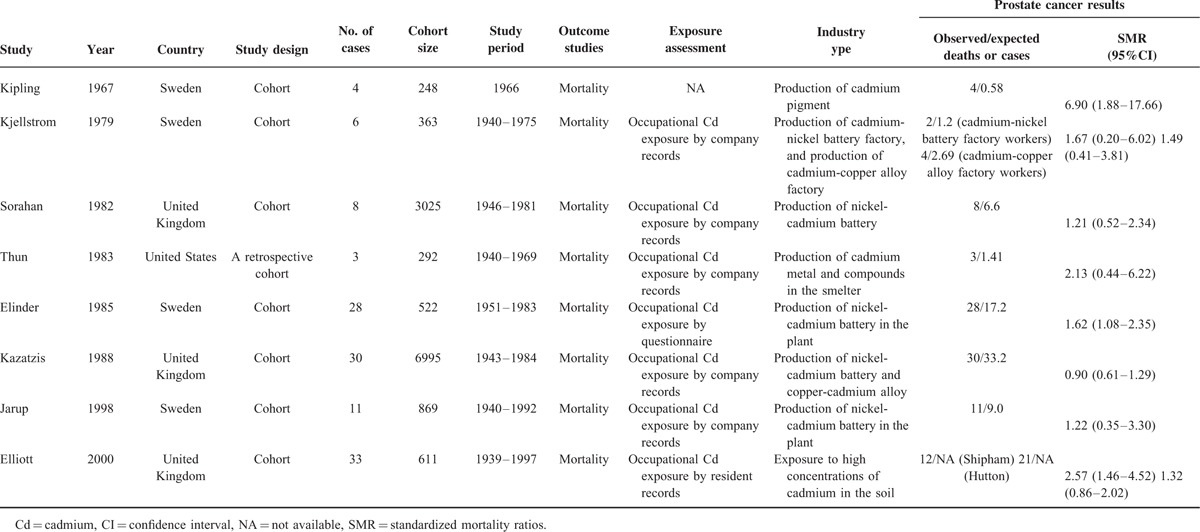
Tables 1–3 present the characteristics of the included studies. A total of 22 studies, comprising 8 case-control and 14 cohort studies, contributed to the meta-analysis. These studies were published from 1967 to 2015. The number of prostate cancer patients ranged from 40 to 358 in the case-control studies and from 3 to 83,085 in the cohort studies. Thirteen studies were conducted in Europe,18–22,29,30,32,35–39 7 in the United States,23,24,26,27,31,33,34 and 2 in Asia.25,28 Thirteen studies reported findings for prostate cancer incidence,18,20,22–25,28,33–37,39 whereas the remaining 9 studies reported results for prostate cancer mortality.19,21,26,27,29–32,38 We included a total of 200 prostate cancer deaths, 6653 prostate cancer cases, and 137,998 participants in the meta-analysis. Eight studies were designed to evaluate OR,20,23–25,33–36 3 evaluated RR,18,37,39 3 evaluated hazard risk,26–28 and 8 evaluated SMR19,21,22,29–32,38. Three articles used urinary Cd (U-Cd) as biomarker for long-term exposure to Cd,25–27 4 articles evaluated Cd levels by estimating the dietary Cd (D-Cd) by using food frequency questionnaires,18,23,28,39 and 2 studies examined Cd in toenails20,33. Twelve studies reported an association between occupational Cd exposure and prostate cancer risk,19,21,22,24,29–32,34–38 whereas 10 studies used nonoccupational populations.18,20,23,25–28,33,36,39 Most of the studies were controlled for some conventional risk factors, including age (n = 6) and smoking (n = 6). Some studies were also controlled for body mass index (n = 3) and alcohol consumption (n = 2), but few studies were adjusted for beef intake, dairy product consumption (n = 1), and intake of vegetable and fruit (n = 1). None of the studies were adjusted for other heavy metals, trace elements of organic pollutants, and intake of grains.
TABLE 1.
ORs/RR of prostate cancer between patients with and without environmental/occupational Cd exposure among included case-control studies
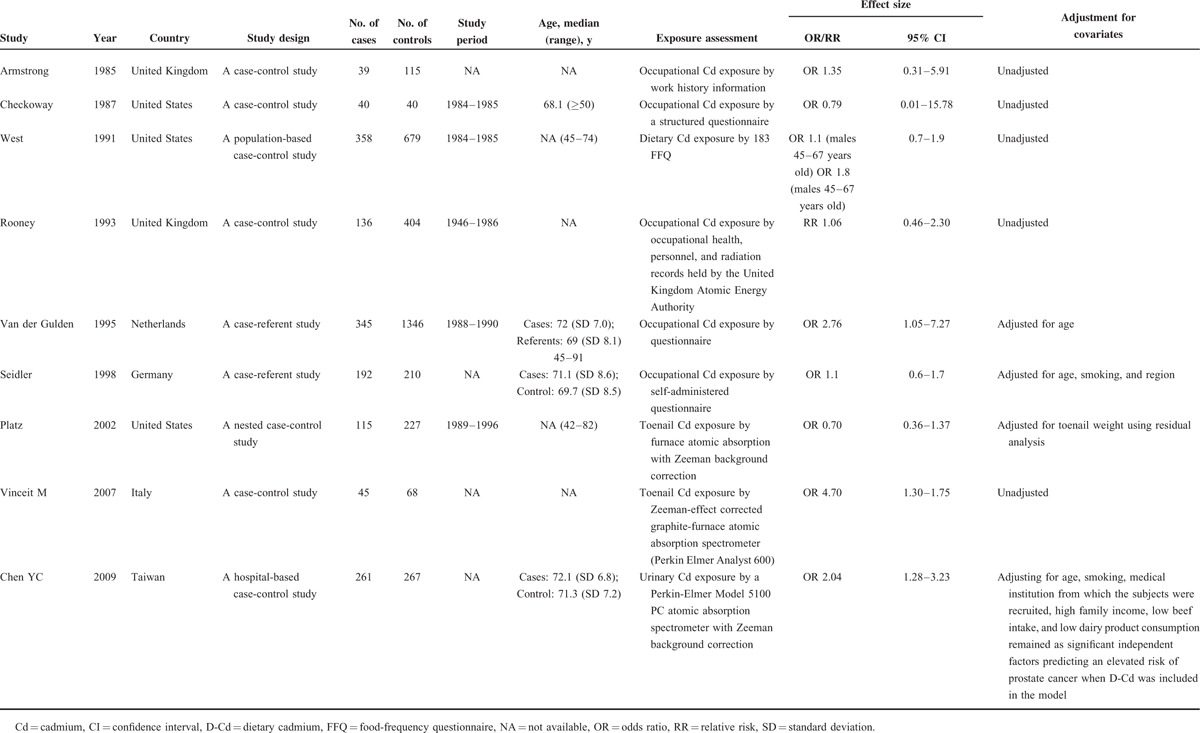
TABLE 3.
OR/RR/HR of prostate cancer between patients with and without environmental/occupational Cd exposure among included cohort studies
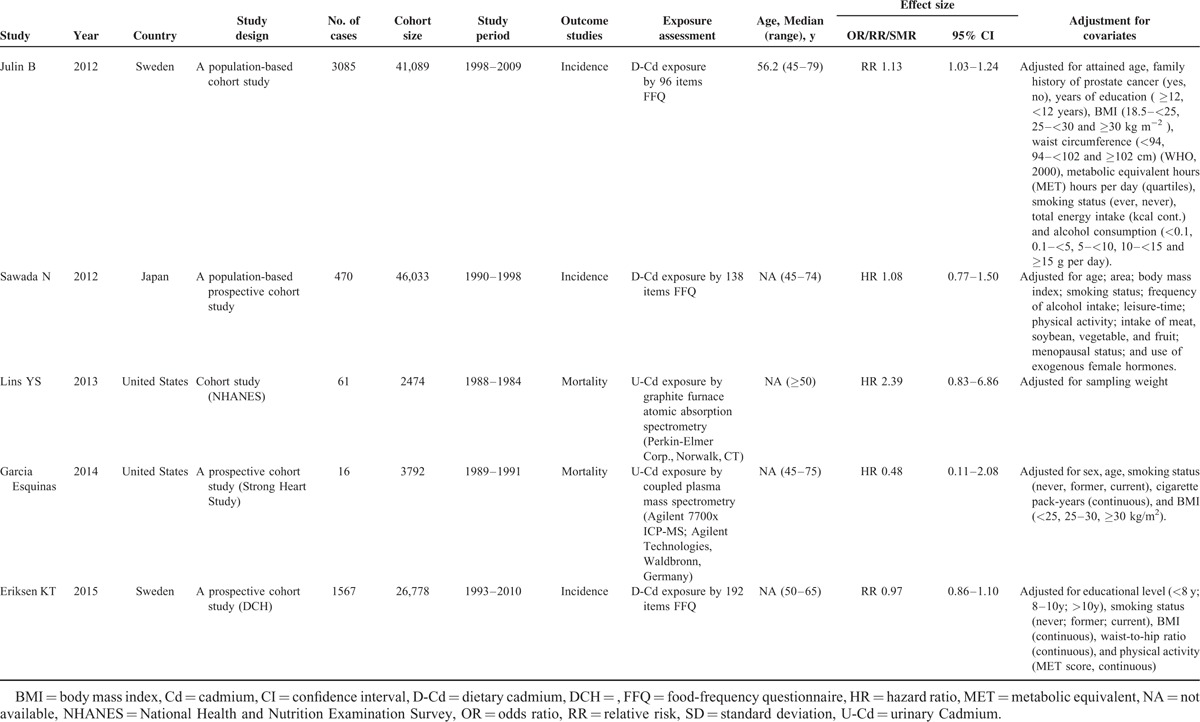
TABLE 2.
Standardized mortality ratios of prostate cancer patients with and without occupational Cd exposure among included cohort studies
NOS was used to assess the quality of studies included in the meta-analysis (Table 4). The median NOS score was 4.8 (range 3–7).
TABLE 4.
Quality assessment of eligible studies based on Newcastle–Ottawa scale
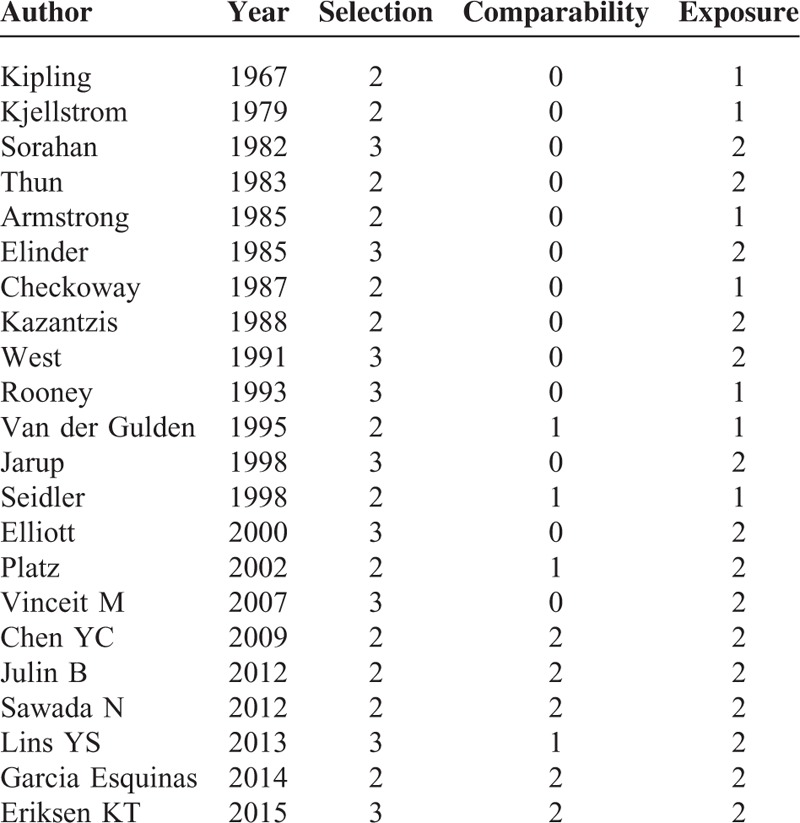
Results From Pooled SMR Estimates With and Without Population Exposed to Occupational Cd
Figure 2 shows the SMR estimates and 95% CI from each study, as well as the pooled SMR estimate based on a random-effects model. Results from the 8 cohort studies indicated that the pooled SMR was 1.66 (95% CI 1.10–2.50) with moderate heterogeneity (Pfor.heterogeneity = 0.002; I2 = 69.9%). In subgroup analyses for exposure type, we restricted each analysis to 7 occupational exposure studies, resulting in a summary SMR of prostate cancer of 1.61 (95% CI 1.04–2.48). Only 1 study was conducted in the United States,31 and the 7 other studies were conducted in Europe. When we stratified the analysis by geographic region, the pooled SMR was 1.63 (95% CI 1.05–2.52) for studies conducted in Europe. Compared with a low NOS score (SMR = 2.08, 95% CI 0.73–5.91), the association was higher among studies with high NOS score (OR = 1.51, 95% CI 1.14–1.98) (Table 5). In a sensitivity analysis, similar results were observed, which ranged from 1.30 (95% CI 1.03–1.64) with low heterogeneity (I2 = 9.0%, heterogeneity P = 0.360) (excluding the study by Kipling et al22) to 1.87 (95% CI 1.23–2.83) with significant heterogeneity (I2 = 58.4%, heterogeneity P = 0.025) (excluding the study by Kazantzis et al30). Egger test (P = 0.241) and funnel plot (Figure 3) showed no publication bias.
FIGURE 2.
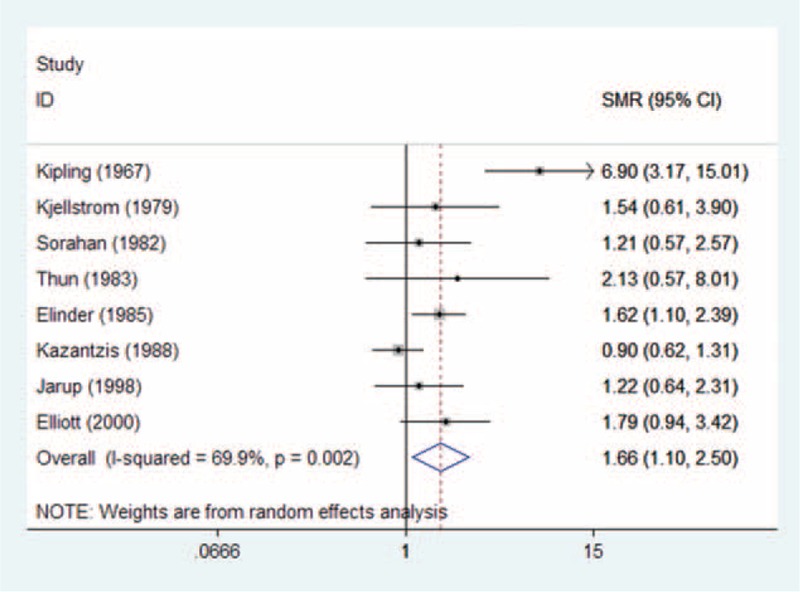
Forest plot of Cd exposure and prostate cancer risk (SMR) in occupational Cd exposure population. Cd = cadmium, SMR = standardized mortality ratio.
TABLE 5.
Results of overall subgroup analysis among occupational Cd exposure populations
FIGURE 3.
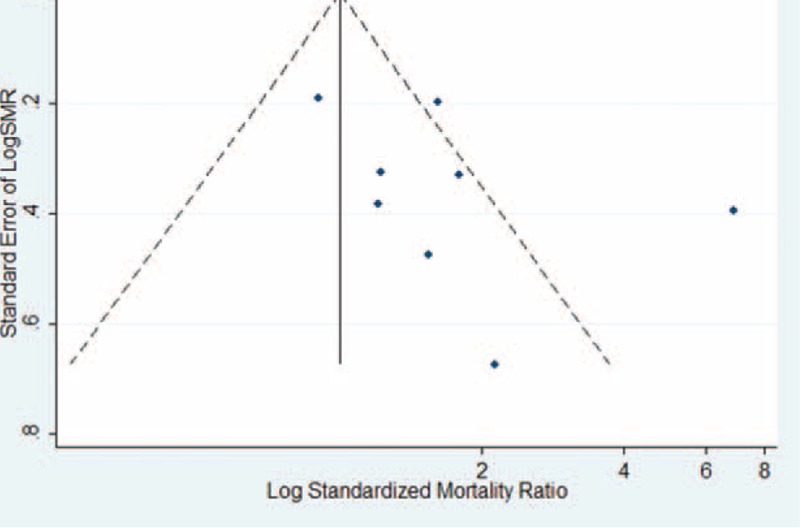
Funnel plot of Cd exposure and prostate cancer risk (SMR) in occupational Cd exposure population. Cd = cadmium, SMR = standardized mortality ratio.
Results From Pooled OR Estimates With and Without Environmental/Occupational Cd-Exposed Population
Figure 4 shows the OR estimates, 95% CI from individual studies, and pooled OR estimate based on a random-effects model. Results from the 14 studies, comprising 9 case-control studies and 6 cohort studies, indicated that the pooled OR was 1.23 (95% CI 0.81–1.88) with significant heterogeneity (Pfor.heterogeneity = 0.000; I2 = 96.2%). In subgroup analyses for study design, we restricted each analysis to 9 case-control studies and 5 cohort studies; the summary ORs of prostate cancer for the highest category of Cd exposure versus the lowest category were 1.31 (95% CI 0.60–2.87) and 1.06 (95% CI 0.99–1.09), respectively. Five studies were conducted in the United States, 6 in Europe, and 2 in Asia. When we stratified the analysis by geographic region, the combined OR was 1.10 (95% CI 0.66–1.84) in studies conducted in the United States, 1.57 (95% CI 0.86–2.84) in studies conducted in Europe, and 0.74 (95% CI 0.34–1.60) in studies conducted in Asia. When stratified by type of Cd exposure, the combined OR of prostate cancer was 1.05 (95% CI 0.97–1.15) for D-Cd, 1.18 (95% CI 0.28–2.34) for U-Cd, and 1.87 (95% CI 0.29–12.06) for toenail Cd. Five studies reported an association between occupational Cd exposure and prostate cancer risk; however, the association was not significant in the occupational exposure population (OR = 1.31, 95% CI 0.79–2.19). When stratified by type of outcome, the combined OR was 1.23 (95% CI 0.78–1.95) for prostate cancer incidence and 1.29 (95% CI 0.51–3.27) for prostate cancer mortality. Compared with studies that presented low NOS scores (OR = 1.27, 95% CI 0.87–1.875), the association was higher among studies with high NOS scores (OR = 1.18, 95% CI 0.71–1.96) (Table 6). Six studies18,25,26,28,35,39 were adjusted for smoking status, resulting in pooled OR of 0.96 (95% CI 0.80–1.16) with moderate heterogeneity (Pforheterogeneity = 0.007; I2 = 68.8%). Sensitivity analysis showed that the overall pooled estimate was not altered substantially, with the exclusion of 1 study. The overall combined OR after sequential exclusion of 1 study at a time ranged from 1.03 (95% CI 0.88–1.21) with significant heterogeneity (Pfor heterogeneity = 0.017; I2 = 51.3%) (excluding the study by Vinceit et al20) to 1.29 (95% CI, 0.84–1.98) with significant heterogeneity (Pfor heterogeneity = 0.000; I2 = 96.5%) (excluding the study by Garcia Esquinas et al 26). No evidence of publication bias resulted from Egger test (P = 0.881) and near-symmetric funnel plot (Figure 5).
FIGURE 4.
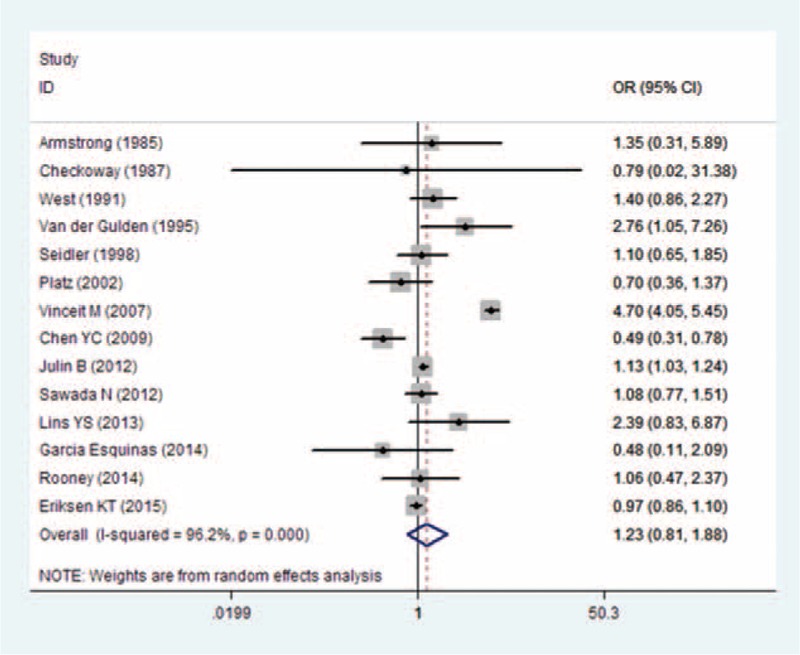
Forest plot of Cd exposure and prostate cancer risk (OR) in occupational/environmental Cd exposure population. Cd = cadmium, OR = odds ratio.
TABLE 6.
Results of overall subgroup analysis among environmental/occupational Cd exposure populations
FIGURE 5.
Funnel plot of Cd exposure and prostate cancer risk (OR) in occupational/environmental Cd exposure population. Cd = cadmium, OR = odds ratio.
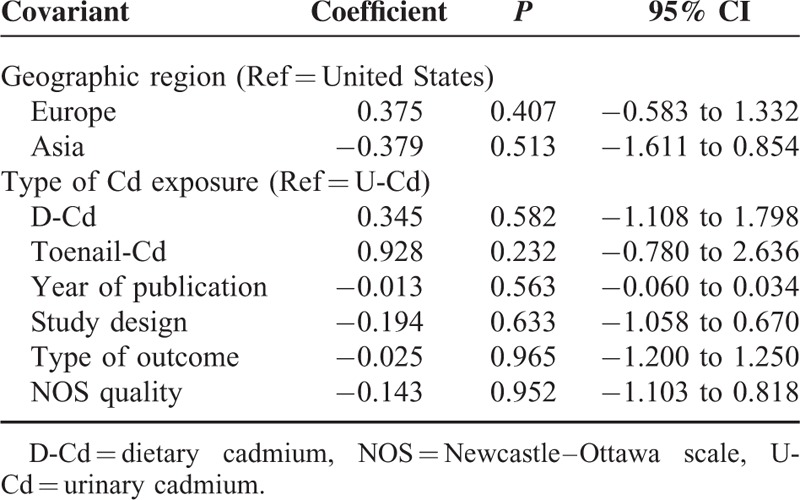
Considering the relatively high heterogeneity observed in the trials, a meta-regression was performed to explore the predefined possible sources of heterogeneity. None of the regression coefficients were statistically significant (Table 7), suggesting that publication year, study design, geographic region, NOS, type of outcome, and type of Cd exposure were insignificant sources of heterogeneity.
TABLE 7.
Effects of study variables on meta-regression
Two studies were included in the dose-response analysis of U-Cd exposure and prostate cancer risk, and a nonlinear association was not observed between them (Pfor nonlinearitytest = 0.47). The summary RR per 0.5 μg/g creatinine increment was 1.07 (95% CI 0.73–1.57) with no evidence of heterogeneity (Pfor heterogeneity = 0.33; I2 = 0.0%). Four studies were included in the dose-response analysis of D-Cd intake and prostate cancer risk. We found no significant departure from a simple linear-response association between Cd exposure and prostate cancer (Pfor nonlinearity test = 0.64). The estimated RR of prostate cancer risk was 1.02 (95% CI 0.99–1.06) for 10 μg/d increase of D-Cd, with little evidence of heterogeneity (Pfor heterogeneity = 0.15; I2 = 40.7%) (Figure 6).
FIGURE 6.
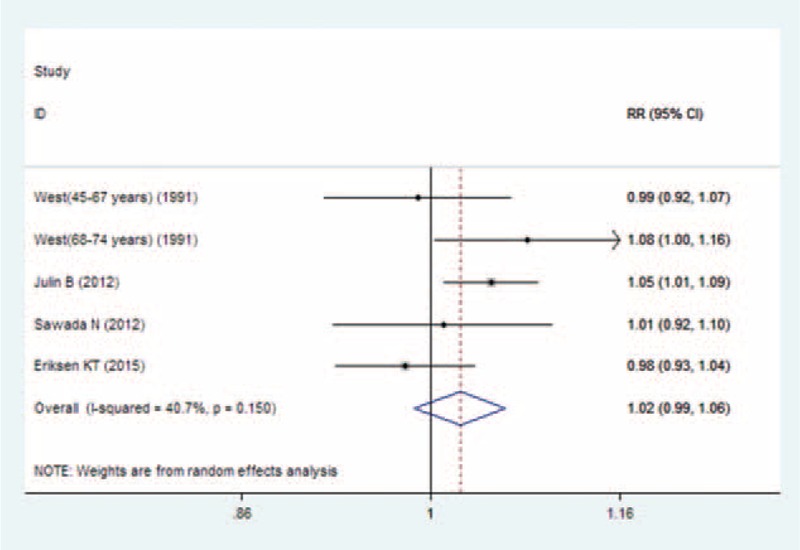
Forest plot of linear trend between dietary Cd intake and risk of prostate cancer (RR), with dose scale of 10 μg/d increase in environmental Cd exposure population. Cd = cadmium, RR = relative risk.
DISCUSSION
Cd is a nonessential metal widely distributed in the environment by industrial and agricultural activities.8 According to accumulated evidence from experimental and epidemiologic studies, Cd has been recognized as a human carcinogen.58,59 Recently, increasing evidence established a link between Cd exposure and prostate cancer,33,39 breast cancer,60–62 pancreatic cancer,63,64 and lung cancer.65,66 Substantial compelling evidence supported that occupational exposure to Cd resulted in lung cancer and showed similar findings regarding prostate and other cancers. A positive association between Cd exposure and prostate cancer mortality was found in 2 cohort studies21,22 and 1 case-control study,36 whereas several studies showed no significant association among occupational populations.19,24,29–32,34–38 These studies were conducted in a Cd-polluted area (eg, nickel batteries, pigments, and soldering alloys). Most nonoccupational Cd exposure studies,23,26–28,33,39 though not all,17,18,20 showed no significant relation between Cd exposure and prostate cancer risk. Verougstraete et al67 conducted a systematic review on Cd-exposed workers and found that Cd exposure was not associated with increased risk of prostate cancer. In another recent meta-analysis based on 8 previous studies, in which D-Cd intake showed no statistically significant association with cancer risk except in stratified analysis by geographic region, a positive association between D-Cd intake and cancer risk was observed.56 However, few studies were included in the meta-analysis, which limited the possibility of drawing robust conclusions, especially in the subgroup analysis. Compared with the 2 previous meta-analyses, the current research presented a more extensive systematic review, which included a large number of studies with more than 6828 cases, 123 deaths, and almost 171,972 participants. Thus, we obtained adequate statistical data to clarify the relation between Cd exposure and risk of prostate cancer. In our meta-analysis, we found a positive association between high Cd exposure and risk of prostate cancer for occupational exposure, but not for nonoccupational exposure. These findings can potentially result in higher Cd exposure levels prevailing in these studies.
No substantial changes were observed in most subgroup analyses because the Cd concentrations in the blood and urine are the most common biomarkers for Cd exposure. U-Cd mainly reflects Cd accumulation in the kidney, as determined by lifelong exposure, whereas D-Cd demonstrates a combination of both current and historical exposure. Results from subgroup analyses stratified by type of Cd exposure showed that both U-Cd and D-Cd were not associated with increased prostate cancer risk. Smoking is a primary source of exposure in the general population and is known to damage health through direct and indirect effects. Thus, we also performed subgroup analyses among studies controlled for smoking status to minimize possible non–Cd-mediated negative effects of tobacco smoking on prostate cancer risk. Six publications were adjusted for smoking status, and the results showed that Cd exposure was not associated with increased prostate cancer risk (OR 0.94, 95% CI 0.77–1.14).
Many studies have demonstrated that the prostate is a target organ for the deposition of Cd,68,69 and numerous experimental studies in vivo and in vitro have indicated that Cd can act as a prostate carcinogen in rats.70 Cd has been recognized as a human carcinogen by the International Agency for Research on Cancer on the basis of mechanistic and epidemiologic evidence from high-exposure occupational settings.9 Several mechanisms are responsible for the carcinogenesis of Cd exposure, including induction of oxidative stress,71 suppression of DNA repair,72 alterations of DNA methylation,73 inhibition of apoptosis proto-oncogene activation,74 tumor suppressor gene inactivation, and cell adhesion disruption.75 In addition, Cd can exert estrogenic activities that play a role in the development of prostate cancer. Cd is suggested to exert estrogenic properties, and direct receptor-mediated effects of estrogen on prostate are plausible.76 Experimental evidence showed that excessive exposure to estrogens can cause prostate cancer. In human prostate epithelial cells, Cd exhibits estrogenic activity, including proliferation of prostate cells and activation of the estrogen receptor-α.77 Substantial evidence showed a positive relation between Cd exposure and risk of endometrial78 and breast cancers.79 Therefore, high Cd exposure can potentially increase the risk of prostate cancer.
The present meta-analysis exhibited several strengths. The first research highlight of this meta-analysis is its large sample size. The large number of total cases provided high statistical power to quantitatively evaluate the association between Cd exposure and prostate cancer risk. Second, publication bias is a potential concern in any meta-analysis because small studies with null results do not get published. However, in our meta-analysis, we found little evidence of publication bias.
Nevertheless, some limitations should be considered in the present meta-analysis. First, observational studies, even if prospective, cannot prove causality. We cannot exclude the possibility that the observed positive relationship between Cd exposure and prostate cancer risk is attributed to confounding factors. Majority of the studies were adjusted for potential confounding factors, but not all potential confounders were adjusted in every study. For instance, a potential confounder such as cigarette smoking is not only a source of Cd but also contains other substances with adverse health effects. In analyses stratified by adjusting the smoking status, similar results were obtained. Most studies were adjusted for some conventional risk factors, including age and smoking status, and some studies were controlled for body mass index and alcohol consumption. However, few studies were adjusted for other dietary variables or nutrients, whereas none of the included studies were controlled for other heavy metals, trace elements, or organic pollutants. Second, an accurate assessment of Cd exposure remains a challenge. Most studies used questionnaires to assess Cd exposure, whereas some research used interviews, company records, and self-reports to evaluate Cd concentration. However, increasing errors in measurements become inevitable. The imprecise measurement of Cd concentration might have attenuated the true associations. Third, the definition of Cd exposure varied across studies. The Cd exposure types differed according to geographical locations, as urine Cd concentration (in μg/g creatinine) ranged from approximately 0.39 to 1.46 in the US and European population. In Asian studies, the mean urine Cd concentration (in μg/g creatinine) ranged from 0.94 to 1.4. The Cd intake from food generally varies between 9 and 25 μg/d in the US and in Europe. In Asian studies, the mean D-Cd intake (in μg/day) ranged from 19.7 to 35.4. These factors can affect our results. However, our subgroup analyses showed that the associations between Cd exposure and prostate cancer risk did not differ significantly in terms of study location. Third, potential sources of between-study heterogeneity, which is common in meta-analyses, should be explored. In sensitivity analyses, the observed heterogeneity was explained by an article22 that reported a significant positive association and yielded a low NOS. Results from subgroup analyses indicated that geographic region, study design, quality of NOS, type of outcome, and type of exposure are potential sources of heterogeneity. Nevertheless, we used meta-regression and sensitivity analysis to explore the potential causes of between-study heterogeneity. Our meta-regression analysis did not find covariates of publication year, study design, geographic region, NOS, type of outcome, and type of Cd exposure as sources of heterogeneity. Finally, although we selected the highest multivariable-adjusted effect estimates in our meta-analysis, we cannot exclude the possibility that the observed increase in association between Cd exposure and prostate cancer risk among occupational populations can be ascribed to unmeasured or residual confounding factors. The unstable results were observed in occupational and environmental populations, indicating that more relevant articles are needed to further explore this association.
In summary, this meta-analysis suggests high Cd exposure as a potential risk factor for prostate cancer in occupational populations but not in nonoccupational populations. However, these results should be carefully interpreted because of the significant heterogeneity among studies. Additional large-scale and high-quality prospective studies are needed to confirm the association between Cd exposure and risk of prostate cancer.
Acknowledgments
The authors thank the reviewers for their helpful comments on this article. They would like to thank editor and anonymous referees for their valuable and informative comments.
Footnotes
Abbreviations: Cd = cadmium, CI = confidence interval, D-Cd = dietary Cd, HR = hazard risk, NOS = Newcastle–Ottawa scale, OR = odds ratio, RR = relative risk, SMR = standardized mortality ratio, U-Cd = urinary Cd.
Wei-De Zhong and Jian-Guo Zhu are the co-corespondence authors (e-mail: zhongwd2009@live.cn).
This work was supported by grants from National Natural Science Foundation of China (81170699, 81360119), Projects of Guangdong Key Laboratory of Clinical Molecular Medicine and Diagnostics, Guangzhou Municipal Science and Technology Key Project (2014J4100072), Outstanding Youth Science and Technology Talent Cultivating Object of Guizhou Province in 2013 (2013–18), International Scientific and Technological Cooperation Projects of Guizhou Province (20137031), China Postdoctoral Science Foundation (2014M562157), Zhejiang provincial medical and health science and technology project (2016KYB265).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015; 65:5–29. [DOI] [PubMed] [Google Scholar]
- 3.Bashir MN. Epidemiology of prostate cancer. APJCP 2015; 16:5137–5141. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Wang HK, Qu YY, et al. Prostate cancer in East Asia: evolving trend over the last decade. Asian J Androl 2015; 17:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allott EH, Masko EM, Freedland SJ. Obesity and prostate cancer: weighing the evidence. Eur Urol 2013; 63:800–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkateswaran V, Klotz LH. Diet and prostate cancer: mechanisms of action and implications for chemoprevention. Nat Rev Urol 2010; 7:442–453. [DOI] [PubMed] [Google Scholar]
- 7.Chan JM, Giovannucci EL. Vegetables, fruits, associated micronutrients, and risk of prostate cancer. Epidemiol Rev 2001; 23:82–86. [DOI] [PubMed] [Google Scholar]
- 8.Jarup L, Akesson A. Current status of Cd as an environmental health problem. Toxicol Appl Pharmacol 2009; 238:201–208. [DOI] [PubMed] [Google Scholar]
- 9.Beryllium, Cadmium, mercury exposures in the glass manufacturing industry. Working Group views and expert opinions, Lyon, 9–16 February 1993. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization. International Agency for Research on Cancer 1993;58: 1–415. [PMC free article] [PubMed] [Google Scholar]
- 10.Nawrot TS, Staessen JA, Roels HA, et al. Cadmium exposure in the population: from health risks to strategies of prevention. Biometals 2010; 23:769–782. [DOI] [PubMed] [Google Scholar]
- 11.Dahl C, Sogaard AJ, Tell GS, et al. Do cadmium, lead, and aluminum in drinking water increase the risk of hip fractures? A NOREPOS study. Biol Trace Elem Res 2014; 157:14–23. [DOI] [PubMed] [Google Scholar]
- 12.Barregard L, Bergstrom G, Fagerberg B. Cadmium, type 2 diabetes, and kidney damage in a cohort of middle-aged women. Environ Res 2014; 135:311–316. [DOI] [PubMed] [Google Scholar]
- 13.Byber K, Lison D, Verougstraete V, et al. Cadmium or cadmium compounds and chronic kidney disease in workers and the general population: a systematic review. Critical reviews in toxicology 2015; 29:1–50. [DOI] [PubMed] [Google Scholar]
- 14.Julin B, Bergkvist C, Wolk A, et al. Cadmium in diet and risk of cardiovascular disease in women. Epidemiology 2013; 24:880–885. [DOI] [PubMed] [Google Scholar]
- 15.Tellez-Plaza M, Jones MR, Dominguez-Lucas A, et al. Cadmium exposure and clinical cardiovascular disease: a systematic review. Current atherosclerosis reports 2013; doi:10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amzal B, Julin B, Vahter M, et al. Population toxicokinetic modeling of cadmium for health risk assessment. Environ Health Perspect 2009; 117:1293–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheung MR, Kang J, Ouyang D, et al. Association between urinary Cd and all cause, all cancer and prostate cancer specific mortalities for men: an analysis of national health and nutrition examination survey (NHANES III) data. Asian Pacific J Cancer Prev 2014; 15:483–488. [DOI] [PubMed] [Google Scholar]
- 18.Julin B, Wolk A, Johansson JE, et al. Dietary cadmium exposure and prostate cancer incidence: a population-based prospective cohort study. Br J Cancer 2012; 107:895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kjellstrom T, Friberg L, Rahnster B. Mortality and cancer morbidity among cadmium-exposed workers. Environ Health Perspect 1979; 28:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vinceti M, Venturelli M, Sighinolfi C, et al. Case-control study of toenail cadmium and prostate cancer risk in Italy. Sci Total Environ 2007; 373:77–81. [DOI] [PubMed] [Google Scholar]
- 21.Elinder CG, Kjellstrom T, Hogstedt C, et al. Cancer mortality of cadmium workers. Br J Ind Med 1985; 42:651–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kipling M, Waterhouse J. Cadmium and prostatic carcinoma. Lancet 1967; 289:730–731. [Google Scholar]
- 23.West DW, Slattery ML, Robison LM, et al. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control 1991; 2:85–94. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong BG, Kazantzis G. Prostatic cancer and chronic respiratory and renal disease in British Cd workers: a case control study. Br J Ind Med 1985; 42:540–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YC, Pu YS, Wu HC, et al. Cadmium burden and the risk and phenotype of prostate cancer. BMC Cancer 2009; 9:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Esquinas E, Pollan M, Tellez-Plaza M, et al. Cadmium exposure and cancer mortality in a prospective cohort: the strong heart study. Environ Health Perspect 2014; 122:363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin YS, Caffrey JL, Lin JW, et al. Increased risk of cancer mortality associated with Cd exposures in older Americans with low zinc intake. J Toxicol Environ Health 2013; 76:1–15. [DOI] [PubMed] [Google Scholar]
- 28.Sawada N, Iwasaki M, Inoue M, et al. Long-term dietary cadmium intake and cancer incidence. Epidemiology 2012; 23:368–376. [DOI] [PubMed] [Google Scholar]
- 29.Jarup L, Bellander T, Hogstedt C, et al. Mortality and cancer incidence in Swedish battery workers exposed to cadmium and nickel. Occup Environ Med 1998; 55:755–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kazantzis G, Lam TH, Sullivan KR. Mortality of cadmium-exposed workers. A five-year update. Scand J Work Environ Health 1988; 14:220–223. [DOI] [PubMed] [Google Scholar]
- 31.Thun MJ, Schnorr TM, Smith AB, et al. Mortality among a cohort of U.S. Cadmium production workers—an update. J Natl Cancer Inst 1985; 74:325–333. [PubMed] [Google Scholar]
- 32.Sorahan T, Waterhouse JA. Mortality study of nickel-cadmium battery workers by the method of regression models in life tables. Br J Ind Med 1983; 40:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platz EA, Helzlsouer KJ, Hoffman SC, et al. Prediagnostic toenail cadmium and zinc and subsequent prostate cancer risk. Prostate 2002; 52:288–296. [DOI] [PubMed] [Google Scholar]
- 34.Checkoway H, DiFerdinando G, Hulka BS, et al. Medical, life-style, and occupational risk factors for prostate cancer. Prostate 1987; 10:79–88. [DOI] [PubMed] [Google Scholar]
- 35.Seidler A, Heiskel H, Bickeboller R, et al. Association between diesel exposure at work and prostate cancer. Scand J Work Environ Health 1998; 24:486–494. [DOI] [PubMed] [Google Scholar]
- 36.van der Gulden JW, Kolk JJ, Verbeek AL. Work environment and prostate cancer risk. Prostate 1995; 27:250–257. [DOI] [PubMed] [Google Scholar]
- 37.Rooney C, Beral V, Maconochie N, et al. Case-control study of prostatic cancer in employees of the United Kingdom Atomic Energy Authority. BMJ 1993; 307:1391–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elliott P, Arnold R, Cockings S, et al. Risk of mortality, cancer incidence, and stroke in a population potentially exposed to cadmium. Occup Environ Med 2000; 57:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eriksen KT, Halkjaer J, Meliker JR, et al. Dietary cadmium intake and risk of prostate cancer: a Danish prospective cohort study. BMC Cancer 2015; 15:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, et al. Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [PMC free article] [PubMed] [Google Scholar]
- 41.Hartling L, Milne A, Hamm MP, et al. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol 2013; 66:982–993. [DOI] [PubMed] [Google Scholar]
- 42.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 43.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 1992; 135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 44.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters JL, Sutton AJ, Jones DR, et al. Performance of the trim and fill method in the presence of publication bias and between-study heterogeneity. Stat Med 2007; 26:4544–4562. [DOI] [PubMed] [Google Scholar]
- 46.Elghany NA, Schumacher MC, Slattery ML, et al. Occupation, cadmium exposure, and prostate cancer. Epidemiology 1990; 1:107–115. [DOI] [PubMed] [Google Scholar]
- 47.Lemen RA, Lee JS, Wagoner JK, et al. Cancer mortality among Cd production workers. Annals N Y Acad Sci 1976; 271:273–279. [DOI] [PubMed] [Google Scholar]
- 48.Armstrong BG, Kazantzis G. The mortality of Cd workers. Lancet 1983; 1:1425–1427. [DOI] [PubMed] [Google Scholar]
- 49.Inskip H, Beral V, McDowall M. Mortality of Shipham residents: 40-year follow-up. Lancet 1982; 1:896–899. [DOI] [PubMed] [Google Scholar]
- 50.Ross RK, Shimizu H, Paganini-Hill A, et al. Case-control studies of prostate cancer in blacks and whites in southern California. J Natl Cancer Inst 1987; 78:869–874. [PubMed] [Google Scholar]
- 51.Li Q, Nishijo M, Nakagawa H, et al. Relationship between urinary Cd and mortality in habitants of a Cd-polluted area: a 22-year follow-up study in Japan. Chin Med J 2011; 124:3504–3509. [PubMed] [Google Scholar]
- 52.Zeng X, Jin T, Jiang X, et al. Effects on the prostate of environmental Cd exposure—a cross-sectional population study in China. Biometals 2004; 17:559–565. [DOI] [PubMed] [Google Scholar]
- 53.Gray MA, Centeno JA, Slaney DP, et al. Environmental exposure to trace elements and prostate cancer in three New Zealand ethnic groups. Int J Environ Res Public Health 2005; 2:374–384. [DOI] [PubMed] [Google Scholar]
- 54.Yaman M, Atici D, Bakirdere S, et al. Comparison of trace metal concentrations in malign and benign human prostate. J Med Chem 2005; 48:630–634. [DOI] [PubMed] [Google Scholar]
- 55.Sarafanov AG, Todorov TI, Centeno JA, et al. Prostate cancer outcome and tissue levels of metal ions. Prostate 2011; 71:1231–1238. [DOI] [PubMed] [Google Scholar]
- 56.Sahmoun AE, Case LD, Jackson SA, et al. Cadmium and prostate cancer: a critical epidemiologic analysis. Cancer Invest 2005; 23:256–263. [DOI] [PubMed] [Google Scholar]
- 57.Van Wijngaarden E, Singer EA, Palapattu GS. Prostate-specific antigen levels in relation to Cd exposure and zinc intake: results from the 2002 National Health and Nutrition Examination Survey. Prostate 2008; 68:122–128. [DOI] [PubMed] [Google Scholar]
- 58.Huff J, Lunn RM, Waalkes MP, et al. Cd-induced cancers in animals and in humans. Int J Occup Environ Health 2007; 13:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartwig A, Schwerdtle T. Interactions by carcinogenic metal compounds with DNA repair processes: toxicological implications. Toxicol Lett 2002; 127:47–54. [DOI] [PubMed] [Google Scholar]
- 60.Strumylaite L, Kregzdyte R, Bogusevicius A, et al. Association between cadmium and breast cancer risk according to estrogen receptor and human epidermal growth factor receptor 2: epidemiological evidence. Breast Cancer Res Treat 2014; 145:225–232. [DOI] [PubMed] [Google Scholar]
- 61.Itoh H, Iwasaki M, Sawada N, et al. Dietary Cd intake and breast cancer risk in Japanese women: a case-control study. Int J Hyg Environment Health 2014; 217:70–77. [DOI] [PubMed] [Google Scholar]
- 62.Nagata C, Nagao Y, Nakamura K, et al. Cd exposure and the risk of breast cancer in Japanese women. Breast Cancer Res Treat 2013; 138:235–239. [DOI] [PubMed] [Google Scholar]
- 63.Luckett BG, Su LJ, Rood JC, et al. Cadmium exposure and pancreatic cancer in south Louisiana. J Environ Public Health 2012; 2012:180186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kriegel AM, Soliman AS, Zhang Q, et al. Serum cadmium levels in pancreatic cancer patients from the East Nile Delta region of Egypt. Environ Health Perspect 2006; 114:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park RM, Stayner LT, Petersen MR, et al. Cadmium and lung cancer mortality accounting for simultaneous arsenic exposure. Occup Environ Med 2012; 69:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demir N, Turksoy VA, Kayaalti Z, et al. The evaluation of arsenic and Cd levels in biological samples of cases with lung cancer. Tuberk Toraks 2014; 62:191–198. [PubMed] [Google Scholar]
- 67.Verougstraete V, Lison D, Hotz P. Cadmium, lung and prostate cancer: a systematic review of recent epidemiological data. J Toxicol Environ Health B Crit Rev 2003; 6:227–255. [DOI] [PubMed] [Google Scholar]
- 68.Brys M, Nawrocka AD, Miekos E, et al. Zinc and Cd analysis in human prostate neoplasms. Biol Trace Elem Res 1997; 59:145–152. [DOI] [PubMed] [Google Scholar]
- 69.Lindegaard PM, Hansen SO, Christensen JE, et al. The distribution of cadmium within the human prostate. Biol Trace Elem Res 1990; 25:97–104. [DOI] [PubMed] [Google Scholar]
- 70.Waalkes MP. Cadmium carcinogenesis. Mutat Res 2003; 533:107–120. [DOI] [PubMed] [Google Scholar]
- 71.Rani A, Kumar A, Lal A, et al. Cellular mechanisms of cadmium-induced toxicity: a review. Int J Environ Health Res 2014; 24:378–399. [DOI] [PubMed] [Google Scholar]
- 72.Lutzen A, Liberti SE, Rasmussen LJ. Cadmium inhibits human DNA mismatch repair in vivo. Biochem Biophys Res Commun 2004; 321:21–25. [DOI] [PubMed] [Google Scholar]
- 73.Pierron F, Baillon L, Sow M, et al. Effect of low-dose cadmium exposure on DNA methylation in the endangered European eel. Environ Sci Technol 2014; 48:797–803. [DOI] [PubMed] [Google Scholar]
- 74.Asara Y, Marchal JA, Carrasco E, et al. Cadmium modifies the cell cycle and apoptotic profiles of human breast cancer cells treated with 5-fluorouracil. Int J Mol Sci 2013; 14:16600–16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hartwig A. Mechanisms in Cd-induced carcinogenicity: recent insights. Biometals 2010; 23:951–960. [DOI] [PubMed] [Google Scholar]
- 76.Johnson MD, Kenney N, Stoica A, et al. Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland. Nat Med 2003; 9:1081–1084. [DOI] [PubMed] [Google Scholar]
- 77.Stoica A, Katzenellenbogen BS, Martin MB. Activation of estrogen receptor-alpha by the heavy metal cadmium. Mol Endocrinol 2000; 14:545–553. [DOI] [PubMed] [Google Scholar]
- 78.Akesson A, Julin B, Wolk A. Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: a population-based prospective cohort study. Cancer Res 2008; 68:6435–6441. [DOI] [PubMed] [Google Scholar]
- 79.Peng L, Huang Y, Zhang J, et al. Cadmium exposure and the risk of breast cancer in Chaoshan population of southeast China. Environ Sci Pollut Res Int 2015; 22:19870–19878. [DOI] [PubMed] [Google Scholar]


