Abstract
Proteins must adopt a defined three-dimensional structure in order to gain functional activity, or must they? An ever-increasing number of intrinsically disordered proteins and amyloid-forming polypeptides challenge this dogma. While molecular chaperones and proteases are traditionally associated with protein quality control inside the cell, it is now apparent that molecular chaperones not only promote protein folding in the “forward” direction by facilitating folding and preventing misfolding and aggregation, but also facilitate protein unfolding and even disaggregation resulting in the recovery of functional protein from aggregates. Here, we review our current understanding of ATP-dependent molecular chaperones that harness the energy of ATP binding and hydrolysis to fuel their chaperone functions. An emerging theme is that most of these chaperones do not work alone, but instead function together with other chaperone systems to maintain the proteome. Hence, molecular chaperones are the major component of the proteostasis network that guards and protects the proteome from damage. Furthermore, while a decline of this network is detrimental to cell and organismal health, a controlled perturbation of the proteostasis network may offer new therapeutic avenues against human diseases.
Keywords: molecular chaperones, chaperones, proteases, protein folding, misfolding, aggregation, ATP-dependent molecular chaperones
Introduction
The vast majority of proteins must fold correctly in order to gain functional activity. While the protein folding information is encoded within the nascent polypeptide chain, newly synthesized polypeptides (or those imported into organelles) are prone to misfolding, causing aggregation and formation of other toxic species 1. Consequently, maintaining protein homeostasis (proteostasis) is essential for cell and organismal health 2. To accomplish this, cells have evolved a sophisticated network of protein quality control machines, consisting of molecular chaperones and proteases, which monitor the folding of proteins and their assembly into functional complexes, and selectively remove excess and damaged proteins from the cell. Challenging the capacity of this proteostasis network increases the risk of human diseases associated with protein misfolding and aggregation 1.
While most proteins adopt a defined three-dimensional structure, several exceptions are known to exist. Notable examples include prions that can adopt multiple, distinct, three-dimensional structures 3– 5, and an ever-increasing number of intrinsically disordered proteins (IDPs), which feature large regions of random coil or lack a defined structure altogether 6– 8. At least in yeast, it is now widely accepted that molecular chaperones play an essential role in prion replication 9, 10 by governing the inheritance and maintenance of yeast prions, and in some cases their elimination by chaperone overexpression 11– 15. However, concrete evidence of an involvement of molecular chaperones in mammalian prion replication, although proposed 16, is missing, and whether molecular chaperones play a role in the stabilization and/or protection of IDPs remains uncertain.
What is a molecular chaperone? A molecular chaperone can be generally defined as any protein that assists other macromolecules in folding and/or assembling into higher order structures, without it being a component of these final structures 17. Thus, while their main function inside the cell is to assist in the folding and maturation of unfolded or partially folded macromolecules and to prevent their misfolding and aggregation, it was widely assumed that molecular chaperones involved in de novo protein folding do not recover functional protein once aggregation has occurred. This concept was challenged by the discovery of a novel stress-inducible molecular chaperone known as Hsp104 18, which functions as an ATP-dependent protein disaggregase that rescues stress-damaged proteins from a previously aggregated state 19, 20. The discovery of Hsp104 has since expanded our definition of molecular chaperones to include those that promote the forward folding or prevent the aggregation of proteins on one hand, and those that recover functional protein from aggregates on the other hand.
At the molecular level, molecular chaperones come in diverse shapes and sizes, and can be broadly separated into two groups: those that depend on metabolic energy to fuel their chaperone activity, and those that do not 21. Examples of the former include all ATP-dependent molecular chaperones 22, while the latter include small heat shock proteins 23, protein disulfide isomerase 24, ribosome-associated chaperones such as trigger factor 25, and conditionally activated chaperones 26.
The focus of this review is on ATP-dependent molecular chaperones that harness the energy from ATP binding and/or hydrolysis to assist protein folding and unfolding (i.e., disaggregation). Their cellular expression can be either constitutive in order to perform vital housekeeping functions, or inducible by short exposure to elevated temperatures or other forms of stress that cause protein denaturation. Those that are stress-inducible are also known as heat-shock proteins or HSPs, while those that are constitutively active are termed heat-shock cognates or HSCs. Different members of both groups are classified according to their molecular weight, for example, HSP of 60-kDa (Hsp60), 70-kDa (Hsp70), 90-kDa (Hsp90), and 100-kDa (Hsp100), although many are better known by their common name that is used to designate each chaperone homolog from eubacteria, for example, GroEL (Hsp60), DnaK (Hsp70), HtpG (Hsp90), and ClpB (Hsp100) ( Figure 1). All of these aforementioned HSPs bind adenine nucleotide and hydrolyze ATP. Furthermore, another common feature is their cooperation with other proteins, termed co-chaperones, which regulate the ATPase and/or chaperone activity, or reset the functional cycle.
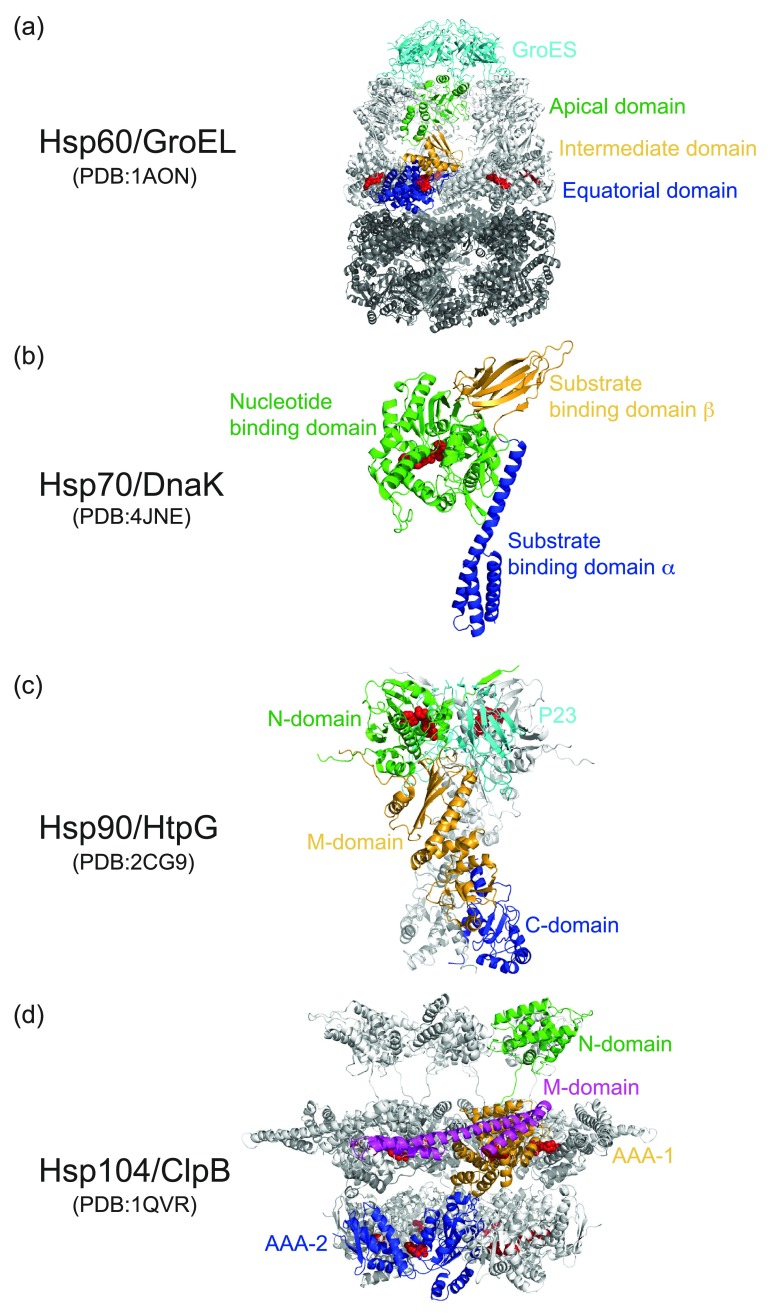
Figure 1. Molecular architecture and domain organization of ATP-dependent molecular chaperones.
Protein is shown as ribbon diagram with the bound nucleotide as red CPK model. For each chaperone, the domains of one subunit are shown in different colors in order of green, orange, and blue from N- to C-termini. Bound co-chaperones are colored cyan. ( a) Hsp60/GroEL: Architecture and domain organization of the E. coli GroEL tetradecamer bound to ADP with a GroES heptamer capping the GroEL cis ring (PDB: 1AON) 33. ( b) Hsp70/DnaK: Architecture and domain organization of the E. coli DnaK monomer in the ATP-bound state (PDB: 4JNE) 54. ( c) Hsp90/HtpG: Architecture and domain organization of the ATP-bound yeast Hsp90 dimer in the closed-state conformation, and its stabilization by p23/Sba1 (PDB: 2CG9) 81. ( d) Hsp104/ClpB: Architecture and domain organization of a yeast Hsp104 hexamer bound to ATP (PDB: 1QVR; EMD-1631) 97, 99. The Hsp104 M-domain that mediates the species-specific interaction with Hsp70 is colored in magenta.
The Hsp60 family
Hsp60 chaperones are known as chaperonins 27, and can be divided into two subgroups. Group I chaperonins are sevenfold symmetric and assemble into a barrel-like structure composed of two rings of seven identical subunits 28. Notable examples include bacterial GroEL and Hsp60 from mitochondria and chloroplasts. Each GroEL subunit consists of an equatorial, intermediate, and apical domain ( Figure 1a) 29– 31. ATP binding triggers a conformational rearrangement of the apical domains followed by GroES binding. The latter is a GroEL co-chaperone that assembles into a heptamer ring 32, and caps one side of the GroEL barrel (the cis ring) 33 to encapsulate the substrate 34, and to promote protein folding 35. The prevailing model suggests that GroEL-ES promotes folding through repetitive binding, encapsulation, and release of the substrate protein 28, 36. Group II chaperonins are homo- or hetero-oligomers forming an eightfold double barrel structure composed of sixteen subunits, and include the eukaryotic chaperonin containing TCP1 complex (CCT), also known as the TCP-1 Ring Complex (TRiC), and the thermosome and Methanococcus maripaludis chaperonin (Mm-Cpn) from Archaea 37. Unlike Group I chaperonins, group II members do not function together with a GroES-like co-chaperone, but instead contain a built-in lid that undergoes an iris-like motion to promote protein folding 38.
Much of our current understanding of chaperonin function comes from seminal work on Escherichia coli GroEL. E. coli GroEL is essential since many vital proteins, including metabolic enzymes and components of the transcription-translation machinery, depend on the GroE system for folding 39. While GroEL’s essential housekeeping function is beginning to be understood, an emerging question is the recent appreciation of multiple copies of GroEL in some bacterial genomes 40, as seen in actinobacteria, which includes Mycobacterium tuberculosis, the causative agent of Tuberculosis (TB). TB accounts for ~2 million deaths annually and is a major public health problem exacerbated by the emergence and rapid spread of new multidrug-resistant M. tuberculosis strains. M. tuberculosis encodes two copies of groEL in its genome 40. While M. tuberculosis GroEL2 is essential for viability, the function of the non-essential GroEL1 paralog remains less clear. The crystal structures of M. tuberculosis GroEL2 and a GroEL1 fragment showed that the apical domains have nearly identical three-dimensional structures 41, 42. While GroEL2 is believed to be the housekeeping chaperonin similar to E. coli GroEL, GroEL1 may function as a specialized chaperonin with a more limited substrate spectrum. Consistent with this notion, it has been proposed that mycobacterial GroEL1 is a dedicated chaperone for biofilm formation 43, which is presumed to confer the extraordinary starvation survival and resistance of M. tuberculosis to known antibiotics 44.
The Hsp70 family
Members of the Hsp70 chaperone family are found in all three surviving domains of life 45. At the molecular level, Hsp70 is a two-domain protein consisting of a nucleotide-binding domain connected by a long and flexible hydrophobic linker to the substrate-binding domain that can be subdivided into a β-sandwich domain and an α-helical domain ( Figure 1b). Furthermore, cytosolic eukaryotic Hsp70s feature a Glu-Glu-Val-Asp or “EEVD” motif at the extreme C-terminus, which is required for the interaction with Hsp70 co-chaperones that regulate the Hsp70 ATPase activity and its ability to bind substrate 46. It has been shown that bacterial Hsp70 recognizes diverse polypeptides mostly in an unfolded or partially unfolded form by binding to a four to five residue stretch of hydrophobic amino acids flanked by regions enriched in basic amino acids 47, which occur on average every 30–40 residues in most proteins. Since Hsp70 binding motifs are typically buried within the correctly folded protein, it provides a means to selectively seek out and bind proteins that are in a non-native conformation. However, Hsp70 chaperones rarely, if ever, function on their own and require the assistance of co-chaperones, which include nucleotide exchange factors, such as bacterial GrpE and eukaryotic Hsp110, and the large family of J-domain-containing Hsp40 co-chaperones, which accelerate ATP hydrolysis, serve as substrate targeting factors, and stabilize Hsp70-substrate interaction 21.
Over the last decade, high-resolution structural information on full-length Hsp70 chaperones has become available 48– 54, providing new insight into the Hsp70 conformational cycle and its allosteric regulation by nucleotide 55. Hsp70 function is controlled by nucleotide binding with ATP, promoting an open-conformation with low substrate-binding affinity, and ADP, promoting a closed-conformation required for tight binding of substrates 21.
In addition to Hsp70’s known role in protein folding, Hsp70 also has other non-chaperone functions. For instance, it was recently shown that Hsp70 functions as an activator of the ring-forming Hsp104 protein disaggregase and is required to unleash the potent protein disaggregating activity 56, 57. While no Hsp104 homolog is known to exist in metazoans, the discovery of a mammalian protein disaggregase, composed of Hsp70, Hsp110, and Hsp40, is exciting and supports functional conservation of a protein disaggregating activity in animal cells 58– 61. However, despite its nomenclature, Hsp110 is not an Hsp100 homolog, but instead belongs to an Hsp70 subfamily that is activated by nucleotide 62, shares structural 49, 63, 64 and perhaps functional conservation with Hsp70 65, and functions as an Hsp70 nucleotide exchange factor 66, 67.
The Hsp90 family
Hsp90 belongs to a conserved group of ATP-dependent molecular chaperones 68– 70 which, together with Hsp70 and a cohort of co-chaperones, facilitates the late-stage folding and maturation of proteins 71, 72. Since Hsp90 substrates are mostly substantially folded proteins, they are known as “client proteins” 68 to distinguish them from other chaperone substrates that lack a defined structure. More than 400 different clients are known to depend on Hsp90 for folding or maturation, and include protein kinases, transcription factors, and E3 ubiquitin ligases 73. The large number of signaling and tumor promoting proteins amongst Hsp90 clients has made Hsp90 a promising drug target 74.
Apart from Hsp90 chaperones in the eukaryotic cytosol, Hsp90 homologs are found in bacteria (HtpG) and eukaryotic organelles, including the endoplasmic reticulum (Grp94), mitochondrion (TRAP1), and chloroplast 75. Interestingly, Hsp90-like domains with chaperone activity have also been found in Sacsin, a 521-kDa protein associated with an autosomal recessive form of spastic ataxia 76, 77. However, an Hsp90 homolog has not been found in Archaea.
Hsp90 chaperones share a similar domain structure consisting of an N-terminal (N-) nucleotide-binding domain, a middle (M-) domain, and a C-terminal (C-) dimerization domain ( Figure 1c). The N-domain is connected to the M-domain via a flexible linker that is often highly charged and, in human Hsp90, is over 60 residues in length. While important to cytosolic eukaryotic Hsp90 function 78– 80, the charged-linker is not universally conserved and is essentially absent in both bacterial and mitochondrial Hsp90s. Crystal structures are now available for full-length members of all Hsp90 subfamilies mostly with bound nucleotide 81– 84, including the recent structure of an asymmetric TRAP1 dimer in the ATP-bound state 84. The latter lends supports for a sequential ATP hydrolysis mechanism 85, 86, although asymmetric binding of nucleotide was not observed 84. Consistent with the prevailing notion, the available structures confirmed that all Hsp90 chaperones form homodimers with the N-domain mediating nucleotide binding. Strikingly, however, apo Hsp90 forms a wide-open, V-shaped dimer with the N-domains separated by over 100 Å 82, while Hsp90 in the ATP-bound state adopts an intertwined, N-terminally closed dimer 81, 84. Since the N-domains are too far apart in the open-state to signal the nucleotide status between neighboring subunits, how ATP-binding induces the closed-state conformation remains an open question. One model suggests that Hsp90 chaperones sample different three-dimensional conformations with different adenine nucleotides stabilizing distinct Hsp90 dimer conformations 87– 89. While not mutually exclusive, the crystal structures of intact Grp94, which were determined in the ATP- and ADP-bound state, revealed a very similar Hsp90 dimer conformation irrespective of the nature of the bound nucleotide 83. Hence, further in vitro and in vivo studies are needed to address the exact roles of ATP and ADP for Hsp90 chaperone function.
The Hsp100 family
Members of the Hsp100 family were first discovered as the protein-activated ATPase components of the protease Ti from E. coli 90, 91, now better known as the ClpAP protease. Members of the Hsp100/Clp family belong to the large superfamily of ATPases Associated with diverse cellular Activities (AAA+) 92, 93. Hsp100/Clp members form a hexameric ring structure and function as the protein-unfolding component of chambered proteases 94, 95. The discovery of yeast Hsp104 that facilitates protein disaggregation 19, as opposed to targeting proteins for degradation, established Hsp104 as the founding member of a new family of ATP-dependent molecular chaperones. In addition to yeast Hsp104, Hsp104 homologs were found subsequently in bacteria (ClpB), plants (Hsp101), and most recently in Dictyostelium discoideum (Hsp101) 96.
Like all Hsp100/Clp proteins, Hsp104 forms an oligomer, with the homohexamer being the functionally active form 97– 100. Hsp104 features two canonical Walker-type ATP-binding domains, known as AAA domains, in addition to several other structural elements that define members of the AAA+ superfamily and include the so-called arginine-finger and the sensor 1 and 2 motifs 101– 106. While the Hsp104 hexamer is stabilized by nucleotide and is an active ATPase in vitro 107, 108, it requires the cooperation of the cognate Hsp70 chaperone system, consisting of Hsp70 and Hsp40 in yeast 20 and DnaK, DnaJ, and GrpE in eubacteria 109– 111, to recover functional protein from aggregates.
At the molecular level, Hsp104 consists of an N-terminal domain, and two tandem AAA+ domains, termed AAA-1 and AAA-2 ( Figure 1d) 97, 103. The AAA-1 domain features an 85-Å long coiled-coil insertion, known as the M-domain, which is located on the outside of the hexamer 99, 100, 112 and distinguishes Hsp104 members from other Hsp100/Clp ATPases. The M-domain is essential for protein disaggregation by mediating the interaction between Hsp104 and Hsp70 113– 115, and may function as a molecular toggle to allosterically control the ATPase and mechanical activities of the Hsp104 motor 116.
How Hsp104 facilitates protein disaggregation has been revealed by the combined efforts of several groups 117. It is now widely accepted that, inside the cell, the Hsp70 system targets the Hsp104 motor to both amorphous and ordered aggregates 15, 118, from which Hsp104 extracts polypeptides using an ATP-driven power stroke involving pore loops present in the AAA-1 and AAA-2 domains 119, and threading the polypeptide through the central channel of the Hsp104 hexamer 120, 121.
While we are beginning to understand the function of the M- and AAA domains, the role of the N-domain is less clear. It was shown that the N-domain is dispensable for Hsp104 function in vitro and in vivo 15, 103, 122– 125. However, others found that the N-domain is essential for bacterial Hsp104 126, 127 and mediates substrate interaction 126, 128– 131. Consistently, the N-domain of yeast Hsp104 enhances protein disaggregation in vitro 114, mediates prion interaction in yeast 132, and is essential for yeast prion dissolution 112 and curing by Hsp104 overexpression 124.
In addition to Hsp104’s role in yeast stress responses and yeast prion replication, new roles are emerging, including the asymmetric distribution of oxidative damaged proteins 133, 134, facilitating the sorting of tail-anchored proteins to the endoplasmic reticulum membrane 135, and septin folding and assembly 136. Hence, future studies will provide a more complete picture as to the extent of Hsp104’s cellular function.
Future perspectives
It is now widely appreciated that molecular chaperones are intimately linked to proteostasis maintenance and are essential to cell and organismal health. Perturbation of the proteostasis network, for instance by “chaperone overload” 137 or polyglutamine expansion 138, invariably disrupts the balance of the protein folding landscape triggering protein misfolding and the formation of aggregates that are hallmarks of neurodegenerative diseases, prion-mediated infections, and amyloidosis. At the same time, a controlled perturbation of the functional interaction between molecular chaperones and proteases could provide new avenues for therapeutic intervention. This could be achieved by using small molecule compounds, or by RNA interference, or restoring the proteostasis network in disease states, for instance with chemical chaperones or by induced chaperone expression 139, 140.
Acknowledgements
We sincerely apologize to all those colleagues whose important work was not cited in this review. We also wish to thank all present and past members of the Francis T.F. Tsai and Sukyeong Lee laboratory for their intellectual contributions towards providing a better mechanistic understanding of molecular chaperone structure and function.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Hays Rye, Department of Biochemistry and Biophysics, Texas A&M University, College Station, TX, USA
Jose Barral, Neuroscience and Cell Biology, University of Texas Medical Branch, Galveston, TX, USA
Funding Statement
Research in the Francis T.F. Tsai and Sukyeong Lee laboratory is supported by grants from the National Institutes of Health (R01-GM111084 and R01-GM104980) and the Robert A. Welch Foundation (Q-1530).
I confirm that the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 1; referees: 2 approved]
References
- 1. Brandvold KR, Morimoto RI: The Chemical Biology of Molecular Chaperones--Implications for Modulation of Proteostasis. J Mol Biol. 2015;427(18):2931–47. 10.1016/j.jmb.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hipp MS, Park SH, Hartl FU: Proteostasis impairment in protein-misfolding and -aggregation diseases. Trends Cell Biol. 2014;24(9):506–14. 10.1016/j.tcb.2014.05.003 [DOI] [PubMed] [Google Scholar]
- 3. Baxa U, Cassese T, Kajava AV, et al. : Structure, function, and amyloidogenesis of fungal prions: filament polymorphism and prion variants. Adv Protein Chem. 2006;73:125–80. 10.1016/S0065-3233(06)73005-4 [DOI] [PubMed] [Google Scholar]
- 4. Toyama BH, Weissman JS: Amyloid structure: conformational diversity and consequences. Annu Rev Biochem. 2011;80:557–85. 10.1146/annurev-biochem-090908-120656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eisenberg D, Jucker M: The amyloid state of proteins in human diseases. Cell. 2012;148(6):1188–203. 10.1016/j.cell.2012.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Varadi M, Kosol S, Lebrun P, et al. : pE-DB: a database of structural ensembles of intrinsically disordered and of unfolded proteins. Nucleic Acids Res. 2014;42(Database issue):D326–35. 10.1093/nar/gkt960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Oldfield CJ, Dunker AK: Intrinsically disordered proteins and intrinsically disordered protein regions. Annu Rev Biochem. 2014;83:553–84. 10.1146/annurev-biochem-072711-164947 [DOI] [PubMed] [Google Scholar]
- 8. Arai M, Sugase K, Dyson HJ, et al. : Conformational propensities of intrinsically disordered proteins influence the mechanism of binding and folding. Proc Natl Acad Sci U S A. 2015;112(31):9614–9. 10.1073/pnas.1512799112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wickner RB, Shewmaker FP, Bateman DA, et al. : Yeast prions: structure, biology, and prion-handling systems. Microbiol Mol Biol Rev. 2015;79(1):1–17. 10.1128/MMBR.00041-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masison DC, Reidy M: Yeast prions are useful for studying protein chaperones and protein quality control. Prion. 2015;9(3):174–83. 10.1080/19336896.2015.1027856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chernoff YO, Lindquist SL, Ono B, et al. : Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science. 1995;268(5212):880–4. 10.1126/science.7754373 [DOI] [PubMed] [Google Scholar]
- 12. Moriyama H, Edskes HK, Wickner RB: [URE3] prion propagation in Saccharomyces cerevisiae: requirement for chaperone Hsp104 and curing by overexpressed chaperone Ydj1p. Mol Cell Biol. 2000;20(23):8916–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shorter J, Lindquist S: Hsp104, Hsp70 and Hsp40 interplay regulates formation, growth and elimination of Sup35 prions. EMBO J. 2008;27(20):2712–24. 10.1038/emboj.2008.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higurashi T, Hines JK, Sahi C, et al. : Specificity of the J-protein Sis1 in the propagation of 3 yeast prions. Proc Natl Acad Sci U S A. 2008;105(43):16596–601. 10.1073/pnas.0808934105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tipton KA, Verges KJ, Weissman JS: In vivo monitoring of the prion replication cycle reveals a critical role for Sis1 in delivering substrates to Hsp104. Mol Cell. 2008;32(4):584–91. 10.1016/j.molcel.2008.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Telling GC, Scott M, Mastrianni J, et al. : Prion propagation in mice expressing human and chimeric PrP transgenes implicates the interaction of cellular PrP with another protein. Cell. 1995;83(1):79–90. 10.1016/0092-8674(95)90236-8 [DOI] [PubMed] [Google Scholar]
- 17. Quinlan RA, Ellis RJ: Chaperones: needed for both the good times and the bad times. Philos Trans R Soc Lond B Biol Sci. 2013;368(1617): 20130091. 10.1098/rstb.2013.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanchez Y, Lindquist SL: HSP104 required for induced thermotolerance. Science. 1990;248(4959):1112–5. 10.1126/science.2188365 [DOI] [PubMed] [Google Scholar]
- 19. Parsell DA, Kowal AS, Singer MA, et al. : Protein disaggregation mediated by heat-shock protein Hsp104. Nature. 1994;372(6505):475–8. 10.1038/372475a0 [DOI] [PubMed] [Google Scholar]
- 20. Glover JR, Lindquist S: Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell. 1998;94(1):73–82. 10.1016/S0092-8674(00)81223-4 [DOI] [PubMed] [Google Scholar]
- 21. Hartl FU, Bracher A, Hayer-Hartl M: Molecular chaperones in protein folding and proteostasis. Nature. 2011;475(7356):324–32. 10.1038/nature10317 [DOI] [PubMed] [Google Scholar]
- 22. Saibil H: Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14(10):630–42. 10.1038/nrm3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haslbeck M, Vierling E: A first line of stress defense: small heat shock proteins and their function in protein homeostasis. J Mol Biol. 2015;427(7):1537–48. 10.1016/j.jmb.2015.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang L, Wang X, Wang CC, et al. : Protein disulfide-isomerase, a folding catalyst and a redox-regulated chaperone. Free Radic Biol Med. 2015;83:305–13. 10.1016/j.freeradbiomed.2015.02.007 [DOI] [PubMed] [Google Scholar]
- 25. Preissler S, Deuerling E: Ribosome-associated chaperones as key players in proteostasis. Trends Biochem Sci. 2012;37(7):274–83. 10.1016/j.tibs.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 26. Bardwell JC, Jakob U: Conditional disorder in chaperone action. Trends Biochem Sci. 2012;37(12):517–25. 10.1016/j.tibs.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hemmingsen SM, Woolford C, van der Vies SM, et al. : Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333(6171):330–4. 10.1038/333330a0 [DOI] [PubMed] [Google Scholar]
- 28. Hayer-Hartl M, Bracher A, Hartl FU: The GroEL-GroES Chaperonin Machine: A Nano-Cage for Protein Folding. Trends Biochem Sci. 2015; pii: S0968-0004(15)00140-1. 10.1016/j.tibs.2015.07.009 [DOI] [PubMed] [Google Scholar]
- 29. Braig K, Otwinowski Z, Hegde R, et al. : The crystal structure of the bacterial chaperonin GroEL at 2.8 Å. Nature. 1994;371(6498):578–86. 10.1038/371578a0 [DOI] [PubMed] [Google Scholar]
- 30. Fukami TA, Yohda M, Taguchi H, et al. : Crystal structure of chaperonin-60 from Paracoccus denitrificans. J Mol Biol. 2001;312(3):501–9. 10.1006/jmbi.2001.4961 [DOI] [PubMed] [Google Scholar]
- 31. Wang J, Boisvert DC: Structural basis for GroEL-assisted protein folding from the crystal structure of (GroEL-KMgATP) 14 at 2.0 Å resolution. J Mol Biol. 2003;327(4):843–55. 10.1016/S0022-2836(03)00184-0 [DOI] [PubMed] [Google Scholar]
- 32. Hunt JF, Weaver AJ, Landry SJ, et al. : The crystal structure of the GroES co-chaperonin at 2.8 Å resolution. Nature. 1996;379(6560):37–45. 10.1038/379037a0 [DOI] [PubMed] [Google Scholar]
- 33. Xu Z, Horwich AL, Sigler PB: The crystal structure of the asymmetric GroEL-GroES-(ADP) 7 chaperonin complex. Nature. 1997;388(6644):741–50. 10.1038/41944 [DOI] [PubMed] [Google Scholar]
- 34. Chen DH, Madan D, Weaver J, et al. : Visualizing GroEL/ES in the act of encapsulating a folding protein. Cell. 2013;153(6):1354–65. 10.1016/j.cell.2013.04.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sigler PB, Xu Z, Rye HS, et al. : Structure and function in GroEL-mediated protein folding. Annu Rev Biochem. 1998;67:581–608. 10.1146/annurev.biochem.67.1.581 [DOI] [PubMed] [Google Scholar]
- 36. Horwich AL, Fenton WA: Chaperonin-mediated protein folding: using a central cavity to kinetically assist polypeptide chain folding. Q Rev Biophys. 2009;42(2):83–116. 10.1017/S0033583509004764 [DOI] [PubMed] [Google Scholar]
- 37. Lopez T, Dalton K, Frydman J: The Mechanism and Function of Group II Chaperonins. J Mol Biol. 2015;427(18):2919–30. 10.1016/j.jmb.2015.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Booth CR, Meyer AS, Cong Y, et al. : Mechanism of lid closure in the eukaryotic chaperonin TRiC/CCT. Nat Struct Mol Biol. 2008;15(7):746–53. 10.1038/nsmb.1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Houry WA, Frishman D, Eckerskorn C, et al. : Identification of in vivo substrates of the chaperonin GroEL. Nature. 1999;402(6758):147–54. 10.1038/45977 [DOI] [PubMed] [Google Scholar]
- 40. Lund PA: Multiple chaperonins in bacteria--why so many? FEMS Microbiol Rev. 2009;33(4):785–800. 10.1111/j.1574-6976.2009.00178.x [DOI] [PubMed] [Google Scholar]
- 41. Qamra R, Mande SC: Crystal structure of the 65-kilodalton heat shock protein, chaperonin 60.2, of Mycobacterium tuberculosis. J Bacteriol. 2004;186(23):8105–13. 10.1128/JB.186.23.8105-8113.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sielaff B, Lee KS, Tsai FT: Structural and functional conservation of Mycobacterium tuberculosis GroEL paralogs suggests that GroEL1 Is a chaperonin. J Mol Biol. 2011;405(3):831–9. 10.1016/j.jmb.2010.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ojha A, Anand M, Bhatt A, et al. : GroEL1: a dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell. 2005;123(5):861–73. 10.1016/j.cell.2005.09.012 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 44. Islam MS, Richards JP, Ojha AK: Targeting drug tolerance in mycobacteria: a perspective from mycobacterial biofilms. Expert Rev Anti Infect Ther. 2012;10(9):1055–66. 10.1586/eri.12.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Powers ET, Balch WE: Diversity in the origins of proteostasis networks--a driver for protein function in evolution. Nat Rev Mol Cell Biol. 2013;14(4):237–48. 10.1038/nrm3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Freeman BC, Myers MP, Schumacher R, et al. : Identification of a regulatory motif in Hsp70 that affects ATPase activity, substrate binding and interaction with HDJ-1. EMBO J. 1995;14(10):2281–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rüdiger S, Germeroth L, Schneider-Mergener J, et al. : Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16(7):1501–7. 10.1093/emboj/16.7.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiang J, Prasad K, Lafer EM, et al. : Structural basis of interdomain communication in the Hsc70 chaperone. Mol Cell. 2005;20(4):513–24. 10.1016/j.molcel.2005.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 49. Liu Q, Hendrickson WA: Insights into Hsp70 chaperone activity from a crystal structure of the yeast Hsp110 Sse1. Cell. 2007;131(1):106–20. 10.1016/j.cell.2007.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Chang YW, Sun YJ, Wang C, et al. : Crystal structures of the 70-kDa heat shock proteins in domain disjoining conformation. J Biol Chem. 2008;283(22):15502–11. 10.1074/jbc.M708992200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bertelsen EB, Chang L, Gestwicki JE, et al. : Solution conformation of wild-type E. coli Hsp70 (DnaK) chaperone complexed with ADP and substrate. Proc Natl Acad Sci U S A. 2009;106(21):8471–6. 10.1073/pnas.0903503106 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 52. Zhuravleva A, Clerico EM, Gierasch LM: An interdomain energetic tug-of-war creates the allosterically active state in Hsp70 molecular chaperones. Cell. 2012;151(6):1296–307. 10.1016/j.cell.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Kityk R, Kopp J, Sinning I, et al. : Structure and dynamics of the ATP-bound open conformation of Hsp70 chaperones. Mol Cell. 2012;48(6):863–74. 10.1016/j.molcel.2012.09.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 54. Qi R, Sarbeng EB, Liu Q, et al. : Allosteric opening of the polypeptide-binding site when an Hsp70 binds ATP. Nat Struct Mol Biol. 2013;20(7):900–7. 10.1038/nsmb.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mayer MP: Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem Sci. 2013;38(10):507–14. 10.1016/j.tibs.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 56. Seyffer F, Kummer E, Oguchi Y, et al. : Hsp70 proteins bind Hsp100 regulatory M domains to activate AAA+ disaggregase at aggregate surfaces. Nat Struct Mol Biol. 2012;19(12):1347–55. 10.1038/nsmb.2442 [DOI] [PubMed] [Google Scholar]
- 57. Lee J, Kim JH, Biter AB, et al. : Heat shock protein (Hsp) 70 is an activator of the Hsp104 motor. Proc Natl Acad Sci U S A. 2013;110(21):8513–8. 10.1073/pnas.1217988110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Shorter J: The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS One. 2011;6(10):e26319. 10.1371/journal.pone.0026319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rampelt H, Kirstein-Miles J, Nillegoda NB, et al. : Metazoan Hsp70 machines use Hsp110 to power protein disaggregation. EMBO J. 2012;31(21):4221–35. 10.1038/emboj.2012.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Song Y, Nagy M, Ni W, et al. : Molecular chaperone Hsp110 rescues a vesicle transport defect produced by an ALS-associated mutant SOD1 protein in squid axoplasm. Proc Natl Acad Sci U S A. 2013;110(14):5428–33. 10.1073/pnas.1303279110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nillegoda NB, Kirstein J, Szlachcic A, et al. : Crucial HSP70 co-chaperone complex unlocks metazoan protein disaggregation. Nature. 2015;524(7564):247–51. 10.1038/nature14884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Andréasson C, Fiaux J, Rampelt H, et al. : Hsp110 is a nucleotide-activated exchange factor for Hsp70. J Biol Chem. 2008;283(14):8877–84. 10.1074/jbc.M710063200 [DOI] [PubMed] [Google Scholar]
- 63. Polier S, Dragovic Z, Hartl FU, et al. : Structural basis for the cooperation of Hsp70 and Hsp110 chaperones in protein folding. Cell. 2008;133(6):1068–79. 10.1016/j.cell.2008.05.022 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Schuermann JP, Jiang J, Cuellar J, et al. : Structure of the Hsp110:Hsc70 nucleotide exchange machine. Mol Cell. 2008;31(2):232–43. 10.1016/j.molcel.2008.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 65. Mattoo RU, Sharma SK, Priya S, et al. : Hsp110 is a bona fide chaperone using ATP to unfold stable misfolded polypeptides and reciprocally collaborate with Hsp70 to solubilize protein aggregates. J Biol Chem. 2013;288(29):21399–411. 10.1074/jbc.M113.479253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Raviol H, Sadlish H, Rodriguez F, et al. : Chaperone network in the yeast cytosol: Hsp110 is revealed as an Hsp70 nucleotide exchange factor. EMBO J. 2006;25(11):2510–8. 10.1038/sj.emboj.7601139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abrams JL, Verghese J, Gibney PA, et al. : Hierarchical functional specificity of cytosolic heat shock protein 70 (Hsp70) nucleotide exchange factors in yeast. J Biol Chem. 2014;289(19):13155–67. 10.1074/jbc.M113.530014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pearl LH, Prodromou C: Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–94. 10.1146/annurev.biochem.75.103004.142738 [DOI] [PubMed] [Google Scholar]
- 69. Krukenberg KA, Street TO, Lavery LA, et al. : Conformational dynamics of the molecular chaperone Hsp90. Q Rev Biophys. 2011;44(2):229–55. 10.1017/S0033583510000314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mayer MP, Le Breton L: Hsp90: breaking the symmetry. Mol Cell. 2015;58(1):8–20. 10.1016/j.molcel.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 71. Pratt WB, Toft DO: Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood). 2003;228(2):111–33. [DOI] [PubMed] [Google Scholar]
- 72. Li J, Buchner J: Structure, function and regulation of the Hsp90 machinery. Biomed J. 2013;36(3):106–17. 10.4103/2319-4170.113230 [DOI] [PubMed] [Google Scholar]
- 73. Taipale M, Krykbaeva I, Koeva M, et al. : Quantitative analysis of HSP90-client interactions reveals principles of substrate recognition. Cell. 2012;150(5):987–1001. 10.1016/j.cell.2012.06.047 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 74. Neckers L, Workman P: Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res. 2012;18(1):64–76. 10.1158/1078-0432.CCR-11-1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Johnson JL: Evolution and function of diverse Hsp90 homologs and cochaperone proteins. Biochim Biophys Acta. 2012;1823(3):607–13. 10.1016/j.bbamcr.2011.09.020 [DOI] [PubMed] [Google Scholar]
- 76. Anderson JF, Siller E, Barral JM: The sacsin repeating region (SRR): a novel Hsp90-related supra-domain associated with neurodegeneration. J Mol Biol. 2010;400(4):665–74. 10.1016/j.jmb.2010.05.023 [DOI] [PubMed] [Google Scholar]
- 77. Anderson JF, Siller E, Barral JM: The neurodegenerative-disease-related protein sacsin is a molecular chaperone. J Mol Biol. 2011;411(4):870–80. 10.1016/j.jmb.2011.06.016 [DOI] [PubMed] [Google Scholar]
- 78. Tsutsumi S, Mollapour M, Graf C, et al. : Hsp90 charged-linker truncation reverses the functional consequences of weakened hydrophobic contacts in the N domain. Nat Struct Mol Biol. 2009;16(11):1141–7. 10.1038/nsmb.1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tsutsumi S, Mollapour M, Prodromou C, et al. : Charged linker sequence modulates eukaryotic heat shock protein 90 (Hsp90) chaperone activity. Proc Natl Acad Sci U S A. 2012;109(8):2937–42. 10.1073/pnas.1114414109 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Jahn M, Rehn A, Pelz B, et al. : The charged linker of the molecular chaperone Hsp90 modulates domain contacts and biological function. Proc Natl Acad Sci U S A. 2014;111(50):17881–6. 10.1073/pnas.1414073111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ali MM, Roe SM, Vaughan CK, et al. : Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature. 2006;440(7087):1013–7. 10.1038/nature04716 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 82. Shiau AK, Harris SF, Southworth DR, et al. : Structural Analysis of E. coli hsp90 reveals dramatic nucleotide-dependent conformational rearrangements. Cell. 2006;127(2):329–40. 10.1016/j.cell.2006.09.027 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 83. Dollins DE, Warren JJ, Immormino RM, et al. : Structures of GRP94-nucleotide complexes reveal mechanistic differences between the hsp90 chaperones. Mol Cell. 2007;28(1):41–56. 10.1016/j.molcel.2007.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lavery LA, Partridge JR, Ramelot TA, et al. : Structural asymmetry in the closed state of mitochondrial Hsp90 (TRAP1) supports a two-step ATP hydrolysis mechanism. Mol Cell. 2014;53(2):330–43. 10.1016/j.molcel.2013.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 85. Richter K, Muschler P, Hainzl O, et al. : Coordinated ATP hydrolysis by the Hsp90 dimer. J Biol Chem. 2001;276(36):33689–96. 10.1074/jbc.M103832200 [DOI] [PubMed] [Google Scholar]
- 86. Mishra P, Bolon DN: Designed Hsp90 heterodimers reveal an asymmetric ATPase-driven mechanism in vivo. Mol Cell. 2014;53(2):344–50. 10.1016/j.molcel.2013.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Southworth DR, Agard DA: Species-dependent ensembles of conserved conformational states define the Hsp90 chaperone ATPase cycle. Mol Cell. 2008;32(5):631–40. 10.1016/j.molcel.2008.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Krukenberg KA, Förster F, Rice LM, et al. : Multiple conformations of E. coli Hsp90 in solution: insights into the conformational dynamics of Hsp90. Structure. 2008;16(5):755–65. 10.1016/j.str.2008.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 89. Partridge JR, Lavery LA, Elnatan D, et al. : A novel N-terminal extension in mitochondrial TRAP1 serves as a thermal regulator of chaperone activity. eLife. 2014;3:e03487. 10.7554/eLife.03487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hwang BJ, Woo KM, Goldberg AL, et al. : Protease Ti, a new ATP-dependent protease in Escherichia coli, contains protein-activated ATPase and proteolytic functions in distinct subunits. J Biol Chem. 1988;263(18):8727–34. [PubMed] [Google Scholar]
- 91. Katayama Y, Gottesman S, Pumphrey J, et al. : The two-component, ATP-dependent Clp protease of Escherichia coli. Purification, cloning, and mutational analysis of the ATP-binding component. J Biol Chem. 1988;263(29):15226–36. [PubMed] [Google Scholar]
- 92. Beyer A: Sequence analysis of the AAA protein family. Protein Sci. 1997;6(10):2043–58. 10.1002/pro.5560061001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Neuwald AF, Aravind L, Spouge JL, et al. : AAA +: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9(1):27–43. [PubMed] [Google Scholar]
- 94. Sauer RT, Baker TA: AAA+ proteases: ATP-fueled machines of protein destruction. Annu Rev Biochem. 2011;80:587–612. 10.1146/annurev-biochem-060408-172623 [DOI] [PubMed] [Google Scholar]
- 95. Alexopoulos JA, Guarné A, Ortega J: ClpP: a structurally dynamic protease regulated by AAA+ proteins. J Struct Biol. 2012;179(2):202–10. 10.1016/j.jsb.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 96. Malinovska L, Palm S, Gibson K, et al. : Dictyostelium discoideum has a highly Q/N-rich proteome and shows an unusual resilience to protein aggregation. Proc Natl Acad Sci U S A. 2015;112(20):E2620–9. 10.1073/pnas.1504459112 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 97. Lee S, Sowa ME, Watanabe YH, et al. : The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell. 2003;115(2):229–40. 10.1016/S0092-8674(03)00807-9 [DOI] [PubMed] [Google Scholar]
- 98. Lee S, Choi J, Tsai FT: Visualizing the ATPase cycle in a protein disaggregating machine: structural basis for substrate binding by ClpB. Mol Cell. 2007;25(2):261–71. 10.1016/j.molcel.2007.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Lee S, Sielaff B, Lee J, et al. : CryoEM structure of Hsp104 and its mechanistic implication for protein disaggregation. Proc Natl Acad Sci U S A. 2010;107(18):8135–40. 10.1073/pnas.1003572107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carroni M, Kummer E, Oguchi Y, et al. : Head-to-tail interactions of the coiled-coil domains regulate ClpB activity and cooperation with Hsp70 in protein disaggregation. eLife. 2014;3:e02481. 10.7554/eLife.02481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Hattendorf DA, Lindquist SL: Cooperative kinetics of both Hsp104 ATPase domains and interdomain communication revealed by AAA sensor-1 mutants. EMBO J. 2002;21(1–2):12–21. 10.1093/emboj/21.1.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hattendorf DA, Lindquist SL: Analysis of the AAA sensor-2 motif in the C-terminal ATPase domain of Hsp104 with a site-specific fluorescent probe of nucleotide binding. Proc Natl Acad Sci U S A. 2002;99(5):2732–7. 10.1073/pnas.261693199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mogk A, Schlieker C, Strub C, et al. : Roles of individual domains and conserved motifs of the AAA+ chaperone ClpB in oligomerization, ATP hydrolysis, and chaperone activity. J Biol Chem. 2003;278(20):17615–24. 10.1074/jbc.M209686200 [DOI] [PubMed] [Google Scholar]
- 104. Yamasaki T, Nakazaki Y, Yoshida M, et al. : Roles of conserved arginines in ATP-binding domains of AAA+ chaperone ClpB from Thermus thermophilus. FEBS J. 2011;278(13):2395–403. 10.1111/j.1742-4658.2011.08167.x [DOI] [PubMed] [Google Scholar]
- 105. Biter AB, Lee J, Sung N, et al. : Functional analysis of conserved cis- and trans-elements in the Hsp104 protein disaggregating machine. J Struct Biol. 2012;179(2):172–80. 10.1016/j.jsb.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zeymer C, Fischer S, Reinstein J: trans-Acting arginine residues in the AAA+ chaperone ClpB allosterically regulate the activity through inter- and intradomain communication. J Biol Chem. 2014;289(47):32965–76. 10.1074/jbc.M114.608828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Parsell DA, Kowal AS, Lindquist S: Saccharomyces cerevisiae Hsp104 protein. Purification and characterization of ATP-induced structural changes. J Biol Chem. 1994;269(6):4480–7. [PubMed] [Google Scholar]
- 108. Watanabe YH, Motohashi K, Yoshida M: Roles of the two ATP binding sites of ClpB from Thermus thermophilus. J Biol Chem. 2002;277(8):5804–9. 10.1074/jbc.M109349200 [DOI] [PubMed] [Google Scholar]
- 109. Motohashi K, Watanabe Y, Yohda M, et al. : Heat-inactivated proteins are rescued by the DnaK.J-GrpE set and ClpB chaperones. Proc Natl Acad Sci U S A. 1999;96(13):7184–9. 10.1073/pnas.96.13.7184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Goloubinoff P, Mogk A, Zvi AP, et al. : Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc Natl Acad Sci U S A. 1999;96(24):13732–7. 10.1073/pnas.96.24.13732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zolkiewski M: ClpB cooperates with DnaK, DnaJ, and GrpE in suppressing protein aggregation. A novel multi-chaperone system from Escherichia coli. J Biol Chem. 1999;274(40):28083–6. 10.1074/jbc.274.40.28083 [DOI] [PubMed] [Google Scholar]
- 112. Sweeny EA, Jackrel ME, Go MS, et al. : The Hsp104 N-terminal domain enables disaggregase plasticity and potentiation. Mol Cell. 2015;57(5):836–49. 10.1016/j.molcel.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Haslberger T, Weibezahn J, Zahn R, et al. : M domains couple the ClpB threading motor with the DnaK chaperone activity. Mol Cell. 2007;25(2):247–60. 10.1016/j.molcel.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 114. Sielaff B, Tsai FT: The M-domain controls Hsp104 protein remodeling activity in an Hsp70/Hsp40-dependent manner. J Mol Biol. 2010;402(1):30–7. 10.1016/j.jmb.2010.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Miot M, Reidy M, Doyle SM, et al. : Species-specific collaboration of heat shock proteins (Hsp) 70 and 100 in thermotolerance and protein disaggregation. Proc Natl Acad Sci U S A. 2011;108(17):6915–20. 10.1073/pnas.1102828108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Mogk A, Kummer E, Bukau B: Cooperation of Hsp70 and Hsp100 chaperone machines in protein disaggregation. Front Mol Biosci. 2015;2:22. 10.3389/fmolb.2015.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Doyle SM, Genest O, Wickner S: Protein rescue from aggregates by powerful molecular chaperone machines. Nat Rev Mol Cell Biol. 2013;14(10):617–29. 10.1038/nrm3660 [DOI] [PubMed] [Google Scholar]
- 118. Winkler J, Tyedmers J, Bukau B, et al. : Chaperone networks in protein disaggregation and prion propagation. J Struct Biol. 2012;179(2):152–60. 10.1016/j.jsb.2012.05.002 [DOI] [PubMed] [Google Scholar]
- 119. Biter AB, Lee S, Sung N, et al. : Structural basis for intersubunit signaling in a protein disaggregating machine. Proc Natl Acad Sci U S A. 2012;109(31):12515–20. 10.1073/pnas.1207040109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Weibezahn J, Tessarz P, Schlieker C, et al. : Thermotolerance requires refolding of aggregated proteins by substrate translocation through the central pore of ClpB. Cell. 2004;119(5):653–65. 10.1016/j.cell.2004.11.027 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 121. Lum R, Tkach JM, Vierling E, et al. : Evidence for an unfolding/threading mechanism for protein disaggregation by Saccharomyces cerevisiae Hsp104. J Biol Chem. 2004;279(28):29139–46. 10.1074/jbc.M403777200 [DOI] [PubMed] [Google Scholar]
- 122. Clarke AK, Eriksson MJ: The truncated form of the bacterial heat shock protein ClpB/HSP100 contributes to development of thermotolerance in the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 2000;182(24):7092–6. 10.1128/JB.182.24.7092-7096.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Beinker P, Schlee S, Groemping Y, et al. : The N terminus of ClpB from Thermus thermophilus is not essential for the chaperone activity. J Biol Chem. 2002;277(49):47160–6. 10.1074/jbc.M207853200 [DOI] [PubMed] [Google Scholar]
- 124. Hung GC, Masison DC: N-terminal domain of yeast Hsp104 chaperone is dispensable for thermotolerance and prion propagation but necessary for curing prions by Hsp104 overexpression. Genetics. 2006;173(2):611–20. 10.1534/genetics.106.056820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Lum R, Niggemann M, Glover JR: Peptide and protein binding in the axial channel of Hsp104. Insights into the mechanism of protein unfolding. J Biol Chem. 2008;283(44):30139–50. 10.1074/jbc.M804849200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Barnett ME, Zolkiewska A, Zolkiewski M: Structure and activity of ClpB from Escherichia coli. Role of the amino-and -carboxyl-terminal domains. J Biol Chem. 2000;275(48):37565–71. 10.1074/jbc.M005211200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Barnett ME, Nagy M, Kedzierska S, et al. : The amino-terminal domain of ClpB supports binding to strongly aggregated proteins. J Biol Chem. 2005;280(41):34940–5. 10.1074/jbc.M505653200 [DOI] [PubMed] [Google Scholar]
- 128. Park SK, Kim KI, Woo KM, et al. : Site-directed mutagenesis of the dual translational initiation sites of the clpB gene of Escherichia coli and characterization of its gene products. J Biol Chem. 1993;268(27):20170–4. [PubMed] [Google Scholar]
- 129. Li J, Sha B: Crystal structure of the E. coli Hsp100 ClpB N-terminal domain. Structure. 2003;11(3):323–8. 10.1016/S0969-2126(03)00030-3 [DOI] [PubMed] [Google Scholar]
- 130. Tanaka N, Tani Y, Hattori H, et al. : Interaction of the N-terminal domain of Escherichia coli heat-shock protein ClpB and protein aggregates during chaperone activity. Protein Sci. 2004;13(12):3214–21. 10.1110/ps.04780704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Chow IT, Barnett ME, Zolkiewski M, et al. : The N-terminal domain of Escherichia coli ClpB enhances chaperone function. FEBS Lett. 2005;579(20):4242–8. 10.1016/j.febslet.2005.06.055 [DOI] [PubMed] [Google Scholar]
- 132. Winkler J, Tyedmers J, Bukau B, et al. : Hsp70 targets Hsp100 chaperones to substrates for protein disaggregation and prion fragmentation. J Cell Biol. 2012;198(3):387–404. 10.1083/jcb.201201074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Erjavec N, Larsson L, Grantham J, et al. : Accelerated aging and failure to segregate damaged proteins in Sir2 mutants can be suppressed by overproducing the protein aggregation-remodeling factor Hsp104p. Genes Dev. 2007;21(19):2410–21. 10.1101/gad.439307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tessarz P, Schwarz M, Mogk A, et al. : The yeast AAA + chaperone Hsp104 is part of a network that links the actin cytoskeleton with the inheritance of damaged proteins. Mol Cell Biol. 2009;29(13):3738–45. 10.1128/MCB.00201-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Wang F, Brown EC, Mak G, et al. : A chaperone cascade sorts proteins for posttranslational membrane insertion into the endoplasmic reticulum. Mol Cell. 2010;40(1):159–71. 10.1016/j.molcel.2010.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 136. Johnson CR, Weems AD, Brewer JM, et al. : Cytosolic chaperones mediate quality control of higher-order septin assembly in budding yeast. Mol Biol Cell. 2015;26(7):1323–44. 10.1091/mbc.E14-11-1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Csermely P: Chaperone overload is a possible contributor to 'civilization diseases'. Trends Genet. 2001;17(12):701–4. 10.1016/S0168-9525(01)02495-7 [DOI] [PubMed] [Google Scholar]
- 138. Gidalevitz T, Ben-Zvi A, Ho KH, et al. : Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311(5766):1471–4. 10.1126/science.1124514 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 139. Balch WE, Morimoto RI, Dillin A, et al. : Adapting proteostasis for disease intervention. Science. 2008;319(5865):916–9. 10.1126/science.1141448 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 140. Lindquist SL, Kelly JW: Chemical and biological approaches for adapting proteostasis to ameliorate protein misfolding and aggregation diseases: progress and prognosis. Cold Spring Harb Perspect Biol. 2011;3(12): pii: a004507. 10.1101/cshperspect.a004507 [DOI] [PMC free article] [PubMed] [Google Scholar]