Abstract
Necrotizing enterocolitis is a devastating intestinal disease that affects ~5% of preterm neonates. Despite advancements in neonatal care, mortality remains high (30–50%) and controversy still persists with regards to the most appropriate management of neonates with necrotizing enterocolitis. Herein, we review some controversial aspects regarding the epidemiology, imaging, medical and surgical management of necrotizing enterocolitis and we describe new emerging strategies for prevention and treatment.
Keywords: Gut, necrotizing enterocolitis, bowel
Introduction
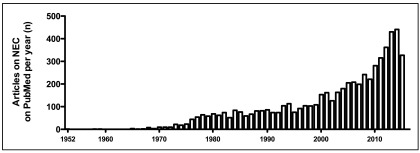
Necrotizing enterocolitis is an inflammatory intestinal disorder primarily seen in premature infants, characterized by variable damage to the intestinal tract, ranging from mucosal injury to full-thickness necrosis and perforation. Reports of infants suffering from necrotizing enterocolitis are found in literature dating back to the nineteenth century 1, but its pathology was described only in 1952, when Schmidt and Quaiser labeled it as enterocolitis ulcerosa necroticans 2, 3. Necrotizing enterocolitis became widely known in the 1960s following an epidemic occurring at the Babies Hospital in New York City between 1955 and 1966 4. The number of neonates collectively treated in New York helped to define the radiological and clinical presentation of this disease. Since then, the interest in and research into necrotizing enterocolitis has risen over the years ( Figure 1), and several randomized controlled trials have been conducted to question or define various aspects of prevention and treatment of this condition. However, controversy still persists with regards to the most appropriate management of neonates with necrotizing enterocolitis. Herein, we review some controversial aspects regarding the epidemiology, imaging, medical and surgical management of necrotizing enterocolitis and we describe new emerging strategies for prevention and treatment.
Figure 1. Number of articles published per year on PubMed since 1952 to 2015 (July).
Epidemiology
Necrotizing enterocolitis is considered to be the most common gastrointestinal emergency among neonates and affects primarily preterm infants. However, the true incidence of necrotizing enterocolitis is unknown, as it is difficult to establish and identify “mild” or “initial” cases, which correspond to stage I Bell’s classification [ Table 1] 5, 6. For this reason, solid epidemiology data are lacking.
Table 1. Bell staging criteria for necrotizing enterocolitis, modified from Walsh and Kliegman 6, 47.
| Stage | I | IIA | IIB | IIIA | IIIB |
|---|---|---|---|---|---|
| Description | Suspected NEC | Mild NEC | Moderate NEC | Severe NEC | Severe NEC |
| Systemic
signs |
Temperature
instability, apnea, bradycardia |
Similar to stage I | Mild acidosis,
thrombocytopenia |
Respiratory and
metabolic acidosis, mechanical ventilation, hypotension, oliguria, DIC |
Further deterioration
and shock |
| Intestinal
signs |
Increased gastric
residuals, mild abdominal distension, occult blood in the stool |
Marked abdominal
distension ± tenderness, absent bowel sounds, grossly bloody stools |
Abdominal wall edema
and tenderness ± palpable mass |
Worsening wall edema
with erythema and induration |
Evidence of
perforation |
| Radiographic
signs |
Normal or mild
ileus |
Ileus, dilated bowel
loops, focal pneumatosis |
Extensive pneumatosis,
early ascites ± PVG |
Prominent ascites, fixed
bowel loop, no free air |
Pneumoperitoneum |
DIC, disseminated intravascular coagulopathy; NEC, necrotizing enterocolitis; PVG, portal venous gas.
In 2010, Rees et al. reported the results of a national study based on a prospective cross-sectional survey administered to 158 level 2 and 3 neonatal intensive care units in the UK between 2005 and 2006 7. A total of 211 infants were diagnosed with necrotizing enterocolitis (45% Bell’s stage I, 21% stage II, and 33% stage III) for a period prevalence of 2% intensive care unit admissions. Data from the Canadian Neonatal Network reported that necrotizing enterocolitis (stage II and III) has an incidence of 5.1% in Canadian infants with a gestational age <33 weeks 8. The incidence of necrotizing enterocolitis has increased in recent decades in Canada and the UK due to more preterm and low birth weight infants being born—a trend that will continue as more preterm infants are treated in neonatal intensive care units.
The development of necrotizing enterocolitis varies also according to geographical and ethnic distribution, with lower frequencies in Japan, Switzerland, and Austria, and higher frequencies in Northern America, the UK and Ireland 8– 14. It is still unknown whether this variability is due to actual genetic and/or environmental factors in the population or whether it is influenced by neonatal care strategies and/or ethnic background.
The overall mortality rate for necrotizing enterocolitis remains high (30–50%), despite advancements in neonatal care. Moreover, a significant proportion of the survivors, particularly those with stage III necrotizing enterocolitis, have profound neurodevelopmental delay 14, resulting in reduced quality of life for the patient and family and in significant costs of ongoing treatment, calculated at between $500 million and $1 billion per year in the US 15. The serious and life-long challenges necrotizing enterocolitis places on patient, family, and society indicate that therapeutic strategies to preserve and/or reconstitute the intestinal structure of necrotizing enterocolitis-affected neonates are urgently needed.
Etiology
Although the exact etiology of necrotizing enterocolitis remains imperfectly understood, it is considered multifactorial, with several contributing causes extensively analyzed over the past 40 years. Some factors are more frequently encountered in babies with necrotizing enterocolitis, thus constituting the classical triggering factors for necrotizing enterocolitis-like bowel damage 16. Prematurity is the most recognized predisposing factor for necrotizing enterocolitis and it rarely occurs in full-term infants 17, where it is usually secondary to congenital diseases, such as cardiac anomalies 18, 19. Conversely, the majority of infants with necrotizing enterocolitis are born preterm, and the risk of developing necrotizing enterocolitis is inversely related to gestational age and birth weight 20– 21. Prematurity implies immaturity of gut motility and digestion, intestinal circulatory regulation, gut barrier function, and immune defense. Another well-recognized predisposing factor is formula feeding. The mechanism of injury may be fluid shift from villus vessels to the bowel lumen, resulting in an ischemic insult to the mucosa. Therefore, not only hyperosmolar formulas but any hyperosmolar fluid, including oral medications using hyperosmolar vehicles or contrast media, could lead to mucosal injury in the bowel 22, 23. Hypoxia is a well-studied phenomenon that can lead to necrotizing enterocolitis: recurrent episodes of apnea, respiratory distress, assisted ventilation, and umbilical vessel catheterization, and can all contribute to hypoxic events in very low birth weight neonates 24. Disruption of commensal bacteria, leading to deficient or abnormal microbial colonization of the gut, has been implicated as a key risk factor in the pathogenesis of necrotizing enterocolitis 25. Whether bacterial infection has a primary inciting role in necrotizing enterocolitis or whether an initial intestinal mucosal injury allows secondary bacterial invasion is unclear. The most commonly identified organisms are: Escherichia Coli, Klebsiella pneumoniae, Proteus, Staphylococcus aureus, S. Epidermidis, Enterococcus spp., Clostridium perfringens, and Pseudomonas aeruginosa.
Imaging
Plain radiography is the cornerstone of necrotizing enterocolitis diagnosis and staging ( Table 1) 5, 6, 26. The pathognomonic radiological finding of necrotizing enterocolitis is that of Pneumatosis intestinalis, defined as gas in the bowel wall originating from pathogenic bacteria. Other findings that are seen in more severe forms of necrotizing enterocolitis are ascites and portal venous gas. Pneumoperitoneum resulting from intestinal perforation is indicative of intestinal perforation and/or necrosis.
Other imaging modalities could provide more information and help diagnosis and follow-up of infants with necrotizing enterocolitis. Doppler ultrasonography, especially aimed at measuring blood flow velocity in the coeliac trunk and superior mesenteric artery, has been used to identify patients at risk of developing necrotizing enterocolitis, as well as to assess the bowel viability in those infants with established necrotizing enterocolitis 27– 30. In particular, sonographic findings of free gas, focal fluid collection, increased bowel wall echogenicity, absent bowel perfusion, portal venous gas, bowel wall thinning or thickening, and Pneumatosis intestinalis, are associated with an adverse outcome. Conversely, the use of magnetic resonance imaging scans in human necrotizing enterocolitis was described only once in the literature and remains anecdotal so far 31.
Near-infrared spectroscopy is a noninvasive real-time method of measuring local tissue oxygenation that is being evaluated as a predictive diagnostic modality for necrotizing enterocolitis. Gay et al. demonstrated that, in a piglet model of necrotizing enterocolitis, splanchnic tissue oxygenation measurements are directly correlated with changes in intestinal blood flow and markedly reduced by necrotizing enterocolitis 32. Patel et al. reported that abdominal near-infrared spectroscopy measurements are lower and have increased variability in preterm infants with necrotizing enterocolitis 33.
Diagnosis and disease progression
Infants with necrotizing enterocolitis present with clinical symptoms of abdominal distension, feeding intolerance, bilious vomiting, and bloody stools, and with laboratory derangements characterized by neutropenia, thrombocytopenia, metabolic acidosis, and high C-reactive protein levels 26.
In some infants, necrotizing enterocolitis progresses to peritonitis, intestinal perforation, septic shock, disseminated intravascular coagulation, and death. However, the majority of patients that require surgical intervention do not present with a fulminant course, resulting in disease progression 34. Clinical parameters alone cannot accurately predict necrotizing enterocolitis progressing to surgical disease in over 40% of patients, and biomarkers have been used to evaluate disease extent and progression 35. Novel biomarkers of necrotizing enterocolitis have been described in serum, urine, feces, buccal swab, or using noninvasive hemodynamic technique (heart rate activity) 35– 38.
Several authors have reported the use of laparoscopy to diagnose necrotizing enterocolitis 39– 42. Pierro et al. reported that laparoscopy can provide important information regarding bowel viability, is feasible and tolerated even in infants weighing less than 1 kg, and can be safely performed on the intensive care unit 39. Moreover, Numanoglu and Millar reported that when bowel ischemia is suspected, fluorescein laparoscopy could be useful to identify the necrotic segments 41.
In 1956, Porter first described a 2-day-old infant who developed a spontaneous intestinal perforation (SIP) 43. SIP most commonly presents as pneumoperitoneum without pneumatosis and, according to some studies, it has distinct, non-ischemic histopathology, different from that of necrotizing enterocolitis 44, 45. Gordon et al. were the first to demonstrate the deleterious relationship between early postnatal steroids and SIP in a retrospective cohort 46. However, despite extensive literature on the subject, there has been an ongoing debate on whether this condition represents a mild form of necrotizing enterocolitis or a distinct entity. In the latter case, many studies on necrotizing enterocolitis could be “contaminated” by infants who instead had SIP.
Medical management
Most infants with suspected (Bell’s stage I) or confirmed (Bell’s stage II) necrotizing enterocolitis are managed non-operatively. Non-operative treatment includes withholding feeds, ventilatory support, fluid resuscitation, inotropic support, correction of acid-base imbalance, coagulopathy and/or thrombocytopenia, bowel rest, and antibiotics.
Currently, there is no consensus and no evidence in the literature on which antibiotic regimen should be prescribed for medically managed infants with necrotizing enterocolitis, and this is reflected by both an international survey and a Cochrane review 48, 49. Therefore, antibiotics are prescribed depending on institutional protocol and changed according to individual culture and sensitivity results. Similarly, the duration of bowel rest with no enteral feeds for medically treated infants with necrotizing enterocolitis is based on tradition and not on evidence-based treatment 48.
Surgical management
A proportion of medically managed infants with necrotizing enterocolitis require acute surgical intervention, due to clinical deterioration or intestinal perforation. Whilst the latter indication is clearly identified with radiologic evidence of pneumoperitoneum, signs of clinical deterioration leading to surgery can be more subtle. These include requirement of inotropes, worsening abdominal findings, hemodynamic instability, worsening laboratory values (intractable acidosis, persistent thrombocytopenia, rising leukocytosis, or worsening leukopenia), and/or sonographic evidence of decreased or absent bowel perfusion.
A laparotomy in high-risk neonates, especially if born with an extremely low weight, can result in serious morbidity or even mortality. To avoid this risk, in 1977 Ein et al. first described the percutaneous insertion of a peritoneal drain in five neonates with bowel perforation as a temporizing measure to delay laparotomy 50. The authors noticed a clinical improvement of these infants within a week, so that they advocated the peritoneal drainage of small infants with perforated necrotizing enterocolitis. In support of this approach, a few years later, the same authors published a bigger series where they showed that 40% of neonates <1500 g treated with the peritoneal drain had complete resolution of their disease without requiring further surgery 51. A similar experience with the peritoneal drain was later reported by other authors 52– 54. However, this surgical approach has been very controversial and two prospective randomized controlled trials comparing the use of peritoneal drain vs. laparotomy in infants with perforated necrotizing enterocolitis were carried out 55, 56. Interestingly, neither of the two trials was able to demonstrate an advantage of one treatment modality over the other 55, 56. Moreover, Rees et al. demonstrated that in neonates with <1000 g body weight and perforated necrotizing enterocolitis, peritoneal drainage was not a definitively effective procedure, as 74% of the infants required a rescue laparotomy 57. It is still debatable whether there is a role for peritoneal drainage in the stabilization of a critically unwell child with perforated necrotizing enterocolitis and/or respiratory compromise, prior to the transfer to another center for laparotomy 57.
The universal principles of surgery in necrotizing enterocolitis are to remove the necrotic intestine and control intra-abdominal sepsis while preserving as much intestinal length as possible 57. Within these principles, there are different surgical options that surgeons favor on the basis of personal experience, rather than evidence-based literature 58. The classical approach to necrotizing enterocolitis has been to resect all areas of the necrotic intestine and fashion a stoma to allow adequate time for healing and growth before restoring intestinal continuity at a later stage. However, stomas, and in particular jejunostomies, are poorly tolerated by preterm infants, as they predispose them to nutritional and metabolic disturbances and poor growth as a consequence of fluid and electrolyte depletion 47. Therefore, some surgeons would resect necrotic bowel and perform a primary anastomosis, even in neonates weighing less than 1000 g 58. To investigate which is the most effective operation for neonates with surgical necrotizing enterocolitis, a multicenter randomized controlled trial of resection with primary anastomosis vs. resection with stoma (STAT: Stoma or Intestinal Anastomosis Trial) is currently underway.
Moreover, there is no consensus among surgeons on the type of stoma to fashion and where to locate it with regard to the surgical wound 58. This is in line with the outcomes of a recent systematic review of the literature that showed no difference in the type or location of colostomy in children with colorectal disease 59.
At laparotomy, some infants are found to have multifocal necrotizing enterocolitis and require multiple resections and multiple anastomoses. In 1996, Vaughan et al. described an alternative approach for such cases: the “clip-and-drop” technique 60. According to this technique, the multiple necrotic areas are resected, the bowel ends are sealed with titanium clips or staples, and the clipped bowel loops are returned to the abdominal cavity. At a second-look laparotomy, the bowel loops can be reassessed and anastomoses can be performed. Since the first description, the “clip-and-drop” technique has been employed for infants with multifocal necrotizing enterocolitis by other authors 60– 62.
When the vast majority of the intestine is affected by severe intestinal damage, the patient is considered to have pancolitis or NEC totalis. This is a very controversial scenario, as the resection of the necrotic bowel may involve almost the whole intestine. Options include closing the abdomen and withdrawing care, or creating a diverting jejunostomy. The latter has been described to rescue a proportion of neonates with extensive necrotizing enterocolitis and to result in enteral autonomy in most patients 63.
Outcome
Despite advancement in medical and surgical treatment over the last 6 decades, the mortality for necrotizing enterocolitis is still very high, especially in extremely low birth weight (ELBW) infants 64. Although the risk and absolute mortality of necrotizing enterocolitis decrease with higher birth weight, necrotizing enterocolitis has a relatively greater impact upon mortality at higher birth weight 64. Moreover, mortality for necrotizing enterocolitis is high even in cases of minimal bowel involvement 65.
Necrotizing enterocolitis survivors are also at high risk of developing severe complications, related either to the intestinal or to the systemic insult. Morbidities include recurrent episodes of necrotizing enterocolitis, development of intestinal strictures, intestinal failure, parenteral nutrition-related complications, and neurodevelopmental disabilities. About 10% of infants who have previously undergone surgery for necrotizing enterocolitis develop a recurrent episode and this results in long-term parenteral nutrition dependency 66. About a quarter of patients who have had necrotizing enterocolitis, especially if treated surgically, will develop one or more intestinal strictures 67, 68. Such strictures are investigated with contrast studies, typically in the form of enemas, due to the higher incidence in the colon, and usually require surgical resection. Strictures are the result of a vascular occlusion or spasm following the initial ischemic episode, as confirmed at histology by Kosloske et al. 68. In this seminal paper, Kosloske et al. reported the death of an infant due to the late diagnosis of post-necrotizing enterocolitis strictures and recommended a barium enema for all infants who had necrotizing enterocolitis about 6 weeks after the acute episode 68.
Longer-term outcome is related to the remaining intestinal length and its capacity for adequate nutrient absorption. The incidence of intestinal failure among infants undergoing surgical treatment for necrotizing enterocolitis is high, and many factors characteristic of severe necrotizing enterocolitis (such as low birth weight, antibiotic use, ventilator use, and greater extent of bowel resection) are associated with the development of intestinal failure 69. However, according to a recent study on a large cohort of children with intestinal failure one year after diagnosis, the diagnosis of necrotizing enterocolitis proved to be a significant predictive factor of enteral autonomy, possibly due to the greater capacity for late adaptation than the residual intestine after resection for other etiologies 70.
Finally, it is being increasingly recognized that approximately 50% of the neonates who developed necrotizing enterocolitis have a deleterious neurodevelopmental effect, although the mechanisms by which this develops is still poorly understood 14, 71. It is known that infants with necrotizing enterocolitis are at greater risk of motor impairment, and this seems to be mediated by white matter abnormalities on magnetic resonance imaging at term 72.
Emerging strategies
Efforts to improve necrotizing enterocolitis outcome are directed towards prevention and treatment of the disease.
Prevention
Probiotics are live microorganisms that increase natural intestinal defenses by regulating inflammatory response, cellular proliferation, and apoptosis. Several studies have demonstrated the efficacy and safety of prophylactic enteral probiotic administration in the prevention of necrotizing enterocolitis in infants with very low birth weight. A Cochrane review on this topic analyzed 24 trials and demonstrated that enteral probiotics supplementation significantly reduced the incidence of severe necrotizing enterocolitis and mortality 73. The probiotics preparations that were found to be effective contained lactobacillus either alone or in combination with bifidobacterium 73. New studies on probiotics in necrotizing enterocolitis are now directed to assess the most effective preparations, timing, and length of therapy to be utilized.
Other preventive strategies are to supplement formulas with prebiotics or synbiotics. Prebiotics are indigestible fiber compounds that stimulate the activity and growth of healthy bacteria within the intestine, whereas synbiotics are a combination of both prebiotics and probiotics, which exert a synergistic effect. Several studies report the beneficial effect of prebiotics or synbiotics on necrotizing enterocolitis incidence in preterm infants with variable outcomes 74– 77.
Treatment
Novel treatment strategies have been tested in experimental models of necrotizing enterocolitis. These include Captopril 78, platelet-activating factor antagonists 79, heparin-binding epidermal growth factor-like growth factor 80, granulocyte colony-stimulating factor and erythropoietin 81. One promising maneuver that was initially tested in animals and then confirmed in a safety and feasibility trial on human infants is moderately controlled hypothermia 82– 87. In animal studies, controlled hypothermia resulted in prolonged survival, prevention of liver energy failure, reduction in neutrophil infiltration of lungs and intestine, attenuation of the derangement in cardiac oxidative metabolism, attenuation of histological damage to the intestine and attenuation of the pro- and anti-inflammatory cytokine response in the portal vein and the systemic circulation 82– 86. In the first trial on preterm neonates with severe necrotizing enterocolitis, controlled hypothermia proved to be feasible and safe for 48 hours 87.
Stem cell therapy, which is nowadays a therapeutic option for refractory inflammatory bowel disease 88, has recently been proposed as a novel strategy for infants with necrotizing enterocolitis. In a neonatal rat model of necrotizing enterocolitis, amniotic fluid stem cells, injected intraperitoneally, proved to integrate into the bowel wall, improve survival, reduce necrotizing enterocolitis incidence, decrease gut damage and improve intestinal function 89– 92. Amniotic fluid stem cell administration was associated with the migration of cyclooxygenase-2 (COX-2) expressing stromal cells from the lamina propria of the small intestinal villi to a position near the base of the intestinal crypts 90. The beneficial effects of amniotic fluid stem cells on the development of necrotizing enterocolitis were blocked by the administration of a selective COX-2 inhibitor, suggesting that the migration of COX-2 cells was involved in the protective effects of the amniotic fluid stem cells. Moreover, the same beneficial effect exerted by amniotic fluid stem cells on rat survival was also obtained when a conditioned medium (supernatant) from amniotic fluid stem cells was administered 90. These findings suggest that amniotic fluid stem cells act via a paracrine mechanism, by secreting factors that stimulate bowel regeneration. Future studies, aimed at identifying those factors secreted by amniotic fluid stem cells, could pave the way for a novel pharmacological therapy for infants with necrotizing enterocolitis.
Acknowledgments
This work was supported by the endowment of the Robert M. Filler Chair of Surgery, The Hospital for Sick Children.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Paul Tam, Division of Paediatric Surgery, Department of Surgery, The University of Hong Kong, Queen Mary Hospital, Hong Kong
Patrick Chung, Division of Paediatric Surgery, Department of Surgery, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Queen Mary Hospital, Hong Kong
Atsuyuki Yamataka, Department of Pediatric Surgery, Juntendo University School of Medicine, Tokyo, Japan
David Wesson, Department of Surgery, Texas Children’s Hospital, Houston, TX, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Obladen M: Necrotizing enterocolitis--150 years of fruitless search for the cause. Neonatology. 2009;96(4):203–10. 10.1159/000215590 [DOI] [PubMed] [Google Scholar]
- 2. Schmid KO: [A specially severe form of enteritis in newborn, enterocolitis ulcerosa necroticans. I. Pathological anatomy]. Osterr Z Kinderheilkd Kinderfuersorge. 1952;8(2):114–136. [PubMed] [Google Scholar]
- 3. Quaiser K: [A specially severe form of enteritis in newborn, enterocolitis ulcerosa necroticans. II. Clinical studies]. Osterr Z Kinderheilkd Kinderfuersorge. 1952;8(2):136–152. [PubMed] [Google Scholar]
- 4. Touloukian RJ, Berdon WE, Amoury RA, et al. : Surgical experience with necrotizing enterocolitis in the infant. J Pediatr Surg. 1967;2(5):389–401. 10.1016/S0022-3468(67)80078-2 [DOI] [Google Scholar]
- 5. Bell MJ, Ternberg JL, Feigin RD, et al. : Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walsh MC, Kliegman RM: Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rees CM, Eaton S, Pierro A: National prospective surveillance study of necrotizing enterocolitis in neonatal intensive care units. J Pediatr Surg. 2010;45(7):1391–7. 10.1016/j.jpedsurg.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 8. Yee WH, Soraisham AS, Shah VS, et al. : Incidence and timing of presentation of necrotizing enterocolitis in preterm infants. Pediatrics. 2012;129(2):e298–304. 10.1542/peds.2011-2022 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Sankaran K, Puckett B, Lee DS, et al. : Variations in incidence of necrotizing enterocolitis in Canadian neonatal intensive care units. J Pediatr Gastroenterol Nutr. 2004;39(4):366–72. 10.1097/00005176-200410000-00012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Holman RC, Stoll BJ, Curns AT, et al. : Necrotising enterocolitis hospitalisations among neonates in the United States. Paediatr Perinat Epidemiol. 2006;20(6):498–506. 10.1111/j.1365-3016.2006.00756.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Kawase Y, Ishii T, Arai H, et al. : Gastrointestinal perforation in very low-birthweight infants. Pediatr Int. 2006;48(6):599–603. 10.1111/j.1442-200X.2006.02282.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Schmolzer G, Urlesberger B, Haim M, et al. : Multi-modal approach to prophylaxis of necrotizing enterocolitis: clinical report and review of literature. Pediatr Surg Int. 2006;22(7):573–80. 10.1007/s00383-006-1709-5 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Guner YS, Friedlich P, Wee CP, et al. : State-based analysis of necrotizing enterocolitis outcomes. J Surg Res. 2009;157(1):21–9. 10.1016/j.jss.2008.11.008 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Rees CM, Pierro A, Eaton S: Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92(3):F193–8. 10.1136/adc.2006.099929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Neu J, Walker WA: Necrotizing enterocolitis. N Engl J Med. 2011;364(3):255–64. 10.1056/NEJMra1005408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lin PW, Stoll BJ: Necrotising enterocolitis. Lancet. 2006;368(9543):1271–83. 10.1016/S0140-6736(06)69525-1 [DOI] [PubMed] [Google Scholar]
- 17. Ng S: Necrotizing enterocolitis in the full-term neonate. J Paediatr Child Health. 2001;37(1):1–4. 10.1046/j.1440-1754.2001.00584.x [DOI] [PubMed] [Google Scholar]
- 18. Bolisetty S, Lui K, Oei J, et al. : A regional study of underlying congenital diseases in term neonates with necrotizing enterocolitis. Acta Paediatr. 2000;89(10):1226–30. 10.1111/j.1651-2227.2000.tb00740.x [DOI] [PubMed] [Google Scholar]
- 19. McElhinney DB, Hedrick HL, Bush DM, et al. : Necrotizing enterocolitis in neonates with congenital heart disease: risk factors and outcomes. Pediatrics. 2000;106(5):1080–7. [DOI] [PubMed] [Google Scholar]
- 20. Guthrie SO, Gordon PV, Thomas V, et al. : Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23(4):278–85. 10.1038/sj.jp.7210892 [DOI] [PubMed] [Google Scholar]
- 21. Hsueh W, Caplan MS, Qu X, et al. : Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol. 2003;6(1):6–23. 10.1007/s10024-002-0602-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tuladhar R, Daftary A, Patole SK, et al. : Oral gastrografin in neonates: a note of caution. Int J Clin Pract. 1999;53(7):565. [PubMed] [Google Scholar]
- 23. Travadi J, Patole S, Simmer K: Gastrointestinal contrast studies in high-risk neonates with suspected necrotising enterocolitis--a note of caution. J Perinat Med. 2003;31(6):523–5. 10.1515/JPM.2003.080 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Palmer SR, Thomas SJ, Cooke RW, et al. : Birthweight-specific risk factors for necrotising enterocolitis. J Epidemiol Community Health. 1987;41(3):210–4. 10.1136/jech.41.3.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patel RM, Denning PW: Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res. 2015;78(3):232–8. 10.1038/pr.2015.97 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Kim SS, Albanese CT: Necrotizing enterocolitis. In: O'Neill JA, Coran AG, Fonkalsrud E, Grosfeld JL. Pediatric Surgery 6th ed. St. Louis: MO Mosby;2003;1427–1452. [Google Scholar]
- 27. Faingold R, Daneman A, Tomlinson G, et al. : Necrotizing enterocolitis: assessment of bowel viability with color doppler US. Radiology. 2005;235(2):587–94. 10.1148/radiol.2352031718 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Murdoch EM, Sinha AK, Shanmugalingam ST, et al. : Doppler flow velocimetry in the superior mesenteric artery on the first day of life in preterm infants and the risk of neonatal necrotizing enterocolitis. Pediatrics. 2006;118(5):1999–2003. 10.1542/peds.2006-0272 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Silva CT, Daneman A, Navarro OM, et al. : Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr Radiol. 2007;37(3):274–82. 10.1007/s00247-006-0393-x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Yikilmaz A, Hall NJ, Daneman A, et al. : Prospective evaluation of the impact of sonography on the management and surgical intervention of neonates with necrotizing enterocolitis. Pediatr Surg Int. 2014;30(12):1231–40. 10.1007/s00383-014-3613-8 [DOI] [PubMed] [Google Scholar]
- 31. Maalouf EF, Fagbemi A, Duggan PJ, et al. : Magnetic resonance imaging of intestinal necrosis in preterm infants. Pediatrics. 2000;105(3 Pt 1):510–4. [DOI] [PubMed] [Google Scholar]
- 32. Gay AN, Lazar DA, Stoll B, et al. : Near-infrared spectroscopy measurement of abdominal tissue oxygenation is a useful indicator of intestinal blood flow and necrotizing enterocolitis in premature piglets. J Pediatr Surg. 2011;46(6):1034–40. 10.1016/j.jpedsurg.2011.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Patel AK, Lazar DA, Burrin DG, et al. : Abdominal near-infrared spectroscopy measurements are lower in preterm infants at risk for necrotizing enterocolitis. Pediatr Crit Care Med. 2014;15(8):735–41. 10.1097/PCC.0000000000000211 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Ji J, Ling XB, Zhao Y, et al. : A data-driven algorithm integrating clinical and laboratory features for the diagnosis and prognosis of necrotizing enterocolitis. PLoS One. 2014;9(2):e89860. 10.1371/journal.pone.0089860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sylvester KG, Ling XB, Liu GY, et al. : A novel urine peptide biomarker-based algorithm for the prognosis of necrotising enterocolitis in human infants. Gut. 2014;63(8):1284–92. 10.1136/gutjnl-2013-305130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stone ML, Tatum PM, Weitkamp JH, et al. : Abnormal heart rate characteristics before clinical diagnosis of necrotizing enterocolitis. J Perinatol. 2013;33(11):847–50. 10.1038/jp.2013.63 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 37. Evennett N, Cerigioni E, Hall NJ, et al. : Smooth muscle actin as a novel serologic marker of severe intestinal damage in rat intestinal ischemia-reperfusion and human necrotising enterocolitis. J Surg Res. 2014;191(2):323–30. 10.1016/j.jss.2014.04.020 [DOI] [PubMed] [Google Scholar]
- 38. Ng PC, Ma TP, Lam HS: The use of laboratory biomarkers for surveillance, diagnosis and prediction of clinical outcomes in neonatal sepsis and necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2015;100(5):F448–52. 10.1136/archdischild-2014-307656 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Pierro A, Hall N, Ade-Ajayi A, et al. : Laparoscopy assists surgical decision making in infants with necrotizing enterocolitis. J Pediatr Surg. 2004;39(6):902–6; discussion 902–6. 10.1016/j.jpedsurg.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 40. Leva E, Di Cesare A, Canazza L, et al. : The role of laparoscopy in newborns affected by NEC. J Laparoendosc Adv Surg Tech A. 2010;20(2):187–9. 10.1089/lap.2009.0073 [DOI] [PubMed] [Google Scholar]
- 41. Numanoglu A, Millar AJ: Necrotizing enterocolitis: early conventional and fluorescein laparoscopic assessment. J Pediatr Surg. 2011;46(2):348–51. 10.1016/j.jpedsurg.2010.11.021 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 42. Smith J, Thyoka M: What role does laparoscopy play in the diagnosis and immediate treatment of infants with necrotizing enterocolitis? J Laparoendosc Adv Surg Tech A. 2013;23(4):397–401. 10.1089/lap.2012.0482 [DOI] [PubMed] [Google Scholar]
- 43. Porter A: Spontaneous pneumoperitoneum in the newborn; report of a case. N Engl J Med. 1956;254(15):694–6. 10.1056/NEJM195604122541504 [DOI] [PubMed] [Google Scholar]
- 44. Aschner JL, Deluga KS, Metlay LA, et al. : Spontaneous focal gastrointestinal perforation in very low birth weight infants. J Pediatr. 1988;113(2):364–7. 10.1016/S0022-3476(88)80285-3 [DOI] [PubMed] [Google Scholar]
- 45. Pumberger W, Mayr M, Kohlhauser C, et al. : Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg. 2002;195(6):796–803. 10.1016/S1072-7515(02)01344-3 [DOI] [PubMed] [Google Scholar]
- 46. Gordon PV, Young ML, Marshall DD: Focal small bowel perforation: an adverse effect of early postnatal dexamethasone therapy in extremely low birth weight infants. J Perinatol. 2001;21(3):156–60. 10.1038/sj.jp.7200520 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Hall NJ, Eaton S, Pierro A: Royal Australasia of Surgeons Guest Lecture. Necrotizing enterocolitis: prevention, treatment, and outcome. J Pediatr Surg. 2013;48(12):2359–67. 10.1016/j.jpedsurg.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 48. Zani A, Eaton S, Puri P, et al. : International survey on the management of necrotizing enterocolitis. Eur J Pediatr Surg. 2015;25(1):27–33. 10.1055/s-0034-1387942 [DOI] [PubMed] [Google Scholar]
- 49. Shah D, Sinn JK: Antibiotic regimens for the empirical treatment of newborn infants with necrotising enterocolitis. Cochrane Database Syst Rev. 2012;8:CD007448. 10.1002/14651858.CD007448.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Ein SH, Marshall DG, Girvan D: Peritoneal drainage under local anesthesia for perforations from necrotizing enterocolitis. J Pediatr Surg. 1977;12(6):963–7. 10.1016/0022-3468(77)90607-8 [DOI] [PubMed] [Google Scholar]
- 51. Janik JS, Ein SH: Peritoneal drainage under local anesthesia for necrotizing enterocolitis (NEC) perforation: a second look. J Pediatr Surg. 1980;15(4):565–6. 10.1016/S0022-3468(80)80774-3 [DOI] [PubMed] [Google Scholar]
- 52. Lessin MS, Luks FI, Wesselhoeft CW, Jr, et al. : Peritoneal drainage as definitive treatment for intestinal perforation in infants with extremely low birth weight (<750 g). J Pediatr Surg. 1998;33(2):370–2. 10.1016/S0022-3468(98)90465-1 [DOI] [PubMed] [Google Scholar]
- 53. Rovin JD, Rodgers BM, Burns RC, et al. : The role of peritoneal drainage for intestinal perforation in infants with and without necrotizing enterocolitis. J Pediatr Surg. 1999;34(1):143–7. 10.1016/S0022-3468(99)90245-2 [DOI] [PubMed] [Google Scholar]
- 54. Goyal A, Manalang LR, Donnell SC, et al. : Primary peritoneal drainage in necrotising enterocolitis: an 18-year experience. Pediatr Surg Int. 2006;22(5):449–52. 10.1007/s00383-006-1670-3 [DOI] [PubMed] [Google Scholar]
- 55. Moss RL, Dimmitt RA, Barnhart DC, et al. : Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med. 2006;354(21):2225–34. 10.1056/NEJMoa054605 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Rees CM, Eaton S, Kiely EM, et al. : Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg. 2008;248(1):44–51. 10.1097/SLA.0b013e318176bf81 [DOI] [PubMed] [Google Scholar]
- 57. Pierro A, Eaton S, Rees CM, et al. : Is there a benefit of peritoneal drainage for necrotizing enterocolitis in newborn infants? J Pediatr Surg. 2010;45(11):2117–8. 10.1016/j.jpedsurg.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 58. Hall NJ, Curry J, Drake DP, et al. : Resection and primary anastomosis is a valid surgical option for infants with necrotizing enterocolitis who weigh less than 1000 g. Arch Surg. 2005;140(12):1149–51. 10.1001/archsurg.140.12.1149 [DOI] [PubMed] [Google Scholar]
- 59. van den Hondel D, Sloots C, Meeussen C, et al. : To split or not to split: colostomy complications for anorectal malformations or hirschsprung disease: a single center experience and a systematic review of the literature. Eur J Pediatr Surg. 2014;24(1):61–9. 10.1055/s-0033-1351663 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Vaughan WG, Grosfeld JL, West K, et al. : Avoidance of stomas and delayed anastomosis for bowel necrosis: the 'clip and drop-back' technique. J Pediatr Surg. 1996;31(4):542–5. 10.1016/S0022-3468(96)90492-3 [DOI] [PubMed] [Google Scholar]
- 61. Ron O, Davenport M, Patel S, et al. : Outcomes of the "clip and drop" technique for multifocal necrotizing enterocolitis. J Pediatr Surg. 2009;44(4):749–54. 10.1016/j.jpedsurg.2008.09.031 [DOI] [PubMed] [Google Scholar]
- 62. Pang KK, Chao NS, Wong BP, et al. : The clip and drop back technique in the management of multifocal necrotizing enterocolitis: a single centre experience. Eur J Pediatr Surg. 2012;22(1):85–90. 10.1055/s-0031-1291287 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Thyoka M, Eaton S, Kiely EM, et al. : Outcomes of diverting jejunostomy for severe necrotizing enterocolitis. J Pediatr Surg. 2011;46(6):1041–4. 10.1016/j.jpedsurg.2011.03.024 [DOI] [PubMed] [Google Scholar]
- 64. Fitzgibbons SC, Ching Y, Yu D, et al. : Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. 2009;44(6):1072–5; discussion 1075–6. 10.1016/j.jpedsurg.2009.02.013 [DOI] [PubMed] [Google Scholar]
- 65. Thyoka M, de Coppi P, Eaton S, et al. : Advanced necrotizing enterocolitis part 1: mortality. Eur J Pediatr Surg. 2012;22(1):8–12. 10.1055/s-0032-1306263 [DOI] [PubMed] [Google Scholar]
- 66. Thyoka M, Eaton S, Hall NJ, et al. : Advanced necrotizing enterocolitis part 2: recurrence of necrotizing enterocolitis. Eur J Pediatr Surg. 2012;22(1):13–6. 10.1055/s-0032-1306264 [DOI] [PubMed] [Google Scholar]
- 67. Schwartz MZ, Hayden CK, Richardson CJ, et al. : A prospective evaluation of intestinal stenosis following necrotizing enterocolitis. J Pediatr Surg. 1982;17(6):764–70. 10.1016/S0022-3468(82)80443-0 [DOI] [PubMed] [Google Scholar]
- 68. Kosloske AM, Burstein J, Bartow SA: Intestinal obstruction due to colonic stricture following neonatal necrotizing enterocolitis. Ann Surg. 1980;192(2):202–7. 10.1097/00000658-198008000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Duro D, Kalish LA, Johnston P, et al. : Risk factors for intestinal failure in infants with necrotizing enterocolitis: a Glaser Pediatric Research Network study. J Pediatr. 2010;157(2):203–208.e1. 10.1016/j.jpeds.2010.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Demehri FR, Stephens L, Herrman E, et al. : Enteral autonomy in pediatric short bowel syndrome: predictive factors one year after diagnosis. J Pediatr Surg. 2015;50(1):131–5. 10.1016/j.jpedsurg.2014.10.011 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Hintz SR, Kendrick DE, Stoll BJ, et al. : Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. 10.1542/peds.2004-0569 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Shah DK, Doyle LW, Anderson PJ, et al. : Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153(2):170–5, 175.e1. 10.1016/j.jpeds.2008.02.033 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 73. AlFaleh K, Anabrees J: Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2014;4: CD005496. 10.1002/14651858.CD005496.pub4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Asmerom M, Crowe L, Marin T: Understanding the Biologic Therapies of Probiotics, Prebiotics, and Synbiotics: Exploring Current Evidence for Use in Premature Infants for the Prevention of Necrotizing Enterocolitis. J Perinat Neonatal Nurs. 2015;29(3):240–7; quiz E2. 10.1097/JPN.0000000000000120 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 75. Patel R, DuPont HL: New approaches for bacteriotherapy: prebiotics, new-generation probiotics, and synbiotics. Clin Infect Dis. 2015;60(Suppl 2):S108–21. 10.1093/cid/civ177 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Timofeev VA: Elektronnomikroskopicheskie dannye ob èkskretsii lipidov Triaenophorus nodulosus (Pall). Tsitologiia. 1967;9:1413–5. [PubMed] [Google Scholar]
- 77. Armanian AM, Sadeghnia A, Hoseinzadeh M, et al. : The Effect of Neutral Oligosaccharides on Reducing the Incidence of Necrotizing Enterocolitis in Preterm infants: A Randomized Clinical Trial. Int J Prev Med. 2014;5(11):1387–95. [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 78. Zani A, Eaton S, Leon FF, et al. : Captopril reduces the severity of bowel damage in a neonatal rat model of necrotizing enterocolitis. J Pediatr Surg. 2008;43(2):308–14. 10.1016/j.jpedsurg.2007.10.022 [DOI] [PubMed] [Google Scholar]
- 79. Lu J, Pierce M, Franklin A, et al. : Dual roles of endogenous platelet-activating factor acetylhydrolase in a murine model of necrotizing enterocolitis. Pediatr Res. 2010;68(3):225–30. 10.1203/PDR.0b013e3181eb2efe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wei J, Besner GE: M1 to M2 macrophage polarization in heparin-binding epidermal growth factor-like growth factor therapy for necrotizing enterocolitis. J Surg Res. 2015;197(1):126–38. 10.1016/j.jss.2015.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. El-Ganzoury MM, Awad HA, El-Farrash RA, et al. : Enteral granulocyte-colony stimulating factor and erythropoietin early in life improves feeding tolerance in preterm infants: a randomized controlled trial. J Pediatr. 2014;165(6):1140–1145.e1. 10.1016/j.jpeds.2014.07.034 [DOI] [PubMed] [Google Scholar]
- 82. Stefanutti G, Pierro A, Parkinson EJ, et al. : Moderate hypothermia as a rescue therapy against intestinal ischemia and reperfusion injury in the rat. Crit Care Med. 2008;36(5):1564–72. 10.1097/CCM.0b013e3181709e9f [DOI] [PubMed] [Google Scholar]
- 83. Vejchapipat P, Williams SR, Proctor E, et al. : Moderate hypothermia ameliorates liver energy failure after intestinal ischaemia-reperfusion in anaesthetised rats. J Pediatr Surg. 2001;36(2):269–75. 10.1053/jpsu.2001.20687 [DOI] [PubMed] [Google Scholar]
- 84. Vejchapipat P, Proctor E, Ramsay A, et al. : Intestinal energy metabolism after ischemia-reperfusion: Effects of moderate hypothermia and perfluorocarbons. J Pediatr Surg. 2002;37(5):786–90. 10.1053/jpsu.2002.32288 [DOI] [PubMed] [Google Scholar]
- 85. Vinardi S, Pierro A, Parkinson EJ, et al. : Hypothermia throughout intestinal ischaemia-reperfusion injury attenuates lung neutrophil infiltration. J Pediatr Surg. 2003;38(1):88–91; discussion 88-91. 10.1053/jpsu.2003.50017 [DOI] [PubMed] [Google Scholar]
- 86. Stefanutti G, Vejchapipat P, Williams SR, et al. : Heart energy metabolism after intestinal ischaemia and reperfusion. J Pediatr Surg. 2004;39(2):179–83; discussion 179-83. 10.1016/j.jpedsurg.2003.10.006 [DOI] [PubMed] [Google Scholar]
- 87. Hall NJ, Eaton S, Peters MJ, et al. : Mild controlled hypothermia in preterm neonates with advanced necrotizing enterocolitis. Pediatrics. 2010;125(2):e300–8. 10.1542/peds.2008-3211 [DOI] [PubMed] [Google Scholar]
- 88. Irhimeh MR, Cooney J: Management of inflammatory bowel disease using stem cell therapy. Curr Stem Cell Res Ther. 2015. [DOI] [PubMed] [Google Scholar]
- 89. Zani A, Cananzi M, Eaton S, et al. : Stem cells as a potential treatment of necrotizing enterocolitis. J Pediatr Surg. 2009;44(3):659–60. 10.1016/j.jpedsurg.2008.12.012 [DOI] [PubMed] [Google Scholar]
- 90. Zani A, Cananzi M, Fascetti-Leon F, et al. : Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut. 2014;63(2):300–9. 10.1136/gutjnl-2012-303735 [DOI] [PubMed] [Google Scholar]
- 91. Zani A, Cananzi M, Lauriti G, et al. : Amniotic fluid stem cells prevent development of ascites in a neonatal rat model of necrotizing enterocolitis. Eur J Pediatr Surg. 2014;24(1):57–60. 10.1055/s-0033-1350059 [DOI] [PubMed] [Google Scholar]
- 92. Eaton S, Zani A, Pierro A, et al. : Stem cells as a potential therapy for necrotizing enterocolitis. Expert Opin Biol Ther. 2013;13(12):1683–9. 10.1517/14712598.2013.849690 [DOI] [PubMed] [Google Scholar]