Abstract
Dexmedetomidine is a commonly used sedative and adjuvant agent to general anesthesia. The present was designed to evaluate the effects of dexmedetomidine on myocardial function by using tissue Doppler echocardiography during general anesthesia in patients with diastolic dysfunction.
Forty patients undergoing orthostatic surgery with ejection fraction preserved diastolic dysfunction grade 2 or 3 were randomly allocated to the Control and Dex group (n = 20, each). In the Dex group, dexmedetomidine was given as an initial loading dose of 1.0 μg/kg over 10 minutes followed by a maintenance dose of 0.5 μg/kg/h. The ratio of peak early diastolic transmitral or transtricuspid inflow velocity to early diastolic mitral or tricuspid annular velocity (LV or RV E/e′) and left or right ventricular myocardial performance index (LV or RV MPI) were measured at before and after the administration dexmedetomidine or saline.
The Dex group showed significant decrease of heart rate (P = 0.038), and increase of mean blood pressure (P < 0.001), LV E/e′ (P = 0.025), and LV MPI (P < 0.001) compared to those of the Control group on a linear mixed model analysis. Also, the Dex group showed significant increase of RV E/e′ (P < 0.001) and RV MPI (P = 0.028) compared to those of the Control group.
Intraoperative dexmedetomidine administration during general anesthesia was appeared to deteriorate biventricular function in patients with diastolic dysfunction. We suggest careful consideration and a need for reducing dosage when administrating dexmedetomidine in patients with diastolic dysfunction.
INTRODUCTION
Dexmedetomidine is a highly selective α2-adrenoreceptor agonist that has gained popularity in the intensive care unit, cardiovascular intervention1 and endoscopic procedures, and as an adjuvant to general anesthesia2 for its sedative and analgesic effects. Although there have been studies suggesting the use of perioperative dexmedetomidine in cardiac surgery improved postoperative morbidity and mortality,3,4 there is also conflict in literature that have reported adverse cardiovascular effects of dexmedetomidine including hypotension or hypertension, bradycardia, and even cardiac arrest.5,6 Even with the amounting evidence that dexmedetomidine has critical cardiovascular effects, few studies have investigated the direct effects of dexmedetomidine on cardiac function. Although our previous study7 presented evidence that dexmedetomidine administration had minimal effects on cardiac function in young healthy patients, there are no current studies assessing the effects of dexmedetomidine administration on biventricular function in patients with cardiac dysfunction. In a recent study,8 64.1% of patients over 65 years were assessed with diastolic dysfunction. Regardless, the importance of diastolic dysfunction has been underestimated in comparison to systolic dysfunction. Because preoperative diastolic dysfunction is highly affiliated with overall postoperative prognosis,9 mortality after acute coronary syndrome,10 and adverse postoperative outcome of patients with myocardial infarction,11 undermining diastolic dysfunction may be a critical mistake. As dexmedetomidine becomes a more ubiquitous agent in the clinical field, we believe a true evaluation of dexmedetomidine on cardiac function in patients with cardiac dysfunction is critically essential.
Tissue Doppler indices are more reliable in estimating cardiac function than 2-dimensional or conventional Doppler echocardiography in patients with preexisting left ventricle (LV) relaxation impairment. The ratio (E/e′) of peak early diastolic transvalvular inflow velocity (E) to early diastolic valvular annular velocity (e′) is a valuable tool in diagnosing diastolic dysfunction independent of preload, in patients with preserved LV ejection fraction (EF) and impaired LV relaxation.12 Tissue Doppler imaging derived myocardial performance index (MPI), estimates combined systolic and diastolic performance to evaluate global cardiac function.13 Its most prominent use is to assess diastolic function. In contrast to Doppler-assessed transvalvular blood flow, tissue Doppler imaging derived MPI is relatively independent of heart rate (HR)14 and loading conditions.15
In this randomized, double-blind, and placebo-controlled trial, we investigated the effects of dexmedetomidine on myocardial function in patients with diastolic dysfunction by using tissue Doppler imaging derived indices including MPI and E/e′ during general anesthesia.
METHODS
Study Population
This study received approval from the institutional review board of Severance Hospital, Yonsei University Health System, Seoul, South Korea (Ref. 4-2015-0284) on May 2015 and was registered at Clinical Trials.gov (NCT02490072). All participants provided written informed consent before participation. Patients undergoing orthopedic surgery in supine position were included. The inclusion criteria were American Society of Anesthesiologists physical status of class II or III, over 40 years of age, and patients with sinus rhythm lateral mitral valvular (MV) e’ velocity < 10 cm/s or septal MV e’ velocity < 8 cm/s and averaged LV E/e′ ≥9 on preoperative transthoracic echocardiographic evaluation. Averaged LV E/e′ = 9–12 was defined as diastolic dysfunction grade 2, and LV E/e′ ≥13 was defined at diastolic dysfunction grade 3.16 The patients with LV systolic function preserved (LV EF ≥50%) diastolic dysfunction were enrolled in this study. For patients without preoperative echocardiographic examination, we performed a transthoracic echocardiography prior to surgery. The patients with lateral MV e′ velocity <10 cm/s or septal MV e′ velocity <8 cm/s were enrolled in our study (Figure 1). The exclusion criteria were the patients with severe functional liver or kidney disease, diagnosed heart failure, regional wall motion abnormality of LV, history of arrhythmia or treatment with antiarrhythmic drugs, bradycardia (HR <45 beats/min) or atrioventricular block, and severe chronic obstructive lung disease. Enrolled patients were randomly allocated to the Control or dexmedetomidine group (Dex group) using a randomized sequence generated by a computer, and the randomization process was centralized. A concealed envelope for random allocation was sent to anesthesia nurses who prepared the dexmedetomidine or saline of comparable volume. Therefore, the anesthesiologist infused the drug in a blind manner. The participating anesthesiologists, nurses, surgeons, and patients were blinded to the treatment allocation.
FIGURE 1.
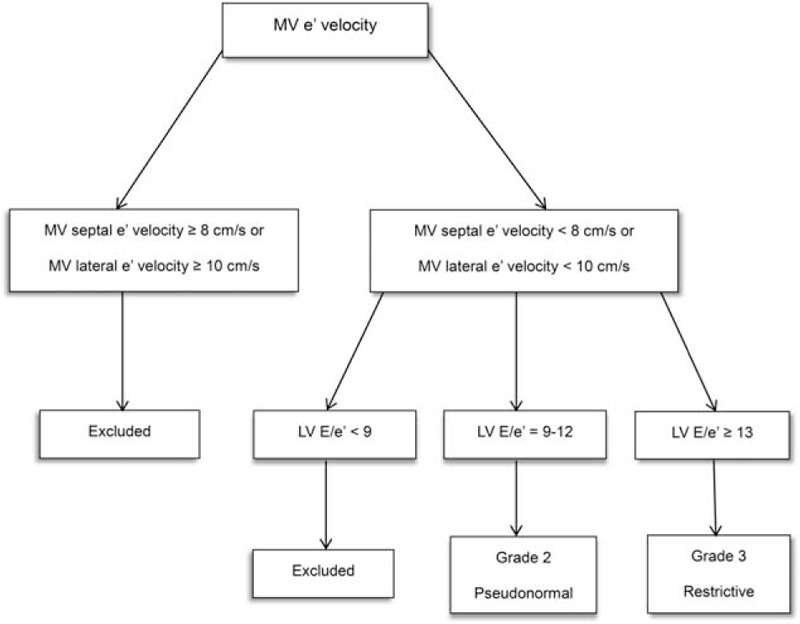
Algorism used for diastolic dysfunction grading. LV = left ventricle, MV e′ = peak early diastolic mitral annular velocity, MV E = peak early diastolic transmitral inflow velocity.
Anesthetic Management
After each patient arrived to the operating room, normal saline 5 mL/kg was administrated to replace the fluid deficit. Patients were not premedicated. Blood pressure, oxygen saturation, electrocardiography, and bispectral index (BIS; A-200 bispectral index monitor, Aspect Medical System Inc., Newton, MA) were monitored noninvasively. Anesthesia was induced by propofol and remifentanil through a target-controlled infusion system (Orchestra; Base Primera, Fresenius Vial, Brezins, France). Following the loss of consciousness, rocuronium 0.8 mg/kg was administered to facilitate tracheal intubation. During the surgery, the dose of propofol and remifentanil were adjusted to maintain BIS range between 40 and 50 in both groups. The effect site concentration and total dose of each administered propofol and remifentanil were recorded. Hemodynamic instability was treated as follows: atropine was administered when the HR decreased to <45 beats/min, while β1-adrenergic antagonist was administered when HR increased to ≥120 beats/min. When mean blood pressure (MBP) decreased to below 20% of baseline value, phenylephrine (50 μg) was administered. When MBP increased up to 120 mmHg, calcium channel blocker (500 μg) was administered. In cases of vasoactive drug administration, measurement was not performed within 5 minutes to minimize its influence on the echocardiographic evaluation.
Intervention
Dexmedetomidine (Precedex; Hospira, Lake Forest, IL) 200 μg was added with normal saline to achieve a total volume of 50 mL. Dexmedetomidine was started once the patient was hemodynamically stable after the induction of anesthesia: a bolus of 1.0 μg/kg over 10 minutes followed by a continuous infusion at 0.5 μg/kg/h infusion for 1 hour in Dex group. A comparable volume of normal saline was administered in the Control.
Echocardiographic Measurements
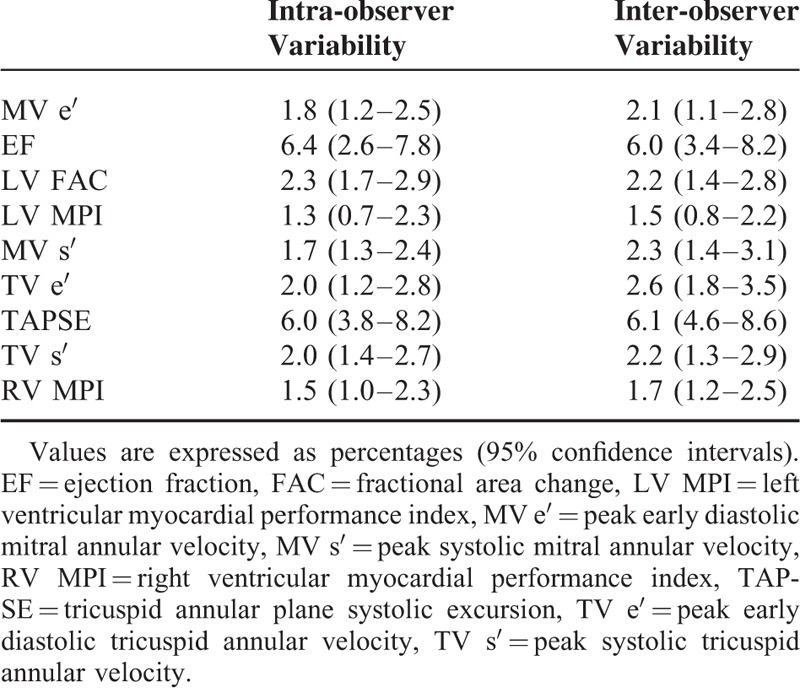
The blinded anesthesiologist inserted a 4–7 MHz multiplane transoesophageal echocardiography (TEE) probe (6TC; GE, Vingmed Ultrasound AS, Horten, Norway) via the oesophagus and connected it to a cardiac ultrasound system (Vivid E9; GE, Vingmed Ultrasound AS, Horten, Norway). The echocardiographic examination was performed by the same anesthesiologist. To assess LV and right ventricle (RV) diastolic function, pulsed-wave Doppler ultrasonography was used to measure transmitral and transtricuspid flow at mid-oesophageal 4-chamber views. The peak early diastolic transmitral inflow velocity (MV E), peak early diastolic (MV e′), and systolic (MV s′) mitral annular velocity were measured at the lateral and septal annular by tissue Doppler imaging. Average LV E/e′ was obtained by averaging the total of lateral and septal LV E/e′. Peak early diastolic (TV e′) and systolic (TV s′) tricuspid annular velocity were also measured at lateral tricuspid annular. The ratio (RV E/e′) of peak early diastolic transtricuspid inflow velocity (TV E) to TV e′ was acquired from these data. LV and RV MPI was defined as follows: (isovolumic contraction time + isovolumic relaxation time)/ejection time. The normal reference value of LV and RV MPI are considered 0.39 ± 0.0517 and 0.28 ± 0.04.18 To assess LV systolic function and dimension, LV end-diastolic area, LV end-systolic area, and EF were measured from the mid-oesophageal 4-chamber view. Fractional area change (FAC) was calculated in the mid-oesophageal 4-chamber view using the following formula: FAC = ([end-diastolic area − end-systolic area]/end-diastolic area) × 100. RV systolic function was assessed by tricuspid annular plane systolic excursion (TAPSE).19 Cardiac output was assessed by stroke volume using pulsed-wave Doppler measurements from the LV outflow tract. The cardiac output was calculated: Cardiac output = stroke volume × HR. All variables were means of the values measured over 3 cardiac cycles during end-expiration. Analysis of the echocardiographic data was performed by 1 anesthesiologist who was blinded to the group assignments. To determine intra- and interobserver variability, a random sample of 25% of all echocardiographic data was submitted twice to a 1st investigator and once to a 2nd investigator. The variabilities were calculated as the mean absolute differences between the 2 readings divided by their mean and expressed as a percentage and their 95% confidence intervals (Table 1). The concentration of propofol and remifentanil, MBP, HR, BIS, and TEE examination were measured after the patient became hemodynamically stable for 10 minutes after induction and before, at 20, 40, and 60 minutes after the administration dexmedetomidine or saline.
TABLE 1.
Inter- and Intra-observer Variability
Statistical Analysis
For justification of numbers, the primary outcome measure was defined as the LV E/e′. A difference of 3.0 between the Control and Dex group was taken as clinically significant in the preliminary results for the 1st 10 patients. Previously, our studies7 have also found a standard deviation (SD) of 3.2 for dexmedetomidine administered group. With α = 0.05 and power of 0.8 at least 18 patients were needed in each group. Assuming a dropout rate of 10%, 20 patients for each group, 40 patients in total were included in this study. Results are expressed as mean ± SD or numbers (proportion). Unpaired Student t-test was used for continuous variables, and Chi-squared or Fisher's exact test was used for categorized variables between 2 groups. The analysis of repeated variables were performed by a linear mixed model for random and fixed effects between the 2 groups. Post hoc analyses with the Bonferroni correction were performed for multiple comparisons when variables with repeated measures showed significant differences between groups. The statistical analyses were performed with SPSS 20.0 software (SPSS Inc., Chicago, IL) and P values less than 0.05 were considered statistically significant.
RESULTS
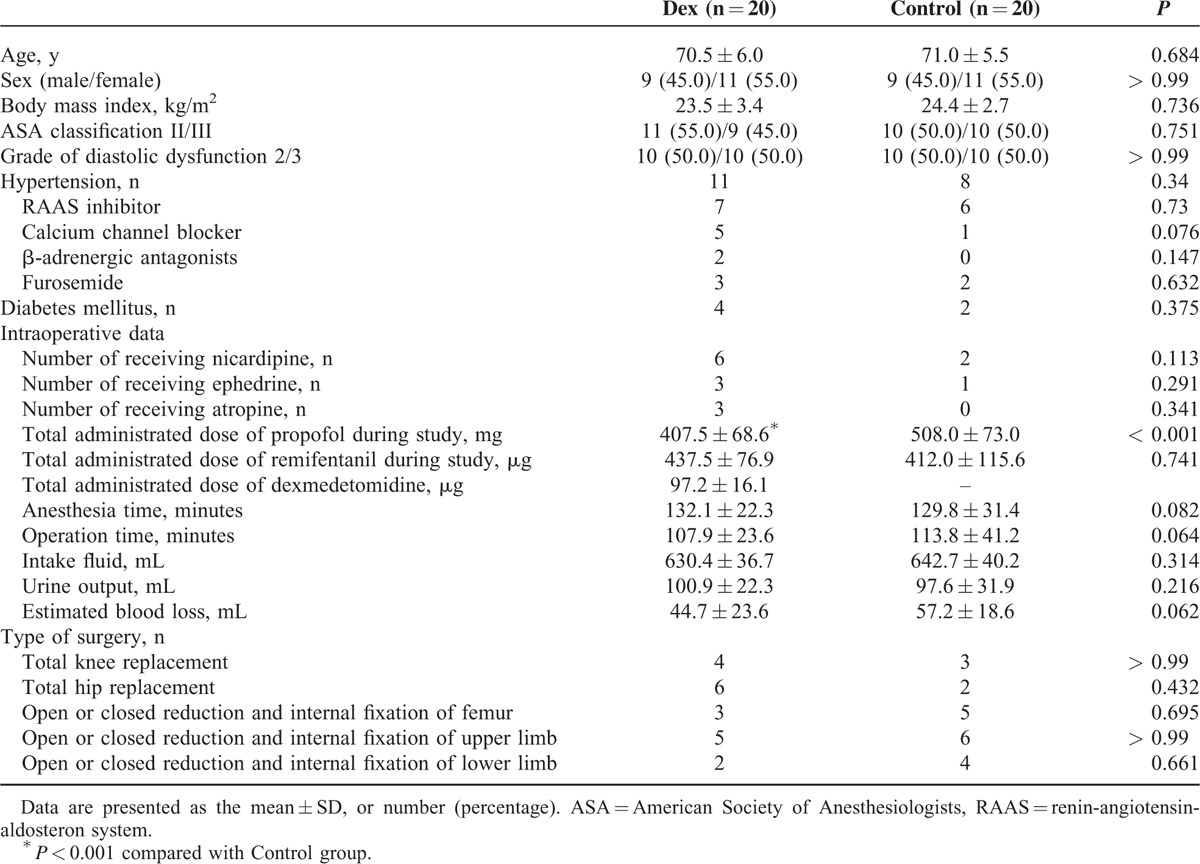
A total of 48 patients were assigned for eligibility. Diastolic dysfunction was confirmed by preoperative echocardiography in 32 patients and transthoracic echocardiography in 16 patients. Six patients did not meet the inclusion criteria, and 2 patients refused participation in this study. Therefore, each group included 20 randomly assigned patients and a total of 40 patients were enrolled in this study (Figure 2). There were no significant demographic characteristic differences between the 2 groups (Table 2). The administration of vasoactive drugs during surgery was more frequent in the Dex group, but not significant different between the 2 groups. In the Dex group, the total administered dose of propofol was significantly decreased, while that of remifentanil was not different between the 2 groups.
FIGURE 2.
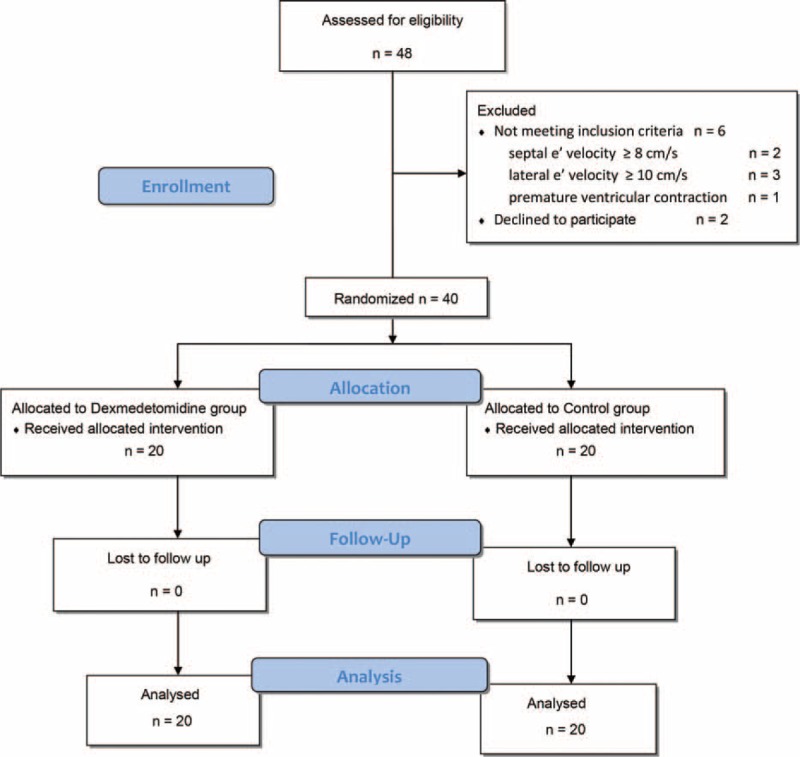
CONSORT flow chart. e′ = peak early diastolic mitral annular velocity.
TABLE 2.
Baseline Demographic and Clinical Characteristics
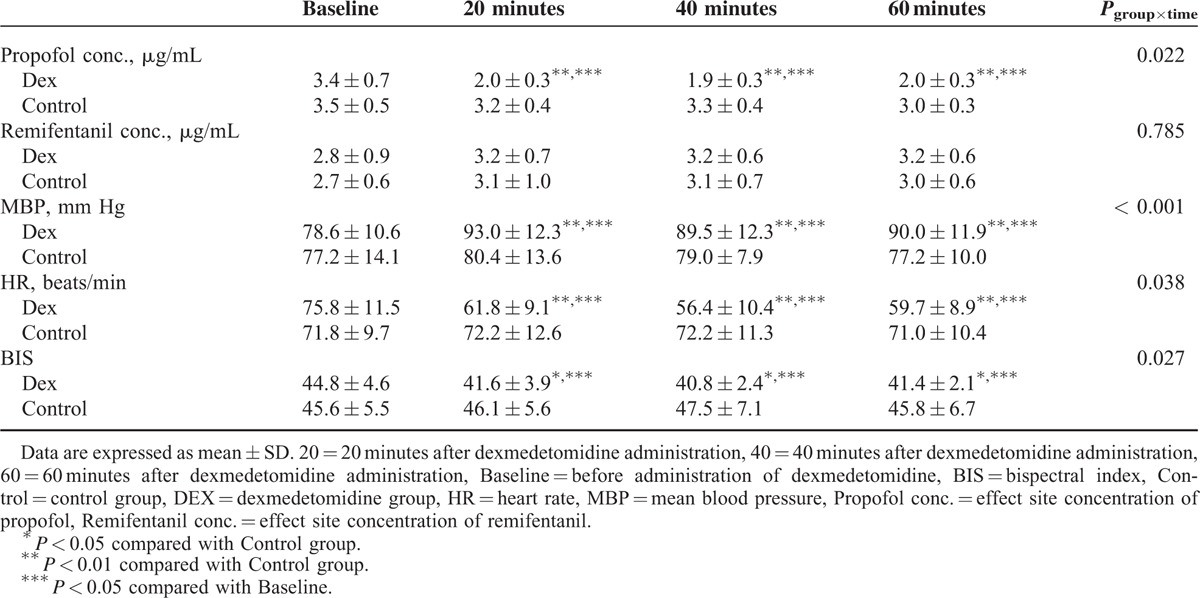
As shown in Table 3, significant differences in MBP, HR, BIS, and effect site concentration of propofol were found between the 2 groups using linear mixed model analyses (P < 0.001, P = 0.038, P = 0.027, and P = 0.022, respectively). MBP of the Dex group was significantly higher than that of the Control group (P < 0.01, respectively), while HR was lower in the Dex group than that of the Control group after 20, 40, and 60 minutes (P < 0.01, respectively).
TABLE 3.
Effect Site Concentration of Anesthetics, Hemodynamics, and BIS Score
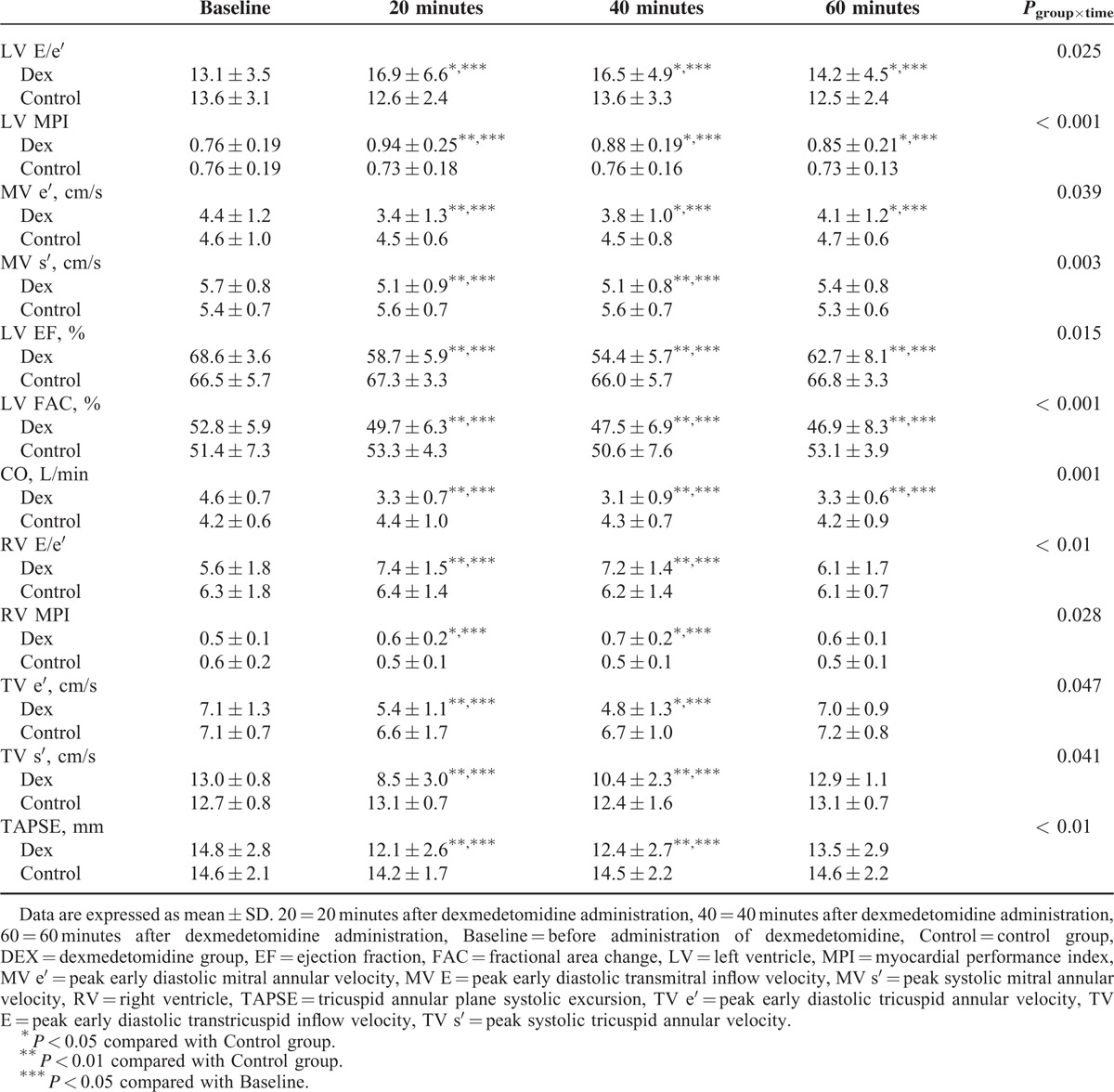
The Dex group showed a significant increase of LV and RV MPI (P < 0.001 and P = 0.028, respectively; Figure 3 and Table 4). As shown in Table 4, the Dex group presented a significant increase of LV E/e′ (P = 0.025), and a significant decrease of MV e′, MV s′, EF, FAC, and cardiac output compared to the Control group (P = 0.039, P = 0.003, P = 0.015, P < 0.001, and P < 0.001, respectively). The Dex group presented a significant increase in RV E/e′ (P < 0.01), and a significant decrease of TV e′, TV s′, and TAPSE (P = 0.047, P = 0.041, and P < 0.01, respectively). There was a significant increase of RV E/e′ and a decrease of TV e′ in the Dex group compared to the Control group after 20 and 40 minutes. RV MPI of the Dex group significantly increased compared to the Control group after 20 and 40 minutes. Also, the Dex group presented decreased TV s′ and TAPSE when compared to the Control group after 20 and 40 minutes.
FIGURE 3.
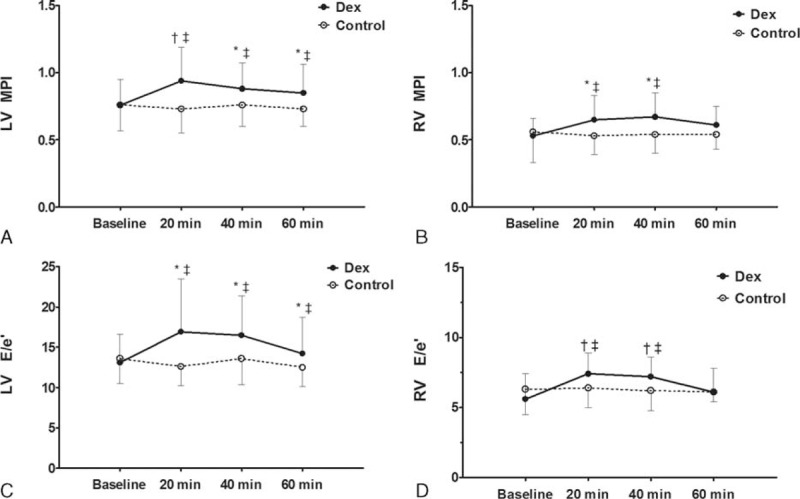
Biventricular function. (A) LV MPI, (B) RV MPI, (C) LV E/e′, and (D) RV E/e′. Data are mean with error bars showing SD. Baseline, before administration of dexmedetomidine; 20 minutes, 20 minutes after dexmedetomidine administration; 40 minutes, 40 minutes after dexmedetomidine administration; 60 minutes, 60 minutes after dexmedetomidine administration. ∗P < 0.05 compared with Control group, †P < 0.01 compared with Control group, and ‡P < 0.05 compared with Baseline. LV E/e′ = the ratio of peak early diastolic transmitral inflow velocity to early diastolic mitral annular velocity, LV MPI = left ventricular myocardial performance index, RV E/e′ = the ratio of peak early diastolic transtricuspid inflow velocity to early diastolic tricuspid annular velocity, RV MPI = right ventricular myocardial performance index.
TABLE 4.
Echocardiographic Variables of Left and Right Ventricle
DISCUSSION
In this study, the administration of dexmedetomidine to the patients with preexisting diastolic dysfunction resulted in a decrease of HR and increase of MBP, MPI, and E/e′ during general anesthesia. Therefore, dexmedetomidine administration during general anesthesia deteriorated biventricular function in patients with underlying diastolic dysfunction.
E/e′ is a relatively load-independent indicator used to estimate LV filling pressure in patients with EF preserved diastolic dysfunction.12 MPI is a comprehensive way to evaluate systolic and diastolic cardiac function within 1 cardiac cycle.17 In our results, the baseline MPI was prolonged, and dexmedetomidine further augmented biventricular MPI prolongation. Also, LV EF, and MV s′ significantly decreased in the Dex group. In patients with EF preserved diastolic dysfunction, dexmedetomidine significantly depressed systolic function as well as diastolic function. Accordingly, when administering dexmedetomidine to patients with diastolic dysfunction, we propose the need to adjust drug dosage and further study is needed to evaluate the relationship between decreased dosage of dexmedetomidine and cardiac function. In our previous study7 of healthy young patients without cardiac dysfunction, transient blood pressure elevation occurred only directly after dose loading of dexmedetomidine. Moreover, the transient increase of blood pressure after loading dexmedetomidine did not affect biventricular diastolic and systolic function. In comparison, in our current study on patients with diastolic dysfunction, the increase of blood pressure persisted throughout the entire dexmedetomidine administration. With this sort of hemodynamic change, dexmedetomidine could induce a rise of LV afterload and have effects on LV relaxation and filling in patients with diastolic dysfunction, which is evidenced by the decrease of e′ and increase of E/e′. These results can be explained by the physiology of diastolic dysfunction. In a diastolic impaired heart, the increased afterload delays onset of relaxation and increases isovolumic relaxation time.20 Although a normal heart is able to react to elevated afterload without change in LV end-systolic volume,21 a heart with decreased afterload reservoir shows marked deterioration of LV relaxation in response to even a slight increase of afterload, thus resulting in an increase of LV systolic and diastolic volume.22
Interestingly, this study revealed that most patients with LV diastolic dysfunction also accompanied RV systolic and diastolic dysfunction. In patients with LV diastolic dysfunction, decreased TV e′ (7.1 ± 1.3 at baseline; normal value, 14.5 ± 3.5)23 represents a depressed RV diastolic function. Because RV is a thin-walled and retrosternal structure, it is difficult to completely visualize RV in a single echocardiographic view. The parameters derived from tissue Doppler imaging are valuable in estimating RV function,24 especially RV MPI can be considered to have powerful prognostic value.25 RV diastolic dysfunction is due to ventricular interdependence as the geometric shape of 1 ventricle directly affects the contralateral ventricle through the septum.26 Elevated LV end-diastolic pressure in patients with chronic LV diastolic dysfunction causes pulmonary venous hypertension and the raised pulmonary vascular resistance causes pulmonary artery hypertension. Pulmonary artery hypertension evokes a rise in RV afterload and subsequently results in RV systolic failure.27 Unfortunately, our study was clinically based, thus we could not evaluate pulmonary artery pressure. Therefore, we were unable to confirm this systematic mechanism of impaired RV function after dexmedetomidine administration.
Another interesting findings made through this study was that the Dex group presented more cases of increased MBP requiring nicardipine administration compared to the Control. It is known that the initial transient increase of blood pressure induced by dexmedetomidine mainly involves vasoconstriction due to vascular smooth muscle contraction by the activation of peripheral α2B-adrenoreceptors.28 This contraction state of the vascular smooth muscles is regulated by Ca2+-dependent29 or Ca2+-sensitization mechanism.30 The vasodilation induced by dexmedetomidine is due to the action of endothelial nitric oxide synthase (eNOS) within the vascular endothelium, and the vasodilation negates the initial vasoconstriction during dexmedetomidine administration.31 However, in patients with diastolic dysfunction, there is a deficit of NO production or action.32 A recent experimental study revealed a deficit in eNOS is largely related to diastolic dysfunction.33 Thus, while the responses to dexmedetomidine of the endothelial components of the blood vessels are suppressed, the response of Ca2+-dependent peripheral vasoconstriction is sustained during dexmedetomidine administration. In literature, there were conflicts of results in changes of blood pressure due to dexmedetomidine administration. In a meta-analysis of randomized, controlled trials of dexmedetomidine in noncardiac surgery, incidence of perioperative hypotension increased.34 On the contrary, in a large cohort study, dexmedetomidine administration did not inflict significant intraoperative hypotension.35 However, these previous studies did not characterize cardiac function of the participants. Therefore, further studies are needed to evaluate the hemodynamic impact of dexmedetomidine administration on not only patients with diastolic dysfunction but atherosclerosis, diabetes and other diseases correlated with abnormal NO production.
The present study might have several limitations. First, since this study was aimed to evaluate the net cardiac performance during dexmedetomidine administration, we minimally controlled the changes of blood pressure within clinically acceptable ranges. We could not differentiate whether dexmedetomidine directly impaired cardiac function or indirectly depressed cardiac function by increase of afterload according to the increase of blood pressure through this study. Thereby, further study regarding the causal relationship between the 2 issues is needed. Second, in this study, dexmedetomidine was used as an adjuvant agent to general anesthesia. Thus, we cannot generalize the results of this study to assume the same results on cardiac function in the case of dexmedetomidine as the sole sedative. We applied general anesthesia using propofol and remifentanil as the baseline anesthetics and monitored the depth of anesthesia by BIS. There is controversy in the effects of intravenous anesthetics on diastolic dysfunction. Propofol administration has been shown to depress MV e′ and subsequently lead to impaired diastolic function in patients with normal cardiac function.36 However, in patients with preexisting diastolic dysfunction, propofol administration did not further aggravate diastolic function.37 Remifentanil did not impair systolic or diastolic function in healthy patients.38 Further study is needed to evaluate the effects of dexmedetomidine as the sole sedative on cardiac function. Third, we could not calculate pulmonary vascular resistance and pulmonary wedge pressure, since we were unable to insert a pulmonary artery catheter due to ethical issues. Therefore, we could not confirm whether decrease in RV function was due to the changes of pulmonary vascular resistance or to direct RV depressant effects of dexmedetomidine. Further study will be needed to assess the direct effects of dexmedetomidine on pulmonary vasculature.
In conclusion, intraoperative dexmedetomidine administration during general anesthesia induced a sustained increase of blood pressure and a deterioration of biventricular function assessed by tissue Doppler imaging, in patients with diastolic dysfunction. Since dexmedetomidine administration has the possibility of aggravating cardiac function in patients with diastolic dysfunction, we suggest careful consideration of its use or a need for reducing its dosage when administrating dexmedetomidine in patients with diastolic dysfunction.
Acknowledgements
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2059283).
Footnotes
Abbreviations: BIS = bispectral index, Dex = dexmedetomidine group, EF = ejection fraction, FAC = fractional area change, HR = heart rate, LV = left ventricle, MBP = mean blood pressure, MPI = myocardial performance index, MV e′ = peak early diastolic mitral annular velocity, MV s′ = peak systolic mitral annular velocity, RV = right ventricle, TAPSE = tricuspid annular plane systolic excursion, TV E = peak early diastolic transtricuspid inflow velocity, TV e′ = peak early diastolic tricuspid annular velocity, TV s′ = peak systolic tricuspid annular velocity.
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2059283).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sairaku A, Yoshida Y, Hirayama H, et al. Procedural sedation with dexmedetomidine during ablation of atrial fibrillation: a randomized controlled trial. Europace 2014; 16:994–999. [DOI] [PubMed] [Google Scholar]
- 2.Ohtani N, Kida K, Shoji K, et al. Recovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgery. Anesth Analg 2008; 107:1871–1874. [DOI] [PubMed] [Google Scholar]
- 3.Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation 2013; 127:1576–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji F, Li Z, Young N, et al. Perioperative dexmedetomidine improves mortality in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth 2014; 28:267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall JE, Uhrich TD, Barney JA, et al. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg 2000; 90:699–705. [DOI] [PubMed] [Google Scholar]
- 6.Ingersoll-Weng E, Manecke GR, Jr, Thistlethwaite PA. Dexmedetomidine and cardiac arrest. Anesthesiology 2004; 100:738–739. [DOI] [PubMed] [Google Scholar]
- 7.Lee SH, Choi YS, Hong GR, et al. Echocardiographic evaluation of the effects of dexmedetomidine on cardiac function during total intravenous anaesthesia. Anaesthesia 2015; 70:1052–1059. [DOI] [PubMed] [Google Scholar]
- 8.Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003; 289:194–202. [DOI] [PubMed] [Google Scholar]
- 9.Flu WJ, van Kuijk JP, Hoeks SE, et al. Prognostic implications of asymptomatic left ventricular dysfunction in patients undergoing vascular surgery. Anesthesiology 2010; 112:1316–1324. [DOI] [PubMed] [Google Scholar]
- 10.Hillis GS, Moller JE, Pellikka PA, et al. Noninvasive estimation of left ventricular filling pressure by E/e′ is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol 2004; 43:360–367. [DOI] [PubMed] [Google Scholar]
- 11.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009; 10:165–193. [DOI] [PubMed] [Google Scholar]
- 12.Kasner M, Westermann D, Steendijk P, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 2007; 116:637–647. [DOI] [PubMed] [Google Scholar]
- 13.Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol 1995; 26:135–136. [PubMed] [Google Scholar]
- 14.Poulsen SH, Nielsen JC, Andersen HR. The influence of heart rate on the Doppler-derived myocardial performance index. J Am Soc Echocardiogr 2000; 13:379–384. [DOI] [PubMed] [Google Scholar]
- 15.Moller JE, Poulsen SH, Egstrup K. Effect of preload alternations on a new Doppler echocardiographic index of combined systolic and diastolic performance. J Am Soc Echocardiogr 1999; 12:1065–1072. [DOI] [PubMed] [Google Scholar]
- 16.McIlroy DR, Lin E, Durkin C. Intraoperative transesophageal echocardiography: a critical appraisal of its current role in the assessment of diastolic dysfunction. J Cardiothorac Vasc Anesth 2015; 29:1033–1043. [DOI] [PubMed] [Google Scholar]
- 17.Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: a simple and reproducible measure of cardiac function—a study in normals and dilated cardiomyopathy. J Cardiol 1995; 26:357–366. [PubMed] [Google Scholar]
- 18.Tei C, Dujardin KS, Hodge DO, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr 1996; 9:838–847. [DOI] [PubMed] [Google Scholar]
- 19.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 20.Gillebert TC, Sys SU, Brutsaert DL. Influence of loading patterns on peak length-tension relation and on relaxation in cardiac muscle. J Am Coll Cardiol 1989; 13:483–490. [DOI] [PubMed] [Google Scholar]
- 21.Gillebert TC, Leite-Moreira AF, De Hert SG. The hemodynamic manifestation of normal myocardial relaxation. A framework for experimental and clinical evaluation. Acta Cardiol 1997; 52:223–246. [PubMed] [Google Scholar]
- 22.De Hert SG, Gillebert TC, Ten Broecke PW, et al. Contraction-relaxation coupling and impaired left ventricular performance in coronary surgery patients. Anesthesiology 1999; 90:748–757. [DOI] [PubMed] [Google Scholar]
- 23.Lindqvist P, Waldenstrom A, Henein M, et al. Regional and global right ventricular function in healthy individuals aged 20–90 years: a pulsed Doppler tissue imaging study: Umea General Population Heart Study. Echocardiography 2005; 22:305–314. [DOI] [PubMed] [Google Scholar]
- 24.Jurcut R, Giusca S, La Gerche A, et al. The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr 2010; 11:81–96. [DOI] [PubMed] [Google Scholar]
- 25.Vizzardi E, D’Aloia A, Bordonali T, et al. Long-term prognostic value of the right ventricular myocardial performance index compared to other indexes of right ventricular function in patients with moderate chronic heart failure. Echocardiography 2012; 29:773–778. [DOI] [PubMed] [Google Scholar]
- 26.Saleh S, Liakopoulos OJ, Buckberg GD. The septal motor of biventricular function. Eur J Cardiothorac Surg 2006; 29:S126–S138. [DOI] [PubMed] [Google Scholar]
- 27.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation 2000; 102:1718–1723. [DOI] [PubMed] [Google Scholar]
- 28.Talke P, Lobo E, Brown R. Systemically administered alpha2-agonist-induced peripheral vasoconstriction in humans. Anesthesiology 2003; 99:65–70. [DOI] [PubMed] [Google Scholar]
- 29.Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 1: basic mechanisms controlling cytosolic Ca2+ concentration and the Ca2+-dependent regulation of vascular tone. J Anesth 2007; 21:220–231. [DOI] [PubMed] [Google Scholar]
- 30.Akata T. Cellular and molecular mechanisms regulating vascular tone. Part 2: regulatory mechanisms modulating Ca2+ mobilization and/or myofilament Ca2+ sensitivity in vascular smooth muscle cells. J Anesth 2007; 21:232–242. [DOI] [PubMed] [Google Scholar]
- 31.Wong ES, Man RY, Vanhoutte PM, et al. Dexmedetomidine induces both relaxations and contractions, via different alpha 2-adrenoceptor subtypes, in the isolated mesenteric artery and aorta of the rat. J Pharmacol Exp Ther 2010; 335:659–664. [DOI] [PubMed] [Google Scholar]
- 32.Paulus WJ, Shah AM. NO and cardiac diastolic function. Cardiovasc Res 1999; 43:595–606. [DOI] [PubMed] [Google Scholar]
- 33.Vignon-Zellweger N, Relle K, Kienlen E, et al. Endothelin-1 overexpression restores diastolic function in eNOS knockout mice. J Hypertens 2011; 29:961–970. [DOI] [PubMed] [Google Scholar]
- 34.Biccard BM, Goga S, de Beurs J. Dexmedetomidine and cardiac protection for non-cardiac surgery: a meta-analysis of randomised controlled trials. Anaesthesia 2008; 63:4–14. [DOI] [PubMed] [Google Scholar]
- 35.Klinger RY, White WD, Hale B, et al. Hemodynamic impact of dexmedetomidine administration in 15,656 noncardiac surgical cases. J Clin Anesth 2012; 24:212–220. [DOI] [PubMed] [Google Scholar]
- 36.Filipovic M, Wang J, Michaux I, et al. Effects of halothane, sevoflurane and propofol on left ventricular diastolic function in humans during spontaneous and mechanical ventilation. Br J Anaesth 2005; 94:186–192. [DOI] [PubMed] [Google Scholar]
- 37.Filipovic M, Michaux I, Wang J, et al. Effects of sevoflurane and propofol on left ventricular diastolic function in patients with pre-existing diastolic dysfunction. Br J Anaesth 2007; 98:12–18. [DOI] [PubMed] [Google Scholar]
- 38.Bolliger D, Seeberger MD, Kasper J, et al. Remifentanil does not impair left ventricular systolic and diastolic function in young healthy patients. Br J Anaesth 2011; 106:573–579. [DOI] [PubMed] [Google Scholar]


