Abstract
Metastasis-associated in colon cancer-1 (MACC1), a newly identified oncogene, is involved in angiogenesis, invasiveness, and metastasis in many cancers. Epidemiological studies have indicated the associations between MACC1 polymorphisms and cancer risk. However, the association between genetic polymorphisms in MACC1 and breast cancer (BC) was not clear. This study aimed to evaluate the relationship between MACC1 polymorphisms and BC risk.
We genotyped 4 single-nucleotide polymorphisms (SNPs) in MACC1 (rs975263, rs1990172, rs3735615, rs4721888) to determine the haplotypes in 560 BC patients and 583 age-, sex-, and ethnicity-matched healthy individuals. Genotypes were determined using the Sequenom MassARRAY method. We estimated the odds ratios (ORs) and 95% confidence intervals (95% CIs) using the chi-square test.
There were significant differences between patients and controls in the MACC1 rs975263 allelic (T vs C: OR = 0.76, 95% CI = 0.61–0.95, P = 0.014) and genotypic groups (TC vs TT: OR = 0.70, 95% CI = 0.54–0.92, P = 0.009; TC+CC vs TT: OR = 0.71, 95% CI = 0.55–0.92, P = 0.008). Analysis of clinical features demonstrated significant associations between rs975263 and Scarff–Bloom–Richardson (SBR) grade 3 cancer (P = 0.006) and postmenopausal women (P = 0.018). Compared with the rs4721888 CC genotype, the frequency of rs4721888 GC and GC+CC variants was higher in patients. Further analysis revealed that the variant genotypes were positively associated with lymph node metastasis. However, we failed to find any relationships between rs1990172 or rs3735615 polymorphism and BC risk. In addition, haplotype analysis indicated that the CTGG and CTCG haplotypes (rs975263, rs1990172, rs3735615, rs4721888) were significantly associated with decreased susceptibility to BC (P = 0.029 and 0.019 respectively).
Our results suggest that rs975263 and rs4721888 polymorphisms in MACC1 are associated with the risk of BC susceptibility and may be involved in the progression of BC in Chinese women.
INTRODUCTION
Breast cancer (BC) is the most common cancer in women and its incidence has increased in recent years worldwide.1 BC is a multifactorial disease caused by complex genetic and environmental factors.2 Metastasis is the most important cause of deaths in breast cancer patients. Candidate genetic risk factors may alter BC onset and outcome may include allele variants in oncogenes.3 Breast cancer susceptibility gene 1 (BRCA1) is a classic tumor suppressor gene involved in basic cellular functions, but its reduced expression increased risk of breast cancer development and associated with familial and sporadic breast cancer.4 The newly identified gene, metastasis-associated in colon cancer 1 (MACC1), is suggested to be related with angiogenesis, invasiveness, and metastasis in many cancers.5
MACC1 gene is located on human chromosome 7 (7p21.1) and contains 7 exons and 6 introns. The coding cDNA contains 2559 nucleotides and is 1 of 21243 sequenced human cDNAs.6 MACC1 was identified in a genome-wide analysis as a differentially expressed gene in primary tumors, metastases, and normal mucosa of subjects with colon cancer.7 MACC1 has been suggested as an independent prognostic indicator of metastasis formation and metastasis-free survival for colon carcinoma patients.8 Subjects with high MACC1 mRNA expression had a 5-year survival rate of 15% compared to 80% for those with low levels of MACC1 mRNA expression, which indicated MACC1 mRNA expression may be prognostic for metastasis-free survival.7 The expression of MACC1, a transcriptional activator, is significantly correlated with clinical staging and TNM classification of breast cancer.9 Advanced hepatocellular carcinoma patients with higher expression of MACC1 mRNA and nuclear protein in tumorous tissues has a shorter post cryoablation median time to progression and overall survival than that with lower MACC1 expression. Therefore, distribution of MACC1 expression in hepatocellular carcinoma cells may help us to select the best appropriate patients for cryotherapy.10 MACC1, as a key regulator of HGF-MET signaling, may be of therapeutic relevance.11 MACC1 induces cell migration, invasion, proliferation, and regulates apoptosis in cancer cells.12,13 In vivo, MACC1 causes tumor growth and metastasis.14
These features designate MACC1 as a gene that can be used to predict the risk of metastasis and guide further diagnostic and therapeutic decisions. Originally discovered in colon cancer, MACC1 overexpression has been demonstrated to promote tumor proliferation, invasion, and metastasis in a wide spectrum of solid tumors including colon cancer,15 gastric carcinoma,16 hepatocellular carcinoma,17,18 osteosarcoma,19 glioma,20,21 lung,22,23 esophageal,24 pancreatic,25 ovarian,26,27 cervical cancer, and BC.9,28
Single nucleotide polymorphisms (SNPs) have been proposed to play an important role in genetic susceptibility to cancer. Numerous SNPs have been identified in the human MACC1 gene using sequence databases. Recently, some studies indicate rs975263, rs1990172 and rs3735615 in MACC1 are associated with clinical outcome for HER-2 positive breast cancer patients and rs975263 and rs1990172 showed a significant high risk of relapse in hepatocellular carcinoma patients after transplantation under the overdominant model.29–31 The 4 MACC1 polymorphisms involved in this study (rs975263, rs1990172, rs3735615, and rs4721888) are annotated in NCBI databases. However, their association with breast cancer risk had not been studied before. To explore their occurrence and frequencies in BC and their association with tumor progression we conducted a case–control study to investigate the association of MACC1 polymorphisms and BC risk in the Chinese Han population.
METHODS
Study Subjects
The characteristics of the BC patients and cancer-free controls in our study are described in Table 1. BC patients (n = 560) were recruited between January 2013 and October 2014 at the Second Affiliated Hospital of Xi’an Jiaotong University, China.32 Inclusion criteria were as follows: (a) patients were recruited without regard to age; (b) patients were pathologically confirmed to have sporadic BC; (c) patients who had another type of cancer were excluded from the study. Cancer-free controls (n = 583) were recruited from individuals seeking health care in the outpatient departments at the hospital and were frequency-matched to the cases by age (±5 years). All subjects were Han Chinese and residents of Northwest China. The study was approved by the institutional review board of Xi’an Jiaotong University (Xi’an, China). All of the participants were interviewed using a self-administered questionnaire includes a complete medical history, demographic data, and physical condition after obtaining written informed consent. We collected blood samples from all participants, and collected blood samples when the patients were pathologically confirmed to have BC before they received the chemotherapy or radiotherapy.
TABLE 1.
Distributions of Select Variables in Breast Cancer Cases and Cancer-Free Controls
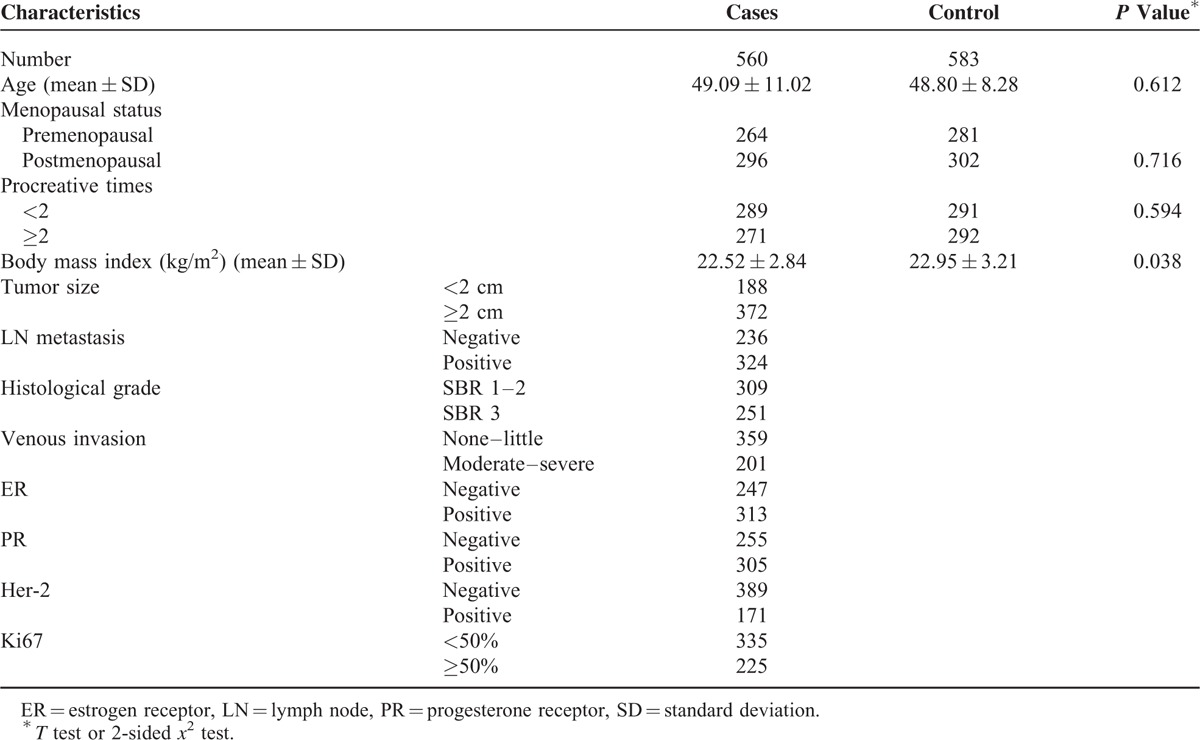
The cases and controls were well matched by age (P = 0.612). There was no significant difference in the distribution of menopausal state between the 2 groups (P = 0.716). However, the BMI (body mass index) was significantly different between BC patients and health controls (P = 0.038). The percentages of patients with tumors <2 cm and ≥2 cm in size were 33.6% and 66.4%, respectively. About 44.8% of the patients had Scarff–Bloom–Richardson (SBR) grade 3 cancer. The percentages of patients with lymph node involvement and venous invasion were 42.1% and 35.9%, respectively. In addition, the percentages of patients with estrogen receptor (ER)-, progesterone receptor (PR)-, human epidermal growth factor receptor 2 (HER2)-, and Ki67-positive disease were 55.9%, 54.5%, 30.5%, and 40.2%, respectively.
Genotyping Assay
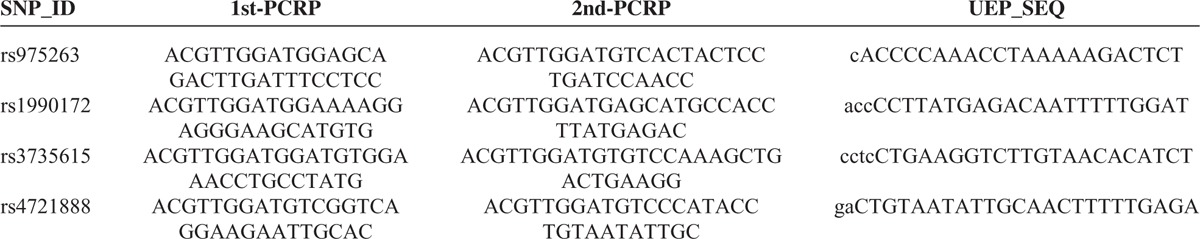
Blood samples were collected in EDTA tubes and stored at −80 °C after centrifugation. DNA extraction carried out using the standard phenol–chloroform extraction method. DNA quantity was evaluated by spectrometry (DU530 UV/VIS spectrophotometer, Beckman Instruments, Fullerton, CA). Four tag SNPs (rs975263, rs1990172, rs3735615 and rs4721888) were selected for our study; these SNPs were with minor allele frequencies >5% in the HapMap Chinese Han Beijing (CHB) population (http://www.hapmap.org). All selected polymorphisms are located in coding regions with the exception of rs1990172, which is located in an intron (Figure 1). Sequenom MassARRAY Assay Design 3.0 Software (Sequenom, Inc, San Diego, CA) was used to design a Multiplexed SNP MassEXTEND assay. We genotype the 4 polymorphisms in all subjects using a Sequenom MassARRAY RS1000 (Sequenom, Inc). Primers of PCR which were used for each SNP in our study are listed in Table 2. Sequenom Typer 3.0 Software (Sequenom, Inc) was used for data analyses.
FIGURE 1.
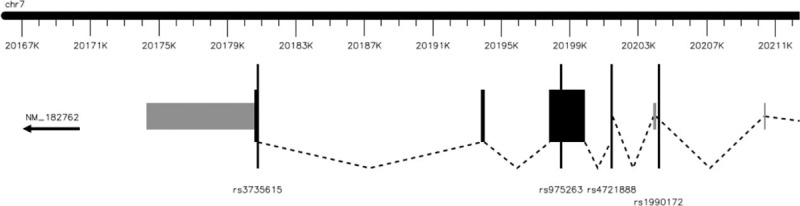
Genomic positions of selected MACC1 SNPs. MACC1 is located on the minus strand of chromosome 7. In the gene structure figure, the exons (coding exons) and untranslated regions (noncoding exons) were separately marked in black and gray, and the arrow under the gene label represented the strand direction. All selected SNPs are located in coding regions, except that rs1990172 is located in the intron region. MACC1 = metastasis-associated in colon cancer-1, SNPs = single nucleotide polymorphisms.
TABLE 2.
Primers Used for this Study
Statistical Analyses
Allele and genotype frequencies of MACC1 polymorphisms were obtained by direct counts. SNP allele frequencies in the control subjects were tested for departure from Hardy–Weinberg Equilibrium (HWE) before analysis. HWE was evaluated by comparing expected and observed frequencies with algorithms in the Alrequin 3.1 program (L. Excoffier, CMPG, University of Bern, Switzerland). The statistical power of the case–control study was calculated using Power and Sample Size Calculation software (available on line: http://biostat.mc.vanderbilt.edu/wiki/Main/PowerSampleSize). Differences between the cases and controls in the distributions of demographic characteristics, selected variables, and genotype frequencies of the 4 SNPs were evaluated using Student's t test or x2 test. Associations between the genotypes of the MACC1 polymorphisms, the risk of BC and the patients’ clinical characteristics were estimated by computing odds ratios (ORs) and 95% confidence intervals (CIs) from unconditional logistic regression analysis with an adjustment for age and body mass index. All of the statistical analyses were performed with the SPSS 18.0 software for Windows (PASW Statistics, SPSS Inc, Chicago, IL). We evaluated the risk in the dominant model (AA+ Aa vs aa; A represents the major allele, a the minor allele) and the recessive model (aa vs Aa+ AA) and the allele model (a vs A). A P value <0.05 was considered statistically significant, and all statistical tests were 2 sided.
RESULTS
Association Between MACC1 Polymorphisms and BC Risk
The genotype and allele frequencies of the MACC1 polymorphisms (rs975263, rs1990172, rs3735615, and rs4721888) are shown in Table 3. All polymorphisms conformed to HWE (rs975263: P = 0.579, rs1990172: P = 0.757, rs3735615: P = 0.406, and rs4721888: P = 0.485).
TABLE 3.
Genotype and Allele Frequencies of MACC1 Polymorphisms Among the Cases and Controls and the Associations With Breast Cancer Risk
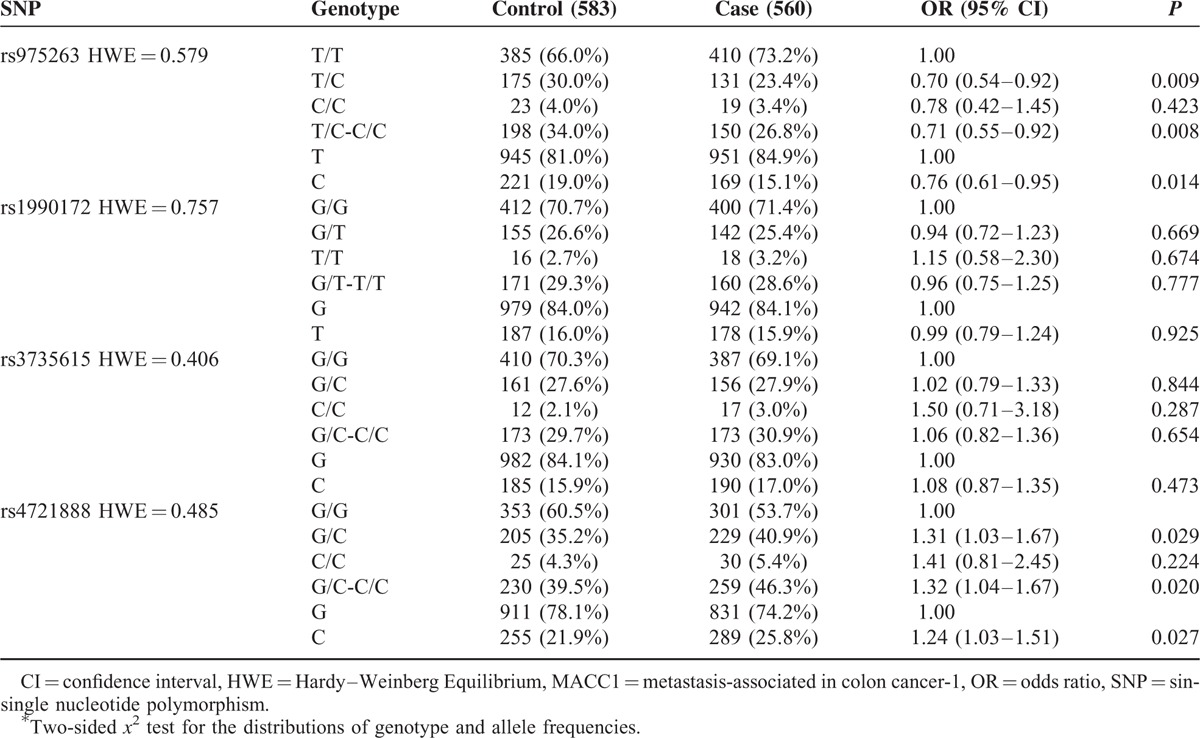
Compared with the TT genotype, the TC and TC+CC frequencies of rs975263 polymorphism among cases were significantly different from the controls (TC vs TT: OR = 0.70, 95% CI = 0.54–0.92, P = 0.009; TC+CC vs TT: OR = 0.71, 95% CI = 0.55–0.92, P = 0.008). The difference in the frequency distributions of T and C alleles among cases and controls was also significant (OR = 0.76, 95% CI = 0.61–0.95, P = 0.014). These results suggested that the MACC1 rs975263 polymorphism had a protective effect on BC risk. Compared with individuals with the rs4721888 GG genotype, individuals with GC and GC+CC genotypes had a significantly increased BC risk (GC vs GG: OR = 1.31, 95% CI = 1.03–1.67, P = 0.029; GC+CC vs GG: OR = 1.32, 95% CI = 1.04–1.67, P = 0.020). In addition, the minor allele C conferred an increased risk of BC in an allele model (C vs G: OR = 1.24, 95%CI = 1.03–1.51, P = 0.027). However, we did not observe significant association between the MACC1 rs1990172 or rs3735615 polymorphisms and BC risk in any genetic model, as shown in Table 3. We also obtained the statistical power of 0.89 and 0.80 for the 2 significant polymorphisms identified, rs975263 and rs4721888, respectively. This showed that our sample size of 1143 was adequate and the study was sufficiently able to detect the true association of these 2 polymorphisms with BC.
Stratified Analysis of MACC1 Polymorphisms and BC Risk
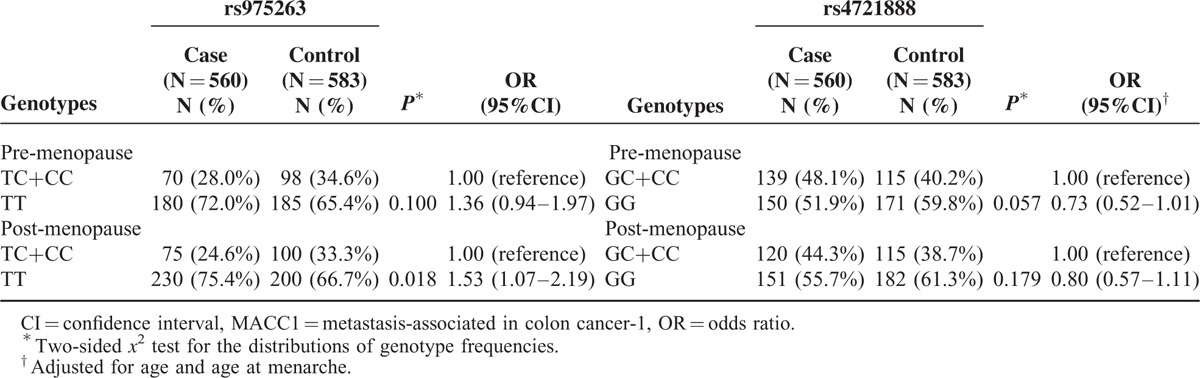
Stratified analysis of the effect of rs975263 and rs4721888 polymorphisms on BC by menopausal status is displayed in Table 4. The results indicated that rs975263 was associated with an increased BC susceptibility in postmenopausal women (OR = 1.53, 95% CI = 1.07–2.19, P = 0.018). However, there was no association between rs4721888 and BC risk in either premenopausal patients or postmenopausal patients.
TABLE 4.
Stratification Analyses by Menopause Status Between MACC1 Polymorphisms and Risk of Breast Cancer
Association Between MACC1 Polymorphisms and Clinical Parameters of BC Patients
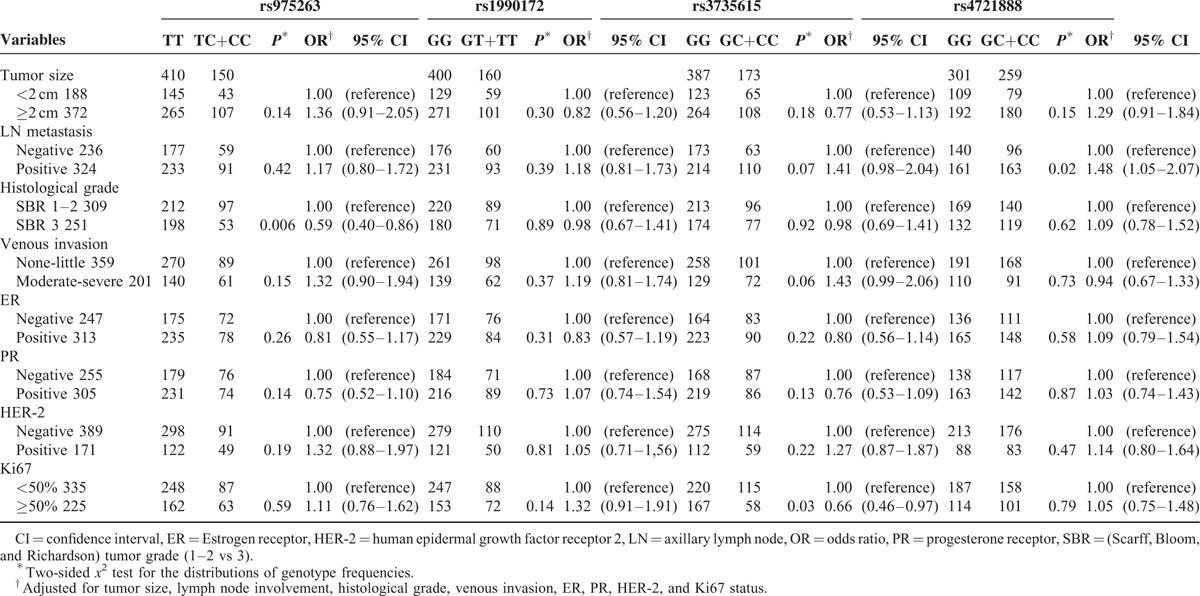
MACC1 gene polymorphisms were also analyzed to establish their associations with clinicopathological features, including tumor size, lymph node metastasis, histological grade, venous invasion and the statuses of ER, PR, HER-2, and Ki67. The positive results are shown in Table 5. For rs975263, compared with the TT genotype, the TC+CC genotype appeared at lower frequencies in SBR grade 3 cases (OR = 0.59, 95% CI = 0.40–0.86, P = 0.006). For rs3735615, we found that the Ki67 value of patients with the GC+CC genotype was more likely to be <50% compared with GG genotype carriers (OR = 0.66, 95% CI = 0.46–0.97, P = 0.03). Moreover, compared with the GG genotype, the GC+CC genotype of rs4721888 had a higher frequency in lymph node involvement (OR = 1.48, 95% CI = 1.05–2.07, P = 0.02). However, no statistical association was detected between the 4 variants and tumor size, venous invasion or the values of ER, PR, and HER-2.
TABLE 5.
The Associations Between MACC1 Polymorphisms and Clinical Characteristics of Breast Cancer Patients
Association of MACC1 Haplotypes With BC Risk
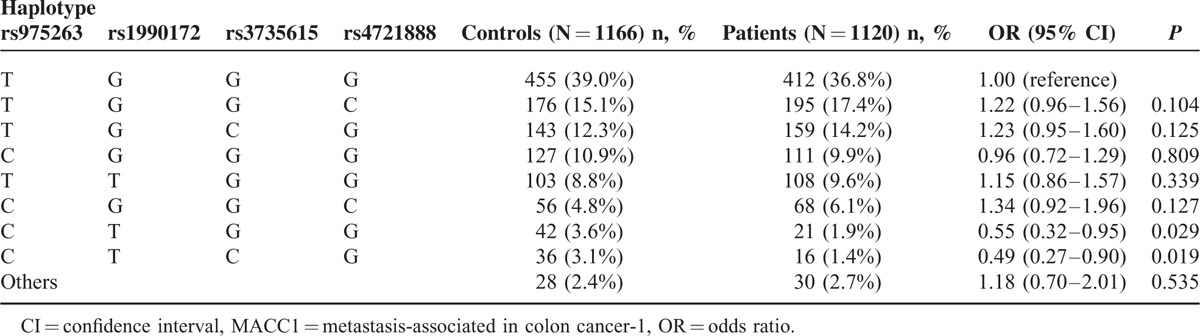
The relationship of MACC1 haplotypes with the risk of developing BC was also evaluated. The frequency distributions of 4 common MACC1 rs975263, rs1990172, rs3735615, and rs4721888 haplotypes are shown in Table 6, with the most frequent haplotype in the controls being chosen as the reference. Haplotype analysis indicated that the frequencies of CTGG and CTCG haplotypes (rs975263, rs1990172, rs3735615, rs4721888) were lower in patients than in controls (1.9% vs 3.6% and 1.4% vs 3.1%, respectively). Compared with the TGGG wild type, carriers of CTGG and CTCG haplotypes had significant associations with decreased susceptibility to BC (CTGG: OR = 0.55, 95% CI = 0.32–0.95, P = 0.029; CTCG: OR = 0.49, 95% CI = 0.27–0.90, P = 0.019).
TABLE 6.
The Haplotype Frequencies of MACC1 Polymorphisms and Breast Cancer Risk
DISCUSSION
Epidemiologic studies have suggested that single nucleotide polymorphisms (SNP) in MACC1 may contribute to individuals’ susceptibility to cancer. Carriers of the G allele of rs1990172 showed a significantly decreased overall survival in colorectal cancer (additive hazard ratios = 1.38, 95%CI = 1.05–1.82, P = 0.023). Multivariate analysis adjusted for age and UICC tumor stage confirmed this result (hazard ratios = 1.49, 95% CI = 1.12–1.98, P = 0.007).33 Other investigated genetic variants (rs3114446, rs10275612, rs3095007, rs3095009, and rs7780032) of the MACC1 gene were not significantly associated with overall survival.33 For the rs975263 polymorphism, younger colon cancer individuals with CT genotype has a reduced survival with stage I or II.29 Zheng et al31 suggested that SNP rs1990172 and SNP rs975263 in MACC1 may be potential genetic markers for hepatocellular carcinoma recurrence in liver transplantation patients. Muendlein et al30 provided the first evidence that MACC1 polymorphisms are associated with clinical outcomes for HER2-positive BC patients.
In our study, we estimated the relationship between rs975263, rs1990172, rs3735615, and rs4721888 in MACC1 and BC susceptibility. Rs1990172 is located in the intronic region of the MACC1 gene and has no impact on coding exon of MACC1 gene.30 Rs975263, rs3735615, and rs4721888 are all missense alterations. Rs975263, which is situated at codon 515, fifth exon, has a nonsynonymous substitution of leucine to serine which may lead to a loss of phosphorylation site.31 Rs3735615 results in a substitution of threonine for arginine that potentially can act as phosphorylation site. Rs4721888 exchanges the amino acid sequence from leucine to valine, which has an unsure effect because leucine and valine are all nonpolar amino acids. Schmid et al29 found rs975263 and rs4721888 variants are possibly benign for they were not in predicted domains of MACC1 structure, whereas rs3735615 variant could be damaging for it lies in a conserved domain.
Overall, we observed that variant genotypes of MACC1 rs975263 and rs4721888, but not rs1990172 or 3735615, were associated with BC risk. Rs975263 was associated with protection from BC, but rs4721888 increased BC susceptibility. The results are partially consistent with other studies. For example, Muendlein et al30 found that carriers of the rs975263 T allele had an adverse effect on cancer prognosis, the rs1990172 G allele was associated with an increased risk of cancer progression or death and the rs3735615 C allele had a protective impact on overall survival.30 We also observed that the variant rs975263 genotypes in the MACC1 gene were inversely associated with SBR grade 3 and that rs4721888 was related to positive lymph node metastasis. Furthermore, the variant genotypes of rs975263 were more frequent in postmenopausal women. The results suggest that rs975263 had a protect effect on BC patients and rs47218888 polymorphism in MACC1 is related to the development and progression of BC and may help to accurately predict the clinical course of BC. In addition, compared with the TGGG wild type, the CTGG and CTCG haplotypes were significantly associated with decreased susceptibility to BC (P = 0.029 and P = 0.019, respectively).
Our study had some limitations. First, the sample size was inadequate for a stratified analysis and for an analysis of these associations in patients with BC. Second, we did not investigate whether predisposing factors, including high-dose radiation exposure, alcohol consumption, and postmenopausal obesity, were associated with the risk of BC because of a lack of such data from both patients with BC and controls. The effect of these factors on BC risk should be assessed in a future study.
In summary, our case–control study indicates that rs975263 and rs4721888 in MACC1 have significant effects on the susceptibility and progression of BC among Chinese women. Further functional studies and larger population-based prospective studies are required to further elucidate the impact of MACC1 polymorphisms on BC.
Footnotes
Abbreviations: BC = breast cancer, BRCA1 = breast cancer susceptibility gene 1, CIs = confidence intervals, ER = estrogen receptor, HER = human epidermal growth factor receptor, HGF = Hepatocyte growth factor, HWE = Hardy–Weinberg equilibrium, LN = lymph node, MACC1 = metastasis-associated in colon cancer-1, ORs = odds ratios, PR = progesterone receptor, SNPs = single nucleotide polymorphisms, SRP = Scarff–Bloom–Richardson.
Z-JD, X-HL, and H-FK contributed equally to this study and share joint first authorship.
Funding: this study was supported by National Natural Science Foundation, China (No. 81471670; 81274136); China Postdoctoral Science Foundation (No. 2014M560791; 2015T81037); the International Cooperative Project of Shaanxi province, People's Republic of China (No. 2013KW-32–01) and the Fundamental Research Funds for the Central Universities, China (No. 2014qngz-04).
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359–386. [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer—analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000; 343:78–85. [DOI] [PubMed] [Google Scholar]
- 3.Wolff MS, Weston A. Breast cancer risk and environmental exposures. Environ Health Perspect 1997; 105 Suppl 4:891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romagnolo AP, Romagnolo DF, Selmin OI. BRCA1 as target for breast cancer prevention and therapy. Anticancer Agents Med Chem 2015; 15:4–14. [DOI] [PubMed] [Google Scholar]
- 5.Sueta A, Yamamoto Y, Yamamoto-Ibusuki M, et al. Differential role of MACC1 expression and its regulation of the HGF/cMet pathway between breast and colorectal cancer. Int J Oncol 2015; 46:2143–2153. [DOI] [PubMed] [Google Scholar]
- 6.Ota T, Suzuki Y, Nishikawa T, et al. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet 2004; 36:40–45. [DOI] [PubMed] [Google Scholar]
- 7.Stein U, Walther W, Arlt F, et al. MACC1, a newly identified key regulator of HGF-MET signaling, predicts colon cancer metastasis. Nat Med 2009; 15:59–67. [DOI] [PubMed] [Google Scholar]
- 8.Hu H, Tian D, Chen T, et al. Metastasis-associated in colon cancer 1 is a novel survival-related biomarker for human patients with renal pelvis carcinoma. PLoS One 2014; 9:e100161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang Y, Zhang H, Cai J, et al. Overexpression of MACC1 and its significance in human breast cancer progression. Cell Biosci 2013; 3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YP, Qu JH, Chang XJ, et al. High intratumoral metastasis-associated in colon cancer-1 expression predicts poor outcomes of cryoablation therapy for advanced hepatocellular carcinoma. J Transl Med 2013; 11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boardman LA. Overexpression of MACC1 leads to downstream activation of HGF/MET and potentiates metastasis and recurrence of colorectal cancer. Genome Med 2009; 1:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arlt F, Stein U. Colon cancer metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem Cell Biol 2009; 41:2356–2359. [DOI] [PubMed] [Google Scholar]
- 13.Kokoszynska K, Krynski J, Rychlewski L, et al. Unexpected domain composition of MACC1 links MET signaling and apoptosis. Acta Biochim Pol 2009; 56:317–323. [PubMed] [Google Scholar]
- 14.Pichorner A, Sack U, Kobelt D, et al. In vivo imaging of colorectal cancer growth and metastasis by targeting MACC1 with shRNA in xenografted mice. Clin Exp Metastasis 2012; 29:573–583. [DOI] [PubMed] [Google Scholar]
- 15.Ge Y, Meng X, Zhou Y, et al. Positive MACC1 expression correlates with invasive behaviors and postoperative liver metastasis in colon cancer. Int J Clin Exp Med 2015; 8:1094–1100. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Wu Y, Lin L, et al. Metastasis-associated in colon cancer-1 upregulation predicts a poor prognosis of gastric cancer, and promotes tumor cell proliferation and invasion. Int J Cancer 2013; 133:1419–1430. [DOI] [PubMed] [Google Scholar]
- 17.Qu JH, Chang XJ, Lu YY, et al. Overexpression of metastasis-associated in colon cancer 1 predicts a poor outcome of hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol 2012; 18:2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao J, Ding F, Liu Q, et al. Knockdown of MACC1 expression suppressed hepatocellular carcinoma cell migration and invasion and inhibited expression of MMP2 and MMP9. Mol Cell Biochem 2013; 376:21–32. [DOI] [PubMed] [Google Scholar]
- 19.Zhang K, Zhang Y, Zhu H, et al. High expression of MACC1 predicts poor prognosis in patients with osteosarcoma. Tumour Biol 2014; 35:1343–1350. [DOI] [PubMed] [Google Scholar]
- 20.Hagemann C, Fuchs S, Monoranu CM, et al. Impact of MACC1 on human malignant glioma progression and patients’ unfavorable prognosis. Neuro Oncol 2013; 15:1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang T, Kong B, Kuang YQ, et al. Overexpression of MACC1 protein and its clinical implications in patients with glioma. Tumour Biol 2014; 35:815–819. [DOI] [PubMed] [Google Scholar]
- 22.Chundong G, Uramoto H, Onitsuka T, et al. Molecular diagnosis of MACC1 status in lung adenocarcinoma by immunohistochemical analysis. Anticancer Res 2011; 31:1141–1145. [PubMed] [Google Scholar]
- 23.Shimokawa H, Uramoto H, Onitsuka T, et al. Overexpression of MACC1 mRNA in lung adenocarcinoma is associated with postoperative recurrence. J Thorac Cardiovasc Surg 2011; 141:895–898. [DOI] [PubMed] [Google Scholar]
- 24.Zhu M, Xu Y, Mao X, et al. Overexpression of metastasis-associated in colon cancer-1 associated with poor prognosis in patients with esophageal cancer. Pathol Oncol Res 2013; 19:749–753. [DOI] [PubMed] [Google Scholar]
- 25.Wang G, Kang MX, Lu WJ, et al. MACC1: A potential molecule associated with pancreatic cancer metastasis and chemoresistance. Oncol Lett 2012; 4:783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang R, Shi H, Chen Z, et al. Effects of metastasis-associated in colon cancer 1 inhibition by small hairpin RNA on ovarian carcinoma OVCAR-3 cells. J Exp Clin Cancer Res 2011; 30:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Zhang H, Zhao S, et al. Overexpression of MACC1 and the association with hepatocyte growth factor/c-Met in epithelial ovarian cancer. Oncol Lett 2015; 9:1989–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou X, Xu CJ, Wang JX, et al. Metastasis-associated in colon cancer-1 associates with poor prognosis and promotes cell invasion and angiogenesis in human cervical cancer. Int J Gynecol Cancer 2015; 25:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid F, Burock S, Klockmeier K, et al. SNPs in the coding region of the metastasis-inducing gene MACC1 and clinical outcome in colorectal cancer. Mol Cancer 2012; 11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muendlein A, Hubalek M, Geller-Rhomberg S, et al. Significant survival impact of MACC1 polymorphisms in HER2 positive breast cancer patients. Eur J Cancer 2014; 50:2134–2141. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Z, Gao S, Yang Z, et al. Single nucleotide polymorphisms in the metastasis-associated in colon cancer-1 gene predict the recurrence of hepatocellular carcinoma after transplantation. Int J Med Sci 2014; 11:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai ZJ, Liu XH, Ma YF, et al. Association between single nucleotide polymorphisms in DNA polymerase kappa gene and breast cancer risk in Chinese Han population: a STROBE-Compliant Observational Study. Medicine (Baltimore) 2016; 95:e2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lang AH, Geller-Rhomberg S, Winder T, et al. A common variant of the MACC1 gene is significantly associated with overall survival in colorectal cancer patients. BMC Cancer 2012; 12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]


