Supplemental Digital Content is available in the text
Abstract
Detection of high-risk subjects in acute myocardial infarction (AMI) by noninvasive means would reduce the need for intracardiac catheterization and associated complications. Liver enzymes are associated with cardiovascular disease risk. A potential predictive value for liver serum markers for the severity of stenosis in AMI was analyzed.
Patients with AMI undergoing percutaneous coronary intervention (PCI; n = 437) were retrospectively evaluated. Minimal lumen diameter (MLD) and percent stenosis diameter (SD) were determined from quantitative coronary angiography. Patients were classified according to the severity of stenosis (SD ≥ 50%, n = 357; SD < 50%, n = 80). Routine heart and liver parameters were associated with SD using random forests (RF). A prediction model (M10) was developed based on parameter importance analysis in RF.
Age, alkaline phosphatase (AP), aspartate aminotransferase (AST), and MLD differed significantly between SD ≥ 50 and SD < 50. Age, AST, alanine aminotransferase (ALT), and troponin correlated significantly with SD, whereas MLD correlated inversely with SD. M10 (age, BMI, AP, AST, ALT, gamma-glutamyltransferase, creatinine, troponin) reached an AUC of 69.7% (CI 63.8–75.5%, P < 0.0001).
Routine liver parameters are associated with SD in AMI. A small set of noninvasively determined parameters can identify SD in AMI, and might avoid unnecessary coronary angiography in patients with low risk. The model can be accessed via http://stenosis.heiderlab.de.
INTRODUCTION
Cardiovascular diseases (CVD) and acute myocardial infarction (AMI) are responsible for about 17.5 million of worldwide deaths per year and are the leading cause of death globally.1 The prognosis of patients surviving the AMI depends on the amount of myocardium that undergoes irreversible injury, that is, the infarct size.2 Early reperfusion is the gold standard for therapy in AMI and the only way to reduce infarct size. However, reperfusion can induce an additional damage as reperfusion injury.3 To identify the extent of stenosis in coronary vessels and thus the necessity for a percutaneous coronary intervention (PCI) in AMI, a cardiac catheterization has to be performed. This procedure is invasive and not without risk for the patients (eg, aortic dissection, aneurysm, arrhythmia, etc.).4 Assessment of the stenosis severity with noninvasive means, that is, based on serum markers of cardiovascular injury, would spare patients without the need for an intervention.
Besides traditional cardiovascular risk factors, clinical studies indicated a potential link between liver disease, primarily nonalcoholic fatty liver disease (NAFLD), and CVD. NAFLD is by now accepted as hepatic manifestation of metabolic syndrome5 and is associated with insulin resistance,6 type 2 diabetes,7,8 and CVD.9,10 NAFLD patients may also have a higher prevalence of subclinical atherosclerosis, independent of the established cardiovascular risk factors.11 To assess subclinical atherosclerosis potent noninvasive procedures are available, such as carotid intima media thickness measurement, brachial artery flow mediated dilatation, and arterial stiffness.12,13 Using coronary imaging, such as multislice computed tomography, studies have also shown, that NAFLD was significantly related to lipid core14 calcified plaques.15–17 Apart from this, elevation of common markers of liver injury [gamma-glutamyltransferase (GGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AP), and bilirubin] are associated with the risk of CVD.18–21 However, it is currently unknown, if liver enzyme concentrations are associated with the severity of a stenosis in AMI.
In the present retrospective study, we have focused on patients with AMI undergoing PCI and aimed to predict the necessity for a PCI with minimally invasive measures. Due to the emergency situation of AMI, quantitative coronary analysis (QCA) remained the only coronary imaging method to assess the severity of the stenosis. To evaluate the use of possible noninvasive predictors in AMI we compared patients with AMI undergoing PCI with a percent stenosis diameter (SD) ≥ 50% and with SD < 50% using QCA. Correlations were calculated for demographic data and classic serum parameters for heart and liver diseases with the SD using a machine learning approach (random forests). Based on those (noninvasive) parameters which were identified as most important by the random forests, a prediction model was developed to identify high-risk subjects in need for PCI.
MATERIALS AND METHODS
Ethics Statement
The study protocol conformed to the ethical guidelines of 1975 Declaration of Helsinki and was approved by the Institutional Review Board (Ethik-Kommission der Medizinischen Fakultät der Universität Duisburg-Essen; Germany; reference number 15-6356-BO). Due to the retrospective nature of the study, the Institutional Review Board cancelled the need for written informed consent.
Study Design and Sample Acquisition
In a retrospective single-center study, a cohort of 437 patients was enrolled [body mass index (BMI): 27.8 ± 0.2 kg/m2; age: 64.8 ± 0.6; males/females: 306 (70.0%)/131 (30.0%)]. All patients had an AMI and underwent coronary angiography in the catheterization laboratory of the West German Heart and Vascular Centre Essen, University Hospital Essen between January 2009 and June 2014. AMI was defined as a troponin value above the 99th percentile of the upper reference level and either with a ST-segment elevation or new left bundle-branch block on the electrocardiogram (STEMI, n = 176)22 or without an ST-segment elevation or new left bundle-branch block on the electrocardiogram (NSTEMI, n = 261).23 Patients were classified according to their calculated SD in 2 groups: SD ≥ 50% (n = 357) and SD < 50% (n = 80). Serum parameters were determined in the central laboratory unit of the University Hospital Essen (Department of Clinical Chemistry and Laboratory Medicine) by standardized methods. Exclusion criteria were a high-grade aortic valve disease, cardiomyopathy, cardiac allograft vasculopathy, endocarditis, hypertensive emergency, myocarditis, pericarditis, tachyarrhythmia absoluta by atrial fibrillation, coronary vasospasm, and survival of sudden cardiac death. In addition, patients with a coronary artery disease after coronary artery bypass graft were excluded from the present study (see Supplementary Figure 1). Patients with noncardiac reasons of troponin elevation were excluded: acute neurological disease (including stroke or cerebral hemorrhage), acute pulmonary embolism, aortic dissection, diseases like amyloidosis, sarcoidosis, or scleroderma, inflammatory myopathies (ie, polymyositis, dermatomyositis), sepsis, and patients, who were on cardiotoxic medication (adriamycin, 5-flurouracil, herceptin).
Quantitative Coronary Angiography
All patients received oral acetylsalicylic acid (100 mg/day) and underwent PCI. All measurements were performed at the Angiography Core Laboratory at the West German Heart and Vascular Centre Essen, University Hospital, University Duisburg-Essen. Coronary angiography was performed using the femoral approach and 6 or 8 F guiding catheters. Stenosis severity was quantified using off-line caliper measurements (QCA-MEDIS, Leiden, NL) before stent implantation.24 The diameter of the catheter tip was measured with digital calipers and used for image calibration. The reference lumen diameter (RLD) and the most narrow point (ie, minimal lumen diameter (MLD)) were calculated. The SD was defined as follows:
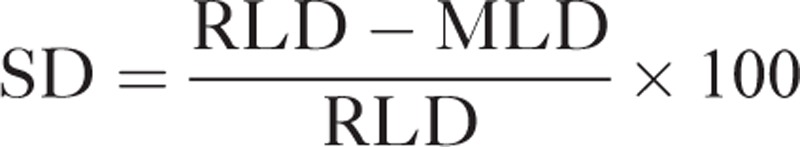 |
Dataset and Statistics
The dataset (437 patients) included the sociodemographic parameters sex and age, BMI, the dichotomous variables STEMI/NSTEMI, smoker/nonsmoker, diabetes mellitus, dyslipidemia, family predisposition, as well as the serum parameters AP, GGT, AST, ALT, C-reactive protein (CRP), bilirubin, creatinine, and troponin. For 302 cases (245 with SD ≥ 50%, 57 with SD < 50%), also information about total cholesterol levels, high-density lipoprotein (HDL), low-density lipoprotein (LDL), and triglyceride levels were available. We refer to these 302 cases as dataset 2.
Predictive Modeling
Statistical data analyses were performed with R (http://www.r-project.org/). All data are presented as mean ± standard error of the mean (SEM) unless specified otherwise. Missing values were imputed by mean imputation. Correlation analysis was performed using Spearman correlation coefficient r.
For building up predictive models, random forests (RFs) implemented in the randomForest package of R were used. An RF is an ensemble learning method that can be used for classification as well as regression,25 which has gained popularity in the recent years.26–28 RFs are classifiers consisting of a collection of decision trees (DTs) that are combined via majority vote. When using the trained RF for prediction, an unseen instance was assigned to the positive class voted for by at least 50% of the trees. Besides being highly accurate classifiers, RFs can be used to estimate variable importance, for example, by measuring the mean decrease in Gini impurity. The importance was calculated based on 100 individual RF models. Due to the imbalance in the dataset, we also build models with repeated (100 times) subsampling.
For evaluation of the classifier performance, a 100-fold leave-one-out cross-validation scheme was used and the receiver operation characteristics (ROC) curve and the corresponding area under the curve (AUC) were calculated with pROC.29 The 95% confidence interval (CI) was computed with 2000 stratified bootstrap replicates. The ROC curve was built by plotting the sensitivity against the specificity for every possible cut-off between the 2 classes. The significance Pσ of the AUC values was calculated based on permutation tests (n = 1000). PU values for comparison between classifiers are based on the Mann–Whitney U test.
RESULTS
Patient Characteristics and Basic Parameters
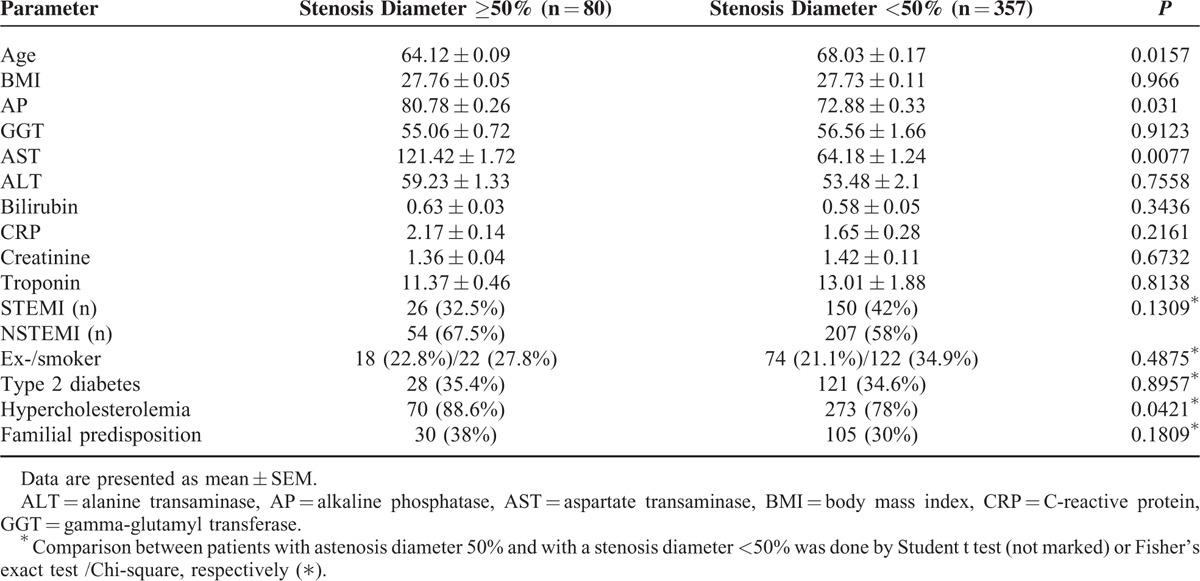
Detailed data of the included patients can be found in Table 1, comprising distribution of demographic parameters (age, BMI) as well as standard parameters of heart (troponin), renal (creatinine), liver damage (AP, GGT, AST, ALT, bilirubin), and risk factors (smoking, diabetes, etc.). The data set was divided into 2 groups, 1 containing patients with a SD ≥ 50% and 1 containing patients with a SD < 50%. This cut-off usually indicates necessity for an intervention (ie, balloon dilation or placement of a stent). For both datasets only a small number of values had to be imputed. For dataset 1 (without HDL, LDL, cholesterol, and triglycerides) the following numbers of values were missing and imputed: BMI: 25 cases (5.7%), smoker/nonsmoker, diabetes mellitus, dyslipidemia, family predisposition (each): 8 cases (1.8%), AP: 52 cases (11.9%), GGT: 1 case (0.2%), bilirubin: 35 cases (8%), CRP: 14 cases (3.2%), creatinine: 6 cases (1.4%), and troponin: 4 cases (0.9%). For dataset 2 (with HDL, LDL, cholesterol, and triglycerides) the following numbers of values were missing and imputed: BMI: 7 cases (2.3%), AP: 14 cases (7.9%), GGT: 1 case (0.3%), bilirubin: 21 cases (6.9%), and CRP: 7 cases (2.3%). Patients with a SD ≥ 50% were younger and had significantly higher AP and AST levels, whereas BMI, ALT, GGT, bilirubin, CRP, troponin, and creatinine did not differ between the groups (Table 1).
TABLE 1.
Demographic and Basic Parameters of the Patient Cohort
Angiographic Data of Minimal Lumen Diameter (MLD), Reference Lumen Diameter (RLD), and Stenosis Diameter (SD) Before Stent Implantation
The RLD did not differ between patients with a SD ≥ 50 (2.84 ± 0.74 mm) and those with a SD < 50 (2.92 ± 0.78 mm) before stent implantation. The group separation by SD resulted in an artificially significant difference in MLD (SD ≥ 50: 0.55 ± 0.46 mm; SD < 50: 1.93 ± 0.67 mm; P < 0.0001) as well as SD (SD ≥ 50: 80.4 ± 0.8%; SD < 50: 34.6 ± 0.5%; P < 0.0001).
Stenosis Diameter and Noninvasively Determined Parameters Correlate Significantly
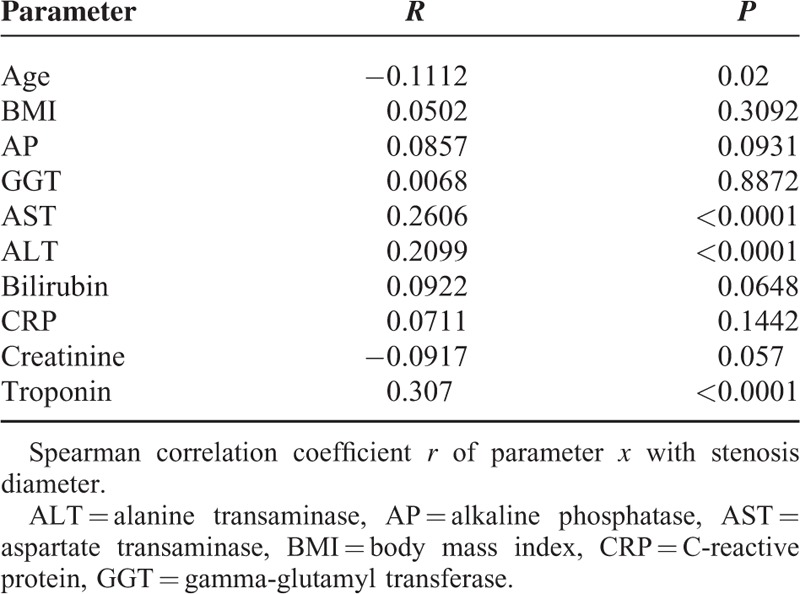
Significant correlations between SD and age (r = −0.1112, P = 0.02), AST (r = 0.2606, P < 0.0001), ALT (r = 0.2099, P < 0.0001), and troponin (r = 0.3104, P < 0.0001) were found. As expected, MLD correlated inversely with SD before stent implantation (Supplementary Figure 2). Other noninvasive parameters did not exhibit significant correlations with the SD (Table 2).
TABLE 2.
Correlation of Noninvasively Determined Parameters With Diameter Stenosis
High Importance of Liver Serum Parameters for Prediction of Stenosis Diameter
As serum liver markers were significantly correlated to the SD, an RF importance analysis for classification into SD ≥ 50 and SD < 50 was performed. A high importance (≥10) of the variables age (12.16 ± 0.03), BMI (13.40 ± 0.03), as well as the serum parameters AP (15.81 ± 0.04), GGT (10.41 ± 0.02), AST (10.98 ± 0.02), ALT (11.84 ± 0.03), creatinine (13.40 ± 0.03), and troponin (15.20 ± 0.03) were identified. The serum parameters bilirubin and CRP showed a relatively high importance (importance >5), while other parameters did not exhibit a high importance for the RF classification and thus were excluded from further analyses (Figure 1).
FIGURE 1.
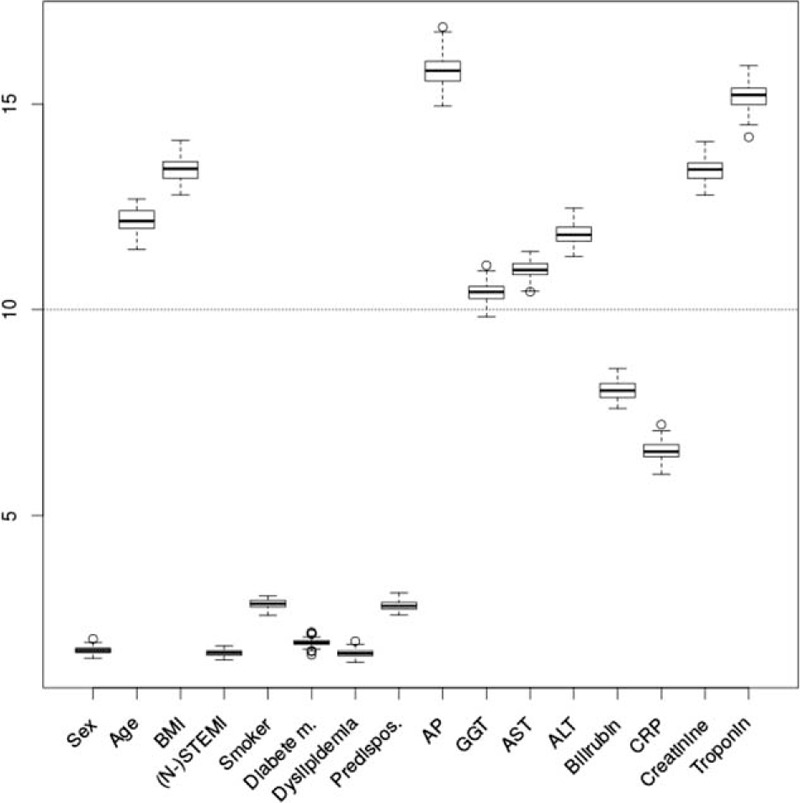
Importance analysis. The y-axis show the estimated importance by mean decrease in Gini impurity for the different parameters (x-axis). ALT = alanine transaminase, AP = alkaline phosphatase, AST = aspartate transaminase, BMI = body mass index, CRP = C-reactive protein, GGT = gamma-glutamyl transferase, NSTEMI = non-ST elevation myocardial infarction, STEMI = ST elevation myocardial infarction.
New Diagnostic Model for Prediction of Stenosis Diameter
Based only on parameters with an importance ≥10 (age, BMI, AP, GGT, AST, ALT, creatinine, troponin) a prediction model (M10) was developed. M10 reached an AUC of 69.7% CI 63.8–75.5% (Pσ < 0.0001) (Figure 2). Addition of the less important parameters (bilirubin, CRP), did not improve the model (M5) in terms of AUC. In fact, the AUC value of M5 was slightly, but significantly lower (Pσ < 0.0001, PU = 0.0015). The AUC gives an overview of the general performance of a classifier. Certain specificities and corresponding sensitivities can be read out directly from the ROC curve, for example, the sensitivity of M10 at a specificity of 90% and 95% is 38.5% and 32.1%, respectively. Repeated subsampling did not improve the prediction performance of the classifiers. In dataset 2, the univariate analyses essentially led to the same results. However, age (P = 0.0745) was no longer significantly correlated with SD. This may be due to the smaller number of patients in dataset 2. Total cholesterol levels, HDL, LDL, and triglyceride levels were not significantly correlated with the SD and no significant differences between SD ≥ 50 and SD < 50 were found. Next, we trained RF models on dataset 2: (i) with the parameters of M10, and (ii) with parameters from M10 and total cholesterol levels, HDL, LDL, and triglyceride levels. We found no significant differences in terms of AUC between (i) and (ii).
FIGURE 2.
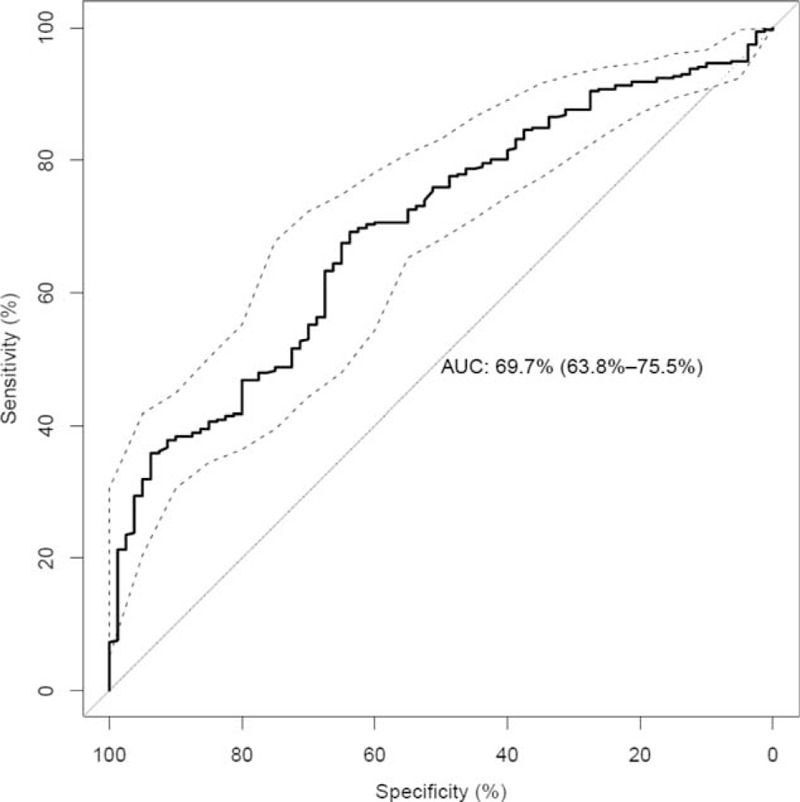
Performance of prediction model (M10) for the prediction of stenosis diameter. On the y-axis the sensitivity and on the x-axis the specificity is shown. The ROC curve is shown as a bold solid line. The AUC of M10 is 69.7% (CI 63.8–75.5%). The confidence interval is shown as dashed lines. The dotted line marks the performance of random guessing.
DISCUSSION
In the present retrospective study, we have investigated the correlation of demographic data and specific serum parameters with the SD in patients with AMI undergoing PCI using a machine learning approach. Based on parameters with an importance of ≥10 in the RF analyses, a prediction model with an AUC of 69.7% was developed. Besides age and troponin, liver transaminases (ALT and AST), and AP were identified as highly important for discrimination of patients with SD ≥ 50 and SD < 50.
The extent and severity of stable CVD is reported to be lower in younger populations,30 and the incidence of ST segment elevation myocardial infarction increases linearly with the age in men, but exponentially in women.31 In contrast, in the presented cohort age was inversely correlated to SD, implying that younger patients had more severe stenosis. In line with previous studies troponin was associated with a higher SD in the present study. Elevated levels of troponin are associated with coronary artery calcification in patients with mild CVD32,33 as well as in non-ST-elevation myocardial infarction.34
It was also possible to confirm an association between the liver transaminases (ALT, AST) and the severity of the SD.19 In a previous study a positive correlation was observed between liver transaminases and the severity of CVD in women, but not in men.35 Saely et al36 also investigated the possible association of ALT and AST with angiographically determined CVD and the presence of metabolic syndrome. A significant association was identified between ALT and ALT/AST ratio with metabolic syndrome, but there was no association between liver transaminases and angiographically determined CVD. This might be due to the currently set normal ranges, which might be too high to detect ongoing liver injury in a metabolic setting.37 Apart from liver diseases reduced arterial perfusion or congestion due to (acute) cardiac failure can also affect liver serum parameters. Within our cohort 100 patients (23%) with AST or ALT concentrations >100 U/L were found, though only 12 of these exhibited signs of right heart burden in echocardiography (Supplementary Table 1). Though, the majority of patients with elevated serum liver enzymes exhibited either confirmed liver disease or signs of metabolic syndrome, which would suggest a NAFLD-type liver injury. Taken together works of other groups and our data suggest that liver damage precedes cardiac manifestations in metabolic syndrome in most cases.
In the present study, AP was identified as the variable with the highest importance to predict SD ≥ 50 or SD < 50. However, this was only detected by the RF approach as the univariate analysis was not able to show a significant association between AP and the SD. AP has been known as predictor of mortality for patients with CVD, who already underwent successful PCI with drug-eluting stent,38 and for those, who survived AMI.39 AP is associated with CVD risk in elderly men40 and correlated with the severity of CVD.41 A possible mechanistic explanation for the high importance of AP in the presented classification could be related to AP acting as a regulator of vascular calcification.42 Shantouf et al20 found a significant association between high AP and the coronary artery calcification score in a cohort of 137 maintenance hemodialysis patients. AP and CVD could also be connected via inflammatory processes,39 which may derive from adipose tissue inflammation observed in obese patients with metabolic syndrome.43,44 This is supported by association of AP with CRP observed in previous studies.40,45 Thus, AP levels may reflect inflammation of hepatic origin, as CRP is also mainly derived from the liver. In vascular disease, atherosclerosis is associated with inflammatory processes, and in advanced atherosclerotic plaque also increased serum AP were found.45
Study Limitations
The presented study has some limitations that need to be addressed in future works. Besides the relatively small number of patients in the whole cohort, one limiting aspect is the different size of subgroups. The cohort consisted of 357 patients with a SD ≥ 50% and the reference group with a SD < 50% consisted of only 80 samples. To reduce the bias in the classification, a subsampling approach was performed, which randomly selects a subset out of the larger group in similar size as the smaller group (n = 80). However, there were no significant differences in the results between the subsampled and the initial computations. Due to the retrospective nature of the study, there were some missing values, especially within the parameters HDL, LDL, triglyceride, and total cholesterol.
To assess atherosclerosis and the SD in coronary vessels a range of methods is available for patients with subclinical or stable CVD. These methods comprise different noninvasive approaches (carotid intima media thickness measurement, brachial artery flow mediated dilatation, arterial stiffness, and multislice computed tomography) and invasive procedures (intravascular ultrasound, QCA). Due to the special emergency situation of AMI and the retrospective nature of the present study, QCA remained the only coronary imaging to assess the severity of the SD. QCA has important limitations: Only lumen, but not the coronary vessel wall can be visualized. The extent of atheroma within the vessel wall is not reliably determined by standard angiographic techniques. Moreover, lumen size is a relatively crude measure of atherosclerotic disease, especially in patients with only mild stenotic lesions.46 Though, in the presented setting (emergency) no other coronary imaging method was feasible. This may change, when noninvasive parameters could predict the severity of stenosis and thus enable a larger time frame until PCI needs to be performed in some AMI cases with less severe stenosis.
Another limitation occurring due to the retrospective nature in a cardiologic emergency setting is a lack of information on liver diseases. Only serum parameters as surrogate indicators for liver injury were available. While our data hint to a connection of liver injury and severity of AMI in metabolic syndrome the exact association cannot be inferred from this study. When reviewing the medical records of the patient cohort only 6 individuals with established/ liver disease (3 with NAFLD, 3 with alcoholic fatty liver disease) were identified.
CONCLUSIONS
Taken together, age and troponin, but also the classic liver enzymes AST and ALT were significantly correlated with the diameter stenosis in patients with AMI undergoing PCI. This adds to the proposed close link between liver and CVD, especially in metabolic syndrome. Moreover, it was possible to build a predictive model from age, BMI, and 6 noninvasively determined serum parameters to classify patients for a SD of < or ≥50. By using a sensitivity cutoff of 90%, the false negative rate is only 10%. The corresponding specificity of the model is 27%. Thus, those patients that have a severe stenosis are reliably detected with our model, however taking into account a moderate number of patients that would undergo catheterization without a clinical need. We implemented a webserver at http://stenosis.heiderlab.de using the aforementioned sensitivity cutoff of 90%, which can be used to predict SD.
Liver parameters may be relevant factors to predict the severity of stenosis in AMI and to identify high-risk subjects in a noninvasive way, sparing patients with less severe stenosis the dangerous procedure of cardiac catheterization and coronary angiography.
Supplementary Material
Acknowledgment
We would like to thank the Dr. Heinz-Horst-Deichmann Foundation, which provided funding for parts of the presented study.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AMI = acute myocardial infarction, AP = alkaline phosphatase, AST = aspartate aminotransferase, AUC = Area under the Curve, BMI = body mass index, CI = confidence interval, CRP = C-reactive protein, CVD = cardiovascular diseases, DT = decision tree, GGT = gamma-glutamyltransferase, HDL = high-density lipoprotein, LDL = low-density lipoprotein, MLD = minimal lumen diameter, NAFLD = nonalcoholic fatty liver disease, PCI = percutaneous coronary intervention, QCA = quantitative coronary analysis, RF = random forest, RLD = reference lumen diameter, ROC = receiver operation characteristics, SD = stenosis diameter, SEM = standard error of the mean.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.WHO. Cardiovascular diseases (CVDs). WHO at http://www.who.int/mediacentre/factsheets/fs317/en/ Accessed May 19, 2015 [Google Scholar]
- 2.Burns RJ, Gibbons RJ, Yi Q, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol 2002; 39:30–36. [DOI] [PubMed] [Google Scholar]
- 3.Heusch G. Reduction of infarct size by ischaemic post-conditioning in humans: fact or fiction? Eur Heart J 2012; 33:13–15. [DOI] [PubMed] [Google Scholar]
- 4.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:e574–e651. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001; 50:1844–1850. [DOI] [PubMed] [Google Scholar]
- 6.Gastaldelli A, Kozakova M, Højlund K, et al. Fatty liver is associated with insulin resistance, risk of coronary heart disease, and early atherosclerosis in a large European population. Hepatology 2009; 49:1537–1544. [DOI] [PubMed] [Google Scholar]
- 7.Adams LA, Waters OR, Knuiman MW, et al. NAFLD as a risk factor for the development of diabetes and the metabolic syndrome: an eleven-year follow-up study. Am J Gastroenterol 2009; 104:861–867. [DOI] [PubMed] [Google Scholar]
- 8.Targher G, Bertolini L, Poli F, et al. Nonalcoholic fatty liver disease and risk of future cardiovascular events among type 2 diabetic patients. Diabetes 2005; 54:3541–3546. [DOI] [PubMed] [Google Scholar]
- 9.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis 2007; 191:235–240. [DOI] [PubMed] [Google Scholar]
- 10.Kim D, Choi S-Y, Park EH, et al. Nonalcoholic fatty liver disease is associated with coronary artery calcification. Hepatology 2012; 56:605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blackett PR, Sanghera DK. Genetic determinants of cardiometabolic risk: a proposed model for phenotype association and interaction. J Clin Lipidol 2013; 7:65–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozturk K, Uygun A, Guler AK, et al. Nonalcoholic fatty liver disease is an independent risk factor for atherosclerosis in young adult men. Atherosclerosis 2015; 240:380–386. [DOI] [PubMed] [Google Scholar]
- 13.Oni ET, Agatston AS, Blaha MJ, et al. A systematic review: burden and severity of subclinical cardiovascular disease among those with nonalcoholic fatty liver; should we care? Atherosclerosis 2013; 230:258–267. [DOI] [PubMed] [Google Scholar]
- 14.Akabame S, Hamaguchi M, Tomiyasu K-I, et al. Evaluation of vulnerable coronary plaques and non-alcoholic fatty liver disease (NAFLD) by 64-detector multislice computed tomography (MSCT). Circ J 2008; 72:618–625. [DOI] [PubMed] [Google Scholar]
- 15.Santos RD, Nasir K, Conceição RD, et al. Hepatic steatosis is associated with a greater prevalence of coronary artery calcification in asymptomatic men. Atherosclerosis 2007; 194:517–519. [DOI] [PubMed] [Google Scholar]
- 16.Assy N, Djibre A, Farah R, et al. Presence of coronary plaques in patients with nonalcoholic fatty liver disease. Radiology 2010; 254:393–400. [DOI] [PubMed] [Google Scholar]
- 17.Chen C-H, Nien C-K, Yang C-C, et al. Association between nonalcoholic fatty liver disease and coronary artery calcification. Dig Dis Sci 2010; 55:1752–1760. [DOI] [PubMed] [Google Scholar]
- 18.Ruttmann E, Brant LJ, Concin H, et al. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 2005; 112:2130–2137. [DOI] [PubMed] [Google Scholar]
- 19.Masoudkabir F, Karbalai S, Vasheghani-Farahani A, et al. The association of liver transaminase activity with presence and severity of premature coronary artery disease. Angiology 2011; 62:614–619. [DOI] [PubMed] [Google Scholar]
- 20.Shantouf R, Kovesdy CP, Kim Y, et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol 2009; 4:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahabadi AA, Lehmann N, Möhlenkamp S, et al. Association of bilirubin with coronary artery calcification and cardiovascular events in the general population without known liver disease: the Heinz Nixdorf Recall study. Clin Res Cardiol 2014; 103:647–653. [DOI] [PubMed] [Google Scholar]
- 22.Levine GN, Bates ER, Blankenship JC, et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: an Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2015; doi:10.1161/CIR.0000000000000336. [Google Scholar]
- 23.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 130:e344–e426. [DOI] [PubMed] [Google Scholar]
- 24.Haude M, Caspari G, Baumgart D, et al. Comparison of myocardial perfusion reserve before and after coronary balloon predilatation and after stent implantation in patients with postangioplasty restenosis. Circulation 1996; 94:286–297. [DOI] [PubMed] [Google Scholar]
- 25.Breiman L. Random forests. Mach Learn 2001; 45:5–32. [Google Scholar]
- 26.Sowa J-P, Heider D, Bechmann LP, et al. Novel algorithm for non-invasive assessment of fibrosis in NAFLD. PLoS ONE 2013; 8:e62439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heider D, Senge R, Cheng W, et al. Multilabel classification for exploiting cross-resistance information in HIV-1 drug resistance prediction. Bioinformatics 2013; 29:1946–1952. [DOI] [PubMed] [Google Scholar]
- 28.Heider D, Appelmann J, Bayro T, et al. A computational approach for the identification of small GTPases based on preprocessed amino acid sequences. Technol Cancer Res Treat 2009; 8:333–341. [DOI] [PubMed] [Google Scholar]
- 29.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011; 12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan HU, Khan MU, Noor MM, et al. Coronary artery disease pattern: a comparison among different age groups. J Ayub Med Coll Abbottabad 2014; 26:466–469. [PubMed] [Google Scholar]
- 31.Kytö V, Sipilä J, Rautava P. Gender, age and risk of ST segment elevation myocardial infarction. Eur J Clin Invest 2014; 44:902–909. [DOI] [PubMed] [Google Scholar]
- 32.Jung HH, Ma KR, Han H. Elevated concentrations of cardiac troponins are associated with severe coronary artery calcification in asymptomatic haemodialysis patients. Nephrol Dial Transplant 2004; 19:3117–3123. [DOI] [PubMed] [Google Scholar]
- 33.Laufer EM, Mingels AMA, Winkens MHM, et al. The extent of coronary atherosclerosis is associated with increasing circulating levels of high sensitive cardiac troponin T. Arterioscler Thromb Vasc Biol 2010; 30:1269–1275. [DOI] [PubMed] [Google Scholar]
- 34.Qadir F, Farooq S, Khan M, et al. Correlation of cardiac troponin I levels (10 folds upper limit of normal) and extent of coronary artery disease in non-ST elevation myocardial infarction. J Pak Med Assoc 2010; 60:423–428. [PubMed] [Google Scholar]
- 35.Adibi P, Sadeghi M, Mahsa M, et al. Prediction of coronary atherosclerotic disease with liver transaminase level. Liver Int 2007; 27:895–900. [DOI] [PubMed] [Google Scholar]
- 36.Saely CH, Vonbank A, Rein P, et al. Alanine aminotransferase and gamma-glutamyl transferase are associated with the metabolic syndrome but not with angiographically determined coronary atherosclerosis. Clin Chim Acta 2008; 397:82–86. [DOI] [PubMed] [Google Scholar]
- 37.Kälsch J, Bechmann LP, Heider D, et al. Normal liver enzymes are correlated with severity of metabolic syndrome in a large population based cohort. Sci Rep 2015; 513058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park J-B, Kang D-Y, Yang H-M, et al. Serum alkaline phosphatase is a predictor of mortality, myocardial infarction, or stent thrombosis after implantation of coronary drug-eluting stent. Eur Heart J 2013; 34:920–931. [DOI] [PubMed] [Google Scholar]
- 39.Tonelli M, Curhan G, Pfeffer M, et al. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation 2009; 120:1784–1792. [DOI] [PubMed] [Google Scholar]
- 40.Wannamethee SG, Sattar N, Papcosta O, et al. Alkaline phosphatase, serum phosphate, and incident cardiovascular disease and total mortality in older men. Arterioscler Thromb Vasc Biol 2013; 33:1070–1076. [DOI] [PubMed] [Google Scholar]
- 41.Sahin I, Karabulut A, Gungor B, et al. Correlation between the serum alkaline phosphatase level and the severity of coronary artery disease. Coron Artery Dis 2014; 25:349–352. [DOI] [PubMed] [Google Scholar]
- 42.O’Neill WC, Sigrist MK, McIntyre CW. Plasma pyrophosphate and vascular calcification in chronic kidney disease. Nephrol Dial Transplant 2010; 25:187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006; 444:860–867. [DOI] [PubMed] [Google Scholar]
- 44.Wree A, Kahraman A, Gerken G, et al. Obesity affects the liver—the link between adipocytes and hepatocytes. Digestion 2011; 83:124–133. [DOI] [PubMed] [Google Scholar]
- 45.Webber M, Krishnan A, Thomas NG, et al. Association between serum alkaline phosphatase and C-reactive protein in the United States National Health and Nutrition Examination Survey 2005–2006. Clin Chem Lab Med 2010; 48:167–173. [DOI] [PubMed] [Google Scholar]
- 46.Ballantyne CM, Raichlen JS, Nicholls SJ, et al. Effect of rosuvastatin therapy on coronary artery stenoses assessed by quantitative coronary angiography: a study to evaluate the effect of rosuvastatin on intravascular ultrasound-derived coronary atheroma burden. Circulation 2008; 117:2458–2466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


