Abstract
The incidence of thyroid cancer is increasing rapidly. A large percentage of new cases identified fall into a low-risk category. As the incidence has increased, clinical experience has confirmed that the majority of patients will have excellent outcomes and that those at risk of doing badly can be reliably identified. Treatment for thyroid cancer is predominantly surgical. The decision about how aggressively this disease should be managed has remained controversial due to the excellent outcomes irrespective of the nature of surgical procedure chosen. This article reviews the developments in our understanding of the biology of thyroid cancer and the evidence that supports the approach to management.
Keywords: Differentiated thyroid cancer, Thyroid surgery, Lymph node surgery, Thyroid lobectomy, Cervical lymphadenectomy, Primary thyroid surgery
Introduction
Differentiated thyroid cancer is the most common endocrine malignancy and rates are increasing worldwide 1– 8. Patients and clinicians are increasingly aware of the diagnosis and, with ever more cases being reported, an understanding of the most appropriate way of managing this condition is essential.
Optimal management of this disease is controversial. The reasons for the controversy include the low rates of disease-specific mortality, relatively low morbidity from therapy, and the gradual evolution in our understanding of the biology of the disease.
Low mortality rates have been a challenge for investigators with an interest in differentiated thyroid cancer. Less than 10% of patients will die of the disease within 10 years of presentation. Indeed, even recurrence rates are low, particularly in the most controversial low-risk groups. As such, randomized controlled trials of therapy have not been considered feasible 9. The lack of prospective trials leads clinicians to rely on retrospective data, which has inherent flaws, no matter how it is collected.
The mainstay of therapy for differentiated thyroid cancer is surgery. Although when initially attempted, thyroidectomy was associated with high rates of mortality, improvements in anaesthetic and surgical technique have resulted in thyroid surgery being extremely safe. Death following thyroid surgery is reported in <0.5% of cases 10. In contrast, rates of injury to the recurrent laryngeal nerve and parathyroid glands are more common (2% nerve palsy and 6% need for calcium supplements at follow up 10). These complications, while not life threatening, result in voice change (and occasionally tracheostomy in cases of bilateral recurrent laryngeal nerve injury) and the need for long term calcium supplementation.
The initial surgical approach to thyroid cancer was radical. Total thyroidectomy and bilateral radical neck dissection achieved macroscopic disease clearance at a cost. In particular, the cervical lymphadenectomy was associated with significant functional and cosmetic impact. The recognition that histology could predict the biological behaviour of tumors was made in the mid-20 th century. Good outcomes were described for patients with differentiated lesions of follicular cell origin (papillary carcinoma/follicular carcinoma/Hurthle cell carcinoma) in comparison with medullary or anaplastic carcinoma 11. This observation led to a significant change in surgical approach, with a move away from aggressive neck surgery in patients with differentiated thyroid cancer.
The reports of large institutions’ retrospective case series provided further insight into disease biology with the recognition of age, tumor size, presence of extra thyroid extension, and distant metastases as risk factors within the differentiated thyroid cancer patient group 12– 16. These observations provided a framework for clinicians to risk stratify their patient group into those at low, intermediate, and high risk of disease-specific death (abbreviated to AMES system of risk stratification).
Along with the interest in predicting features of patients and their tumors, which predicted the biology of disease, came scrutiny of outcomes related to management of the disease itself. Until this point, no universally agreed approach had been accepted. In 1977, a report of outcomes for 576 patients with papillary thyroid cancer (the most common differentiated thyroid cancer) recorded in the US Air Force Central Tumor Registry found that total thyroidectomy and post-operative radioactive iodine therapy were associated with lower rates of recurrence and higher survival 17. Clinicians would use this evidence to support an approach of total thyroidectomy and radioactive iodine for all cases of differentiated thyroid cancer. The report also found high rates of surgical morbidity following neck surgery with no impact on outcome. This finding supported an approach of primary thyroid surgery without neck dissection in those patients without evidence of regional metastases.
In contrast, other groups found that, in lower risk patients, outcomes were similar following total thyroidectomy and thyroid lobectomy 18, 19. The conflicting findings of the impact of the extent of initial therapy on outcome continue to this day. The surgical debate has focused on the need for aggressive primary thyroid surgery (total thyroidectomy versus lobectomy) 20– 25 and the approach to the clinically uninvolved central neck (prophylactic dissection versus observation) 26– 33.
Much research has followed since the early reports. However, prospective trials are still lacking and most recommendations are based upon retrospective data, which have been analysed by expert authors with long standing biases.
The aim of this article is to examine the contemporary approach to surgical management of differentiated thyroid cancer by analysing arguments for primary thyroid surgery and cervical lymphadenectomy.
The aims of surgical management of differentiated thyroid cancer
The primary aim of surgical oncology is to prevent death from disease. However, for the overwhelming majority of patients with differentiated thyroid cancer, the risk of death is minimal. In addition, for many patients, occult disease will be present even after successful treatment. So the goals of therapy must be seen in the appropriate context. Risk stratification is paramount. High risk patients are treated aggressively, while low risk patients may be suitable for a less aggressive approach, and some select patients with the lowest risk disease (micropapillary carcinoma distant from the recurrent nerve or trachea) may even be candidates for an observational approach. In addition to preventing disease specific death, minimizing the chance of recurrence and preventing iatrogenic injury are key objectives for the treatment team.
Primary thyroid surgery
The most important aim of primary surgery is to achieve complete macroscopic disease clearance and to minimize the chance that ipsilateral thyroid bed surgery will ever be required again. This requires a thyroid lobectomy as a minimum (other than for the occasional patient with isolated isthmic disease). An extra capsular thyroidectomy with preservation of the recurrent laryngeal nerve and parathyroid glands should be the standard. This will achieve disease clearance and minimize the risk of thyroid bed recurrence.
A second aim of primary surgery is to render the patient suitable for adjuvant radioactive iodine by removing all thyroid tissue. The approach to adjuvant therapy is now changing. Previously, radioactive iodine was recommended for the majority of patients with tumors of 1cm or greater. Particularly in health care systems that do not biopsy lesions smaller than 1cm, this meant that all patients were candidates for total thyroidectomy. More recently, however, the role of radioactive iodine in intermediate and low risk patients has been questioned. There is a trend away from the blanket approach of total thyroidectomy and radioactive iodine for all towards a risk-adapted, individualized approach. Although radioactive iodine is still recommended in patients with aggressive primary lesions or metastatic disease to the neck or beyond, low risk patients with small-volume primary disease and without evidence of spread have little to gain from adjuvant therapy 34.
Therefore, appropriately selected patients are suitable for thyroid lobectomy rather than total thyroidectomy. While preserving excellent oncological outcomes, this approach has significant potential benefits. Rates of recurrent laryngeal nerve injury, hypocalcaemia, and tracheostomy are significantly lower following such unilateral surgery.
The ideal candidate for such an approach is a young patient with uninodular disease limited to the thyroid. The risk of permanent post-operative hypocalcaemia and tracheostomy is more or less 0% following thyroid lobectomy. Recurrent nerve injury is most commonly a temporary palsy, but is permanent in around 2% of cases 10. Operating on one side rather than both is, unsurprisingly, associated with lower rates of morbidity 35. However, these patients, by definition, have a residual lobe following treatment. They are not suitable for radioactive iodine and require monitoring of the contralateral lobe in the post-operative period by ultrasound. In the long term, 5–10% of such patients will require completion thyroidectomy at some point during follow up. This is mainly due to the development of nodular disease in the residual lobe. Such disease is malignant approximately half of the time 21.
A number of factors make the decision of which primary procedure to offer complex. Many expert authors report extremely low complication rates following total thyroidectomy. In contrast, reports from a community setting suggest that complication rates are significantly higher for the majority of patients who are operated on outside centers of excellence 35, 36. Following initial therapy, the tumor marker thyroglobulin can be used during follow up to detect recurrence. This tumor marker is produced both from native thyroid tissue and persistent disease. Therefore it is less useful in patients who have had thyroid lobectomy. Due to high rates of multifocal disease within the thyroid, most authors do not recommend thyroid lobectomy if there are nodules in the contralateral lobe, even if they appear benign. This is particularly relevant in areas with a high incidence of multinodular thyroid disease and is an issue increasingly encountered due to improvements in ultrasound imaging, which now detect nodular disease in over 50% of otherwise healthy individuals 37.
When making a decision about primary thyroid surgery, the clinician must consider a number of factors. Risk stratification should be performed for each patient and used as a guide to selection of therapy. Tumor factors are critical, and total thyroidectomy remains the treatment of choice for those high-risk patients who will be candidates for adjuvant radioactive iodine. Surgical factors must also be considered. A patient presenting to a high-volume thyroid surgeon in a center of excellence has a significantly lower risk of suffering a complication than one who presents to a surgeon with little experience. Although an ideal solution would be the centralization of thyroid surgery to minimize the number of surgeons performing this procedure, this is not feasible for most patients. Patient factors must also be considered. Differentiated thyroid cancer commonly affects young women. This patient group is often well read, motivated, and anxious. Patients may have an idea of what they consider the treatment of choice long before they arrive in the surgical clinic.
For many patients, total thyroidectomy is the treatment of choice. When performed well, it provides excellent oncological outcomes safely. It facilitates radioactive iodine if required, allows optimal follow up using thyroglobulin as a tumor marker, and addresses concerns about multifocal disease within the gland.
Clinicians should also be aware that thyroid lobectomy offers equal oncological outcomes in appropriately selected patients. There are significant advantages, particularly when patients are managed outside high-volume surgical departments. In addition, although the use of thyroglobulin in follow up is less accurate, it can still be used effectively. It should be remembered that “low-risk” patients have a mortality rate of <5% at 20 years and recurrences are extremely rare, so the value of a highly accurate tumor marker is questionable.
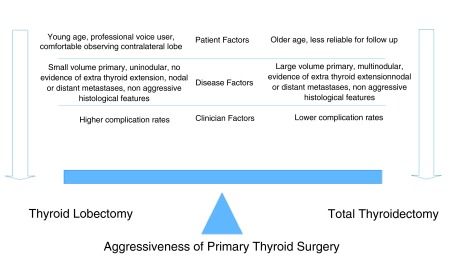
Disease management teams should consider the issues listed above when counselling patients with differentiated thyroid cancer. By balancing tumor, clinician, and patient factors, an individualized plan can be tailored for each patient using a risk-adapted approach to optimize outcome on a case by case basis ( Figure 1).
Figure 1. An individualized approach to selecting the aggressiveness of primary thyroid surgery.
Lymph node surgery
Differentiated thyroid cancer and in particular papillary thyroid cancer commonly metastasizes to the neck. The most common site of metastasis is the central neck (levels VI and VII), which surrounds the thyroid gland. The second echelon of lymph nodes is the lateral neck (most commonly levels III and IV). As stated earlier, radical neck dissection was considered the treatment of choice at one point 38. This was an operation that was relatively quick, safe, and resulted in macroscopic disease clearance. However, it was associated with high rates of morbidity. A vogue for a much less aggressive “berry picking” approach to the removal of macroscopically involved neck nodes has largely been abandoned due to unacceptably high recurrence rates. As experience in neck surgery has improved, a compartment oriented neck dissection is recommended by most authors as the operation of choice for patients with evidence of neck disease 39.
Critically, the surgeon must ensure the neck has been properly evaluated prior to embarking on surgery. Ultrasound is a reliable way of assessing the lateral neck and is also the investigation of choice for the thyroid. If lateral nodal disease is encountered, imaging of the central neck may be considered using CT or MRI. Cross sectional imaging is preferable to ultrasound in assessing the central neck, particularly the mediastinal component (level VII), which is poorly visualized using ultrasound.
Those patients considered N1a or N1b (metastatic disease in the central or lateral neck respectively) following investigation should have surgery planned to remove all involved levels, and any other levels considered at risk (therapeutic neck dissection). So, those patients with disease in the central neck alone should have a central neck dissection (almost always with a total thyroidectomy as radioactive iodine is likely to be indicated).
Those patients with lateral neck involvement should have clearance of levels II-V, which are at the highest risk of metastatic involvement 38. Involvement of the neck above the accessory nerve and in the submental/submandibular region (level I) is uncommon, so these levels are routinely spared. In addition, differentiated thyroid cancer rarely presents with aggressive nodal disease and extra nodal extension. Therefore, in almost all patients, the sternocleidomastoid muscle, internal jugular vein, and accessory nerve can be spared. This significantly limits the morbidity of surgery and has become the standard of care for patients with lateral neck disease.
In contrast, the approach to the clinically negative neck is highly controversial. The reasons for the controversy are multiple, and again, without prospective evidence may never be resolved.
There are authors who recommend prophylactic lateral neck dissection (surgery without pre-operative evidence of involved nodes) 28. However, they are in the minority and the vast majority do not consider the morbidity of lateral neck surgery worth the “benefit” 39, 40. Despite this, if one chooses to dissect the apparently uninvolved lateral neck, metastatic disease will often be found on histology 28. This disease rarely manifests and if it does it can safely be salvaged at a later date. In addition, entering the lateral neck requires an extended incision and places structures at risk that are not routinely encountered in thyroid and central neck surgery (accessory nerve, marginal mandibular nerve, carotid sheath and thoracic duct).
In contrast, when performing a thyroidectomy, the central neck is, by definition, entered. The recurrent laryngeal nerves and parathyroid glands are encountered during the dissection and revision central neck surgery carries higher risks than primary procedures.
Authors who argue for prophylactic central neck surgery highlight the fact that the central neck is exposed during primary thyroid surgery. They also cite high rates of occult histopathological metastases and that such metastases “upstage” patients when identified, which gives the treating team an effective way of further risk stratifying patients to rationalize the approach to adjuvant radioactive iodine. There is some evidence that excision of the involved nodes in the central neck results in lower post-operative thyroglobulin levels, which may result in lower recurrence rates. No author has ever proven that prophylactic central neck dissection results in improved survival, as almost no patient without metastatic disease dies during follow up.
In contrast, those authors who do not support prophylactic surgery highlight the higher surgical morbidity of the procedure versus thyroidectomy alone, and the fact that patients who have observation rather than central neck dissection have extremely good outcomes with low rates of recurrence and extremely low rates of death. With such good outcomes enjoyed by this group of low-risk patients, the need for radioactive iodine is questionable. In addition, the approach to a central neck dissection is probably variable. Prophylactic central neck surgery involves removing tissue that lies between the recurrent laryngeal nerves. However, those centers with experience of re-operative central neck surgery find high rates of disease in areas not normally included in prophylactic surgery, such as dorsal to the recurrent laryngeal nerve or low at the thoracic inlet, which are high-risk areas for dissection and hence not included in the primary surgical field 41.
The controversy has resulted in ambiguity in international guidelines 39, 40. Such documents recommend an individualization of approach dependant on risk factors for involvement. Patients with large volume tumors and those with evidence of extra thyroid extension are at higher risk and, in such patients, guidelines recommend considering prophylactic central neck surgery without definite evidence that such an approach results in improved outcomes. In general, there is a move away from an aggressive approach where central neck dissection should be considered for all patients (with papillary thyroid cancer) and towards selecting on a case by case basis, recognizing that the degree of potential benefit cannot be calculated.
Again, in making a decision about the surgical approach to the central neck, patient, tumor, and surgeon factors must be considered. Older male patients with large volume, multicentric disease and extra thyroid extension are at higher risk of metastatic disease to the central neck, even if it is not evident on pre-operative investigation. Such patients may be considered for prophylactic central neck surgery. However, the experience of the surgeon involved should also be weighed in the decision. Morbidity for high-volume neck surgeons is lower than that for those with less experience. The potential for benefit is small, and this must be weighed against the increased risks of damage to the parathyroids and recurrent laryngeal nerves when giving advice to patients without evidence of metastatic disease in the lymph nodes (cN0).
Conclusions
Outcomes for patients with differentiated thyroid cancer are excellent in comparison with other human cancers. Those at high risk can be easily identified from patient and tumor factors. Based upon a risk-stratified approach to managing differentiated thyroid cancer, therapeutic decisions in regard to surgery can be broken down into primary thyroidectomy and regional lymphadenectomy.
Total thyroidectomy is an operation associated with high cure rates and has been considered the gold standard internationally for years. However, thyroid lobectomy is now recognized as equally oncologically effective and is associated with lower morbidity in properly selected patients.
Compartment-oriented neck dissection has replaced both radical neck dissection and berry picking as the favoured approach to therapeutic lymphadenectomy. Outcomes for patients who have no evidence of regional metastases are now recognized as excellent, irrespective of the surgical approach. This has led to a less aggressive prophylactic surgical approach to the central neck in recent guidelines. This move away from routine prophylactic surgery has continued with the updated 2015 American Thyroid Association guidelines 34.
Although many treatment recommendations are now available, definitive prospective evidence to guide the thyroid surgeon is lacking in most cases. For this reason the surgical approach to this increasingly common disease remains controversial. By understanding the pros and cons of more and less aggressive approaches to the thyroid gland and the regional lymph nodes, with an understanding of the disease biology, and by applying risk stratification to all patients with differentiated thyroid cancer, appropriate decisions can be made that result in excellent oncological and surgical outcomes for the treatment team and their patients.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Sebastiano Bonventre, Department of General, Emergency and Transplant Surgery, Azienda Ospedaliera Universitaria Policlinico Paolo Giaccone Palermo, Palermo, 90127, Italy
Martin Schlumberger, Department of Nuclear Medicine and Endocrine Oncology, Institut Gustave-Roussy and University Paris-Sud, Villejuif, 94800, France
Brendan Stack, Department of Otolaryngology-Head and Neck Surgery, University of Arkansas for Medical Sciences, Little Rock, Arkansas, 72205, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Amphlett B, Lawson Z, Abdulrahman GO, Jr, et al. : Recent trends in the incidence, geographical distribution, and survival from thyroid cancer in Wales, 1985–2010. Thyroid. 2013;23(11):1470–8. 10.1089/thy.2012.0573 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Aschebrook-Kilfoy B, Ward MH, Sabra MM, et al. : Thyroid cancer incidence patterns in the United States by histologic type, 1992–2006. Thyroid. 2011;21(2):125–34. 10.1089/thy.2010.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 3. Chen AY, Jemal A, Ward EM: Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115(16):3801–7. 10.1002/cncr.24416 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 4. Davies L, Welch HG: Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295(18):2164–7. 10.1001/jama.295.18.2164 [DOI] [PubMed] [Google Scholar]
- 5. Enewold L, Zhu K, Ron E, et al. : Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18(3):784–91. 10.1158/1055-9965.EPI-08-0960 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 6. Kilfoy BA, Zheng T, Holford TR, et al. : International patterns and trends in thyroid cancer incidence, 1973–2002. Cancer Causes Control. 2009;20(5):525–31. 10.1007/s10552-008-9260-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. McNally RJ, Blakey K, James PW, et al. : Increasing incidence of thyroid cancer in Great Britain, 1976–2005: age-period-cohort analysis. Eur J Epidemiol. 2012;27(8):615–22. 10.1007/s10654-012-9710-x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 8. Olaleye O, Ekrikpo U, Moorthy R, et al. : Increasing incidence of differentiated thyroid cancer in South East England: 1987–2006. Eur Arch Otorhinolaryngol. 2011;268(6):899–906. 10.1007/s00405-010-1416-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 9. Carling T, Carty SE, Ciarleglio MM, et al. : American Thyroid Association design and feasibility of a prospective randomized controlled trial of prophylactic central lymph node dissection for papillary thyroid carcinoma. Thyroid. 2012;22(3):237–44. 10.1089/thy.2011.0317 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 10. Chadwick D, Kinsman R, Walton P: The British Association of Endocrine & Thyroid Surgeons Fourth National Audit Report. Dendrite Clinical Systems Ltd, The Hub, Station Road, Henley-on-Thames, Oxfordshire RG9 1AY, United Kingdom.2012. Reference Source [Google Scholar]
- 11. Graev M, Panunzi CA: [Findings on one hundred cases of thyroid carcinoma; incidence, frequency of various histological types, prognosis on the basis of histology, survival]. Arch De Vecchi Anat Patol. 1957;27(1):31–63. [PubMed] [Google Scholar]
- 12. Cady B, Sedgwick CE, Meissner WA, et al. : Risk factor analysis in differentiated thyroid cancer. Cancer. 1979;43(3):810–20. [DOI] [PubMed] [Google Scholar]
- 13. McConahey WM, Hay ID, Woolner LB, et al. : Papillary thyroid cancer treated at the Mayo Clinic, 1946 through 1970: initial manifestations, pathologic findings, therapy, and outcome. Mayo Clin Proc. 1986;61(12):978–96. 10.1016/S0025-6196(12)62641-X [DOI] [PubMed] [Google Scholar]
- 14. Shah JP, Loree TR, Dharker D, et al. : Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg. 1992;164(6):658–61. 10.1016/S0002-9610(05)80729-9 [DOI] [PubMed] [Google Scholar]
- 15. Byar DP, Green SB, Dor P, et al. : A prognostic index for thyroid carcinoma. A study of the E.O.R.T.C. Thyroid Cancer Cooperative Group. Eur J Cancer. 1979;15(8):1033–41. 10.1016/0014-2964(79)90291-3 [DOI] [PubMed] [Google Scholar]
- 16. Hay ID, Bergstralh EJ, Goellner JR, et al. : Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114(6):1050–7; discussion 1057–8. [PubMed] [Google Scholar]
- 17. Mazzaferri EL, Young RL, Oertel JE, et al. : Papillary thyroid carcinoma: the impact of therapy in 576 patients. Medicine (Baltimore). 1977;56(3):171–96. 10.1097/00005792-197705000-00001 [DOI] [PubMed] [Google Scholar]
- 18. Hay ID, Grant CS, Taylor WF, et al. : Ipsilateral lobectomy versus bilateral lobar resection in papillary thyroid carcinoma: a retrospective analysis of surgical outcome using a novel prognostic scoring system. Surgery. 1987;102(6):1088–95. [PubMed] [Google Scholar]
- 19. Shah JP, Loree TR, Dharker D, et al. : Lobectomy versus total thyroidectomy for differentiated carcinoma of the thyroid: a matched-pair analysis. Am J Surg. 1993;166(4):331–5. 10.1016/S0002-9610(05)80326-5 [DOI] [PubMed] [Google Scholar]
- 20. Bilimoria KY, Bentrem DJ, Ko CY, et al. : Extent of surgery affects survival for papillary thyroid cancer. Ann Surg. 2007;246(3):375–81; discussion 381–4. 10.1097/SLA.0b013e31814697d9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nixon IJ, Ganly I, Patel SG, et al. : Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery. 2012;151(4):571–9. 10.1016/j.surg.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 22. Vaisman F, Momesso D, Bulzico DA, et al. : Thyroid Lobectomy Is Associated with Excellent Clinical Outcomes in Properly Selected Differentiated Thyroid Cancer Patients with Primary Tumors Greater Than 1 cm. J Thyroid Res. 2013;2013: 398194. 10.1155/2013/398194 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Mendelsohn AH, Elashoff DA, Abemayor E, et al. : Surgery for papillary thyroid carcinoma: is lobectomy enough? Arch Otolaryngol Head Neck Surg. 2010;136(11):1055–61. 10.1001/archoto.2010.181 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Adam MA, Pura J, Goffredo P, et al. : Impact of extent of surgery on survival for papillary thyroid cancer patients younger than 45 years. J Clin Endocrinol Metab. 2015;100(1):115–21. 10.1210/jc.2014-3039 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Matsuzu K, Sugino K, Masudo K, et al. : Thyroid lobectomy for papillary thyroid cancer: long-term follow-up study of 1,088 cases. World J Surg. 2014;38(1):68–79. 10.1007/s00268-013-2224-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Nixon IJ, Ganly I, Patel SG, et al. : Observation of clinically negative central compartment lymph nodes in papillary thyroid carcinoma. Surgery. 2013;154(6):1166–72; discussion 1172–3. 10.1016/j.surg.2013.04.035 [DOI] [PubMed] [Google Scholar]
- 27. Monchik JM, Simon CJ, Caragacianu DL, et al. : Does failure to perform prophylactic level VI node dissection leave persistent disease detectable by ultrasonography in patients with low-risk papillary carcinoma of the thyroid? Surgery. 2009;146(6):1182–7. 10.1016/j.surg.2009.10.024 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Hartl DM, Leboulleux S, Al Ghuzlan A, et al. : Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg. 2012;255(4):777–83. 10.1097/SLA.0b013e31824b7b68 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 29. Ywata de Carvalho A, Chulam TC, Kowalski LP: Long-term Results of Observation vs Prophylactic Selective Level VI Neck Dissection for Papillary Thyroid Carcinoma at a Cancer Center. JAMA Otolaryngol Head Neck Surg. 2015;141(7):599–606. 10.1001/jamaoto.2015.0786 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 30. Sosa JA: Is routine prophylactic central neck dissection indicated for low-risk papillary thyroid cancer: can we determine cost-effectiveness if we are unsure about its effectiveness and safety? Surgery. 2013;154(6):1146–7. 10.1016/j.surg.2013.06.026 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Hartl DM, Mamelle E, Borget I, et al. : Influence of prophylactic neck dissection on rate of retreatment for papillary thyroid carcinoma. World J Surg. 2013;37(8):1951–8. 10.1007/s00268-013-2089-3 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 32. Dralle H: [Prophylactic central lymph node dissection improves prognosis for papillary thyroid cancer]. Chirurg. 2013;84(2):149. 10.1007/s00104-012-2463-y [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Zanocco K, Elaraj D, Sturgeon C: Routine prophylactic central neck dissection for low-risk papillary thyroid cancer: a cost-effectiveness analysis. Surgery. 2013;154(6):1148–55; discussion 1154–5. 10.1016/j.surg.2013.06.016 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Haugen BR, Md, Alexander EK, Bible KC, et al. : 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2015. 10.1089/thy.2015.0020 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Zerey M, Prabhu AS, Newcomb WL, et al. : Short-term outcomes after unilateral versus complete thyroidectomy for malignancy: a national perspective. Am Surg. 2009;75(1):20–4. [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Rosato L, Avenia N, Bernante P, et al. : Complications of thyroid surgery: analysis of a multicentric study on 14,934 patients operated on in Italy over 5 years. World J Surg. 2004;28(3):271–6. 10.1007/s00268-003-6903-1 [DOI] [PubMed] [Google Scholar]
- 37. Ezzat S, Sarti DA, Cain DR, et al. : Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med. 1994;154(16):1838–40. 10.1001/archinte.1994.00420160075010 [DOI] [PubMed] [Google Scholar]
- 38. Stack BC, Jr, Ferris RL, Goldenberg D, et al. : American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid. 2012;22(5):501–8. 10.1089/thy.2011.0312 [DOI] [PubMed] [Google Scholar]
- 39. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, . Cooper DS, Doherty GM, et al. : Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–214. 10.1089/thy.2009.0110 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 40. Perros P, Boelaert K, Colley S, et al. : Guidelines for the management of thyroid cancer. Clin Endocrinol (Oxf). 2014;81(Suppl 1):1–122. 10.1111/cen.12515 [DOI] [PubMed] [Google Scholar]
- 41. Clayman GL, Agarwal G, Edeiken BS, et al. : Long-term outcome of comprehensive central compartment dissection in patients with recurrent/persistent papillary thyroid carcinoma. Thyroid. 2011;21(12):1309–16. 10.1089/thy.2011.0170 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation