Abstract
Nasal intermittent positive pressure ventilation (NIPPV) and nasal continuous positive airway pressure (NCPAP) have proven to be effective modes of noninvasive respiratory support in preterm infants. Although they are increasingly used in neonatal intensive care, their hemodynamic consequences have not been fully evaluated. The aim of this study was to investigate the hemodynamic changes between NIPPV and NCPAP in preterm infants.
This prospective observational study enrolled clinically stable preterm infants requiring respiratory support received NCPAP and nonsynchronized NIPPV at 40/minute for 30 minutes each, in random order. Cardiac function and cerebral hemodynamics were assessed by ultrasonography after each study period. The patients continued the study ventilation during measurements.
Twenty infants with a mean gestational age of 27 weeks (range, 25–32 weeks) and birth weight of 974 g were examined at a median postnatal age of 20 days (range, 9–28 days). There were no significant differences between the NCPAP and NIPPV groups in right (302 vs 292 mL/kg/min, respectively) and left ventricular output (310 vs 319 mL/kg/min, respectively), superior vena cava flow (103 vs 111 mL/kg/min, respectively), or anterior cerebral artery flow velocity.
NIPPV did not have a significant effect on the hemodynamics of stable preterm infants. Future studies assessing the effect of NIPPV on circulation should focus on less stable and very preterm infants.
INTRODUCTION
Although mechanical ventilation through an endotracheal tube is a standard life-supporting procedure in most preterm infants, it is associated with an increased risk of various complications, and this has led to the use of noninvasive respiratory support. Nasal intermittent positive pressure ventilation (NIPPV) augments the benefits of nasal continuous positive airway pressure (NCPAP) with the addition of positive-pressure breaths. Although NIPPV is being increasingly used in preterm infants,1 published data on the hemodynamic consequences are lacking. In comparison, the mechanisms by which invasive mechanical positive pressure ventilation affect hemodynamics have been studied in newborns.2,3 It has been suggested that intermittent positive pressure or distended airway pressure can increase intrathoracic pressure and alveolar volume. Alveolar expansion can increase pulmonary vascular resistance, and a high intrathoracic pressure, when transmitted to the heart and major blood vessels, may impede venous return to the heart. This then decreases cardiac contractility and output, and consequently cerebral blood flow. However, the interaction between respiratory support and cardiac output is complex. In particular, any hemodynamic effects are associated with pulmonary compliance, and therefore additional positive airway pressure may also optimize lung volume. Higher lung volume and better oxygenation will improve pulmonary blood flow in lung areas that were previously collapsed or hypoxic. NIPPV provides an increase in positive pressure compared to NCPAP, however whether or not this affects hemodynamic findings has not been adequately studied.
Cardiac output is a determinant of systemic blood flow and can be measured by functional echocardiography.4 The most commonly measured flows in newborn infants are right ventricular output (RVO), left ventricular output (LVO), and superior vena cava (SVC) flow.5,6 Doppler ultrasound applied to the major cerebral arteries, such as the anterior cerebral artery, can provide a valuable estimate of cerebral blood flow. The aim of this prospective observational study was to investigate the hemodynamic changes between NIPPV and NCPAP in preterm infants. It was hypothesized that NIPPV may impede venous return and cardiac output when compared to NCPAP.
MATERIALS AND METHODS
Patient Selection
Clinically stable preterm infants born at less than 34 weeks of gestation and weighing under 1500 g at birth who required NCPAP or NIPPV with less than 30% oxygen were eligible for this study. The infants with major congenital anomalies, requiring sedation, ongoing sepsis, or a significant shunt such as a patent ductus arteriosus (PDA), or atrial septal defect/persistent foramen ovale were excluded. Significant PDA was diagnosed when there was color Doppler echocardiographic evidence of left to right ductal shunt, ductal diameter >1.5 mm/kg, left atrial/aortic root ratio >1.4.7 A significant atrial septal defect/patent foramen ovale was defined as a diameter of >3 mm measured with color flow Doppler echocardiography. The study was conducted at the Hsinchu Mackay Memorial Hospital Neonatal Intensive Care Unit, and it was approved by MacKay Memorial Hospital's Institutional Review Board (IRB number: 10MMHIS049). All infants were recruited and studied after written informed consent had been obtained from their parents.
Study Protocol
The infants were studied in their incubators while they were left undisturbed after standard nursing procedures had been completed. The infants were in the supine position throughout the study. During the study, each infant underwent 2 study periods of 30 minutes each with NCPAP and nonsynchronized NIPPV at 40/min, in random order. A 10-minute period was used for hemodynamics measurements while the infants were asleep or in a quiet awake state after each study periods. Both modes of ventilation were provided by a time cycled, pressure limited neonatal ventilator (Babylog 8000 plus, Dräger Medizintechnik GmbH, Germany). A short bi-nasal prong (Hudson RCI, Temecula, CA) was used as the nasal interface. NCPAP pressure and positive end-expiratory pressure of NIPPV were set at the same level as before the study, and peak inspiratory pressure was set at 15 to 20 cm H2O according to chest excursion. In addition, inspiratory time was set at 0.45 seconds, and inspired oxygen concentration was set to maintain preductal oxygen saturation between 88% and 95%. We ensured mouth closure by using a pacifier with tape to prevent any fluctuation in pressure.
Hemodynamic Measurements
Hemodynamics measurements were assessed immediately after each study period by echocardiography and head ultrasound using a Philips Sonos 4500 ultrasound machine (Philips Medical Systems, Andover, MA) with a 5 to 12 MHz transducer (S12, 21380A, Philips Medical Systems, Andover, MA). The patients continued the study ventilation during measurements. The scans were recorded on a hard disk, and measurements were analyzed offline to minimize patient handling time. The investigator performing the offline analyses was blinded to the treatment mode and not involved in the randomization or the recording of the intervention.
Echocardiographic Data Collection
In each echocardiographic evaluation, the following variables were measured according to previously published methodology: SVC flow, LVO, RVO, and diameter of and shunt across the PDA. All flow measurements were calculated and indexed to the infant's weight using the following formula: flow = [velocity time integral (VTI) × (π × (mean diameter2/4) × heart rate]/body weight.5,6 A mean of the maximum and minimum internal diameters was used for the flow calculation. Diameter measurements were averaged from 3 cardiac cycles. This averaged diameter was used constantly for each flow measurement. Each VTI was calculated from the area under the curve of the spectral trace. To minimize respiratory cycle-related variation, averages of 6 to 10 consecutive cardiac cycles were recorded for analysis of the VTI. SVC flow, LVO, and RVO were expressed in mL/kg/min.
Cranial Ultrasound Data Collection
The anterior cerebral artery was visualized using color Doppler in the middle plane through the anterior fontanelle. The resistance index was calculated as (S-D)/S of the mean of 5 waveforms, where S and D are the maximum and minimum blood flow velocities, respectively, during 1 cardiac cycle.
Clinical data were collected from medical records, and included demographic data, details of the birth, and clinical course. The following physiological variables were obtained from the Agilent monitor (Agilent M1205A, Philips Medical Systems, Andover, MA) throughout each mode of respiratory support: heart rate, respiratory rate, and transcutaneous oxygen saturation (SpO2). Blood pressure (systolic, diastolic, and mean) was measured with an appropriate cuff every 10 minutes. Respiratory support variables of oxygen requirement, peak pressure, positive end-expiratory pressure, rate, and mean airway pressure were obtained from the ventilator (Babylog 8000 plus, Dräger Medizintechnik GmbH).
Statistical Analysis
As there are no preliminary data on the primary outcome measurements of cardiac output (SVC flow, RVO, and LVO) between NIPPV and NCPAP, we estimate a sample size of 20 patients to perform this pilot study. The collected data are presented as means ± SD. Comparisons between paired variables in the NIPPV and NCPAP groups were performed using the Wilcoxon signed-rank test. Each included infant was used as his or her own control. Significance was defined as a 2-sided P value of <0.05. Data were statistically analyzed using SAS software version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
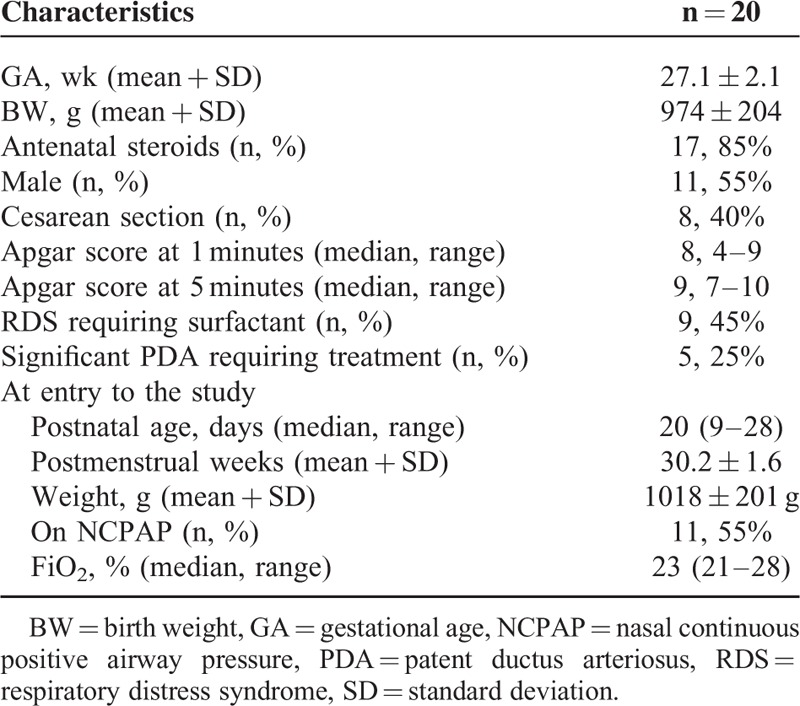
A total of 20 preterm infants (11 males and 9 females) were studied (Table 1). The mean gestational age at birth was 27.1 ± 2.1 weeks, and the mean birth weight was 974 ± 204 g. Antenatal steroids were administered to 85% (n = 17) of the mothers, and all infants were inborn. Nine (45%) infants received ventilation and received early rescue surfactant therapy. None of them had severe intraventricular hemorrhage. Five infants had clinically significant PDA and received treatment (2 intravenous ibuprofen, 3 surgical ligation) before entry to this study. At entry to the study, the median postnatal age was 20 (range 9–28) days, with a mean postmenstrual age of 30.2 ± 1.6 weeks, and birth weight of 1018 ± 201 g. All infants were stable on noninvasive respiratory support due to respiratory distress syndrome. The median FiO2 before the study was 23% (range 21%–28%). Eleven (55%) infants were receiving NCPAP with an NCPAP level of 5 cm H2O. Before the study, of the 9 infants receiving NIPPV, the mean peak inspiratory pressure was 18.2 cm H2O. None of the enrolled infants received inotropic support during the study period, however 5 infants received methylxanthines.
TABLE 1.
Characteristics of the Study Population
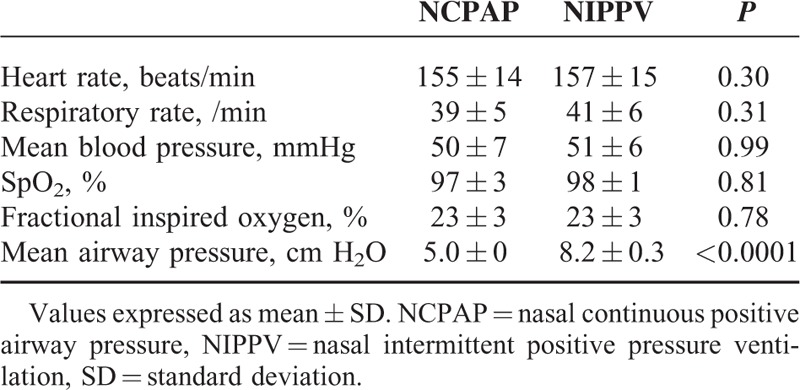
The hemodynamic and respiratory measurements are shown in Tables 2 and 3. A hemodynamically insignificantly small PDA (diameter <1 mm) was found in 3 patients during the study. The mean airway pressure was significantly higher in the NIPPV group compared with the NCPAP group (8.2 cm H2O versus 5 cm H2O, P < 0.0001). There were no significant differences in the hemodynamic parameters including RVO, LVO, SVC flow, anterior cerebral artery flow velocity between the NIPPV and NCPAP groups (Table 2). There were also no significant differences in systolic, diastolic, and mean arterial blood pressure between the 2 groups (Table 3).
TABLE 2.
Changes in Hemodynamic Parameters for NCPAP and NIPPV (N = 20)
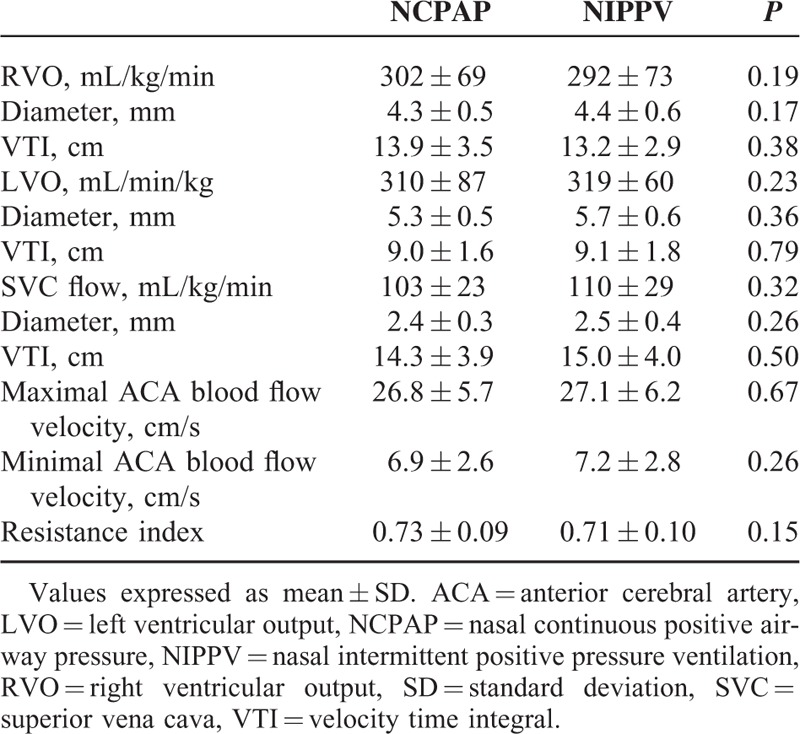
TABLE 3.
Changes in Clinical Variables for NCPAP and NIPPV (N = 20)
DISCUSSION
To the best of our knowledge, this is the first study to compare the circulatory impact of NCPAP and NIPPV in stable preterm infants. The results showed that there were no significant changes in central blood flow in any measured parameter between NCPAP and NIPPV. Impairment of cardiac output by nasal positive pressure ventilation had been reported in several adults studies.8–10 In theory, these hemodynamic effects should be significant in stable preterm infants when lung compliance has improved and most of the airway pressure can be transmitted to the heart and major blood vessels. However, in contrast to the findings in adults, our results showed that there were no overall differences in the hemodynamic measurements with NIPPV. There are several possible explanations for this. First, an increase of 5 to 8 cm H2O in mean airway pressure may not have been large enough to cause a negative effect on blood flow. Second, the mechanism of action of NIPPV is not fully understood, and it is still unclear whether the advantage provided by NIPPV in clinical studies is due to pressure changes, reduced work of breathing, or simply the provision of a higher mean airway pressure than with NCPAP.11 Previous studies have shown that NIPPV has little or no effect on tidal volume,12,13 and therefore the application of NIPPV may not result in significant hemodynamic changes. Third, the lack of an effect of NIPPV on hemodynamics may be due to the fact that these infants were stable on noninvasive respiratory support prior to the study, and that the lung volumes were already optimal. Studies of smaller, more preterm, or more symptomatic infants may produce different results.
Functional echocardiography has been recommended to obtain information on cardiac function and systemic and pulmonary blood flow in critically ill preterm infants.5,6 The most common measurements of central blood flow are LVO, RVO, and SVC flow. Central blood flow velocity is influenced by various factors such as changes in pulmonary vascular resistance, heart rate, and the intrinsic properties of the left and right ventricles. Vessel diameter, VTI, heart rate, and body weight are also determinants of blood flow.14 In addition, LVO is often influenced by shunting via the ductus arteriosus, and RVO is influenced by shunting via the patent foramen ovale. Due to the unreliability of LVO and RVO, SVC flow, of which approximately 80% is estimated to be venous return from the brain, can be used as a surrogate marker of systemic blood flow.6 Reference values for flow in preterm infants of this postnatal age are limited.15 Low SVC flow, defined as less than 40 mL/kg/minute, is associated with an increased risk for intraventricular hemorrhage and adverse neurodevelopmental outcomes.16–18 Previous studies have shown that a higher mean airway pressure is associated with a low SVC flow in preterm infants.18 Whether this is a direct effect of positive pressure ventilation on reducing systemic venous return, or a reflection of severity of lung disease is unknown.2 Although our study showed no difference in hemodynamics with NIPPV, long-term neurodevelopmental follow-up in preterm infants use NIPPV is warranted.
Studies evaluating hemodynamic changes with NCPAP in neonates are also conflicting. No significant hemodynamic changes with NCPAP have been observed in some studies,19,20 whereas others demonstrated that NCPAP at a pressure of 5 cm H2O significantly decreased RVO and SVC flow.21 Another study related the use of NCPAP pressure up to 10 cm H2O with compromised cardiac output in neonates,22 and another study showed that invasive intermittent positive pressure can reduce cerebral blood volume compared to endotracheal continuous positive airway pressure.23 Our findings are consistent with another report in which noninvasive respiratory support had no effect on cerebral circulation.24
There are several possible limitations to our study such as the small sample size, which may have limited our ability to detect intergroup differences. A study to compare the hemodynamic changes between NIPPV, NCPAP, and off nasal respiratory support would have been preferable. However, because these infants still required noninvasive respiratory support, the ethics committee of our hospital did not allow us to remove them from noninvasive respiratory support for research purposes. In addition, this study only recorded short-term hemodynamics effects, and whether the impact increases with time could not be excluded. The wide range of postnatal age (9–28 days) in our subjects could also have theoretically affected our findings, because cardiac vascular flow and resistance may change over the early postnatal period.15,25 The delivered peak inspiratory pressure in NIPPV has been reported to be variable and frequently lower than the set peak inspiratory pressure,26 and NIPPV pressure peaks or NCPAP pressure may not effectively reach the lungs due to variable gas leaks around the prongs or mouths. We used a sealed method to prevent pressure drops in this study; however, we did not use esophageal pressure to estimate changes in intrathoracic pressure. In addition, we used nonsynchronized NIPPV in the present study because synchronized NIPPV is not available in our institution. Synchronized ventilation could in theory enhance transmission of the positive pressure, and the hemodynamic effect of synchronized NIPPV requires further investigation. Furthermore, there are also limitations in our echocardiography analysis. Central blood flow assessments should also be interpreted with caution, as marked intra- and interobserver variability has been demonstrated.27 The main cause of variability is the measurement of vessel diameter, because it varies through the cardiac cycle and is difficult to measure accurately.27 Repeated measurements of the minimum and maximum diameter from several consecutive cardiac cycles may minimize this problem. In addition, the same observer should preferably perform sequential measurements and analysis to prevent interobserver variability.27 Because ultrasound evaluation cannot be available all the time, real-time and continuous hemodynamic measurements during different respiratory strategies may be more practical for clinician in the future. This may help clinicians to determine the most suitable ventilatory support, especially during the golden minutes after birth.
CONCLUSIONS
This study advances the understanding of the hemodynamic effect of NIPPV. We conclude that NIPPV does not affect central blood flow and can be used safely in stable preterm infants. However, our results should be interpreted with caution as they may not apply to infants with cardiovascular compromise. Future studies assessing the effect of NIPPV on circulation should focus on less stable and very preterm infants. In addition, more and larger studies are needed to confirm whether similar or more exaggerated hemodynamic changes occur with the use of NIPPV over a longer period of time.
Acknowledgments
The authors thank Nelson Claure, PhD, Division of Neonatology, Department of Pediatrics, University of Miami Miller School of Medicine for helpful comments and criticism on the manuscript.
Footnotes
Abbreviations: LVO = left ventricular output, NCPAP = nasal continuous positive airway pressure, NIPPV = nasal intermittent positive pressure ventilation, PDA = patent ductus arteriosus, RVO = right ventricular output, SVC = superior vena cava, VTI = velocity time integral.
H-YC, K-SC, C-YL, and H-CL conceptualized and designed the study, drafted the initial manuscript, and revised the manuscript. K-SC and S-TL performed the ultrasounds and interpreted the data. H-LL, C-HL, and H-FH analyzed and interpreted the data and wrote of the initial report. All authors read and approved the final manuscript.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Tang S, Zhao J, Shen J, et al. Nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure in neonates: a systematic review and meta-analysis. Indian Pediatr 2013; 50:371–376. [DOI] [PubMed] [Google Scholar]
- 2.Mirro R, Busija D, Green R, et al. Relationship between mean airway pressure, cardiac output, and organ blood flow with normal and decreased respiratory compliance. J Pediatr 1987; 111:101–106. [DOI] [PubMed] [Google Scholar]
- 3.Kluckow M, Evans N. Relationship between blood pressure and cardiac output in preterm infants requiring mechanical ventilation. J Pediatr 1996; 129:506–512. [DOI] [PubMed] [Google Scholar]
- 4.de Waal K, Kluckow M. Functional echocardiography; from physiology to treatment. Early Hum Dev 2010; 86:149–154. [DOI] [PubMed] [Google Scholar]
- 5.Evans N, Kluckow M. Early determinants of right and left ventricular output in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed 1996; 74:F88–F94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kluckow M, Evans N. Superior vena cava flow in newborn infants: a novel marker of systemic blood flow. Arch Dis Child Fetal Neonatal Ed 2000; 82:F182–F187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su BH, Watanabe T, Shimizu M, et al. Echocardiographic assessment of patent ductus arteriosus shunt flow pattern in premature infants. Arch Dis Child Fetal Neonatal Ed 1997; 77:F36–F40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz O, Iglesia R, Ferrer M, et al. Effects of noninvasive ventilation on pulmonary gas exchange and hemodynamics during acute hypercapnic exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997; 156:1840–1845. [DOI] [PubMed] [Google Scholar]
- 9.Thorens JB, Ritz M, Reynard C, et al. Haemodynamic and endocrinological effects of noninvasive mechanical ventilation in respiratory failure. Eur Respir J 1997; 10:2553–2559. [DOI] [PubMed] [Google Scholar]
- 10.Philip-Joet FF, Pagnelli FF, Dutau HL, et al. Hemodynamic effects of bilevel nasal positive airway pressure ventilation in patients with heart failure. Respiartion 1999; 66:136–143. [DOI] [PubMed] [Google Scholar]
- 11.Bancalari E, Claure N. The evidence for non-invasive ventilation in the preterm infant. Arch Dis Child Fetal Neonatal Ed 2013; 98:F98–F102. [DOI] [PubMed] [Google Scholar]
- 12.Chang HY, Claure N, D’Ugard C, et al. Effects of synchronization during nasal ventilation in clinically stable preterm infants. Pediatr Res 2011; 69:84–89. [DOI] [PubMed] [Google Scholar]
- 13.Owen LS, Morley CJ, Dawson JA, et al. Effects of non-synchronised nasal intermittent positive pressure ventilation on spontaneous breathing in preterm infants. Arch Dis Child Fetal Neonatal Ed 2011; 96:F422–F428. [DOI] [PubMed] [Google Scholar]
- 14.de Waal K, Kluckow M, Evans N. Weight corrected percentiles for blood vessel diameters used in flow measurements in preterm infants. Early Hum Dev 2013; 89:939–942. [DOI] [PubMed] [Google Scholar]
- 15.Sloot SC, de Waal K, van der Lee JH, et al. Central blood flow measurements in stable preterm infants after the transitional period. Arch Dis Child Fetal Neonatal Ed 2010; 95:F369–F372. [DOI] [PubMed] [Google Scholar]
- 16.Hunt RW, Evans N, Rieger I, et al. Low superior vena cava flow and neurodevelopment at 3 years in very preterm infants. J Pediatr 2004; 145:588–592. [DOI] [PubMed] [Google Scholar]
- 17.Miletin J, Dempsey EM. Low superior vena cava flow on day 1 and adverse outcome in the very low birthweight infant. Arch Dis Child Fetal Neonatal Ed 2008; 93:F368–F371. [DOI] [PubMed] [Google Scholar]
- 18.Kluckow M, Evans N. Low superior vena cava flow and intraventricular haemorrhage in preterm infants. Arch Dis Child Fetal Neonatal Ed 2000; 82:F188–F194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moritz B, Fritz M, Mann C, et al. Nasal continuous positive airway pressure (n-CPAP) does not change cardiac output in preterm infants. Am J Perinatol 2008; 25:105–109. [DOI] [PubMed] [Google Scholar]
- 20.Beker F, Rogerson SR, Hooper SB, et al. The effects of nasal continuous positive airway pressure on cardiac function in premature infants with minimal lung disease: a crossover randomized trial. J Pediatr 2014; 164:726–729. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Hady H, Matter M, Hammad A, et al. Hemodynamic changes during weaning from nasal continuous positive airway pressure. Pediatrics 2008; 122:e1086–e1090. [DOI] [PubMed] [Google Scholar]
- 22.Hsu HS, Chen W, Wang NK. Effect of continuous positive airway pressure on cardiac output in neonates. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi 1996; 37:353–356. [PubMed] [Google Scholar]
- 23.Palmer KS, Spencer SA, Wickramasinghe YA, et al. Effects of positive and negative pressure ventilation on cerebral blood volume of newborn infants. Acta Paediatr 1995; 84:132–139. [DOI] [PubMed] [Google Scholar]
- 24.Dani C, Bertini G, Cecchi A, et al. Brain haemodynamic effects of nasal continuous airway pressure in preterm infants of less than 30 weeks’ gestation. Acta Paediatr 2007; 96:1421–1425. [DOI] [PubMed] [Google Scholar]
- 25.Noori S, Wlodaver A, Gottipati V, et al. Transitional changes in cardiac and cerebral hemodynamics in term neonates at birth. J Pediatr 2012; 160:943–948. [DOI] [PubMed] [Google Scholar]
- 26.Owen LS, Morley CJ, Davis PG. Pressure variation during ventilator generated nasal intermittent positive pressure ventilation in preterm infants. Arch Dis Child Fetal Neonatal Ed 2010; 95:F359–F364. [DOI] [PubMed] [Google Scholar]
- 27.Lee A, Liestøl K, Nestaas E, et al. Superior vena cava flow: feasibility and reliability of the off-line analyses. Arch Dis Child Fetal Neonatal Ed 2010; 95:F121–F125. [DOI] [PubMed] [Google Scholar]


