Abstract
Cervical cancer (CC) is the second most frequent neoplasia among women worldwide. Cancer prevention programs around the world have used the Papanicolaou (Pap) smear as the primary diagnostic test to reduce the burden of CC. Nevertheless, such programs have not been effective in developing countries, thus leading to research on alternative tests for CC screening. During the virus life cycle and in the process toward malignancy, different human papillomavirus (HPV) proteins are expressed, and they induce a host humoral immune response that can be used as a potential marker for different stages of the disease. We present a new Slot blot assay to detect serum antibodies against HPV16 E4, E7, and VLPs-L1 antigens. The system was validated with sera from a female population (n = 485) aged 18 to 64 years referred to the dysplasia clinic at the General Hospital in Cuautla, Morelos, Mexico. To evaluate the clinical performance of the serological markers, the sensitivity, specificity, positive, and negative predictive values and receiver-operating characteristic curves (for antibodies alone or in combination) were calculated in groups of lesions of increasing severity. The results showed high prevalence of anti-E4 (73%) and anti-E7 (80%) antibodies in the CC group. Seropositivity to 1, 2, or 3 antigens showed associations of increasing magnitude with CC (odds ratio [OR] = 12.6, 19.9, and 58.5, respectively). The highest association with CC was observed when the analysis was restricted to only anti-E4+E7 antibodies (OR = 187.7). The best clinical performance to discriminate CC from cervical intraepithelial neoplasia 2 to 3 was the one for the combination of anti-E4 and/or anti-E7 antibodies, which displayed high sensitivity (93.3%) and moderate specificity (64.1%), followed by anti-E4 and anti-E7 antibodies (73.3% and 80%; 89.6% and 66%, respectively). In addition, the sensitivity of anti-E4 and/or anti-E7 antibodies is high at any time of sexual activity (TSA), which suggests they can be biomarkers for the early detection of CC. The sensitivity of anti-E4 antibodies was low (<10%) when the TSA was <10 years, and it increased up to 100% in relation to the TSA, suggesting that anti-E4 antibodies can be useful as HPV exposure markers at early stages of the disease.
INTRODUCTION
Human papillomavirus (HPV) infections are a necessary cause but not sufficient for cervical cancer (CC) to develop.1,2 Women with persistent infections with high risk (HR) HPVs, especially HPV16, are at increased risk of developing precancerous lesions that may ultimately progress to CC.3–7
CC is the second most frequent neoplasia among women worldwide. In developed countries, there is a marked decrease in CC incidence and mortality rates due to efficient Papanicolaou (Pap)-based screening programs. However, in developing countries these programs are not that effective, thus leading to the search for alternative tests for CC screening.8 For instance, DNA testing for HR-HPV provides a more reliable identification of women with cervical precancerous lesions and cancer than Pap testing.9,10 The limitations of DNA testing for HR-HPV include its complexity, its cost, and its inability to detect a productive or persistent infection that could progress to CC.
During the virus life cycle and in the process toward malignancy, different HPV proteins are expressed, and they can induce a host humoral immune response.11 Some women with HPV infections will become seropositive, as an antibody response can take at least 18 months to develop.12,13 The L1 major capsid protein is considered a marker of cumulative exposure to HPV, and it has been the study target for the development of vaccines against the virus. Early proteins E6 and E7 interact with proteins involved in the cell cycle control system and the DNA repair process (p53 and pRb, respectively); therefore, they have been implicated in the induction and maintenance of malignant cell transformation. The anti-E6 and anti-E7 antibodies have been identified as markers of CC.11,14–16 The E4 protein binds to the cytoskeleton to promote the release of viral particles and assembles into stable amyloid-like fibers to finally accumulate in the lesion at different extents, depending on lesion grade.17 In consequence, it has been suggested that the detection of E4 protein and/or antibodies against it in cervical biopsy tissues or serum can be markers for cervical lesions.18–20
We present a new immunoenzymatic assay (Slot blot) to detect serum antibodies against HPV16 capsid protein L1 and early proteins E4 and E7. The system was validated in women with precancerous lesions and CC, and it pointed to the benefit of using antibodies against E4 and E7 to detect CC at early stages of the disease.
MATERIALS AND METHODS
Study Population
Between 2007 and 2009, 1000 women that were referred to the dysplasia clinic “Dr. Mauro Belauzaran Tapia” General Hospital in Cuautla, Morelos, Mexico, were invited to participate in our prospective serological study. Women who agreed to participate in the study (n = 516) provided written informed consent, answered a risk factor questionnaire, and donated a blood sample to detect antibodies against HPV antigens (E4, E7, and L1 VLPs [virus-like particles]). Women underwent clinical examination, and the presence of uterine cervical lesions was confirmed by histopathology, except for 31 women whose biopsy sample was insufficient or inadequate (Figure 1). Women whose cervical lesion was confirmed by histopathology underwent treatment at the clinic according to the medical doctor.
FIGURE 1.
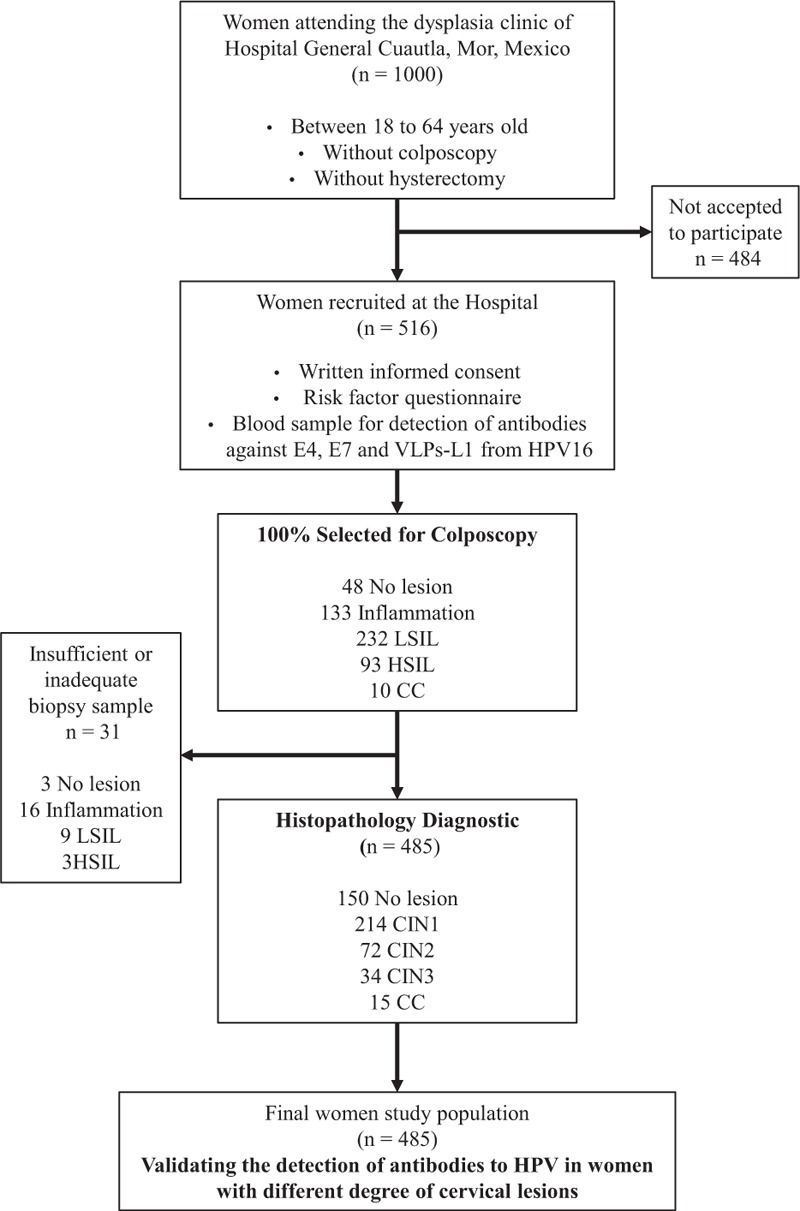
Flow diagram of the selection of the female study population. CC = cervical cancer, CIN = cervical intraepithelial neoplasia grade 1, 2, or 3, HSIL = high-grade squamous intraepithelial lesion, LSIL = low-grade squamous intraepithelial lesion, VLPs-L1 = virus-like particles from L1 protein.
The Ethical Research Committee at the National Institute of Public Health in Mexico revised and approved the study.
Production of HPV16 Antigens for the Slot Blot Assay
HPV16 antigens (E4 and E7) were produced in the in vitro Rapid Translation System (RTS, Roche appliced Science, Germany) at high concentrations and low contaminating proteins from bacterial extract. Protein production of E4 and E7 was performed at 22°C for 16 hours with continuous stirring, and it was purified on a Ni-NTA resin (Qiagen, Germany). The VLPs-L1 from HPV16 were produced in High Five cells infected with recombinant baculoviruses harboring the corresponding L1 gene. After production, the VLPs-L1 was purified using a cesium chloride gradient as described elsewhere.21 Viral antigens (E4, E7, and VLPs-L1) were analyzed by immune Western blot to verify the presence and identity of the proteins. In the case of E4 and E7 proteins, an anti-His monoclonal antibody generated in our laboratory was used to identify the 6-His present in the recombinant proteins produced in vitro. The HPV16 L1 protein was tested with an anti-HPV1 mouse monoclonal antibody (DAKO, Denmark) that recognizes a linear L1 epitope from several types of HPV including HPV16. The antigen-antibody reaction was detected using the Western Lightning Chemiluminescence Reagent Plus (Perkin Elmer), and it was analyzed with the Odyssey Fc system (LI-COR GmbH, Germany). Protein concentrations from the different viral antigens were calculated from the blots by using a standard protein curve of previously purified E7 protein, and they were analyzed using the Image Studio Lite 4.0.21 software.
The green fluorescent protein (GFP) was prepared under the same in vitro conditions as the HPV antigens to discard the background of the Slot blot system for each one of the sera tested.
Slot Blot for Antibody Detection
Ten nanograms of E4, 10 ng of E7, 200 ng of VLPs-L1, or 200 ng of GFP proteins were placed per well onto a PROTRAN nitrocellulose membrane using the Hybri-Slot Manifold (Biometra, Germany), previously equilibrated with phosphae buffer solution (PBS). Membranes were blocked with 10% nonfat milk in PBS-0.05% Tween 20, and strips containing E4, E7, VLPs-L1, and GFP proteins were cut and tested with the women's sera. The sera were diluted in blocking solution at 1:2,500 and incubated at 4oC for 16 hours with continuous shaking. The biotinylated secondary antibody (goat anti-human immunoglobulins; dilution 1:10,000) (Jackson Immunoresearch Laboratories, USA) was diluted in blocking solution and incubated at 4oC for 1.5 hours. To develop the system, Streptavidin-horseradish peroxidase (DAKO, Germany) diluted in PBS-0.05% Tween 20 was incubated at 4oC for 1.5 hours, and it was visualized by chemiluminescence in the Odyssey Fc system. The images obtained from the slot blot assay were analyzed with the Image Studio Lite 4.0.21 software (Figure 2).
FIGURE 2.
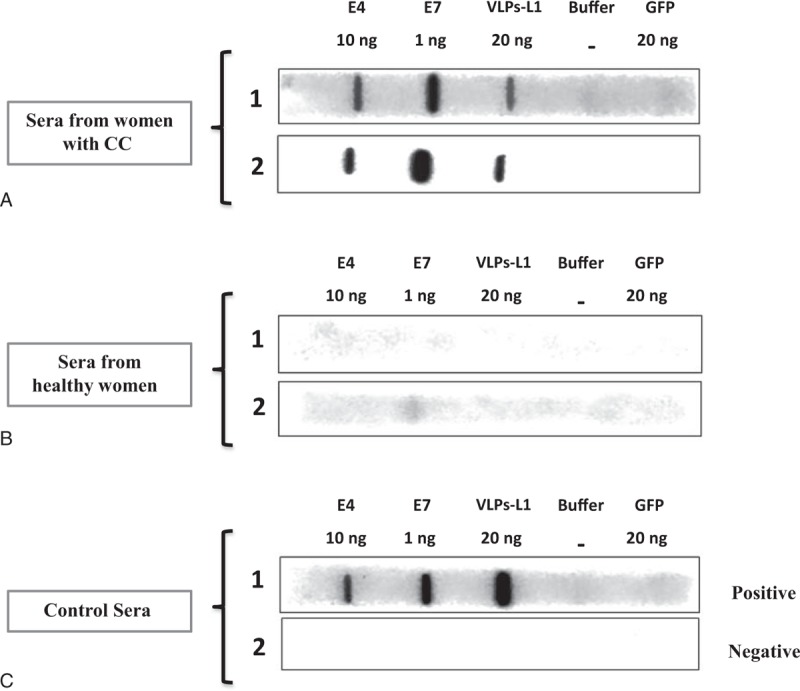
Seropositivity to E4, E7, and VLPs-L1 in serum of healthy women and women with CC by Slot blot system. In vitro purified HPV antigens were immobilized in PROTRAN membranes using the Hybri-Slot system, and membranes were fixed and blocked as described in “Materials and Methods.” Strips containing the 3 HPV16 antigens (E4 10 ng, E7 10 ng, VLPs-L1 200 ng) and negative controls (buffer alone and GFP 200 ng) were incubated with dilutions (1:2,500) of sera from women with CC (A), sera from healthy women (B), and positive and negative female serum controls for anti-HPV antibodies (C). Biotinylated secondary antibody (dil 1:10,000) and Streptavidin-horseradish peroxidase (1:8,000) were used to develop the system, and the bands were finally visualized by chemiluminescence in the Odyssey Fc system. CC = cervical cancer, GFP = green fluorescent protein, HPV = human papillomavirus, VLPs-L1 = virus-like particles from L1 protein.
Internal controls were used for each membrane strip: slot without antigen to control for reagents background, and GFP protein, an irrelevant antigen, to control for contaminants from the antigen production and purification processes. To control for sera background and for system reproducibility in the case of the 3 antigens, a negative and a positive female serum were introduced in the slot blot system for every 20 sera tested (Figure 2). Although the difference between positive and negative signals was visible, we used the Image Studio software to measure the optical density, and the cutoff points for each one of the antibodies tested were established.
The cutoff points for anti-E4, anti-E7, and anti-VLPs-L1 for total immunoglobulins expressed as arbitrary units (AU/mL) were calculated from a standard curve for each antibody using the 4-parameter equation. The standard curves for each antibody were derived from a pool of 10 serum samples from women with CC, HPV16 DNA-positive women, and anti-HPV antibody-positive women by means of Enzyme Link Immunosorbent Assay (ELISA). Serial dilutions of the pooled sera were carried out in the Slot blot system, and the standard curves were constructed by measuring the number of pixels per area of the specific antigen bands visualized in the Slot blot strips as described above. The cutoff values were estimated by using a control seruam bank (n = 81) from naive female adolescents (aged between 9 and 13 years), and they were defined as the geometric mean + 2SD and established as 7 AU/mL for anti-E4, 2 AU/mL for anti-E7, and 4 AU/mL for anti-VLPs-L1.
Statistical Analysis
The research associate and the PhD student carrying out the Slot blot were trained for 6 months to standardize, prepare, develop, and read the Slot blot system. The personnel validated the system with a control non–HPV-exposed young female population and a female population with CC using the histopathological diagnostic as the gold standard to discriminate true cases. The personnel were blinded to the clinical information of all the patients recruited in this study.
For the analysis, we compared the distributions of sociodemographic and sexual behavior characteristics by pathological diagnosis group. To identify the behavior of anti-HPV antibodies during the development of CC, the prevalence of each one of the serum antibodies against HPV antigens was compared among the different cervical lesions and the time of sexual activity (TSA). Logistic regression models were used to estimate the multivariate odds ratios (ORs) and the 95% confidence intervals (CIs) for the association of serological antibody profiles against HPV16 antigens with precancerous lesions and CC, adjusted by TSA. Profiles were determined in the sera from women that presented only anti-E4, anti-E7, or anti-VLPs-L1 antibodies alone or in combination (anti-E4+E7, antiE4+VLPs-L1, anti-E7+VLPs-L1, and anti-E4+E7+VLPs-L1). The anti-E4+VLPs-L1 profile was not present in the sera of the female population studied. There were no seropositives in the case of only anti-E4, only anti-L1, anti VLPS-E7+VLPs-L1, and anti-E4+E7+VLPs-L1 profiles in the reference group (group of women seronegative to all antigens), and the OR could not be calculated for these categories. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV), and areas under the receiver-operating characteristics curve (AUC) for the 3 serological markers alone and for the combined anti-E4 and/or anti-E7 marker were calculated in the groups of lesions with increasing severity (cervical intraepithelial neoplasia [CIN 1] vs control group; CIN 2–3 vs CIN 1; CC vs CIN 2–3). Finally, we evaluated the clinical performance of the serological markers by comparing their prevalence across the categories of TSA. All the tests were 2-sided and the level of significance was 5%. The statistical analysis was carried out using Stata 12 statistical software (Stata Corp, College Station, TX).
RESULTS
Population Characteristics
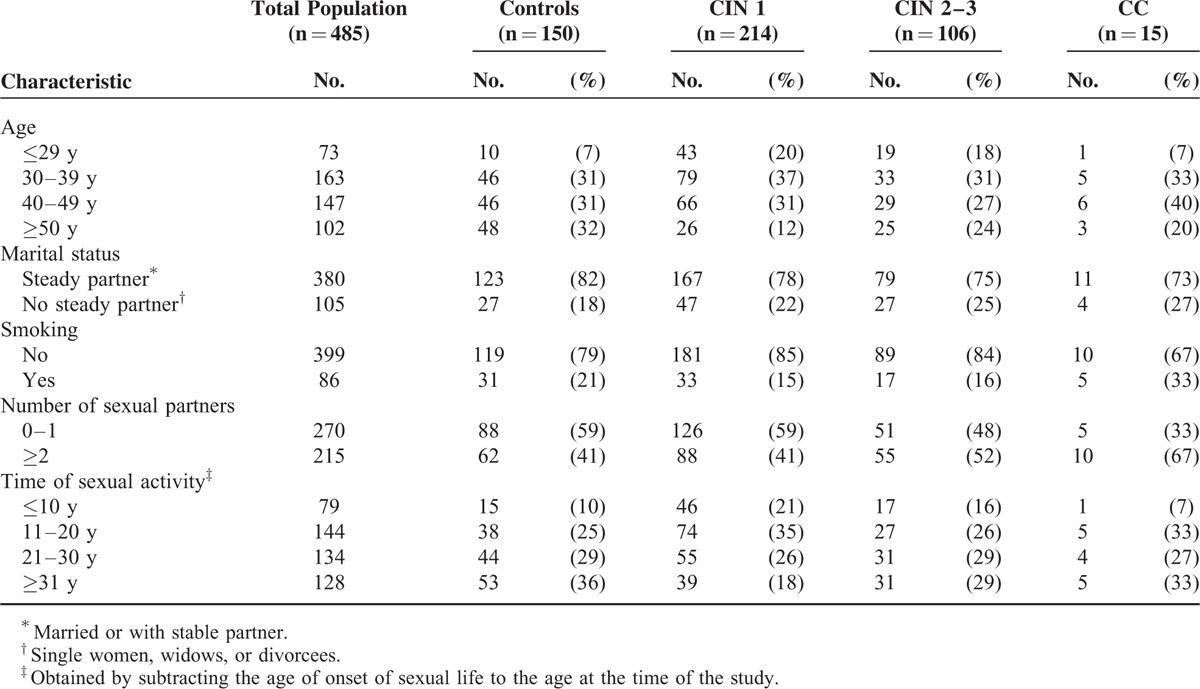
The mean age of the final study population (n = 485) was 40 years (range 18–64 years). Out of the total population, 214 women were diagnosed with CIN 1, 72 with CIN 2, 34 with CIN 3, and 15 women with CC. A group of 150 women finally diagnosed without cervical lesions accepted to participate as the control group (mean age 43.6 years) (Table 1). The study population's overall sociodemographic and sexual behavior characteristics did not show statistically significant differences by pathological diagnosis groups. The highest prevalence of any cervical lesion was in the 30 to 39 and 40 to 49 age groups (33% and 40%, respectively) (Table 1). Most women were married or had a steady partner (>75%) and had never smoked (82%). A high prevalence of sexual behavior risk factors was present only in the CIN 2 to CIN 3 and CC groups where >50% of the women reported >2 sexual partners and >58% reported >20 years of initiated sexual activity. A parallel analysis of the sociodemographic and sexual behavior characteristics of the 31 women that were not included in the study, due to an insufficient or inadequate biopsy, did not show a statistical difference with the female population studied.
TABLE 1.
Sociodemographic and Sexual Behavior Characteristics of the Female Population Studied
Prevalence of Sera Antibodies Against HPV16 Proteins From Women With Different Cervical Lesions
In an attempt to identify the behavior of the anti-HPV antibodies during the development of CC, we compared the prevalence of each one of the serum antibodies against HPV antigens among the different cervical lesion groups (Figure 3A). The prevalence of anti-E4 and anti-VLPs-L1 was low (17% and 9%, respectively) in the control and in all the CIN groups. However, a substantial increase of the prevalence of anti-E4 antibodies (73%) was observed in the CC group (Figure 3A). Anti-E7 antibodies were highly prevalent even in normal women (39%) as well as in CIN 2 to CIN 3 patients (34%), whereas women with CC showed the highest prevalence of anti-E7 antibodies (80%) (Figure 3A).
FIGURE 3.
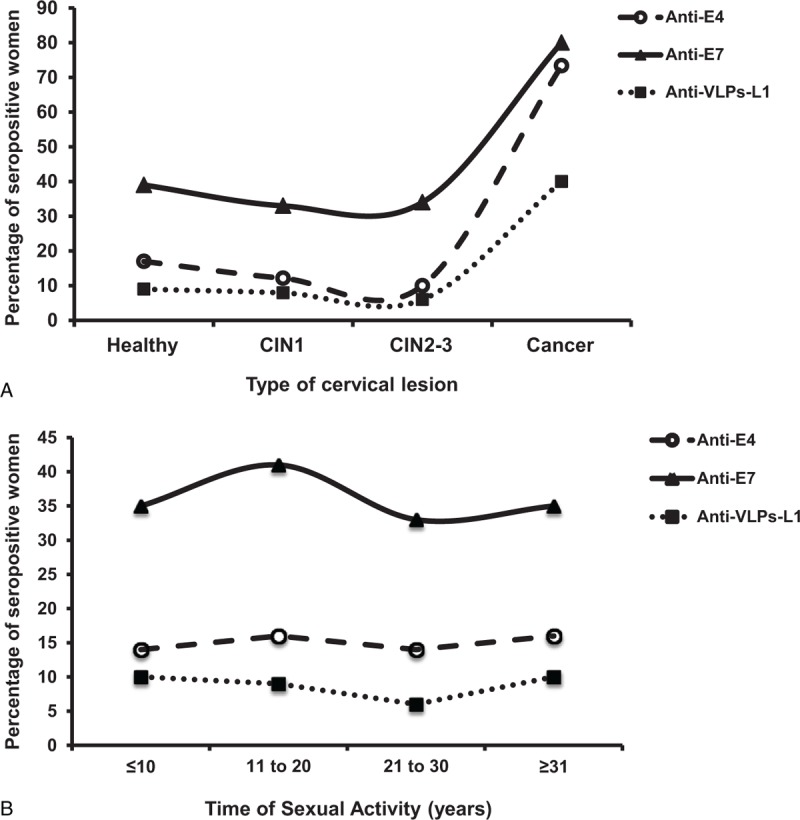
Prevalence distribution of HPV16 anti-E4, anti-E7 and anti-VLPs-L1 antibodies detected in the sera of healthy women and of patients with different cervical lesions. The Slot blot system was used with sera from 150 healthy women and 335 women with different-degree of cervical lesions and CC to detect the presence of anti-E4 (O), anti-E7 (Δ) and anti-VLPs-L1 (■) antibodies. The frequency of antibodies against each one of the antigens was plotted against the degree of the cervical lesion (A) or against the time of sexual activity (B). CC = cervical cancer, HPV = human papillomavirus, VLPs-L1 = virus-like particles from L1 protein.
We compared the prevalence of anti-HPV antibodies among the TSA groups, as a measure of exposure to HPV and as a risk factor to develop CC (Figure 3B). The prevalence of anti-E7 antibodies was especially high (41%) among those in the group with 11 to 20 years of TSA, and it remained around 30% for the rest of the TSA groups. In the case of anti-E4 and anti-VLPs-L1 antibodies, the prevalence was around 15% and 9%, respectively, in the different TSA groups (Figure 3B).
Association of Antibodies Against HPVl6 E4, E7, and VLPs-L1 in Patients With Premalignant and Cancer of Lesions of the Uterine Cervix
The presence of serum antibodies against HPV16 E4, E7, and VLPs-L1 was correlated with the lesion degree as shown in Table 2. Initially, antibody profiles were analyzed as seropositive for only 1 antigen or for the combination of 2 or 3 serological markers, and the reference group included all those women that were negative to all antigens (n = 296). A statistically significant association was observed between seropositivity to any antigen and CC (OR = 21.7; 95% CI 2.8–170.2), whereas seropositivity to 1, 2, or 3 antigens showed increasing statistically significant associations with CC (OR = 12.6, 19.9, and 58.5, respectively) (Table 2). On the contrary, when serological markers were analyzed as antibody profiles (specific antibody combinations), the strongest association was shown between seropositivity to anti-E4+E7 antibodies and CC (OR = 187.7; 95% CI 8.3–4232.9). A marginal association of seropositivity with only anti-E7 antibodies and CIN 2 to CIN 3 lesions (OR = 1.92, 95% CI 0.9–4.0) was also observed (Table 2). No statistically significant associations were found between the rest of the specific antibody profiles and the CIN 1 and CIN 2 to CIN 3 groups (Table 2).
TABLE 2.
Association of Antibodies Against E4, E7, and VLPs-L1 of HPVl6 in Patients With Premalignant and Cancer Lesions From Uterine Cervix
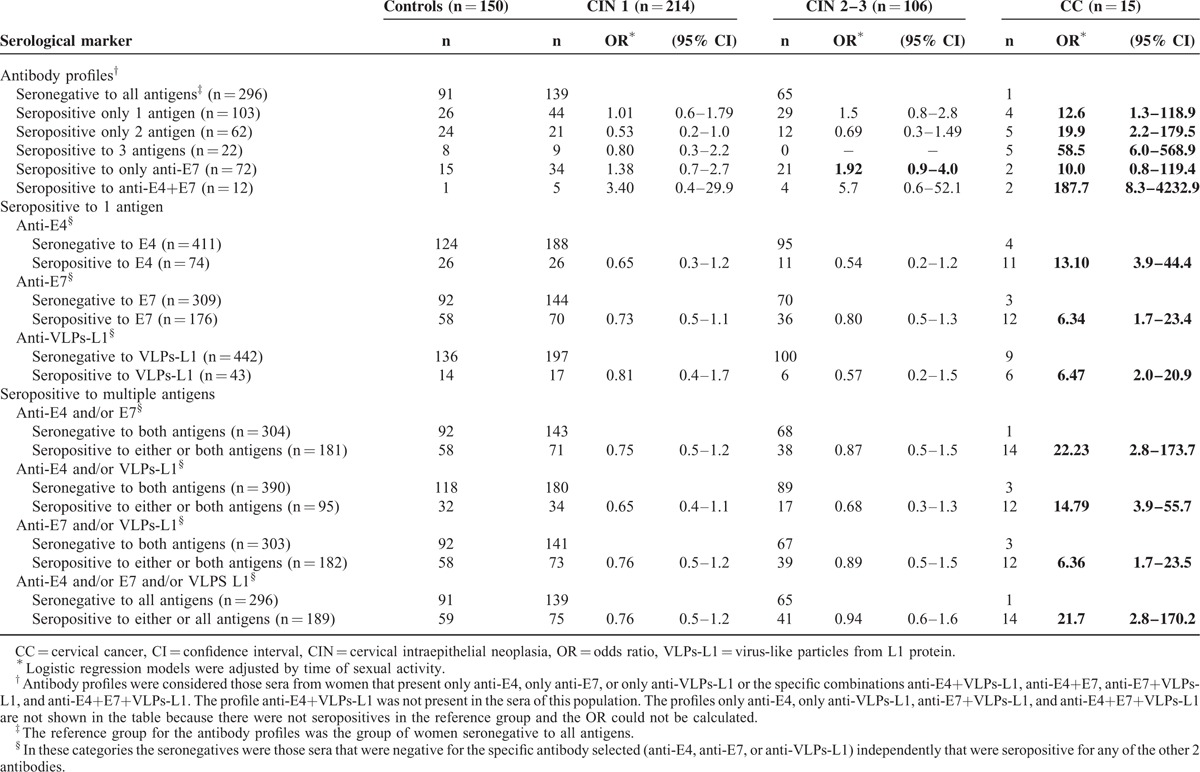
In addition, when serological markers were analyzed as seropositive for 1 antigen (seropositive for one specific marker alone and/or in combination with others) and the reference group included women who were exclusively seronegative for that specific marker category, the ORs for the association between antibodies and CC ranged between 6.34 (anti-E7 and anti-VLPs-L1) and 13.10 (anti-E4) (Table 2). When the analysis was carried out for seropositivity to multiple antigens (combination of 2 or 3 antibodies together or separately) and the reference groups included women who were seronegative to 2 or 3 antigens, the results showed that anti-E7 and/or anti-VLPs-L1 seropositivity was associated to CC (OR = 6.36). However, a higher association with CC was observed in the case of anti-E4 and/or anti-E7 seropositivity (OR = 22.23) and anti-E4 and/or anti-E7 and/or anti-VLPs-L1 seropositivity (OR = 21.7). All of these associations were statistically significant (Table 2).
Clinical Performance of Serological Markers by Degree of Cervical Lesion
The clinical performance (sensitivity, specificity, PPV, NPV, and AUC) was determined for the 3 serological markers separately and for the combined serological marker anti-E4 and/or anti-E7 in the different groups of lesions of increasing severity (CIN 1 vs control group; CIN 2–3 vs CIN 1; CC vs CIN 2–3) as shown in Table 3. Anti-E4 and anti-E7 antibodies, as separate markers, were the most sensitive to detect CC (73.3% and 80%, respectively) and to differentiate it from CIN 2 to CIN 3. However, the highest sensitivity to differentiate CIN 2 to CIN 3 from CC was shown by the combined anti-E4 and/or anti-E7 marker (93.3%). The sensitivity displayed by the separated and combined serological markers in the remaining comparison groups was low (<40%) (Table 3).
TABLE 3.
Comparison of HPV16 Serological Markers as Diagnostic Test for Cervical Cancer
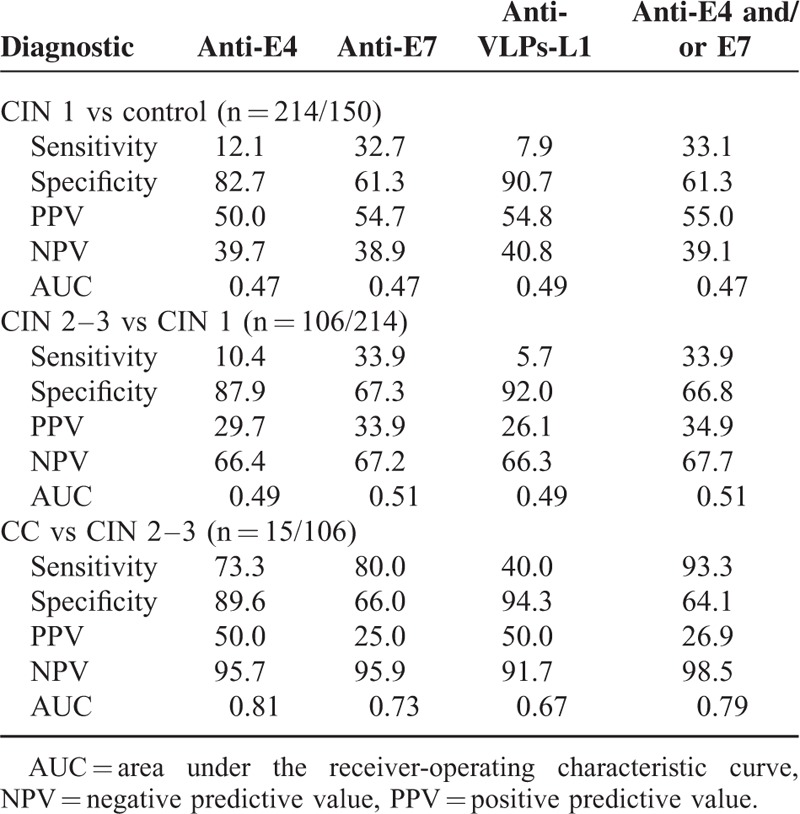
The anti-E7 marker was the less specific (61.3%–67.3%) in all the comparison groups, followed by the anti-E4 (82.7%–89.6%). The serological marker with the highest specificity was the anti-VLPs-L1, according to the lesion degree (90.7%–94.3%) (Table 3). The anti-VLPs-L1 marker showed the highest PPV (54.8%) when comparing the CIN 1 group and the controls. The combined anti-E4 and/or anti-E7 marker displayed the highest NPV (98.5%) when comparing CIN 2 to CIN 3 and CC.
In terms of the comparison between CIN 2 to CIN 3 and CC, the AUCs ranged from 0.67 (anti-VLPs-L1) to 0.81 (anti-E4). The remaining comparison AUCs of each serological markers were close to 0.50.
Finally, we evaluated the clinical performance of the serological markers by comparing their prevalence across the TSA categories (Figure 4). When comparing the early stages of the disease (CIN 2–3 vs CIN 1), the higher sensitivity was observed for serological markers anti-E7 and anti-E4 and/or anti-E7 (approximately 40%) with 2 slight peaks at ages ≤10 and 21 to 30 years (Figure 4A) and a maximum PPV of 47% for women with a TSA ≥21 years (Figure 4B). On the contrary, the sensitivity to discriminate CC from CIN 2 to CIN 3 had an increasing trend for serological markers anti-VLPs-L1 (0%–60%) and anti-E4 (0%–100%) with increasing years of TSA. The sensitivity shown by serological markers anti-E7 and anti-E4 and/or E7 was between 80% and 100% across the different TSA categories, although a low sensitivity was observed in the 21 to 30 years TSA group (50%) (Figure 4C). The best PPVs for CC vs CIN 2 to CIN 3 were observed for markers anti-E4 and anti-VLPs-L1 (83% and 60%, respectively) at TSA ≥31 years. On the contrary, serological markers anti-E7 and anti-E4 and/or anti-E7 displayed fluctuating PPVs with a maximum of 35% for both TSA categories 11 to 20 and ≥31 years (Figure 4D). Therefore, serological markers anti-E4 and/or anti-E7 displayed the best performance to differentiate CC from CIN 2 to CIN 3, with a high sensitivity and a PPV of around 35%, from early HPV exposure (TSA ≤10 years).
FIGURE 4.
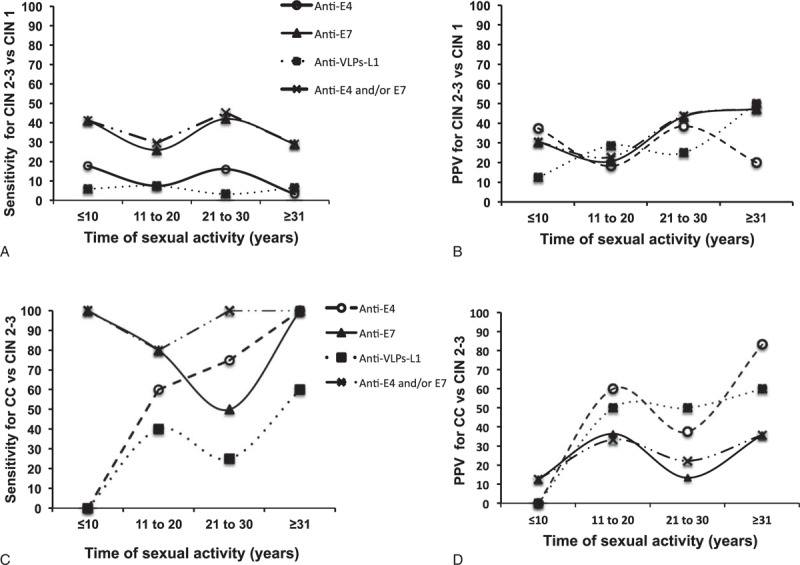
Clinical performance of anti-E4, anti-E7, and anti-VLPs-L1 antibodies in relation to the time of sexual activity. The sera from 150 healthy women and 335 women with different-degree of cervical lesions and CC were tested by Slot blot system for the presence of anti-E4 (O), anti-E7 (▲), anti-VLPs-L1 (■) antibodies and the combined anti-E4 and/or anti-E7 marker (X). The Slot blot sensitivity and positive predictive values were calculated for each one of the serological markers and plotted against groups of lesions of increasing severity such as CIN 2 to CIN 3 vs CIN 1 (A and B) and CC versus CIN 2 to CIN 3 (C and D). CC = cervical cancer, VLPs-L1 = virus-like particles from L1 protein
DISCUSSION
We have hereby evaluated the clinical performance (sensitivity, specificity, PPV, NPV, and AUC) of serum antibodies against HPV16 E4, E7, and VLPs-L1 (alone or in combination) to detect women with CC by means of a novel immunoenzymatic assay (Slot blot). We found positive associations between virtually all serological markers and CC, and this novel Slot blot showed a high ability to discriminate CC from CIN 2 to CIN 3 through the detection of anti-E4, anti-E7, and anti-E4 and/or anti-E7 serological markers (AUC = 0.81, 0.73, and 0.79, respectively). The anti-E4 and/or anti-E7 antibodies displayed the best clinical performance with high sensitivity (93%) and moderate specificity (64%). In addition, the sensitivity of anti-E4 and/or anti-E7 antibodies was high for all categories of TSA, which suggests that these antibodies can be used as biomarkers for the early detection of CC.
The analysis of the humoral immune response against the HPV infection has been mainly used to study current and past HPV infections and more recently to follow the efficacy of HPV vaccines.22 Some groups have also investigated the presence of antibodies to HPV antigens in patients with different degree of cervical lesions associated to the viral infection, and it has been suggested that these antibodies can be markers of disease progression and of CC diagnosis.18,19,23,24 However, these results are still inconclusive, more detailed, and larger clinical studies needs to be carried out to support the use of anti-HPV antibodies for the diagnosis of CC.
It was previously demonstrated that there is a high prevalence of anti-E7 antibodies among women with CIN 1 to CIN 2 (50%), CIN 3 (70%), and CC (75%), but the main association of these antibodies was observed with respect to CC (OR = 108),18,19 thus establishing the importance of these antibodies for the detection of CC. These results are in agreement with our observations because we found the highest prevalence of anti-E7 antibodies in the CIN 2 to CIN 3 and CC groups (34% and 80%, respectively), as well as an increasing association between these antibodies and these 2 cervical lesion groups (OR = 1.92 and 10.0, respectively). In contrast, there were differences observed in terms of the prevalence of anti-E7 antibodies between the CIN 1 and the control groups because our results showed higher prevalence (33% and 39%, respectively) than those previously reported (CIN 1–3 = 14% and controls = 3%).18 These variations could be ascribed to the different antigens used (in vitro translation vs bacterial expression), as well as to the antigen purification methods (affinity chromatography vs electro-elution). Overall, these results suggest that anti-E7 antibodies are generated early during the development of the cervical lesion, and they remain detectable at all the different stages of the disease with a substantial increase in the case of CC, which correlates with the overexpression of the E7 protein during the late stages of the disease. This is in agreement with the clinical performance of anti-E7 antibodies observed in the Slot blot assay that showed a moderate ability to discriminate CC from CIN 2 to CIN 3 lesions (AUC = 0.73), with high sensitivity (80%) and a low false negative rate (20%), which corroborates the usefulness of anti-E7 antibodies as markers of CC.
E4 HPV protein is highly expressed in low-grade lesions (coinciding with viral replication), and it is almost absent in high-grade lesions; thus, E4 was proposed as a viral replication marker.17,20,25 The detection of anti-E4 antibodies has become an indirect amplified way to detect this important marker. Several epidemiological studies have shown a higher prevalence of anti-E4 antibodies in women with premalignant lesions than among those with CC or in the general population.11,18,26,27 Previously, Pedroza-Saavedra et al18 showed that anti-E4 antibodies were present at low prevalence in healthy women (10%), but they were elevated in women with CIN 1 to CIN 3 lesions (58%–66%) and among CC patients (60%), suggesting an early immune recognition of this protein. On the contrary, other groups have shown a low prevalence of anti-E4 antibodies in healthy women (4%–24%), as well as among CC patients (17%–29%).11,27,28 Our results are partially in agreement with Pedroza-Saavedra et al's findings because we reported a low prevalence of anti-E4 antibodies in the control population (17%) and a high prevalence (73%) among CC patients; on the contrary, the prevalence of these anti-E4 antibodies remained low in the CIN 1 and CIN 2 to CIN 3 groups (12% and 10%, respectively). The different results among the CIN 1 to CIN 3 patients could be due to the highly purified HPV viral antigens used in the Slot blot, which allow the use of reduced amounts of viral antigens, a reduction of background and a better estimate of the cutoff points, all of which could help to detect the real anti-E4 seropositive patients.
It is noteworthy that we identified the sensitivity of anti-E4 antibodies to be low (<10%) when the TSA was <10 years, but it increased up to 100% when the TSA was >31 years. This suggests that anti-E4 antibodies can be useful as HPV exposure markers at early stages of the disease.
The normal immune response against L1 protein generates neutralizing antibodies, with seroconversion taking place between 8 to 9 months after the first HPV DNA detection; antibody titers are low, and between 50% and 70% of women seroconvert.13 This protective anti-L1 response persists for at least 10 years and it has been used as marker of past HPV exposure.22 Recently, the generation of VLPs-L1 has allowed the characterization of the anti-VLPs-L1 antibody response in different female populations. It was found that anti-VLPs-L1 antibodies increase with the severity of the cervical lesion, and that they are probably not neutralizing, as cervical lesions are able to progress.29–34 In another study, anti-VLPs-L1 antibodies were associated to CIN 3 and CC.35 Our results are consistent with these data because the prevalence of anti-VLPs-L1 antibodies in our population was low (6%–9%) at early stages of the disease, and it increased importantly at the stage of CC (40%), during which a statistically significant association was also observed with CC (OR = 6.47) but not with high-grade lesions as has been reported before.
The clinical performance of this anti-VLPs-L1 marker was low: the sensitivity was only 40%, but it displayed high specificity (94%). A likely explanation is that only HPV16 antigens were used in the Slot blot system and cross-reactivity with other HPV types is very low in the case of VLPs-L1. However, both sensitivity and specificity can be increased if several L1 antigens from different HPV types are introduced in the system, as was demonstrated in the Luminex Multiplex system.36
During our analysis, it became clear that seropositivity to E4 and/or E7 has an additive effect to discriminate women with CC from those with CIN 2 to CIN 3 (AUC = 0.79), with a high sensitivity (93%) and moderate specificity (64%). The sequential expression of viral proteins depends upon the viral cycle, allowing the identification of different infection stages such as replication (associated to E4 protein), transformation (associated to E6/E7 proteins), and past infections (associated to L1 protein). In this context, the immune system generates an antibody response against the different HPV antigens, as they appear in the system, allowing the indirect identification of the different infection stages. Thus, the expression of anti-E4 antibodies has been related to viral replication, whereas anti-E7 antibodies are considered markers of a current HPV-related malignancy.23,37–39 In this context, the use of the combined anti-E4 and/or anti-E7 serological markers in the Slot blot system allowed us to identify CC patients more precisely than when markers were used separately.
For the study of the humoral immune response, the ELISA system has been the most frequently used method to detect antibodies against HPV antigens. The ELISA has shown high specificity but low sensitivity, and this could fluctuate depending on the type of antigen, its purity, the serum dilution, and the antigen concentration.19,23,40–47 On the contrary, the Western blot technology has been used to detect anti-E4 and anti-E7 antibodies, but the sensitivity varies depending on the developing methods such as the colorimetric or radioactive ones. The latter showed a specificity of 78% and a sensitivity of 67%.18,42,48 Despite the good performance of the Western blot system, its implementation in large epidemiological studies is hampered by its technical complexity. Therefore, we decided to develop a Slot blot system to reduce the complexity of the Western blot, without compromising the sensitivity and specificity of the assay. Thus, we used highly purified viral antigens, the biotin-streptavidin-HRP system enhancer and developed by chemiluminescence to maximize the sensitivity of the method. These modifications allow the use of low concentrations of viral antigens and high dilutions of sera and secondary antibodies, making the reduction of the background and a better estimate of the cutoff points possible. The novel Slot blot system that we developed in our laboratory was standardized to detect serum antibodies against HPV16 antigens (E4, E7, and VLPs-L1), and it displayed a high clinical performance for the identification of women at risk of developing CC. The performance of the Slot blot system could be improved by adding the detection of antibodies against HPV18 antigens, as it has been shown that HPV types 16 and 18 account for >70% of the CC cases.
Finally, the improvement of CC screening programs is an important task in low- and middle-income countries as the mortality rates have not decreased in the past 20 years as compared with high-income countries.49,50 The main problem is the inefficient set up of the Pap test in low-income countries due to insufficient laboratory facilities, transportation and tracking of specimens, trained cytopathologists, patients follow-up, with a final increase in costs due to treatment of real patients that were not identified during the screening.50–52 On the contrary, the sensitivity of the Pap test is very variable (50%–84%),39,53,54 and on top of this, there is a high number of false positives that are referred to colposcopy with the concomitant saturation of this health service.55 In this sense, the serological test for anti-E4 and anti-E7 antibodies could potentially be used after a positive Pap test or in co-testing with this, helping to decrease the false positives of the Pap test and discriminate the real CC cases as the NPV of this serological test is very high (98%). However, further prospective studies are necessary to confirm the usefulness of anti-E4 and anti-E7 antibodies as biomarkers for early detection of CC.
In conclusion, our novel Slot blot displayed a good clinical performance to detect CC and to discriminate it from premalignant lesions. More generally, our results point to the benefit of using anti-E4 and anti-E7 antibodies to detect CC at early stages of the disease at the level of the general population.
Acknowledgments
The authors thank the subjects for their patience and time spent participating in this study; thank nurse Cristina García Morales for helping in sample collection and questionnaire application during the recruitment phase of the study; and thank the technical support of the personnel from the Epidemiological Interaction Department at the National Institute of Public Health. The authors also thank Adela Iglesias Morineau for editing the English version.
Footnotes
Abbreviations: AU = arbitrary units, AUC = area under the receiver-operating characteristics curve, CC = cervical cancer, CI = confidence interval, CIN = cervical intraepithelial neoplasia, ELISA = Enzyme Link Immunosorbent Assay, GFP = green fluorescent protein, HPV = human papillomavirus, HR = high risk, NPV = negative predictive value, OR = odds ratio, Pap = Papanicolaou, PBS = phosphae buffer solution, PPV = positive predictive value, TSA = time of sexual activity, VLPs = virus-like particles.
This article was carried out within the PhD program in Infectious Diseases of the National Institute of Public Health. This research was supported by the National Institute of Public Health and funded through CONACYT grants 87075, 168635, and 205707, and PROMEP-PIFI (UAEMOR-CA-26). The CONACYT postgraduate fellowship program (No. 218122) sponsored Salazar-Piña A.
The CONACYT and PROMEP funders did not participate in the study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cogliano V, Baan R, Straif K, et al. Carcinogenicity of human papillomaviruses. Lancet Oncol 2005; 6:204. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine 2006; 24 Suppl 3:S11–S25. [DOI] [PubMed] [Google Scholar]
- 3.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2002; 2:342–350. [DOI] [PubMed] [Google Scholar]
- 4.Munoz N, Bosch FX, de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907. [DOI] [PubMed] [Google Scholar]
- 6.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19. [DOI] [PubMed] [Google Scholar]
- 7.Bosch FX, Lorincz A, Munoz N, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunnell AS, Ylitalo N, Sandin S, et al. A longitudinal Swedish study on screening for squamous cell carcinoma and adenocarcinoma: evidence of effectiveness and overtreatment. Cancer Epidemiol Biomarkers Prev 2007; 16:2641–2648. [DOI] [PubMed] [Google Scholar]
- 9.Cuzick J, Mayrand MH, Ronco G, et al. Chapter 10: new dimensions in cervical cancer screening. Vaccine 2006; 24 Suppl 3:S90–S97. [DOI] [PubMed] [Google Scholar]
- 10.Castle PE, Fetterman B, Poitras N, et al. Variable risk of cervical precancer and cancer after a human papillomavirus-positive test. Obstet Gynecol 2011; 117:650–656. [DOI] [PubMed] [Google Scholar]
- 11.Combes JD, Pawlita M, Waterboer T, et al. Antibodies against high-risk human papillomavirus proteins as markers for invasive cervical cancer. Int J Cancer 2014; 135:2453–2461. [DOI] [PubMed] [Google Scholar]
- 12.Xi LF, Carter JJ, Galloway DA, et al. Acquisition and natural history of human papillomavirus type 16 variant infection among a cohort of female university students. Cancer Epidemiol Biomarkers Prev 2002; 11:343–351. [PubMed] [Google Scholar]
- 13.Carter JJ, Koutsky LA, Hughes JP, et al. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J Infect Dis 2000; 181:1911–1919. [DOI] [PubMed] [Google Scholar]
- 14.Achour M, Zeghal D, Kochbati L, et al. Antibody response for L1, E6, E7 HPV 16, and HPV 18 antigens in Tunisian women with cervical cancer. J Immunoassay Immunochem 2009; 30:82–96. [DOI] [PubMed] [Google Scholar]
- 15.Ravaggi A, Romani C, Pasinetti B, et al. Correlation between serological immune response analyzed by a new ELISA for HPV-16/18 E7 oncoprotein and clinical characteristics of cervical cancer patients. Arch Virol 2006; 151:1899–1916. [DOI] [PubMed] [Google Scholar]
- 16.Luevano M, Bernard HU, Barrera-Saldana HA, et al. High-throughput profiling of the humoral immune responses against thirteen human papillomavirus types by proteome microarrays. Virology 2010; 405:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Middleton K, Peh W, Southern S, et al. Organization of human papillomavirus productive cycle during neoplastic progression provides a basis for selection of diagnostic markers. J Virol 2003; 77:10186–10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pedroza-Saavedra A, Cruz A, Esquivel F, et al. High prevalence of serum antibodies to Ras and type 16 E4 proteins of human papillomavirus in patients with precancerous lesions of the uterine cervix. Arch Virol 2000; 145:603–623. [DOI] [PubMed] [Google Scholar]
- 19.Vazquez-Corzo S, Trejo-Becerril C, Cruz-Valdez A, et al. Association between presence of anti-Ras and anti-VPH16 E4/E7 antibodies and cervical intraepithelial lesions. Salud Publica Mex 2003; 45:335–345. [PubMed] [Google Scholar]
- 20.Griffin H, Wu Z, Marnane R, et al. E4 antibodies facilitate detection and type-assignment of active HPV infection in cervical disease. PLoS One 2012; 7:e49974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller M, Zhou J, Reed TD, et al. Chimeric papillomavirus-like particles. Virology 1997; 234:93–111. [DOI] [PubMed] [Google Scholar]
- 22.Stanley M. HPV—immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meschede W, Zumbach K, Braspenning J, et al. Antibodies against early proteins of human papillomaviruses as diagnostic markers for invasive cervical cancer. J Clin Microbiol 1998; 36:475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silins I, Avall-Lundqvist E, Tadesse A, et al. Evaluation of antibodies to human papillomavirus as prognostic markers in cervical cancer patients. Gynecol Oncol 2002; 85:333–338. [DOI] [PubMed] [Google Scholar]
- 25.Doorbar J. Latent papillomavirus infections and their regulation. Curr Opin Virol 2013; 3:416–421. [DOI] [PubMed] [Google Scholar]
- 26.Suchankova A, Krchnak V, Vagner J, et al. Epitope mapping of the human papillomavirus type 16 E4 protein by means of synthetic peptides. J Gen Virol 1992; 73:429–432. [DOI] [PubMed] [Google Scholar]
- 27.Lang Kuhs KA, Anantharaman D, Waterboer T, et al. Human papillomavirus 16 E6 antibodies in individuals without diagnosed cancer: a pooled analysis. Cancer Epidemiol Biomarkers Prev 2015; 24:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh AK, Smith NK, Stacey SN, et al. Serological response to HPV 16 in cervical dysplasia and neoplasia: correlation of antibodies to E6 with cervical cancer. Int J Cancer 1993; 53:591–596. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y, Eluf-Neto J, Bosch FX, et al. Serum antibodies to human papillomavirus 16 proteins in women from Brazil with invasive cervical carcinoma. Cancer Epidemiol Biomarkers Prev 1999; 8:935–940. [PubMed] [Google Scholar]
- 30.Marais D, Rose RC, Williamson AL. Age distribution of antibodies to human papillomavirus in children, women with cervical intraepithelial neoplasia and blood donors from South Africa. J Med Virol 1997; 51:126–131. [DOI] [PubMed] [Google Scholar]
- 31.Combita AL, Bravo MM, Touze A, et al. Serologic response to human oncogenic papillomavirus types 16, 18, 31, 33, 39, 58 and 59 virus-like particles in colombian women with invasive cervical cancer. Int J Cancer 2002; 97:796–803. [DOI] [PubMed] [Google Scholar]
- 32.Skiba D, Mehlhorn G, Fasching PA, et al. Prognostic significance of serum antibodies to HPV-16 L1 virus-like particles in patients with invasive cervical cancer. Anticancer Res 2006; 26:4921–4926. [DOI] [PubMed] [Google Scholar]
- 33.Urquiza M, Sanchez R, Amaya J, et al. Specificity of L1 peptides versus virus-like particles for detection of human papillomavirus-positive cervical lesions in females attending Engativa Hospital, Bogota, Colombia. J Clin Microbiol 2008; 46:3714–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JS, Park DC, Kim CJ, et al. HPV-16-related proteins as the serologic markers in cervical neoplasia. Gynecol Oncol 1998; 69:47–55. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, Wang LL, Chen LF, et al. Expression of human papillomavirus type 6 L1 and L2 isolated in China and self assembly of virus-like particles by the products. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2003; 35:27–34. [PubMed] [Google Scholar]
- 36.Skjeldestad FE, Mehta V, Sings HL, et al. Seroprevalence and genital DNA prevalence of HPV types 6, 11, 16 and 18 in a cohort of young Norwegian women: study design and cohort characteristics. Acta Obstet Gynecol Scand 2008; 87:81–88. [DOI] [PubMed] [Google Scholar]
- 37.Dillner J. The serological response to papillomaviruses. Semin Cancer Biol 1999; 9:423–430. [DOI] [PubMed] [Google Scholar]
- 38.Lehtinen M, Pawlita M, Zumbach K, et al. Evaluation of antibody response to human papillomavirus early proteins in women in whom cervical cancer developed 1 to 20 years later. Am J Obstet Gynecol 2003; 188:49–55. [DOI] [PubMed] [Google Scholar]
- 39.Gutierrez-Xicotencatl L, Plett-Torres T, Madrid-Gonzalez CL, et al. Molecular diagnosis of human papillomavirus in the development of cervical cancer. Salud Publica Mex 2009; 51 Suppl 3:S479–S488. [DOI] [PubMed] [Google Scholar]
- 40.Muller M, Gausepohl H, de Martynoff G, et al. Identification of seroreactive regions of the human papillomavirus type 16 protein E4, E6, E7 and L1. J Gen Virol 1990; 71:2709–2717. [DOI] [PubMed] [Google Scholar]
- 41.Plett-Torres T, Cruz-Valdez A, Esquivel-Guadarrama F, et al. Frequency of antibodies against E4 and E7 from human papillomavirus type 16 in Mexican soldiers. Arch Virol 2007; 152:97–114. [DOI] [PubMed] [Google Scholar]
- 42.Kanda T, Onda T, Zanma S, et al. Independent association of antibodies against human papillomavirus type 16 E1/E4 and E7 proteins with cervical cancer. Virology 1992; 190:724–732. [DOI] [PubMed] [Google Scholar]
- 43.Onda T, Kanda T, Zanma S, et al. Association of the antibodies against human papillomavirus 16 E4 and E7 proteins with cervical cancer positive for human papillomavirus DNA. Int J Cancer 1993; 54:624–628. [DOI] [PubMed] [Google Scholar]
- 44.Sehr P, Zumbach K, Pawlita M. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. J Immunol Methods 2001; 253:153–162. [DOI] [PubMed] [Google Scholar]
- 45.Di Bonito P, Grasso F, Mochi S, et al. Serum antibody response to Human papillomavirus (HPV) infections detected by a novel ELISA technique based on denatured recombinant HPV16 L1, L2, E4, E6 and E7 proteins. Infect Agent Cancer 2006; 1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krchnak V, Vagner J, Suchankova A, et al. Synthetic peptides derived from E7 region of human papillomavirus type 16 used as antigens in ELISA. J Gen Virol 1990; 71:2719–2724. [DOI] [PubMed] [Google Scholar]
- 47.Bleul C, Muller M, Frank R, et al. Human papillomavirus type 18 E6 and E7 antibodies in human sera: increased anti-E7 prevalence in cervical cancer patients. J Clin Microbiol 1991; 29:1579–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jochmus-Kudielka I, Schneider A, Braun R, et al. Antibodies against the human papillomavirus type 16 early proteins in human sera: correlation of anti-E7 reactivity with cervical cancer. J Natl Cancer Inst 1989; 81:1698–1704. [DOI] [PubMed] [Google Scholar]
- 49.Yeoh GP, Tse MP, Chan KW, et al. Human papillomavirus DNA and liquid-based cervical cytology cotesting in screening and follow-up patient groups. Acta Cytol 2006; 50:627–631. [DOI] [PubMed] [Google Scholar]
- 50.Bobdey S, Balasubramanium G, Kumar A, et al. Cancer screening: should cancer screening be essential component of primary health care in developing countries? Int J Prev Med 2015; 6:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flores Y, Shah K, Lazcano E, et al. Design and methods of the evaluation of an HPV-based cervical cancer screening strategy in Mexico: The Morelos HPV Study. Salud Publica Mex 2002; 44:335–344. [DOI] [PubMed] [Google Scholar]
- 52.Huchko MJ, Sneden J, Leslie HH, et al. A comparison of two visual inspection methods for cervical cancer screening among HIV-infected women in Kenya. Bull World Health Organ 2014; 92:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albert S, Oguntayo O, Samaila M. Comparative study of visual inspection of the cervix using acetic acid (VIA) and Papanicolaou (Pap) smears for cervical cancer screening. Ecancermedicalscience 2012; 6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hegde D, Shetty H, Shetty PK, et al. Diagnostic value of acetic acid comparing with conventional Pap smear in the detection of colposcopic biopsy-proved CIN. J Cancer Res Ther 2011; 7:454–458. [DOI] [PubMed] [Google Scholar]
- 55.Beal CM, Salmeron J, Flores YN, et al. Cost analysis of different cervical cancer screening strategies in Mexico. Salud Publica Mex 2014; 56:429–501. [PubMed] [Google Scholar]


