Abstract
The aim of this study was to evaluate the risk of depression in and effect of l-thyroxine therapy on patients with Hashimoto thyroiditis (HT) in Taiwan.
In this retrospective, nationwide cohort study, we retrieved data from the Longitudinal Health Insurance Database 2000. We collected data of 1220 patients with HT and 4880 patients without HT for the period 2000 to 2011. The mean follow-up period for the HT cohort was 5.77 years. Univariate and multivariate Cox proportional hazards regression models were used to estimate the risk of depression in the HT cohort.
In the HT cohort, 89.6% of the patients were women. Compared with the non-HT cohort, the HT cohort exhibited a higher prevalence of diabetes mellitus, hyperlipidemia, and coronary artery disease. Furthermore, the HT cohort showed a higher overall incidence of depression compared with the non-HT cohort (8.67 and 5.49 per 1000 person-year; crude hazard ratio [HR] = 1.58, 95% confidence interval [CI] = 1.18–2.13). The risk of depression decreased after administration of l-thyroxine treatment for more than 1 year (adjusted HR = 1.02; 95% CI = 0.66–1.59).
In Taiwan, the overall incidence of depression was greater in the young HT cohort. l-thyroxine treatment reduced the risk of depression.
INTRODUCTION
According to a World Health Organization study of 245,400 patients with chronic physical diseases in 60 countries,1 the 1-year prevalence of depression among the patients was 23%, whereas that in healthy control patients it was 3.2%. Compared with healthy control patients, the risk of depression was 2-fold higher in patients with diabetes (DM), hypertension (HTN), and coronary artery disease (CAD) and 3-fold higher in patients with end-stage renal failure and cerebrovascular disease.2 Hashimoto thyroiditis (HT) is an autoimmune thyroid disease that causes primary hypothyroidism by destroying thyroid tissue.3 Hypothyroidism or subclinical hypothyroidism may cause coronary heart disease through hyperlipidemia, HTN, DM, or obesity.4–7 Previous studies have reported that thyroid hormones are critical in the development of mood disturbances, cognitive impairment, and other neuropsychiatric manifestations.8 A previous study revealed that patients older than 55 years and with depression exhibited high prevalence rates of thyroid disease.9 Furthermore, another study indicated that patients with major depressive disorder had high prevalence rates of thyroid disease.10 However, 1 study reported that patients with depression did not exhibit high prevalence rates of thyroid disease.11 These inconsistent results necessitate conducting additional clinical or basic studies to confirm the relationships between depression and thyroid dysfunction. Therefore, the present study investigated the risk of depression and other chronic morbidities among patients with HT and examined the interaction between depression and the effect of l-thyroxine therapy period on patients in Taiwan.
METHODS
Data Source
In this retrospective, nationwide cohort study, we retrieved data from the Longitudinal Health Insurance Database 2000 (LHID 2000). The LHID 2000 was established through the cooperation of the Taiwan National Health Insurance Administration and the National Health Research Institute (NHRI). It contains original inpatient and outpatient claims data of 1,000,000 randomly sampled beneficiaries of the National Health Insurance (NHI) program in the year 2000 (23.75 million citizens). The NHI program is a nationwide, single-payer program that was established in March 1995, and it has covered nearly 99% of Taiwan residesnts.12 The LHID 2000 research database contains patient medical orders, operative procedures, and clinical diagnoses, and the diagnostic codes are based on the International Classification of Diseases, Ninth Revision, Clinical Modification Code (ICD-9-CM). All patient data in this database have been anonymized, and the NHRI approves access to the data. This study was approved to fulfill the condition for exemption by the Institutional Review Board (IRB) of China Medical University (CMUH104-REC2-115). The IRB also specifically waived the consent requirement.
Sampled Patients
We identified patients ages 20 years and older and newly diagnosed with HT (ICD-9-CM code 245.2) according to the data in the LHID2000 from 2000 to 2011. The date of HT diagnosis was defined as the index date. Patients with a history of depression (ICD-9-CM codes 296.2–296.3, 300.4, 311) before the index date were excluded. The non-HT cohort was formed by randomly selecting patients without a history of HT and depression from the LHID 2000. The selected patients were frequency matched with the patients with HT according to sex, age (in 5-year bands), and index year at a ratio of 1:4.
Outcomes and Comorbidities
All study patients were followed-up until they were given a diagnosis of depression or censored because of loss to follow-up, withdrawal from the insurance program, death, or the end of 2011. Furthermore, we defined the following baseline comorbidities: HTN (ICD-9-CM codes 401–405), DM (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), stroke (ICD-9-CM codes 430–438), CAD (ICD-9-CM codes 410–414), and cancer (ICD-9-CM codes 140–208).
Statistical Analysis
The distributions of sex, age, and comorbidities between the HT and non-HT cohorts were compared and examined using the chi-squared test. The mean ages (standard deviations, SDs) and follow-up periods (SDs) were measured and examined using a t test. The incidence rates of depression (per 1000 person-year) were calculated in the 2 cohorts. Univariate and multivariate Cox proportional hazards regression models were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for assessing the effects of HT on the risk of depression. The multivariate models were simultaneously adjusted for age and sex and the comorbidities of HTN and CAD. Further analysis was conducted to assess whether l-thyroxine treatment affected depression outcomes. Cumulative incidence curves of depression were computed using the Kaplan–Meier method, and the differences in the cumulative incidence curves between the 2 cohorts were tested using a log-rank test. All analyses were executed using SAS statistical software for Windows (Version 9.4, SAS Institute Inc., Cary, NC). A 2-tailed probability level of P < 0.05 was considered statistically significant.
RESULTS
In this retrospective cohort study, we collected data on 1220 patients with HT and 4880 patients without HT. In the HT cohort, 89.6% of the patients were women and 71.4% of the patients were ages 49 years or younger (Table 1). The mean age of the study patients was 42.7 ± 13.8 years for the HT cohort and 42.5 ± 14.1 years for the non-HT cohort. Compared with the non-HT cohort, the HT cohort exhibited a higher prevalence of DM, hyperlipidemia, and CAD. The mean follow-up periods for the HT and non-HT cohorts were 5.77 and 5.83 years, respectively. The Kaplan–Meier analysis results revealed that during the follow-up periods, the HT cohort had a higher cumulative incidence of depression than did the non-HT cohort (log-rank test, P = 0.002; Figure 1).
TABLE 1.
Demographic Characteristics and Comorbidity in Patient With and Without Hashimoto's Thyroiditis
FIGURE 1.
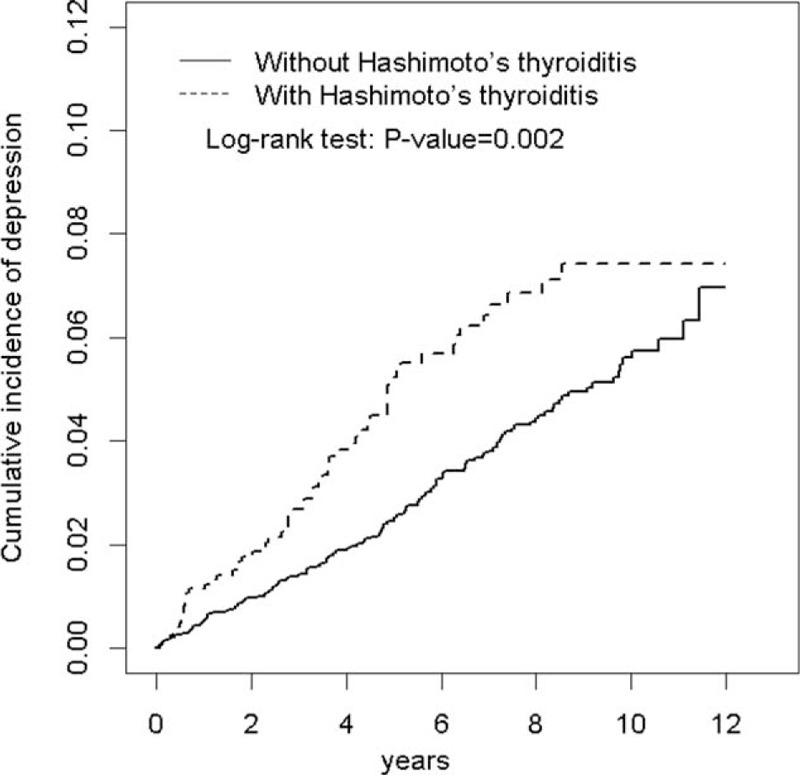
Cumulative incidence of depression for patients with and without Hashimoto's thyroiditis.
After adjustments for age, sex, and comorbidities (namely HTN and cancer), the overall incidence of depression was greater in the HT cohort than that in the non-HT cohort (8.67 and 5.49 per 1000 person-year, crude HR = 1.58, 95% CI = 1.18–2.13), with an adjusted HR (aHR) of 1.55 (95% CI = 1.16–2.09; Table 2). In both cohorts, the women had a higher depression incidence than the men did. Furthermore, the sex-specific depression aHR was greater for the women (aHR = 1.62, 95% CI = 1.20–2.19) compared with that for the men in both cohorts.
TABLE 2.
Comparison of Incidence and Hazard Ratio of Depression Stratified by Sex, Age, and Comorbidity Between With and Without Hashimoto's Thyroiditis Patients
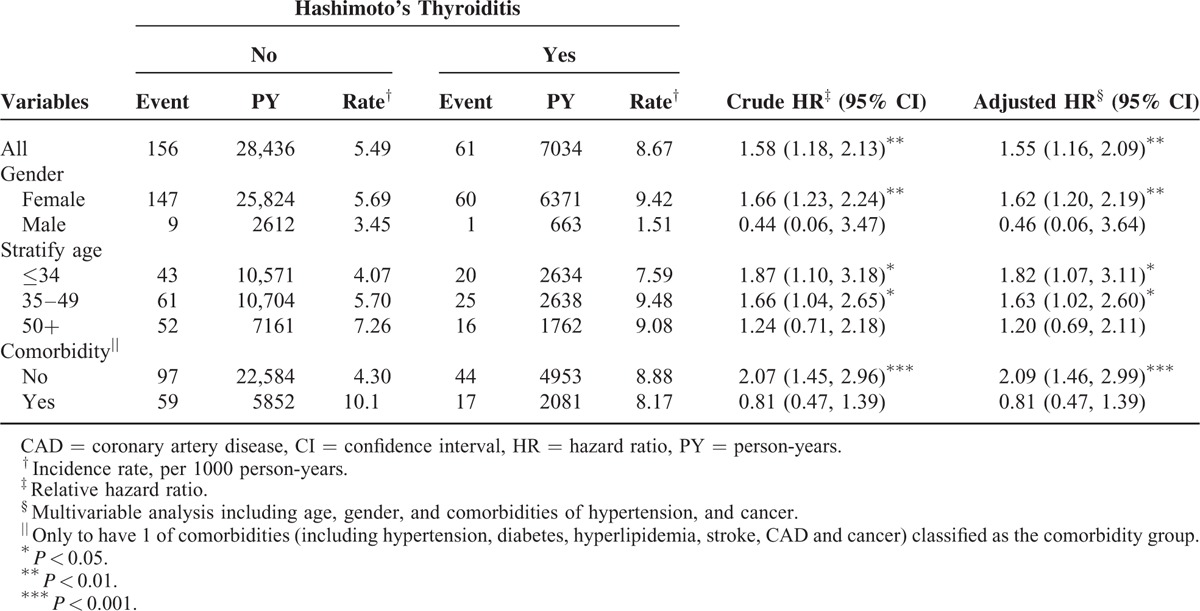
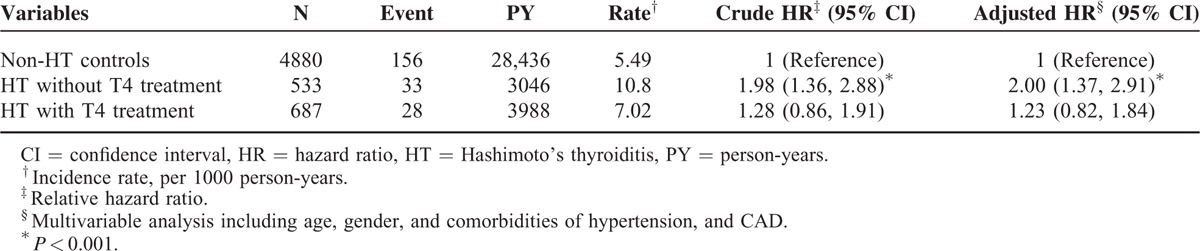
Except for patients older than 50 years, the increase in the risk of depression in patients with HT was greater for 2 age groups (i.e., ≤34 years: aHR = 1.82, 95% CI = 1.07–3.11; 35–49 years: aHR = 1.63, 95% CI = 1.02–2.60) than it was for those without HT. Patients with HT and with no comorbidity had a higher risk of depression than did those without HT and with no comorbidity (aHR = 2.09, 95% CI = 1.46–2.99). In the multivariate model, the risk of depression was 2.34-fold higher in women compared with that in men (95% CI = 1.24–4.44). The risk of developing depression was higher in patients with the HTN comorbidity (HR = 1.55, 95% CI = 1.03–2.31; Table 3). Table 4 shows the relationship between l-thyroxine treatment and the risk of depression. Compared with those in the non-HT cohort, patients in the HT cohort without l-thyroxine treatment were associated with a higher risk of depression (those without l-thyroxine treatment: aHR = 2.00, 95% CI = 1.37–2.91). By contrast, the risk of depression was decreased after treatment with thyroxine and did not differ from that of the non-HT cohort (aHR = 1.23, 95% CI = 0.82–1.84).
TABLE 3.
Hazard Ratios of Depression in Association With Age, Gender, and Comorbidities in Univariable and Multivariable Cox Regression Models
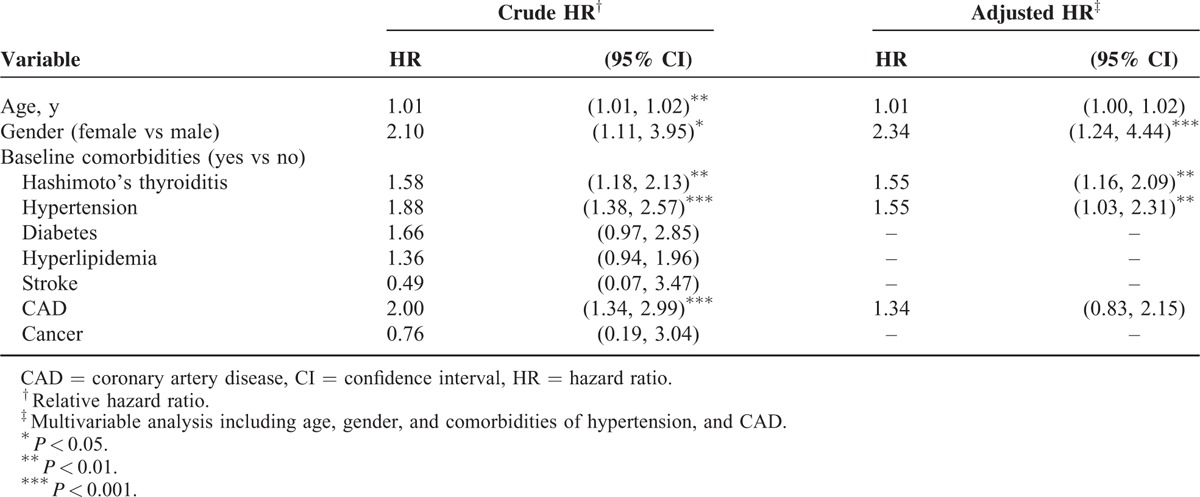
TABLE 4.
Incidence, Crude, and Adjusted Hazard Ratio of Depression Compared Among HT Patients With and Without Thyroxine (T4) Treatment and Non-HT Controls
DISCUSSION
HT and Chronic Morbidities
HT is an autoimmune thyroid disease that causes primary hypothyroidism.3 Previous studies have reported that hypothyroidism, which reflects metabolic effects, was attributable to a higher incidence of DM and hyperlipidemia.13–15 Another study mentioned that HT caused coronary heart disease.16 In the present study, the HT cohort exhibited a higher prevalence of DM, hyperlipidemia, and CAD compared with that in the non-HT cohort. Previous studies have reported that in addition inducing hypothyroidism, HT causes thyroid autoimmunity. HT is considerably influenced by hereditary factors, such as insulin-dependent DM and pernicious anemia.17 A previous study reported that HT altered the cell-mediated immunity through a genetic defect, resulting in a defective suppression of T cell functions,18 which causes patients with HT to produce various cytokines such as interferon-γ and tumor necrosis factor-α.19 The interferon-γ and tumor necrosis factor-α cytokines can regulate fat inflammation or promote lipogenesis in mice.20 These cytokines may also cause weight gain and lipolysis in humans.20
HT, l-Thyroxine Treatment, and Depression
Our results revealed that the overall incidence of depression was greater in the HT cohort and the aHR was 1.55 after adjustments for age, sex, and comorbidities (HTN and cancer).
Several studies have examined the association between depression and hypothyroidism.21,22 HT is an autoimmune thyroid disease that causes primary hypothyroidism.3 However, thyroid immunity has been reported to have a higher association with mood disorders in populations of patients with endocrine and psychiatric disorders compared with that in general populations.23–25 Except for patients older than 50 years, the increase in the risk of depression for patients with HT was more significant in 2 age groups (i.e., ≤34 years: aHR = 1.82, 95% CI = 1.07–3.11; 35–49 years: aHR = 1.63, 95% CI = 1.02–2.60) than that for patients without HT. Our finding is consistent with those of other studies that have reported that the prevalence of hypothyroidism increased with age and was higher among patients older than 65 years.26,27 In the present study, patients with HT and without comorbidities had a higher risk of depression than did those without HT and comorbidities; thus, HT is a possible independent risk factor for depression in younger Asian populations. In the multivariate model, the risk of depression was 2.34-fold higher in women compared with that in men, a result that is consistent with that of a previous study.27 Studies have commonly reported hypercortisolism in patients with depression. This disorder results in the modification of the HPT axis, which may reduce the secretion of serum thyroid-stimulating hormone (TSH) and cause a weakened TSH response to thyrotrophin-releasing hormone (TRH).28,29 Cortisol can also diminish the production of thyroid hormones by inhibiting the pathway of T4 conversion to active T3.30 A previous study indicated that compared with people in the general population, patients with depression had a lower level of T3.31 This possibly explains the l-thyroxine treatment results for patients with HT. Studies on the preventive effects of using l-thyroxine treatment in patients with HT to prevent CAD or reduce mortality have reported inconsistent results. Some studies have shown that l-thyroxine treatment could improve coronary flow or reduce hyperlipidemia in patients with HT.32,33 By contrast, another study reported that l-thyroxine treatment increased coagulation factor levels and inhibited fibrinolysis, increasing the risk of ischemic stroke.34 In the present study, an insufficient period of l-thyroxine treatment affected the outcome, whereas l-thyroxine treatment reduced the risk of depression in Taiwan.
CONCLUSION
In Taiwan, patients with HT demonstrated a higher prevalence of DM, hyperlipidemia, and CAD. Furthermore, in the HT cohort, younger patients exhibited a higher overall incidence of depression compared with that in other patients. l-thyroxine treatment can reduce the risk of depression.
LIMITATIONS
There are certain study limitations: No individual subject's information including the tobacco use, alcohol amount, stress evaluation, obesity status, exercise habit, income data, and family history of thyroid disease. The NHIRD could not provide the patients’ thyroid functions (such as TSH, TRH, T4, or T3) and images (such as thyroid echo or scans). These records in the NHIRD are majorly from billing of insurance uses, not for study uses, although all insurance claims in the NHIRD were already strictly surveyed by specialists’ peer review under the standard regulations. Because the identification for every patient in the NHIRD is anonymous under the laws for the personal information protection in Taiwan, we could not directly check individual subject's medical chart.
Footnotes
Abbreviations: CAD = coronary artery disease, CI = confidence interval, HT = Hashimoto thyroiditis, HTN = hypertension, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, NHIRD = National Health Insurance Research Database.
H-HC and C-HK contributed equally to this work.
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript: Study concept and design: H-HC, I-CL, C-HK; acquisition of data: all authors; analysis and interpretation of data: all authors; drafting of the manuscript: all authors; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: C-LL; obtained funding: C-HK; administrative, technical, or material support: all authors; study supervision: C-HK.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019); China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037); NRPB Stroke Clinical Trial Consortium (MOST 104-2325-B-039-005); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; and Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Moussavi S, Chatterji S, Verdes E, et al. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007; 370:851–858. [DOI] [PubMed] [Google Scholar]
- 2.Egede LE. Major depression in individuals with chronic medical disorders: prevalence, correlates and association with health resource utilization, lost productivity and functional disability. Gen Hosp Psychiatry 2007; 29:409–416. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V. Kumar V, Abbas KA, Fausto N, et al. The endocrine system. Robbins and Cotran Pathologic Basis of Disease, 8th ed. Philadelphia, PA: Elsevier; 2010. 1111–1205. [Google Scholar]
- 4.Hak AE, Pols HA, Visser TJ, et al. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med 2000; 132:270–278. [DOI] [PubMed] [Google Scholar]
- 5.Imaizumi M, Akahoshi M, Ichimaru S, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab 2004; 89:3365–3370. [DOI] [PubMed] [Google Scholar]
- 6.Rodondi N, Newman AB, Vittinghoff E, et al. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 2005; 165:2460–2466. [DOI] [PubMed] [Google Scholar]
- 7.Ochs N, Auer R, Bauer DC, et al. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med 2008; 148:832–845. [DOI] [PubMed] [Google Scholar]
- 8.Bauer M, Goetz T, Glenn T, et al. The thyroid–brain interaction in thyroid disorders and mood disorders. J Neuroendocrinol 2008; 20:1101–1114. [DOI] [PubMed] [Google Scholar]
- 9.Lobo-Escolar A, Saz P, Marcos G, et al. Somatic and psychiatric comorbidity in the general elderly population: results from the ZARADEMP Project. J Psychosom Res 2008; 65:347–355. [DOI] [PubMed] [Google Scholar]
- 10.Farmer A, Korszun A, Owen MJ, et al. Medical disorders in people with recurrent depression. Br J Psychiatry 2008; 192:351–355. [DOI] [PubMed] [Google Scholar]
- 11.Patten SB, Williams JV, Lavorato DH, et al. Major depression as a risk factor for chronic disease incidence: longitudinal analyses in a general population cohort. Gen Hosp Psychiatry 2008; 30:407–413. [DOI] [PubMed] [Google Scholar]
- 12.Database NHIR. Taiwan. http://nhird.nhri.org.tw/en/Background.html. (cited in 2015). [Google Scholar]
- 13.Diekman T, Lansberg PJ, Kastelein JJ, et al. Prevalence and correction of hypothyroidism in a large cohort of patients referred for dyslipidemia. Arch Intern Med 1995; 155:1490–1495. [PubMed] [Google Scholar]
- 14.Morris MS, Bostom AG, Jacques PF, et al. Hyperhomocysteinemia and hypercholesterolemia associated with hypothyroidism in the third US National Health and Nutrition Examination Survey. Atherosclerosis 2001; 155:195–200. [DOI] [PubMed] [Google Scholar]
- 15.Shah JH, Motto GS, Papagiannes E, et al. Insulin metabolism in hypothyroidism. Diabetes 1975; 24:922–925. [DOI] [PubMed] [Google Scholar]
- 16.Chen WH, Chen YK, Lin CL, et al. Hashimoto's thyroiditis, risk of coronary heart disease, and l-thyroxine treatment: a nationwide cohort study. J Clin Endocrinol Metab 2015; 100:109–114. [DOI] [PubMed] [Google Scholar]
- 17.Davies TF, Greenberg D, Tomer Y. The genetics of the autoimmune thyroid diseases. Ann Endocrinol (Paris) 2003; 64:28–30. [PubMed] [Google Scholar]
- 18.Volpe R. Autoimmune Thyroiditis. In: Braverman LE, Utiger RD. eds. Werner and Ingbar's the Thyroid. Philadelphia: JB Lippincott Co; 1991:921–941. [Google Scholar]
- 19.Jackson IMD, Hennessey JV. Thyroiditis. In: Becker KL, ed. Principles and Practice of Endocrinology and Metabolism. Philadelphia: Lippincott Williams & Wilkins; 2001:456–459. [Google Scholar]
- 20.Sultan A, Strodthoff D, Robertson AK, et al. T cell-mediated inflammation in adipose tissue does not cause insulin resistance in hyperlipidemic mice. Circ Res 2009; 104:961–968. [DOI] [PubMed] [Google Scholar]
- 21.Gold MS, Pottash AL, Extein I. Hypothyroidism and depression. Evidence from complete thyroid function evaluation. JAMA 1981; 245:1919–1922. [DOI] [PubMed] [Google Scholar]
- 22.Thvilum M, Brandt F, Almind D, et al. Increased psychiatric morbidity before and after the diagnosis of hypothyroidism: a nationwide register study. Thyroid 2014; 24:802–808. [DOI] [PubMed] [Google Scholar]
- 23.Carta MG, Loviselli A, Hardoy MC, et al. The link between thyroid autoimmunity (antithyroid peroxidase autoantibodies) with anxiety and mood disorders in the community: a field of interest for public health in the future. BMC Psychiatry 2004; 4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bunevicius R, Velickiene D, Prange AJ., Jr Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves’ disease. Gen Hosp Psychiatry 2005; 27:133–139. [DOI] [PubMed] [Google Scholar]
- 25.Fountoulakis KN, Iacovides A, Grammaticos P, et al. Thyroid function in clinical subtypes of major depression: an exploratory study. BMC Psychiatry 2004; 4:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderpump MP. The epidemiology of thyroid diseases. Br Med Bull 2011; 99:39–51. [DOI] [PubMed] [Google Scholar]
- 27.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87:489–499. [DOI] [PubMed] [Google Scholar]
- 28.Jackson IM. The thyroid axis and depression. Thyroid 1998; 8:951–956. [DOI] [PubMed] [Google Scholar]
- 29.Brouwer JP, Appelhof BC, Hoogendijk WJG, et al. Thyroid and adrenal axis in major depression: a controlled study in outpatients. Eur J Endocrinol 2005; 152:185–191. [DOI] [PubMed] [Google Scholar]
- 30.Stratakis CA, Chrousos GP. Neuroendocrinology and pathophysiology of the stress system. Ann N Y Acad Sci 1995; 771:1–18. [DOI] [PubMed] [Google Scholar]
- 31.Stipcevic T, Pivac N, Kozarić-Kovacić D, et al. Thyroid activity in patients with major depression. Coll Antropol 2008; 32:973–976. [PubMed] [Google Scholar]
- 32.Oflaz H, Kurt R, Sen F, et al. Coronary flow reserve after l-thyroxine therapy in Hashimoto's thyroiditis patients with subclinical and overt hypothyroidism. Endocrine 2007; 32:264–270. [DOI] [PubMed] [Google Scholar]
- 33.Tagami T, Tamanaha T, Shimazu S, et al. Lipid profiles in the untreated patients with Hashimoto thyroiditis and the effects of thyroxine treatment on subclinical hypothyroidism with Hashimoto thyroiditis. Endocr J 2010; 57:253–258. [DOI] [PubMed] [Google Scholar]
- 34.Squizzato A, Gerdes VE. Comment: does l-thyroxine prevent or cause stroke in hypothyroidism? Neurology 2014; 82:1650. [DOI] [PubMed] [Google Scholar]


