Abstract
Reduction of cardiovascular death might have a significant effect on the long-term survival rates of renal transplant recipients (RTRs). The aim of the study was to assess the relation between arterial stiffness and graft function, adipose tissue content, and hydration status in patients after kidney transplantation (KTx).
The study included 83 RTR patients (mean age: 55 ± 13 years) who had been admitted to a nephrology-transplantation outpatient clinic 0.5 to 24 years after KTx. Clinical and laboratory data were analyzed and eGFR was calculated with the CKD-EPI formula. Arterial stiffness was assessed in all RTRs with pulse wave propagation velocity (PWV) with the use of a complior device. In addition, fluid and nutritional status was assessed with a Tanita BC 418 body composition analyzer. The control group consisted of 31 hospital workers who received no medication and had no history of cardiovascular disease.
Multivariable linear regression analysis, with PWV as a dependent variable, retained the following independent predictors in the final regression model: red blood cell distribution width (RDW) (B = 0.323; P = 0.004), age (B = 0.297; P = 0.005), tacrolimus therapy (B = −0.286; P = 0.004), and central DBP (B = 0.185; P = 0.041). Multivariable linear regression analysis with eGFR as a dependent variable retained the following independent predictors in the final regression model; creatinine concentration (B = −0.632; P = 0.000), hemoglobin (B = 0.280; P = 0.000), CRP (B = −0.172; P = 0.011), tacrolimus therapy (B = 0.142; P = 0.039), and triglycerides (B = −0.142; P = 0.035).
Our data indicates that: kidney transplant recipients can present modifiable CVD risk factors linked to increased arterial stiffness, DBP, waist circumference, SCr, time on dialysis, CyA therapy, and visceral fat mass; RDW is a parameter associated with arterial stiffness; and parameters such as CyA therapy, time on dialysis, PWV, RDW, and triglycerides show negative associations with the allograft function assessed with eGFR.
INTRODUCTION
Kidney transplantation (KTx) significantly reduces cardiovascular disease (CVD) risk factors in patients with end-stage renal disease (ESRD),1 yet these factors still remain the main cause of death in patients with a functioning allograft,2,3 for which the risk is 50 times higher than in the general population.4 The reduction of death due to CVD could significantly improve the long-term survival rates of patients after KTx.
Apart from traditional cardiovascular risk factors characteristic of chronic kidney disease (CVD), patients after KTx also present with other factors affecting the status of the cardiovascular system including past history of dialysis treatment prior to KTx, immunosuppressive therapy, deteriorating allograph function, proteinuria, chronic inflammation, or anemia. Accumulation of the factors listed leads to complications such as arterial hypertension, lipid disturbances, and diabetes. Many of the factors listed also contribute to increased arterial stiffness.5 Other recognized risk factors determining arterial stiffness in renal transplant recipients (RTRs) include age, arterial hypertension, smoking, history of acute withdrawal incidents, allograft function assessed with estimated glomerular filtration rate (eGFR), development of diabetes de novo after KTx, and the use of immunosuppresive medication.6,7
Arterial stiffness assessment methods include measurement of pulse wave propagation velocity (PWV),5,8 overhydration, and nutritional status measured with bioelectrical impedance analysis (BIA). There are no reports in the literature assessing PWV in patients a short or long time after KTx. The majority of clinical studies have focused on the short-term (up to 36 months) effects and have not included nutritional and hydration status. There are major discrepancies in research findings regarding the assessment of changes to arterial stiffness indicators after renal transplantation. Although some report improvement, others indicate an absence of changes to arterial stiffness parameters in the early post-KTx period.9–11
Moreover, over the past few years immunosuppressive therapy schemes have changed; tacrolimus (TAC) has largely replaced cyclosporine A (CyA)2 and a new formula chronic kidney disease epidemiology collaboration (CKD-EPI) has replaced modification of diet in renal disease (MDRD). According to 2012 Kidney Disease: Improving Global Outcomes (KDIGO) recommendations, the MDRD formula commonly applied in eGFR calculation should be replaced with the CKD-EPI formula.12 The new formula recommended by clinicians is more precise when the value of eGFR is >60 mL/min/1.73 m2 as it makes it possible to verify the moment of CKD diagnosis. The use of MDRD has been proved to be responsible for overestimating the incidence of CKD in the general population.13
In an attempt to seek new, modified CVD risk factors which could become potential targets in RTR therapy, it seems well justified to assess the connection between hydration and nutritional status and increased arterial stiffness.
The aim of the study was to assess the relation between arterial stiffness and graft function, adipose tissue content, and hydration status of patients after KTx.
TRIAL REGISTRATION: clinicaltrials.gov Identifier: NCT02443454.
MATERIALS AND METHODS
Study Population
The observational, cross-sectional, cohort, single-center study was carried out between May and August 2015. The 83 patients (mean age: 55 ± 13 years, 0.5–24 years after KTx) who qualified for the study had been admitted to the nephrology and transplantology clinic of the Infant Jesus Teaching Hospital in Warsaw (Poland). The patients who qualified for the study were informed about the principles, aims and benefits of the study through oral, and written instructions and gave written consent to their participation. The inclusion criteria stipulated age ≥18 years and time from KTx ≥6 months, in order to exclude the effect of immunosuppression and stable allograft function, that is, creatinine clearance not changed by more than 5 mL/min/1.73 m2 for at least 3 months. Excluded from the study were patients with active infections, combined organ transplants, metal valves, stents, metal sutures or metal prosthesis, and atrial fibrillation, which makes PWV-testing uninterpretable. The control group consisted of 31 hospital workers who were not on any medication and had no history of CVDs.
Study Design
The study protocol was approved by the local Research Ethics Committee at the Medical University of Warsaw (Approval No. KB/70/2015) and registered in the Clinical Trials Register (www.clinicaltrials.gov, identifier NCT02443454). The investigation conformed to the principles outlined in the Declaration of Helsinki. PWV and BIA measurements were performed during routine patient visits to the nephrology and transplantology clinic. All PWV measurements were performed by a trained researcher. RTR data included age, gender, pretransplant dialysis, cause of ESRD, cold ischemia time, human leukocyte antigen (HLA) mismatches, presence of delayed graft function (DGF), acute rejection (AR) status, hepatitis (B and C virus), pretransplantation diabetes mellitus, new-onset diabetes after transplantation (NODT), and donation type and immunosuppressant. Body mass, height, waist circumference, and body mass index (BMI) were all assessed. BMI was calculated as body weight (kg) divided by the square of body height (m). Abdominal obesity was defined as waist circumference of 102 cm in men and 88 cm in women.8 The diagnostic criteria for NODT, as recommended by the 2003 international consensus guidelines for NODT, were used.14 DGF was defined as the need for dialysis within the 1st week after transplantation.
Hemodynamic
Results of laboratory examinations (serum creatinine [SCr] concentration, C-reactive protein [CRP], hemoglobin [Hb], and red blood cell distribution width [RDW]) were measured on the same day as PWV and BIA measurements. Results of laboratory examinations (hemoglobin A1c, lipoprotein profile, and vitamin D) were obtained from the patients’ medical records up to 6 months after PWV and BIA measurement. The eGFR was calculated with the CKD-EPI formula.12 CKD in the study was defined as an eGFR <60 mL/min/1.73 m2. The patients were divided into 3 groups according to the eGFR indicator: group 1 contained patients with eGFR >60 mL/min/1.73 m2, n: 27; group 2 contained patients with eGFR >30 < 60 mL/min/1.73 m2, n: 47; and group 3 contained patients with eGFR <30 mL/min/1.73 m2, n:13. For the purpose of analyzing the associations with arterial stiffness, the patients were divided into 3 groups according to the PWV cut-off values: group 1 contained patients with PWV <8.0 m/s, n: 46; group 2 contained patients with PWV ≥8.0 < 10 m/s, n: 19; and group 3 contained patients with PWV ≥10 m/s, n: 18. Because the group of RTRs with PWV >12 m/s (n = 5) was too small, we combined this group with patients with PWV >10 m/s. The European Society of Hypertension-European Society of Cardiology hypertension guidelines classify PWV >10 m/s as target organ damage.8 In a prospective study including 1040 RTRs by Dahle et al,3 a PWV in the highest quartile (≥12 m/s) was independently associated with mortality (hazard ratio [HR], 2.48; 95% confidence interval [95% CI] 1.29–4.79; P = 0.01). A PWV in the medium quartile (10.0–11.9 m/s) was associated with mortality (HR 1.37, 95% CI 0.68–2.73, P = 0.38). A PWV in the lowest quartile (<8.0 m/s) was associated with mortality (HR 0.23, 95% CI 0.05–1.05, P = 0.06). In the cited study, PWV >12 m/s was significantly associated with mortality (HR 1.36, 95% CI 1.14–1.62, P = 0.001).
Peripheral Blood Pressure Measurement
Blood pressure was recorded in the dominant or nonfistula arm using a validated oscillometric device (BR-102 plus Schiller AG, Baar, Switzerland) recommended by the European Society of Hypertension (ESH). The measurement provided systolic blood pressure (SBP) and diastolic blood pressure (DBP) values. The values are reported as the mean of 2 stable readings.
Pulse Wave Analysis and Central Blood Pressure Measurement
PWV measurements were performed with a Complior device (Artech Medical, Pantin, France). RTR studies were carried out on an empty stomach from 08:00 to 10:00 am, in a quiet air-conditioned room after a 15-minute rest in a supine position. In brief, 2 device sensors were placed: the 1st in a place of palpable pulse on the carotid artery; the 2nd in a place of palpable pulse on the femoral artery. The time (t) between the appearance of the pulse wave on the carotid artery and the femoral artery was measured automatically in 10 successive cycles (successive beats) and averaged. Once the result of the measurement of the distance between the sensors (m) was introduced, PWV was calculated by the device according to the equation PWV = m/t and expressed in [m/s]. The complior device was also used to measure central systolic blood pressure (cSBP) and central diastolic blood pressure (cDBP). Intra- and intersession variability of PWV, cSBP, and cDBP obtained during reproducibility studies were acceptable (<5%).
Body Composition and Volume Measurement
The study made use of a Tanita BC 418 body composition analyzer (Tanita Corp., Tokyo, Japan), a technique based on BIA measurement with the use of a single frequency current of 50 kHz (single frequency BIA – SF-BIA) and an 8-contact electrode system. The analyzer measured: basal metabolic rate in kcal, the volume of the total body water (TBW) in the system (%); the volume of adipose tissue in the organism as a whole in relation to body mass expressed in percent (fat mass [FAT%]) and in the abdominal cavity (visceral fat mass [Visc.FAT%]).
Statistical Analysis
Results concerning quantitative variables were presented as average values ± standard deviation. In the comparative characteristics of PWV and eGFR, a one-way analysis of variance (ANOVA) and post-hoc NIR test were used. In the comparative analysis of PWV and eGFR, as well as the clinical results, laboratory examinations, and anthropometric examination results, simple linear regression analysis (Pearson) was applied to detect and describe the strength and direction of correlations of PWV and eGFR to clinical, laboratory, and body composition data. In the multivariable linear regression, ANOVA was applied with PWV and eGFR as dependable variables. Qualitative variables (age, sex) were presented as quantity (n) and percentage values of the whole group (%), while proportions in groups were assessed with a Chi-squared test. In the comparative characteristics of RTRs and the control group, a Student t-test was used. Statistica 12 software (StatSoft Inc., Tulsa, OK) was used in the statistical analysis. P < 0.05 was adopted as the significance level.
RESULTS
The causes of chronic kidney disease in the study population were: glomerulonephritis (n = 42; 51%), diabetes (n = 3; 4%), hypertension (n = 8; 10%), polycystic kidney disease (n = 14; 16%), unknown (n = 12; 14%), and other (n = 3; 4%). The immunosuppressive medication included TAC (n = 43; 52%), CyA (n = 40; 48%), mycophenolate mofetil (n = 78; 94%), prednisone (n = 71; 86%), and sirolimus (n = 3; 4%). Seventy patients (84%) had been on hemodialysis, 10 (12%) had been on peritoneal dialysis prior to transplantation, and 3 (4%) were preemptive. The mean duration of dialysis before transplantation was 28.6 ± 23.7 months. NODT was diagnosed in 11 RTRs (13%). Before KTx, diabetes had been diagnosed in 9 patients (11%). Seven patients (8%) were recipients of a kidney from a living donor and 76 patients (92%) had received a deceased donor graft. Eleven patients (13%) had developed at least 1 AR episode. Twelve RTRs (15%) had developed DGF. The mean number of HLA mismatches was 3.2 ± 1.4. Mean cold ischemia time was 19.1 ± 9.1 hours. Hepatitis B virus had been diagnosed in 9 (11%) patients and C virus in 3 (4%) RRTs.
The study included 83 RTRs with a mean age of 54.7 years, 57% male, mean eGFR of 52.9 mL/min/1.73 m2, mean PWV of 8.0 m/s, median time since transplantation of 7 years, and mean waist circumference of 96 cm. Our study revealed significantly higher PWV in RTRs on CyA, based immunosuppression compared to TAC (9.1 ± 2.8 vs 7.0 ± 1.8 m/s, P < 0.01). Compared to RTRs, the control group consisted of 31 healthy volunteers, mean age was 43 years (P = < 0.001), 31% male (P < 0.001), mean PWV of 5.9 m/s (P < 0.001, mean waist circumstance of 83 cm (P < 0.001), mean SBP of 129 mm Hg (P < 0.001), and mean cSBP of 122 mm Hg (P < 0.001).
Simple Linear Regression Analysis (Pearson) and Univariate Comparison of RTRs According to Tertiles of PWV and Biological Factors, Laboratory Measurements, and Body Composition
The one-way ANOVA confirmed the existence of statistically significant differences (all P for trend < 0.05) between tertiles of PWV and age, SBP, DBP, cDBP, SCr, eGFR, RDW, CRP, TAC therapy, time on dialysis, waist circumstance, Visc.FAT(%), and vitamin D Table 1. In simple linear regression analysis, age, DBP, cDBP, SCr, RDW, CRP, time on dialysis, waist circumstance, FAT(%), and Visc.FAT(%) were positively correlated with PWV (all P for trend <0.05) while TAC therapy, eGFR, HDL-cholesterol, and vitamin D were negatively correlated (both P for trend < 0.05), see Table 2, Figure 1. The other parameters did not correlate with PWV.
TABLE 1.
Univariate Comparison of Renal Transplant Recipients According to Pulse Wave Velocity Ranges and Biological Factors, Laboratory Measurements, and Body Composition
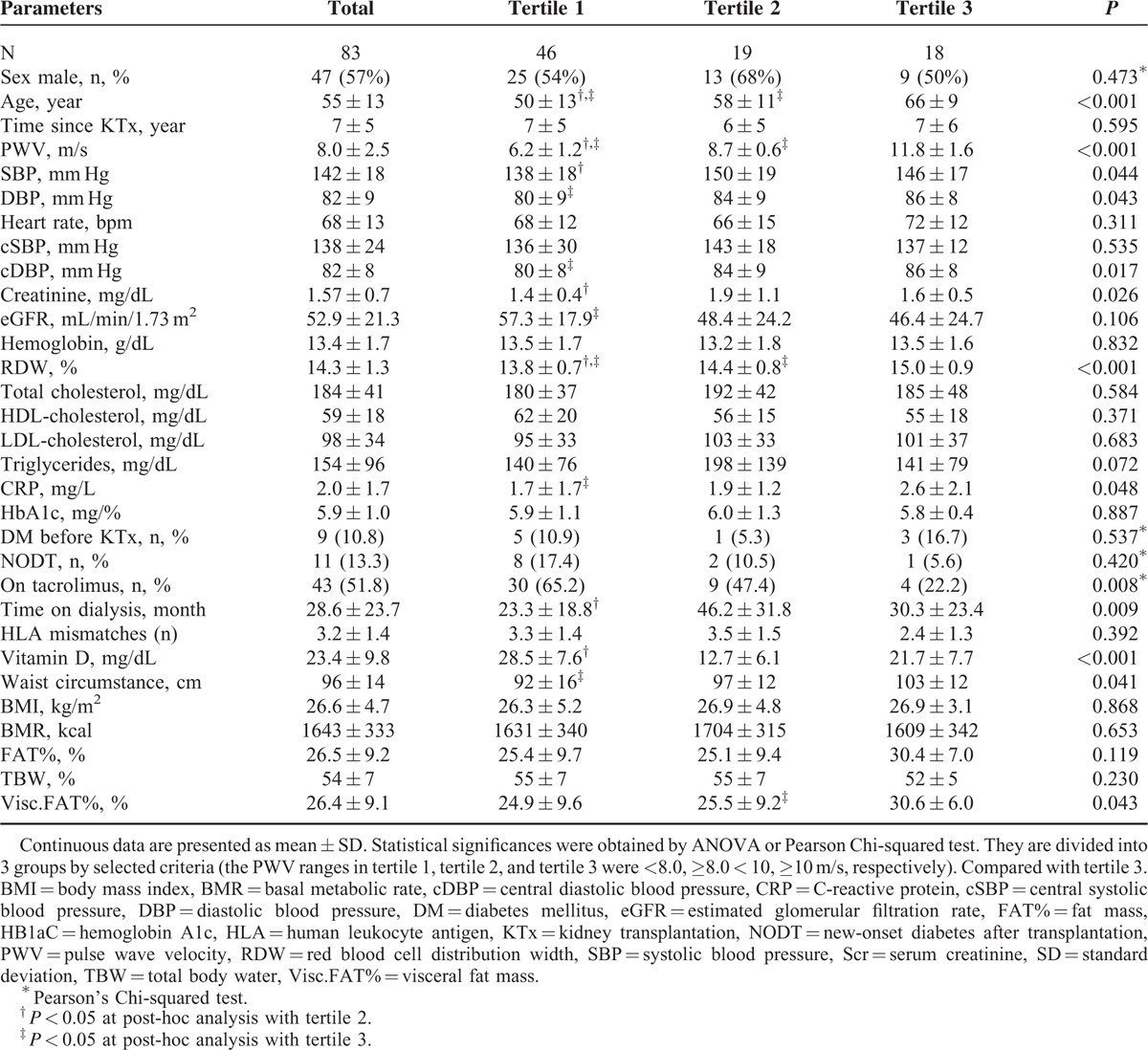
TABLE 2.
Results of Simple Regression Analyses Between Pulse Wave Velocity, Estimated Glomerular Filtration Rate and Clinical, Laboratory, and Body Composition Data in Renal Transplant Recipients
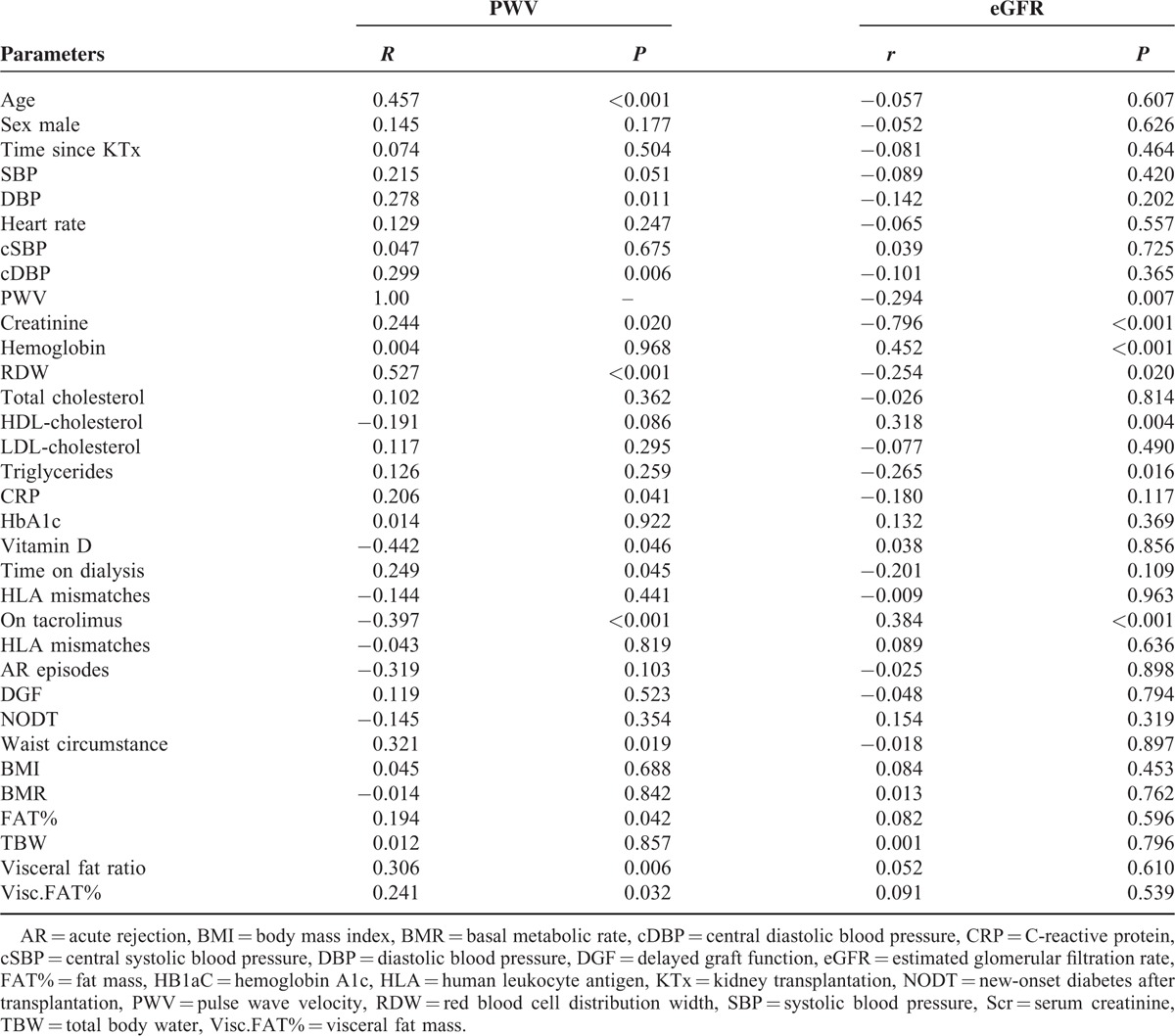
FIGURE 1.
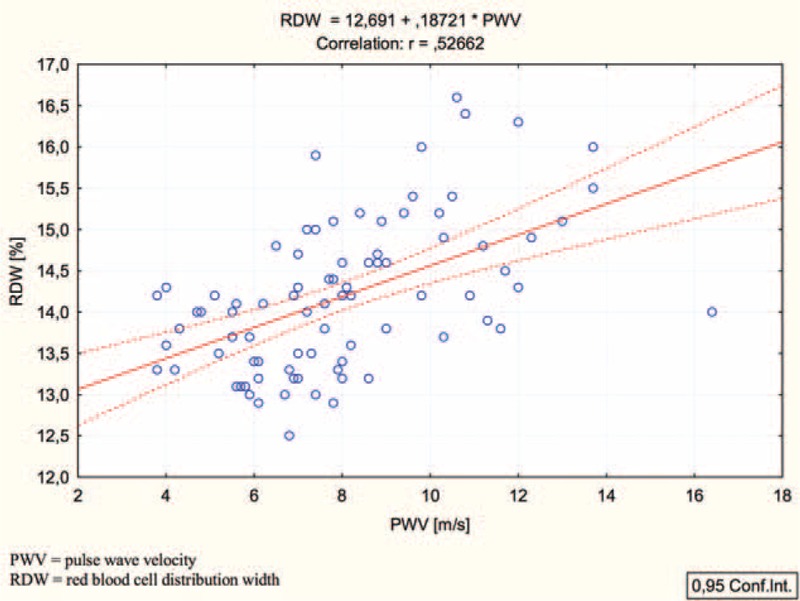
Simple linear regression analysis (Pearson) between pulse wave velocity and red blood cell distribution width.
Simple Linear Regression analysis (Pearson) and Univariate Comparison of RTRs According to Tertiles of eGFR and Biological Factors, Laboratory Measurements, and Body Composition
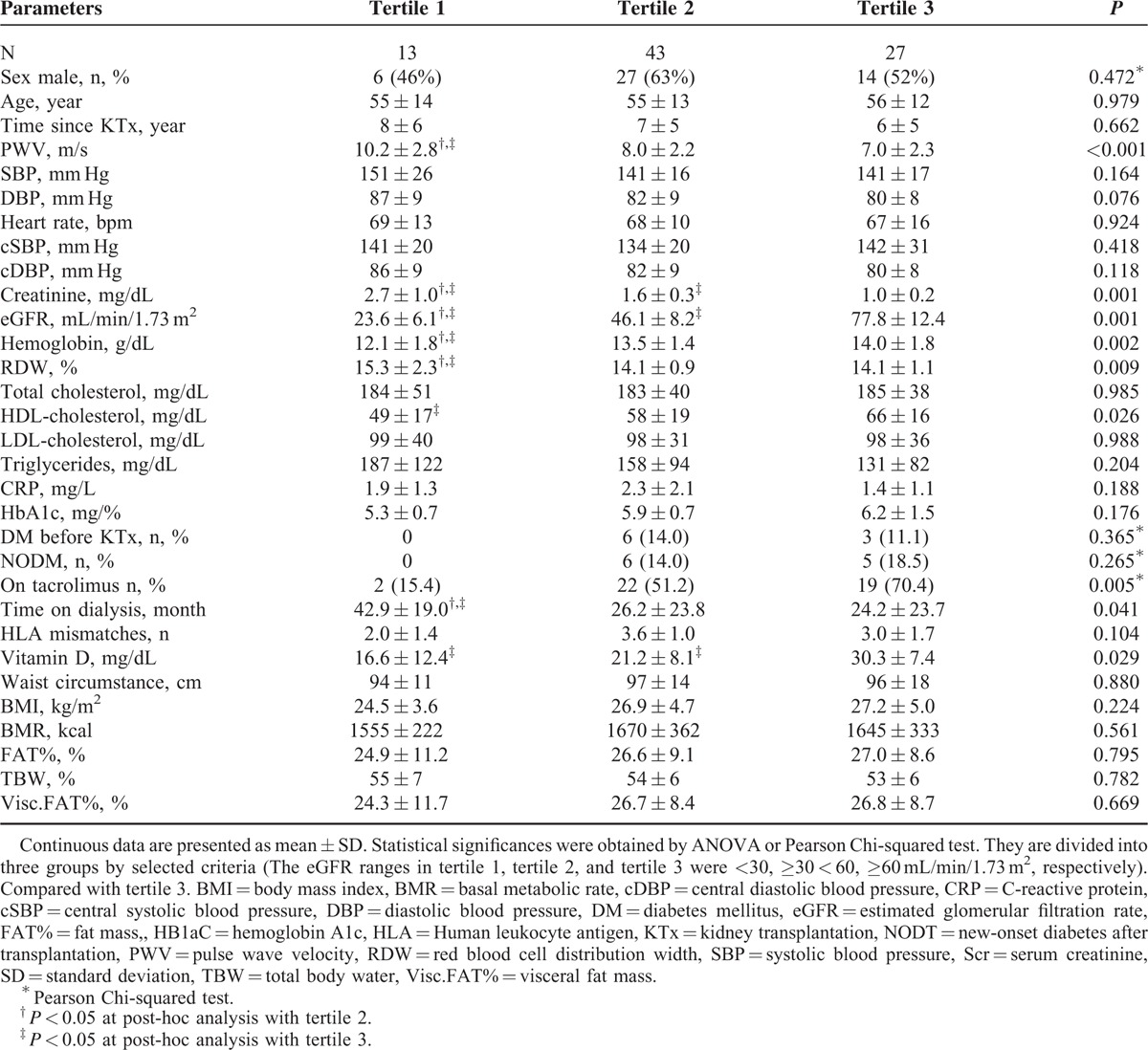
The one-way ANOVA confirmed the existence of statistically significant differences (all P for trend < 0.05) between tertiles of eGFR and PWV, TAC therapy, time on dialysis, Hb, RDW, HDL-cholesterol, and vitamin D, see Table 3. In simple linear regression analysis, TAC therapy, Hb, and HDL-cholesterol were positively correlated with eGFR (all P for trend < 0.05) while time on dialysis, PWV, RDW, and triglycerides (TGs) were negatively correlated (both P for trend < 0.05), see Table 2. No association was observed between eGFR and body composition in RTRs.
TABLE 3.
Univariate Comparison of Renal Transplant Recipients According to Estimated Glomerular Filtration Rate Ranges and Biological Factors, Laboratory Measurements, and Body Composition
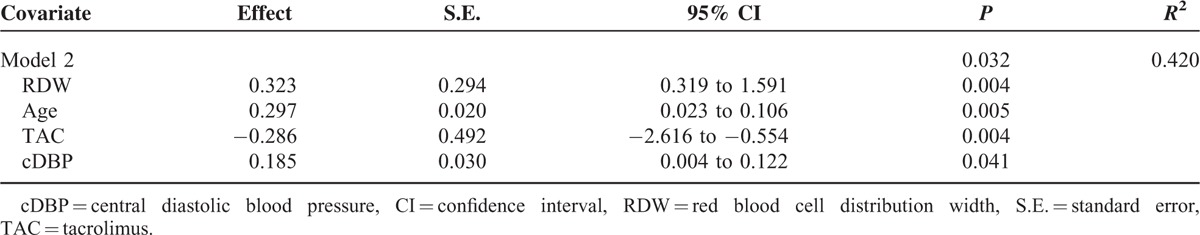
Multivariable Linear Regression Analysis With PWV as a Dependent Variable
The independent predictors retained in the final regression model were RDW, age, TAC therapy, and cDBP. The remaining factors were eliminated, see Table 4.
TABLE 4.
Multivariable Linear Regression Model of Pulse Wave Velocity in Renal Transplant Recipients
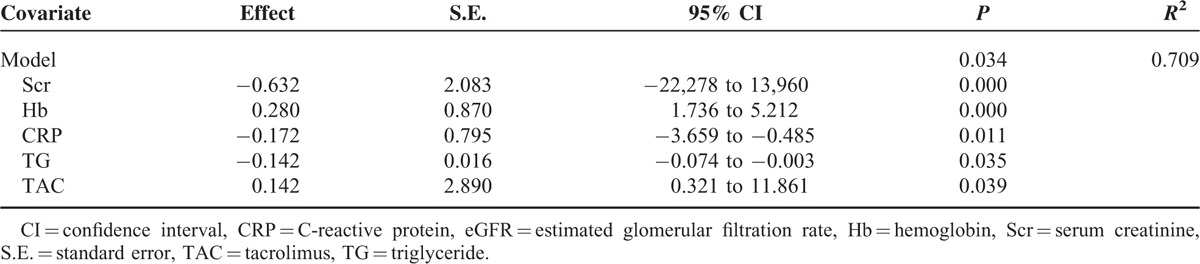
Multivariable Linear Regression Analysis With eGFR as an Dependent Variable
The independent predictors retained in the final regression model were SCr, Hb, CRP, TGs, and TAC therapy. The remaining factors were eliminated, see Table 5.
TABLE 5.
Multivariable Linear Regression Model of Estimated Glomerular Filtration Rate in Renal Transplant Recipients
DISCUSSION
CVDs are the main cause of death (37%) in patients after kidney implantation.2 Compared to dialyzed patients, patients after KTx show a reduced CVD risk. This suggests that KTx can reduce the risk of CVD. Even though this positive effect is dependent on numerous factors, this study assessed mutual relations between nutritional status, arterial stiffness, and the eGFR-assessed function of the implanted kidney. In ESRD patients, PWV-assessed arterial stiffness is a recommended procedure in CVD risk assessment.3 In a prospective study, Seibert et al15 showed during a 52-month follow-up of 64 RTRs that there was a significant correlation between PWV and CVD appearance (10.1 ± 3.6 m/s in patients reaching the endpoint vs 8.5 ± 1.5 m/s in patients not reaching the endpoint; P = 0.048), where the endpoint was defined as the incidence of either death, myocardial infarction, stroke, or admission for symptomatic intermittent claudication or decompensated congestive heart failure. Interestingly, aortic SBP (115 ± 28 vs 118.7 mm Hg; P = 0.635), peripheral SBP (130.5 ± 29.9 vs 131.7 ± 17 mm Hg; P = 0.408), and pulse pressure (62.3 ± 17.3 vs 56.8 ± 12.1 mm Hg; P = 0.128) did not show any significant correlation with CVD appearance.
Our study revealed that the age of the patients contributed to an increase in arterial stiffness assessed with PWV. The fact that increasing age, DBP, cDBP, SCr, RDW, CPR, time on dialysis, waist circumference, FAT(%), and Visc.FAT(%) and decreasing eGFR, CyA therapy, HDL-cholesterol, and vitamin D were associated with increased PWV score may help identify patients with increased arterial stiffness.
Among others, RDW is a relatively new prognostic parameter in cardiac insufficiency.16 An elevated RDW value is evidence of a great diversity of erythrocytes (anisocytosis). The prognostic value of RDW in patients with cardiac insufficiency is comparable to NT-proBNP.17 In addition, the parameter is given in every complete blood count which makes it cheap and generally available. The normal level of RDW ranges from 11.5% to 14.5%. In a cross-sectional study, Lippi et al18 showed that in a group of 8585 adult outpatients over a 3-year period the percentage share of patients with eGFR <60 mL/min/1.73 m2 increased when the RDW value exceeded 13.9% (from 5% in patients with normal eGFR to 19%; P < 0.0001). The conducted analysis confirmed that an elevated RDW value constitutes a parameter of prognostic significance in the assessment of arterial stiffness. This finding suggested that the measurement and interpretation of RDW might represent a useful screening test in predicting CVD after KTx.
Recent research findings indicate that arterial stiffness in patients after KTx decreases when compared to the period of dialysis treatment. Moreover, the longer the allograft-functioning period, the more reduced the arterial stiffness. A study of Birdwell et al11 conducted on a group of 66 newly implanted RTRs over a 12-month follow-up did not reveal PWV increase (median change of – 0.07, P = 0.7). In the aforementioned study, a multivariable regression analysis showed that the age of the patient (P = 0.008) and diabetes (P = 0.002) were related to PWV growth.
RTRs are exposed to an increased risk of metabolic disturbances. Immunosuppressive treatment, hormonal changes, hyperlipidemia, dyslipidemia, and chronic inflammation affect the vessel walls and the function of vascular endothelium. To assess the CVD risk, it is worth assessing the influence of nutritional and fluid status on arterial stiffness in RTRs. A large number of studies have examined the relationship between the nutritional and fluid status and hypertension in the general population19 or in patients treated with dialysis;20 however, these have not been studied in RTRs. In the general population, increases in trunk mass and decreases in peripheral FAT% are associated with accelerated arterial stiffening.19 It has been shown that excess fluid plays an important role in the development of arterial stiffness in dialyzed patients by increasing arterial distension and SBP.20 Body mass growth in RTRs is a common phenomenon caused, among others, by steroid administration and correction of uremia. Although overweight hemodialyzed patients can have a cardioprotective action,21 in RTRs the results of clinical examinations are not consistent.22–24 The available research findings indicate that 15% of RTRs show symptoms of malnutrition.25 Sezer et al26 showed in their study that TBW was found to be a predictor of PWV (r = 0.216; P < 0.05). In addition, it was found that mean TBW (31.0 ± 11.1 L) was significantly correlated with mean SBP (117.4 ± 27.3 mm Hg; P = 0.001). Lean tissue mass (15.8 ± 0.4 vs 38.1 ± 2.5 kg; P = 0.002) and TBW (45.1 ± 1.0 vs 23.3 ± 0.3 L; P = 0.001) were significantly higher in patients with eGFR 15–49 mL/min/1.73 m2 compared to patients with eGFR >50 mL/min/1.73 m2. In another study, Saxena and Sharma27 showed that SBP was associated with TBW (P = 0.016) and DBP with TBW (P = 0.003), dry weight (r = 0.76), and intracellular water percent (r = 0.79). Patients with uncontrolled blood pressure had higher FAT% and low fat free mass. Our own study demonstrated that TBW was not a determinant of PWV in RTRs.
The results of our study clearly demonstrate that eGFR was not associated with obesity incidence in RTRs. In their study on 189 RTRs, Tutal et al23 showed that eGFR values positively correlate with visceral fat ratio, BMI, fat, fat-free mass, and muscle masses, suggesting that graft function predicts the nutritional status.
Several other studies have investigated stiffness markers in renal transplant patients. Various immunosuppressants have an impact on cardiovascular risk. Drugs such as calcineurin inhibitors and steroids increase traditional cardiovascular risk factors including hypertension, diabetes, and hyperlipidemia. Immunosuppression therapy after KTx can directly affect the process of atherosclerosis, for example, acute and chronic calcineurin inhibitor toxicity.28 Several studies revealed increased arterial stiffness in RTRs on CyA immunosuppression compared to TAC-based immunosuppressive protocol. This is probably due to the fact that CyA therapy is associated with increased blood pressure, while CyA leads to decreased endothelium-mediated vasodilatation.29
Stróżcki et al30 showed that PWV was significantly higher in the CyA group compared to TAC group of RTRs (9.33 ± 2.10 vs 8.54 ± 1.35 m/s, respectively; P < 0.01). In the CyA group, Stróżecki et al found significant correlations between PWV and age (r = 0.40; P < 0.001), fasting blood glucose (r = 0.31; P < 0.01), SBP (r = 0.45; P < 0.001), DBP (r = 0.28; P < 0.02), and pulse pressure (r = 0.41; P < 0.001). In the TAC group, a correlation between PWV and age (r = 0.46; P < 0.001) was found. No relationship was found between eGFR and PWV.
NODM is a common complication of immunosuppression therapy after KTx and is associated with increased PWV. NODM is linked to a 1.9-fold increase in mortality and 1.6-fold increase in graft failure.31 In our study, diabetic RTRs had increased PWV. Opazo Saez et al32 showed that out of 318 RTRs, 57 had NODM. Compared to nondiabetic transplant controls, transplant patients with NODM showed a significantly higher PWV (8.7 vs 10.5 m/s, P = 0.0002). In multiple regression analysis, PWV was significantly correlated to age (B = 0.516, P < 0.0001) and SBP (B = 0.134, P = 0.0081).
Interestingly, our study revealed that a higher PWV also exhibited a higher brachial DBP (r = 0.278; P = 0.011) and central DBP (r = 0.299; P = 0.006). PWV was not significantly statistically associated with higher brachial SBP (r = 0.215; P = 0.051) and central SBP (r = 0.047; P = 0.675). Although DBP is a major determinant of PWV in healthy males (B = 0.279, P = 0.0072),33 SBP is more closely related to PWV in older individuals.34
The majority of studies of RTRs have revealed that a higher PWV also exhibits a higher SBP.32,34 Kneifel et al35 showed a significant correlation between increased large artery stiffness and higher SBP (r = 0.41; P < 0.01), higher DBP (r = 0.31; P = 0.04), reduced eGFR (r = −0.45; P < 0.01), and the older age of the kidney donor of RTRs (r = 0.38; P = 0.03). Dahle et al3 showed that PWV was more closely correlated with pulse pressure (r = 0.50, P < 0.001) than with SBP (r = 0.39, P < 0.001) or DBP (r = −0.07, P = 0.04). Bahous et al7 followed up 106 RTRs (mean duration 54.3 ± 28.9 months) and found that age (B = 9.37; P < 0.0001), MBP (B = 7.46; P < 0.0001), smoking (B = 0.02; P = 0.025), and acute renal rejection (B = 1.15; P = 0.01) influence aortic stiffness as measured by PWV. The same factors influenced the decrease in eGFR observed after KTx.
DGF is a common complication of KTx. In the study by Muth et al,36 of 697 deceased donor kidney transplants, the incidence of DGF was 30.1%, depending on many factors. Patients with DGF were less likely to be female (41.8% vs 31.0%, P = 0.009), have a higher risk of rejection (P < 0.0001), graft loss (P = 0.02), death with a functioning graft, higher likelihood of readmission within the first 30 days after KTx (18% vs 12%; P = 0.02), have higher BMI (28.7 ± 5.1 vs 27.3 ± 5.2, P = 0.001), and were more likely to receive a kidney from a donor after circulatory death (56% vs 19%, P < 0.001) or expanded criteria donor (26% vs 17%, P = 0.04). One year serum creatinine was similar between RTRs with DGF and no DGF (1.4 ± 1.7 and 1.6 ± 0.8; respectively, P = 0.12).
Calcineurin inhibitors are an important cause of posttransplant renal function decline and graft loss.37 TAC seems to be less toxic than CyA.38 Cheung et al39 showed in paired kidney analysis of TAC and CyA treatment that mean calculated creatinine clearance was significantly higher in patients receiving TAC-based therapy. The rate of biopsy-proven AR was lower in the TAC group (18.4% vs 42.1%, P = 0.03). The patient and graft survival were comparable in both treatment arms. In a study by Artz et al,40 after 6 months of follow-up conversion from CyA to TAC resulted in a significant decrease in the serum creatinine level from 137 ± 30 to 131 ± 29 mmol/L (P < 0.01). After 3 months of follow-up, SBP and DBP were significantly decreased in the TAC group from 144 ± 21 mm Hg to 138 ± 18 mm Hg and 84 ± 12 mm Hg to 80 ± 11 mm Hg, respectively.
Incidence of AR within the 1st posttransplantation year has declined to less than 15%.41 Reduction in the incidence of AR has not resulted in better long-term renal allograft survival.42 The impact of AR on long-term graft survival is debated. In the study by El Ters et al,43 during the 1st year posttransplant 15.2% patients had AR episodes which were associated with reduced graft survival (HR 3.07, 95% CI 1.92–4.94, P < 0.0001). Compared to patients with no AR, those with AR during the 1st year were more likely to be recipients of deceased donor kidneys (20.3% vs 31.4%; P = 0.008) and have a higher number of HLA mismatches (2.8 ± 1.9 vs 3.2 ± 1.7; P = 0.029). In multivariate logistic regression analysis, the risk of AR during the 1st year related to deceased donors (OR 1.36, 95% CI 1.07–1.73, P = 0.012) and an increased number of HLA mismatches (HR 1.16, 95% CI 1.03–1.29, P = 0.015). Of the 8036 RTRs in a study by Lim et al,44 59% had between 2 and 4 HLA mismatches. Compared with 0 HLA mismatches, increasing HLA mismatches were associated with a higher risk of graft failure, risk of rejection, and patient death in the adjusted models.
Chronic hepatitis B, chronic hepatitis C, and cytomegalovirus infections are common viral infections after KTx and can cause an impairment or loss of allograft function and increases in patient morbidity and mortality.45 Treatment of cytomegalovirus, hepatitis B, and chronic hepatitis C infections in RTRs represents a challenge for physicians. After KTx, levels of HCV-RNA rise as a consequence of immunosuppressive therapy. In a study by Kasiske et al,31 the prevalence of HCV infection in RTRs is 5.6%. Hepatitis C virus infection is associated with an increased PTDM risk at 3, 12, and 36 months of 15.6%, 25.6%, and 35.4%, respectively, compared with 8.8%, 15.4%, and 23.4% for patients who were hepatitis C antibody negative at transplantation (P < 0.0001). Furthermore, hepatitis C infection is an independent risk factor PTDM OR = 1.33 (1.15–1.55) P < 0.0001.
Currently, the 5 to 10 year survival rate of KTx recipients with HBV is approaching that of HBsAg negative patients.46
This study has some limitations. First, it is not a prospective study, but a cross-sectional and observational study. Second, pretransplantation PWV measurements were unavailable. Despite these limitations, the results of our study suggest that PWV, BIA, and laboratory tests (especially RDW) can be used as one of the screening tests for predicting CVD in RTRs. Therefore, we recommend PWV, BIA, and RDW as a screening tool after KTx.
In conclusion, our data indicate that: RTRs manifest modified CVD risk factors linked to increased arterial stiffness, and, among others, DBP, waist circumference, SCr, time on dialysis, CyA therapy, and Visc.FAT%; the red blood cells distribution width (RDW) is a parameter associated with arterial stiffness; and it was shown that parameters such as CyA therapy, time on dialysis, PWV, RDW, and TGs show negative associations with the allograft function assessed with eGFR.
Acknowledgments
The authors thank the Reynolds Medical Sp. z o.o. for making the Complior device available.
Footnotes
Abbreviations: AR = acute rejection, BIA = bioelectrical impedance analysis, BMI = body mass index, cDBP = central diastolic blood pressure, CI = confidence interval, CKD = chronic kidney disease, CKD-EPI = chronic kidney disease epidemiology collaboration, CRP = C-reactive protein, cSBP = central systolic blood pressure, CVD = cardiovascular disease, CyA = cyclosporine A, DBP = diastolic blood pressure, DGF = delayed graft function, eGFR = estimated glomerular filtration rate, ESRD = end-stage renal disease, FAT% = fat mass, Hb = hemoglobin, HLA = human leukocyte antigen, KTx = kidney transplantation, MDRD = modification of diet in renal disease, NODT = new-onset diabetes after transplantation, PWV = pulse wave propagation velocity, RDW = red blood cell distribution width, RTR = renal transplant recipient, SBP = systolic blood pressure, SCr = serum creatinine, TAC = tacrolimus, TBW = total body water, TG = triglyceride, Visc.FAT% = visceral fat mass.
Conception and design: LC, JW, EC; Analysis and interpretation: LC, JS, LS; and Drafting the manuscript for important intellectual content: LC, AK, ZT.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 1999; 341:1725–1730. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Chavers B, et al. 2013 USRDS Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Am J Kidney Dis 2014; 63:e283–e294. [DOI] [PubMed] [Google Scholar]
- 3.Dahle DO, Eide IA, Åsberg A, et al. Aortic stiffness in a mortality risk calculator for kidney transplant recipients. Transplantation 2015; 99:1730–1737. [DOI] [PubMed] [Google Scholar]
- 4.Aakhus S, Dahl K, Widerøe TE. Cardiovascular disease in stable renal transplant patients in Norway: morbidity and mortality during a 5-yr. Clin Transplant 2004; 18:596–604. [DOI] [PubMed] [Google Scholar]
- 5.Boutouyrie P, Fliser D, Goldsmith D, et al. Assessment of arterial stiffness for clinical and epidemiological studies: methodological considerations for validation and entry into the European Renal and Cardiovascular Medicine registry. Nephrol Dial Transplant 2014; 29:232–239. [DOI] [PubMed] [Google Scholar]
- 6.Ferro CJ, Savage T, Pinder SJ, et al. Central aortic pressure augmentation in stable renal transplant recipients. Kidney Int 2002; 62:166–171. [DOI] [PubMed] [Google Scholar]
- 7.Bahous SA, Stephan A, Barakat W, et al. Aortic pulse wave velocity in renal transplant patients. Kidney Int 2004; 66:1486–1492. [DOI] [PubMed] [Google Scholar]
- 8.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34:2159–2219. [DOI] [PubMed] [Google Scholar]
- 9.Ignace S, Utescu MS, De Serres SA, et al. Age-related and blood pressure–independent reduction in aortic stiffness after kidney transplantation. J Hypertens 2011; 29:130–136. [DOI] [PubMed] [Google Scholar]
- 10.Westhoff TH, Straub-Hohenbleicher H, Basdorf M, et al. Time-dependent effects of cadaveric renal transplantation on arterial compliance in patients with end-stage renal disease. Transplantation 2006; 81:1410–1414. [DOI] [PubMed] [Google Scholar]
- 11.Birdwell KA, Jaffe G, Bian A, et al. Assessment of arterial stiffness using pulse wave velocity in tacrolimus users the first year post kidney transplantation: a prospective cohort study. BMC Nephrol 2015; 16:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150. [Google Scholar]
- 13.Inker LA, Levey AS. Pro: estimating GFR using the chronic kidney disease epidemiology collaboration (CKD–EPI) 2009 creatinine equation: the time for change is now. Nephrol Dial Transplant 2013; 28:1390–1396. [DOI] [PubMed] [Google Scholar]
- 14.Davidson J, Wilkinson A, Dantal J, et al. New-onset diabetes after transplantation: 2003 International consensus guidelines Proceedings of an international expert panel meeting Barcelona, Spain, 19 February 2003. Transplantation 2003; 75 (10 Suppl):S3–S24. [DOI] [PubMed] [Google Scholar]
- 15.Seibert FS, Behrendt C, Pagonas N, et al. Prediction of cardiovascular events after renal transplantation. Transplant Proc 2015; 47:388–393. [DOI] [PubMed] [Google Scholar]
- 16.Tonelli M, Sacks F, Arnold M, et al. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation 2008; 117:163–168. [DOI] [PubMed] [Google Scholar]
- 17.Förhécz Z, Gombos T, Borgulya G, et al. Red cell distribution width in heart failure: prediction of clinical events and relationship with markers of ineffective erythropoiesis, inflammation, renal function, and nutritional state. Am Heart J 2009; 158:659–666. [DOI] [PubMed] [Google Scholar]
- 18.Lippi G, Targher G, Montagnana M, et al. Relationship between red blood cell distribution width and kidney function tests in a large cohort of unselected outpatients. Scand J Clin Lab Invest 2008; 68:745–748. [DOI] [PubMed] [Google Scholar]
- 19.Schouten F, Twisk JW, de Boer MR, et al. Increases in central fat mass and decreases in peripheral fat mass are associated with accelerated arterial stiffening in healthy adults: the Amsterdam Growth and Health Longitudinal Study. Am J Clin Nutr 2011; 94:40–48. [DOI] [PubMed] [Google Scholar]
- 20.Wizemann V, Wabel P, Chamney P, et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009; 24:1574–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Czyżewski L, Sańko-Resmer J, Wyzgał J, et al. Comparative analysis of hypertension and its causes among renal replacement therapy patients. Ann Transplant 2014; 19:556–568. [DOI] [PubMed] [Google Scholar]
- 22.Pischon T, Sharma AM. Obesity as a risk factor in renal transplant patients. Nephrol Dial Transplant 2001; 16:14–17. [DOI] [PubMed] [Google Scholar]
- 23.Tutal E, Sezer S, Uyar ME, et al. Evaluation of nutritional status in renal transplant recipients in accordance with changes in graft function. Transplant Proc 2013; 45:1418–1422. [DOI] [PubMed] [Google Scholar]
- 24.Nicoletto BB, Fonseca NK, Manfro RC, et al. Effects of obesity on kidney transplantation outcomes: a systematic review and meta-analysis. Transplantation 2014; 98:167–176. [DOI] [PubMed] [Google Scholar]
- 25.Djukanović L, Lezaić V, Blagojević R, et al. Co-morbidity and kidney graft failure-two main causes of malnutrition in kidney transplant patients. Nephrol Dial Transplant 2003; 18 Suppl 5:v68–v70. [DOI] [PubMed] [Google Scholar]
- 26.Sezer S, Gurlek Demirci B, Guliyev O, et al. Graft function and arterial stiffness: can bioimpedance analysis be useful in renal transplant recipients? Transplant Proc 2015; 47:1182–1185. [DOI] [PubMed] [Google Scholar]
- 27.Saxena A, Sharma RK. Hypertension in post-renal transplant patients: pilot study. Saudi J Kidney Dis Transpl 2014; 25:22–28. [DOI] [PubMed] [Google Scholar]
- 28.Hornum M, Clausen P, Idorn T, et al. Kidney transplantation improves arterial function measured by pulse wave analysis and endothelium-independent dilatation in uraemic patients despite deterioration of glucose metabolism. Nephrol Dial Transplant 2011; 26:2370–2377. [DOI] [PubMed] [Google Scholar]
- 29.Cohen DL, Warburton KM, Warren G, et al. Pulse wave analysis to assess vascular compliance changes in stable renal transplant recipients. Am J Hypertens 2004; 17:209–212. [DOI] [PubMed] [Google Scholar]
- 30.Stróżecki P1, Adamowicz A, Włodarczyk Z, et al. The influence of calcineurin inhibitors on pulse wave velocity in renal transplant recipients. Ren Fail 2007; 29:679–684. [DOI] [PubMed] [Google Scholar]
- 31.Kasiske BL, Snyder JJ, Gilbertson D, et al. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant 2003; 3:178–185. [DOI] [PubMed] [Google Scholar]
- 32.Opazo Saez A, Kos M, Witzke O, et al. Effect of new-onset diabetes mellitus on arterial stiffness in renal transplantation. Transpl Int 2008; 21:930–935. [DOI] [PubMed] [Google Scholar]
- 33.Nürnberger J, Dammer S, Opazo Saez A, et al. Diastolic blood pressure is an important determinant of augmentation index and pulse wave velocity in young, healthy males. J Hum Hypertens 2003; 17:153–158. [DOI] [PubMed] [Google Scholar]
- 34.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006; 27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 35.Kneifel M, Scholze A, Burkert A, et al. Impaired renal allograft function is associated with increased arterial stiffness in renal transplant recipients. Am J Transplant 2006; 6:1624–1630. [DOI] [PubMed] [Google Scholar]
- 36.Muth BL, Astor BC, Turk J, et al. Outpatient management of delayed graft function is associated with reduced length of stay without an increase in adverse events. Am J Transplant 2015; doi: 10.1111/ajt.13689. (Accepted Article). [DOI] [PubMed] [Google Scholar]
- 37.Nankivell BJ, Borrows RJ, Fung CL, et al. The natural history of chronic allograft nephropathy. N Engl J Med 2003; 349:2326–2333. [DOI] [PubMed] [Google Scholar]
- 38.Kaplan B, Schold JD, Meier–Kriesche HU. Long–term graft survival with neoral and tacrolimus: a paired kidney analysis. J Am Soc Nephrol 2003; 14:2980–2984. [DOI] [PubMed] [Google Scholar]
- 39.Cheung CY, Chan HW, Liu YL, et al. Long–term graft function with tacrolimus and cyclosporine in renal transplantation: paired kidney analysis. Nephrology (Carlton) 2009; 14:758–763. [DOI] [PubMed] [Google Scholar]
- 40.Artz MA, Boots JM, Ligtenberg G, et al. Improved cardiovascular risk profile and renal function in renal transplant patients after randomized conversion from cyclosporine to tacrolimus. J Am Soc Nephrol 2003; 14:1880–1888. [DOI] [PubMed] [Google Scholar]
- 41.Nankivell BJ, Alexander SI. Rejection of the kidney allograft. N Engl J Med 2010; 363:1451–1462. [DOI] [PubMed] [Google Scholar]
- 42.Meier–Kriesche HU, Schold JD, Srinivas TR, et al. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004; 4:378–383. [DOI] [PubMed] [Google Scholar]
- 43.El Ters M, Grande JP, Keddis MT, et al. Kidney allograft survival after acute rejection, the value of follow-up biopsies. Am J Transplant 2013; 13:2334–2341. [DOI] [PubMed] [Google Scholar]
- 44.Lim WH, Chadban SJ, Clayton P, et al. Human leukocyte antigen mismatches associated with increased risk of rejection, graft failure, and death independent of initial immunosuppression in renal transplant recipients. Clin Transplant 2012; 26:E428–E437. [DOI] [PubMed] [Google Scholar]
- 45.Carbone M, Mutimer D, Neuberger J, et al. C virus and nonliver solid organ transplantation. Transplantation 2013; 95:779–786. [DOI] [PubMed] [Google Scholar]
- 46.Mahmoud IM, Elhabashi AF, Elsawy E, et al. The impact of hepatitis C virus viremia on renal graft and patient survival: a 9-year prospective study. Am J Kidney Dis 2004; 43:131–139. [DOI] [PubMed] [Google Scholar]


