Abstract
Infections are a major cause of morbidity and mortality in patients with chronic lymphocytic leukemia (cll), who typically have increased susceptibility because of hypogammaglobulinemia (hgg) related to their disease and its treatment. Immunoglobulin replacement therapy (igrt) has been shown to reduce the frequency of bacterial infections and associated hospitalizations in patients with hgg or a history of infection, or both. However, use of igrt in cll is contentious. Studies examining such treatment were conducted largely before the use of newer chemoimmunotherapies, which can extend lifespan, but do not correct the hgg inherent to the disease. Thus, the utility of igrt has to be re-evaluated in the current setting. Here, we discuss the evidence for the use of igrt in cll and provide a practical approach to its use in the prevention and management of infections.
Keywords: Chronic lymphocytic leukemia, hypogammaglobulinemia, immunoglobulins, immunoglobulin replacement therapy, infection, ivig, scig, immunodeficiency
BACKGROUND
Chronic lymphocytic leukemia (cll) is a hematopoietic neoplasia, marked by the proliferation and accumulation of small, mature-appearing, immunologically incompetent B lymphocytes in blood, bone marrow, lymph nodes, and spleen1. Chronic lymphocytic leukemia is the most common leukemia in adults in North America, with a median age at diagnosis ranging between 67 and 72 years1. In Canada, 2165 individuals were diagnosed with cll in 2010, and another report based on data collected during 1998–2003 suggested that as many as 7.99 people are diagnosed per 100,000 population per year2,3.
The prognosis of patients with cll depends on a number of factors (for example, immunophenotype, molecular genetics, stage of disease, etc.), and survival ranges from 1–2 years to more than 15 years4.
INFECTIONS AND HYPOGAMMAGLOBULINEMIA
Infection is a frequent cause of morbidity and mortality in patients with cll. The germinal description of the natural history of cll published by Wintrobe and Hasenbush5 in 1939 established that point, estimating that 38.2% of cll patients develop infections (usually more than one type of infection over the course of their disease), with an attributable mortality rate of 66.7%. Subsequent studies have estimated that approximately one third to one half of all cll patients develop at least 1 infectious complication during the course of their disease6. Those infections have traditionally been classified as moderate (that is, requiring oral antibiotics and no hospitalization) or severe (that is, requiring parenteral antibiotics or hospitalization, or both). Before the advent of novel therapeutics, mortality rates of 25%–50% were found to be directly attributable to such infections7,8.
Infections in patients with cll are paradigmatically bacterial in origin and tend to occur in the respiratory tract; however, they can also affect the skin, gastrointestinal tract, and bloodstream7. Before the use of purine analogues, the most frequent bacterial infections included Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyo-genes, and Escherichia coli8. Infections with opportunistic organisms such as Listeria, Nocardia, Candida, Aspergillus, Pneumocystis jiroveci, Histoplasmosis, Cryptococcus, and non-tuberculous (“atypical”) mycobacteria can also occur in these patients if they are sufficiently immunosuppressed from specific chemotherapeutic regimens8.
Although no clinical trials have examined the use of prophylactic antibiotics in patients with cll, guidelines for their use in preventing opportunistic infections are associated with certain treatments8–15. In addition, certain antineoplastic agents (for example, purine analogues, alkylating agents, alemtuzumab, and combination chemo-therapy) can also increase the risk of select viral diseases (herpes simplex, cytomegalovirus, Epstein–Barr virus, and human herpesvirus 8, for instance)7,8. Furthermore, novel therapies have recently changed the landscape of cll treatment. Among them, Bruton kinase inhibitors such as ibrutinib and idelalisib have shown promising efficacy in the treatment of cll. A recent review of prolonged therapy with ibrutinib suggested a decline in the infection rate during treatment16. Two mechanisms are proposed: first, inhibition of the interleukin-associated T-cell kinase, which can promote T-helper cell type 1 CD4 T-cell outgrowth, leading to a reduction of morbidity from infection in animal models; and second, some return of the humoural immunity function (mainly the level of immunoglobulin A), although the exact mechanisms of immunoglobulin (Ig) stabilization or improvement remain unknown17. Thus, the spectrum of infections in patients with cll varies according to disease stage (early vs. late) and treatment history, with a core susceptibility to bacterial respiratory tract infections, but potentially extending to other organisms as a result of cll-directed therapy.
The primary factor contributing to core susceptibility is impaired antibody production, which most commonly manifests as hypogammaglobulinemia (hgg)7. Over the course of the disease, hgg occurs in approximately 85% of patients, becoming more prevalent in advanced cll7. The mechanisms contributing to the development of hgg in cll are multifactorial. Because cll is a malignant clonal disorder of B cells, the resulting dysfunctional B lymphocytes have an impaired ability to produce Ig, at least in vitro18. Additionally, the malignant B cells have the capacity to directly induce apoptosis of healthy Ig-producing plasma cells19. Lastly, cll appears to be associated with dysfunctional T-helper cells, which are normally involved in the generation of antibody responses20. Thus, an impaired capacity to produce functionally diverse Igs appears to be an intrinsic general feature of cll.
Although not all patients with hgg will necessarily develop problematic infections, studies to date have demonstrated that such infections more frequently occur when IgG levels fall below 6 g/L7,21. Conversely, not all patients with cll and quantitatively normal levels of serum IgG are free of infection. The basis for the discordance is probably multifactorial and relates to the disease-induced immune dysfunction. Monoclonal Igs are produced in some patients with cll and might contribute to a false “normal” level22–24. In cll, hgg is progressive, typically worsening as the disease evolves, and hgg is associated with a reduced probability of survival6,25. Compounding that issue is the knowledge that certain treatments for cll (for example, fludarabine, rituximab, stem-cell transplantation, radiation, glucocorticoids) also appear to reduce Ig levels26.
Primary immunodeficiency disorders (pids) marked by hgg are caused by inborn errors of B-cell development or maturation and are associated with increased susceptibility to the same types of infections associated with hgg in cll27. Decades of experience have established Ig replacement therapy (igrt) as the standard of care for the prevention of such infections in pids with hgg. There is no reason to believe that cll patients with hgg would not similarly benefit from the same approach. Although this topic has historically been contentious, cll therapies are improving control of the disease, and accordingly, the use of igrt to mitigate the risk of infection in patients with cll has to be revisited.
Assessing the Risk of Infection
Given that patients with cll are at increased risk of infection, particularly bacterial respiratory tract infections, their risk should be routinely evaluated. Evaluation should consist of regular clinical assessments that include a detailed history of recent infections, focusing particularly on frequency, severity, required treatment, and sequelae. Infections requiring hospitalization or prolonged or repeated courses of antimicrobial therapy are obviously concerning.
In episodes of infection, microbiologic confirmation provides insight into whether the infection is likely to be related to hgg (for example, encapsulated bacteria, viruses) or to the chemotherapies being used (for example, Pneumocystis jiroveci with steroids); such confirmation should be pursued7,26. The clinical evaluation should be performed concomitantly with monitoring of serum Ig levels. Given that hgg worsens with disease duration, clinical (infection history) and laboratory (Ig level) evaluations should be performed at least every 6–12 months, although the timing should be tailored to each patient. Because treatment for cll can itself worsen hgg and lead to infections, it is also advisable to measure serum Ig levels and circulating CD19+ B cells before treatment with immunomodulatory agents is started28. For some agents (rituximab, for instance), delayed B-cell recovery (>9 months) and treatment-induced neutropenia could be associated with an increased risk of serious infections28,29. Thus, periodic measurement of Ig levels and CD19+ B cells could aid in identifying patients at risk.
In the investigation of suspected pids, antibody responses to protein and polysaccharide antigens are routinely used to characterize the integrity of B-cell immunity (Table i)7. Whether, based on residual B-cell function, those responses can similarly be used to stratify cll patients into low-risk and high-risk categories for infection is not clear. Older studies assessing vaccine responses in patients with cll have demonstrated that bacterial polysaccharides are generally ineffective in antibody formation: It is thought that hgg reflects impaired antibody responses to both primary immunization and re-immunization and likely reflects a similar phenomenon in response to primary infection or re-infection6,25,31. An additional pragmatic hurdle in evaluating vaccine response is the time required for seroconversion, which encompasses both the time required for the body to generate a peak antibody response to the vaccine challenge and the time for the diagnostic laboratory to perform the tests; the resulting delays can be prohibitive. Further, the interpretation of serologic results can be straightforward for some vaccines (tetanus and Haemophilus influenzae type b, for instance), but perhaps less so for others (for example, Pneumococcus serotypes). Lastly, vaccines have evolved since the original studies, and vaccine immunogenicity in patients with cll is not well defined. In discerning which patients with cll would benefit from igrt, further research is therefore needed to determine the utility of vaccine responses.
TABLE I.
Immunologic characteristics of the major diagnostically applied vaccinesa
| Vaccine | T cell–independent or –dependent | Timing of peak antibody level | Protective level |
|---|---|---|---|
| Hib conjugate | Dependent | 6 Months (3–4 weeks after 3rd dose) | 1.0 μg/mL |
| Meningococcal conjugate | Dependent | 2–4 Weeks | 2.0 μg/mL |
| Meningococcal polysaccharide | Independent | 2–4 Weeks | 2.0 μg/mL |
| Pneumococcal conjugate | Dependent | 4 Weeks | 1.3 μg/mL |
| Pneumococcal polysaccharide | Independent | 4 Weeks | 1.3 μg/mL |
| Rabies | Dependent | 21 Days after 3rd dose for pre-exposure prophylaxis | 0.5 IU |
| Tetanus | Dependent | 2–3 Weeks after initial series | 0.15 IU/mL |
Adapted from Orange et al., 201230.
Hib = Haemophilus influenzae type b.
Ig PROPHYLAXIS
In patients who have an impaired ability to produce antibodies and who require prophylaxis, igrt is the standard of care. Preparations for igrt are derived by pooling normal polyvalent IgG antibodies from large numbers of healthy donors32,33. Antibodies to foreign antigens (microbes, for example), to self-antigens (natural autoantibodies, for instance), and to other antibodies (for example, anti-idiotypic antibodies) are also included in the preparation. Immunoglobulin replacement therapy is available either as an intravenous infusion (ivig) or as a subcutaneous injection (scig).
Current Indications for IGRT
In Canada, ivig is currently indicated for the treatment of patients with pid and secondary immunodeficiency disorders33. Those disorders include, but are not limited to, common variable immunodeficiency, X-linked agammaglobulinemia, congenital agammaglobulinemia, secondary hgg, Wiskott–Aldrich syndrome, and severe combined immunodeficiencies. Treatment with ivig is also indicated for patients with immune thrombocytopenic purpura (to rapidly raise platelet counts for the prevention of bleeding) and for patients with chronic inflammatory demyelinating polyneuropathy (to provide immunomodulation). Treatment with scig is currently indicated for adult and pediatric patients with pid who require igrt. It is considered equivalent in efficacy to ivig34,35. Examples of currently approved ivig preparations include Privigen (CSL Behring AG, King of Prussia, PA, U.S.A.), Gamunex (Bayer HealthCare, Leverkusen, Germany), IGIVnex (Grifols Therapeutics, Research Triangle Park, NC, U.S.A.), and Gammagard S/D (Baxter International, Deerfield, IL, U.S.A.). Current scig preparations include Hizentra (CSL Behring AG) and IGIVnex33–37.
Evidence for IGRT in CLL
The compelling association between hgg and risk of infection prompted Fairley and Scott38 to pioneer igrt in 3 cll patients in 1961. One patient remained free of severe infection on replacement; the second could not tolerate the intramuscular injections and died of infection upon stopping replacement; and in the third, the sentinel cll-related infection was fatal (and did not improve when replacement was started during the infection). Admittedly, the cases were anecdotal, and other studies of intramuscular replacement suggested no benefit; however, that lack of benefit might have been a result of the difficulties associated with administering sufficient quantities of Ig intramuscularly.
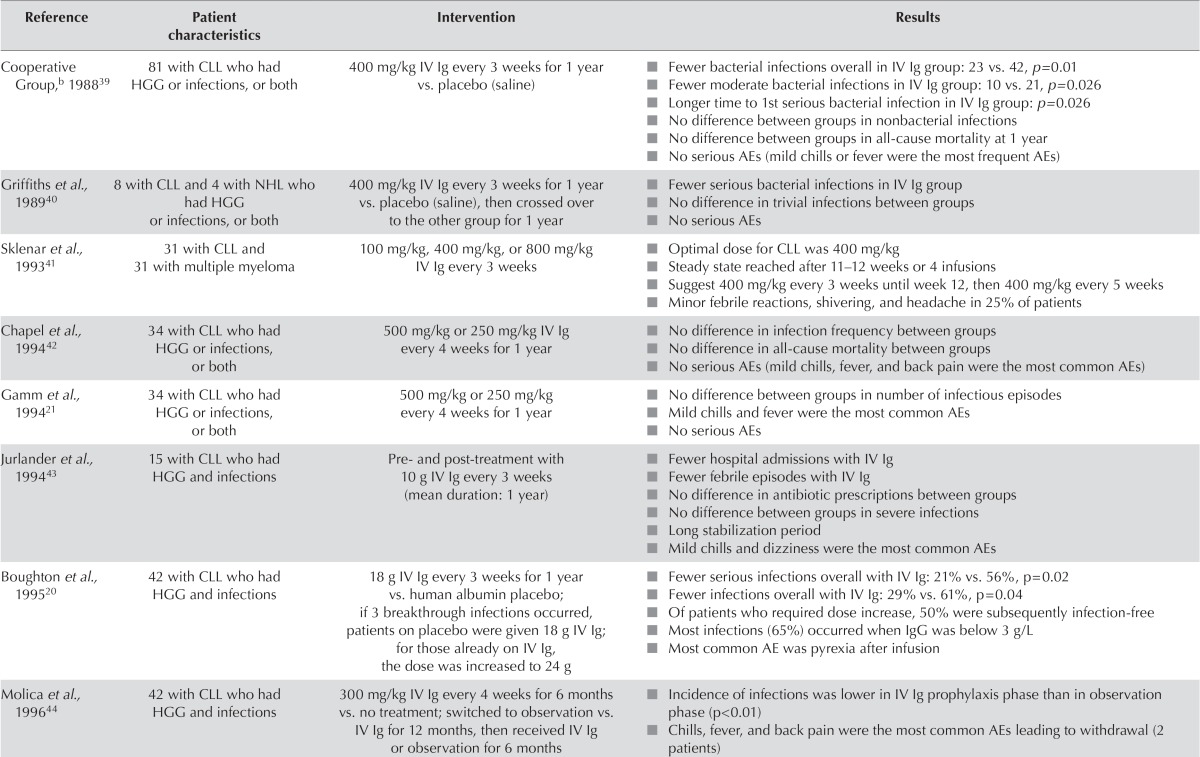
The beneficial effect of ivig was later demonstrated in 1988 in a randomized controlled double-blind clinical trial conducted by the Cooperative Group for the Study of Immunoglobulin in CLL39, whereby, compared with placebo, use of ivig was associated with fewer bacterial infections (p = 0.01, Table ii). The effect on serious infections was seen early, with segregation of the Kaplan–Meier curves at approximately 25 days after ivig initiation. Additionally, compared with the control group, patients who received igrt remained free of serious bacterial infection for a longer period after entering the study. Not unexpectedly, ivig had no effect on viral (that is, herpes simplex virus or varicella zoster virus) or fungal infections; the same lack of effect is also seen in pid patients with hgg. A subsequent double-blind crossover follow-up study in a cohort of the same patients confirmed the earlier findings40. Other studies have consistently demonstrated the beneficial effect of igrt in cll patients with respect to a decreased incidence of severe bacterial infection (Table ii). In addition, a study by Jurlander et al.43 demonstrated a significant reduction in febrile episodes and hospital admissions related to infections in patients given ivig. Moreover, all studies have shown that ivig is relatively well tolerated in patients with cll, with mild chills and fever being the most common toxicities (Table ii). Such reactions can be managed with premedications such as acetaminophen and antihista-mines as needed; steroids are also occasionally used in cases of severe reactions39,45.
TABLE II.
Key studies investigating immunoglobulin (Ig) replacement therapy in chronic lymphocytic leukemia (CLL)a
| Reference | Patient characteristics | Intervention | Results |
|---|---|---|---|
| Cooperative Group,b198839 | 81 with CLL who had HGG or infections, or both | 400 mg/kg IV Ig every 3 weeks for 1 year vs. placebo (saline) |
|
| Griffiths et al., 198940 | 8 with CLL and 4 with NHL who had HGG or infections, or both | 400 mg/kg IV Ig every 3 weeks for 1 year vs. placebo (saline), then crossed over to the other group for 1 year |
|
| Sklenar et al., 199341 | 31 with CLL and 31 with multiple myeloma | 100 mg/kg, 400 mg/kg, or 800 mg/kg IV Ig every 3 weeks |
|
| Chapel et al., 199442 | 34 with CLL who had HGG or infections, or both | 500 mg/kg or 250 mg/kg IV Ig every 4 weeks for 1 year |
|
| Gamm et al., 199421 | 34 with CLL who had HGG or infections, or both | 500 mg/kg or 250 mg/kg every 4 weeks for 1 year |
|
| Jurlander et al., 199443 | 15 with CLL who had HGG and infections | Pre- and post-treatment with 10 g IV Ig every 3 weeks (mean duration: 1 year) |
|
| Boughton et al., 199520 | 42 with CLL who had HGG and infections | 18 g IV Ig every 3 weeks for 1 year vs. human albumin placebo; if 3 breakthrough infections occurred, patients on placebo were given 18 g IV Ig; for those already on IV Ig, the dose was increased to 24 g |
|
| Molica et al., 199644 | 42 with CLL who had HGG and infections | 300 mg/kg IV Ig every 4 weeks for 6 months vs. no treatment; switched to observation vs. IV Ig for 12 months, then received IV Ig or observation for 6 months |
|
| Compagno et al., 201445 | (A) 61 with lymphoproliferative disorders and HGG (B) 33 previously treated with IV Ig | 75 mg/kg weekly 16% or 20% SC Ig vs. previous IV Ig 300 mg/kg every 4 weeks Mean duration: 19 months SC Ig vs. 42 months IV Ig |
|
Adapted from Dhalla et al., 20147.
Full name: Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia.
HGG = hypogammaglobulinemia; IV = intravenous; AE = adverse event; NHL = non-Hodgkin lymphoma; IgG = immunoglobulin G; SC = subcutaneous.
Dose and Administration
A number of trials have investigated the ideal dose of ivig in cll (Table ii). In studies examining ivig, the initial dose has ranged from 100 mg/kg to 800 mg/kg or a total dose of 10–24 g per infusion. The study by Sklenar et al.41 found the ideal dose to be 400 mg/kg every 3 weeks until a steady state is reached, followed by a maintenance dose of 400 mg/kg every 5 weeks. However, the study by Boughton et al.20 that used a dose of 18 g every 3 weeks found that, by increasing the dose in patients with breakthrough infections, 50% were kept infection-free.
In pid, a meta-analysis of studies evaluating IgG trough levels showed a 27% reduction in the incidence of pneumonia with each 100 mg/dL increment in trough IgG, and individualized dosing has proved to be ideal in preventing infections46,47. Similar results have been observed in dose evaluation studies in secondary immunodeficiency disorders, suggesting the need for regular medical evaluation of patients20,41.
Studies to date have demonstrated that infections tend to occur when IgG levels are below approximately 6 g/L; however, not all patients with hgg develop infections21. That observation suggests that other factors—such as neutrophil count, functional ability, and natural killer cells— could play a part in the development of infections21. The study by Gamm et al.21 concluded that igrt should be given at a lower dose (250 mg/kg monthly) in patients with either hgg (IgG < 6 g/L) or a history of serious infections and that the dose should be increased if breakthrough infections occur. Patients should therefore undergo regular monitoring for IgG level and titration of igrt dosing to achieve an optimal IgG serum level that allows for freedom from recurrent infection7. As happens in pid, maintenance of higher IgG trough levels could be beneficial in patients with underlying comorbidities, particularly bronchiectasis7.
In addition to variability in dose, frequency of treatment has also varied, with igrt being given every 3 weeks in five studies and every 4 weeks in four studies (Table ii)20,21,39–45. With respect to duration of therapy, most studies have been given ivig for a period of 1 year (Table ii)20,21,39,40,42,43. However, cll is a chronic and progressive disease, with hgg becoming more prevalent with advanced disease. It stands to reason that igrt might be required for more than the artificial 1 year cut-off used in experimental study designs.
More recently, subcutaneous formulations of igrt have been developed that ease administration, reduce adverse effects, and improve quality of life (qol). Table iii presents a comparison of the advantages and disadvantages of ivig and scig. The scig formulation was examined in a study by Compagno et al.45 of patients with lymphoproliferative disorders and hgg (Table ii). Compared with ivig, the scig formulation resulted in higher IgG trough levels, fewer infections, and less need for antibiotics. In addition, a reduction in the number of adverse events and an improvement in qol parameters were seen with scig compared with ivig. In the pid setting, scig has proved to be as effective as ivig, with reduced variations in peak and trough IgG levels and a better safety profile27. In addition, qol is improved with scig because of improved convenience, making it suitable for self-injection by patients who are unable to travel or who have vascular access issues (Table iii).
TABLE III.
Advantages and disadvantages of intravenous and subcutaneous immunoglobulin
| Variable | Intravenous | Subcutaneous |
|---|---|---|
| Administration | Once every 3–4 weeks by nurse in hospital | Flexible: weekly dose (or double dose every 2 weeks) administered by patient at home and when travelling |
| Efficacy | Reduces frequency and severity of serious bacterial infections equally | |
| Venous access required? | Yes | No |
| Nursing required? | Yes, to administer in medical facility | Yes, for initial training of patient |
| Systemic AEsa | More common | Infrequent |
| Local AEs | Infrequent | Expected and mild |
| Training required? | No special skills required by patient or family | Requires training of patient or family, good dexterity, good vision, capacity to learn new technique |
| Costs | Patient: Loss of work, travel, parking | Saves patient: approximately $1000–$1500 annually |
| Hospital: Nursing hours, equipment | Saves government: approximately $2000–$2600 annually | |
For example, anaphylactoid reaction.
AE = adverse event.
Cost-Effectiveness of Ig Therapy
Despite multiple studies demonstrating efficacy, a common concern precluding the routine use of igrt in patients with cll pertains to cost-effectiveness. The concern stems, at least in part, from a paper published in 1991 by Weeks et al.48 that analyzed the costs and benefits of igrt in patients with cll. In their economic model, which was based on data from the Cooperative Group trial39, the statistically significant decrease in bacterial infections was offset by two variables: the detrimental impact of igrt on qol, and the absence of effect on 1-year survival.
The effect of ivig on qol was driven primarily by the inconvenience associated with intravenous infusion, which must be given in a hospital setting over several hours every 3 or 4 weeks. When the inconvenience of the treatment modality was not considered, ivig resulted in a gain of 0.8 quality-adjusted days per patient per year of therapy at a cost of $6 million per quality-adjusted life-year gained. Additionally, the absence of benefit on mortality at 1 year should be viewed with caution, given that cll is a chronic, progressive disease and the effect of interventions on survival might not be detected at the censored 1-year mark. For example, Molica et al.49 estimated the 5-year risk of developing severe infection in patients with low IgG (<6.5 g/L) to be 57.1%. The cost of infections can be substantial, with one U.S. study showing a cost of $38,574 per hospitalized patient50. Given the risk of infections in cll patients with hgg, the improved survival of such patients with emerging chemotherapies, and the development of more convenient scig formulations, the cost-effectiveness of igrt should be reconsidered.
A number of studies have shown that, compared with ivig, scig results in reduced resource utilization and improved cost-effectiveness. A study by Haddad et al.51 in pid showed that higher doses of scig (mean: 213 mg/kg vs. 120 mg/kg weekly) resulted in lower rates of nonserious infections (2.76 episodes vs. 5.18 episodes annually, p < 0.0001), hospitalization (0.20 vs. 3.48 days annually, p < 0.0001), antibiotic use (48.50 days vs. 72.75 days annually, p < 0.001), and missed work or school activities (2.10 vs. 8.00 days annually, p < 0.001). In addition, studies comparing the cost-effectiveness of scig and ivig demonstrated savings of $2000–$2500 per patient per year with scig (Table iii)52,53. A study evaluating the cost savings of scig in a Canadian setting found the net economic gain from switching 1 patient with pid or secondary immunodeficiency disorder to home-based scig care to be $2,603 in the first year and $2,948 in each subsequent year54. In addition, every 37 patients treated with scig instead of ivig resulted in the gain of 1 nursing full-time equivalent. Although no similar pharmacoeconomic study has been formally conducted for scig in patients with cll, the consistent savings with the scig formulation argue that that formulation could be a viable option for those who see ivig as being cost-prohibitive.
CANADIAN PERSPECTIVE
Although the evidence is based primarily on studies that pre-date the modern chemotherapies used for cll, the data indicate a consistent beneficial effect of igrt in reducing serious bacterial infections in cll. The utility of igrt in reducing viral infections is unclear and is not currently supported by the evidence. Despite the evidence of benefit, the major ongoing debate in this area relates to the type of cll patient who should be considered for igrt. That debate requires a re-analysis of the inclusion criteria used in the studies.
The pivotal work by the Cooperative Study Group was explicit in stating that their data do not indicate that all patients with cll should receive Ig therapy39. That conclusion was based on the fact that not all cll patients receiving ivig were prevented from having infections; however, that conclusion might not be any more realistic than assuming that chemotherapy cures all patients with leukemia. The Cooperative Study Group’s enrolment criteria included cll patients with an increased susceptibility to infection, defined either by an IgG level at 50% or less of the lower limit of normal (typically <4 g/L) or a history of 1 or more serious infections39. In the era of evidence-based medicine, patients meeting those criteria are the ones to whom the study data could (or should) be applied. The beneficial effect noted in the crossover follow-up study by Griffiths et al.40 used the same definitions, as did the studies by Chapel et al.42 and Molica et al.44.
On the other hand, the prevailing paradigm, iterated by Rai and Sawitsky55 in 1991 and used in the studies by Jurlander et al.20 and Boughton et al.43, suggests that ivig should be used only for cll patients with reduced serum IgG levels and a history of 1 major infection. However, the caveat to that condition is that the sentinel major infection could be fatal, because the hgg might lead to any one or more of severe respiratory tract infection, extrapulmonary dissemination (for example, bacteremia, meningitis), and sepsis56. Prophylaxis against such infections might be preferable to a preemptive strategy of selecting a cohort of patients who have survived a severe infection for eventual igrt.
Although igrt does not improve survival in patients with cll, various chemoimmunotherapeutic regimens have shown a survival advantage and can be associated with significant immunosuppression. Some of those agents predispose patients to distinct opportunistic infections (for example, cytomegalovirus with alemtuzumab), necessitating routine antimicrobial prophylaxis. However, none restore humoural immunity, which is the underlying problem in patients with cll. Given the improvement in survival with newer cll regimens and the profound B-cell depletion after B cell–directed therapy (for example, monoclonal antibodies, chimeric antigen receptor T cells), re-evaluation of immunoprophylaxis with igrt should be considered.
With respect to the cost-effectiveness of igrt, Weeks et al.48 justifiably argued that the inconvenience and costs associated with ivig are a major barrier to its use. On the other hand, because of the availability of scig, igrt need no longer be given intravenously; in fact, scig is now the mainstay of administration in patients with a genetic basis for hgg57. In such patients, scig is considered equally as efficacious as ivig with respect to a reduction in the frequency and severity of respiratory tract infections57. Additionally, scig achieves steady serum levels, avoiding the peaks and troughs associated with ivig administration; whether that lesser variation is associated with fewer periods at risk for infection remains plausible, but unproven34,45.
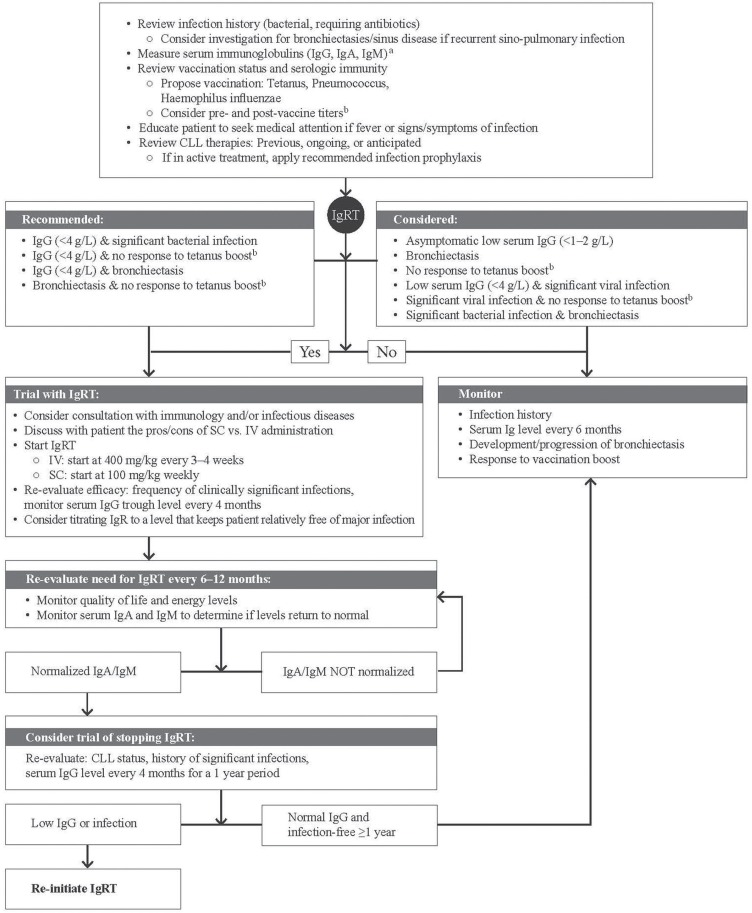
Suggested Algorithm for the Use of IGRT in CLL
The protocol presented in Figure 1 outlines a proposed algorithm for the use of igrt in patients with cll. It is based on current evidence and the clinical experience of the authors.
FIGURE 1.
Treatment algorithm for the use of immunoglobulin replacement therapy in chronic lymphocytic leukemia (CLL). a Where a patient has “normal” levels of immunoglobulin G (IgG), but a phenotype consistent with humoural immunodeficiency, the patient should be evaluated for monoclonal gammopathy. b Response to vaccination before and after boost. For example, for tetanus, obtain serum for a pre-vaccine titer, then administer the vaccine (same day), and 4 weeks later, measure the response to boost. IgA/M = immunoglobulin A/M; IGRT = immunoglobulin replacement therapy; SC = subcutaneous; IV = intravenous; IgR = immunoglobulin replacement.
CONCLUSIONS
Infections are a major cause of morbidity and mortality in patients with cll and are largely associated with the hgg related to cll and its treatment. The use of igrt reduces the frequency of bacterial infections and associated hospitalizations in patients with hgg or a history of infection, or both. Patients with cll should therefore be monitored to determine the potential benefit of igrt in reducing their risk of infection.
Data for the use of igrt in cll are limited and largely based on studies conducted before the advent of standard chemoimmunotherapy. Given that the newer treatment regimens do not correct the hgg associated with cll, the use of igrt has to be re-evaluated in the current setting. In addition, the availability of subcutaneous formulations of igrt appears to reduce the cost and inconvenience of hospital-based intravenous administration, suggesting that the cost-effectiveness of igrt should be reassessed. Finally, as the development of novel cll treatments continues, the impact of therapy on humoural immunity and infection risk will have to be closely monitored and periodically reassessed.
ACKNOWLEDGMENTS
CSL Behring Canada Inc. supported the development of this paper and the medical writing services provided by Anna Christofides of New Evidence.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: All authors received an honorarium from CSL Behring for their participation in this paper. EH has received honoraria for advisory board participation and has received funding for an investigator-initiated trial from CSL Behring Canada. DCV is supported by a Chercheur-boursier clinicien award from the Fonds de recherche du Québec en Santé (frqs) and has received honoraria from CSL Behring Canada, Pfizer Canada, and Sunovion for advisory board participation and from CSL Behring Canada, Sunovion, and Merck Canada for speaking events. DCV has also received funding from Astellas Canada and CSL Behring Canada for an investigator-initiated trial. CLT and CS have received honoraria from CSL Behring Canada for advisory board participation. JKL has received grants and research support from CSL Behring, Sanofi, AstraZeneca, Novartis, GlaxoSmithKline, and ALK-Abello Pharmaceuticals. JKL has also received honoraria for speaking events and consulting fees from Pfizer, Merck, Teva, Meda, AstraZeneca, Novartis, Sanofi, CSL Behring, Takeda, ALK-Abello Pharmaceuticals, Hollister-Stier Laboratories, and Alcon. BCR has provided unpaid consulting advice to Baxter, Bayer, Boehringer Ingelheim, Bristol–Myers Squibb, Canadian Blood Services, Canadian Hemophilia Society, Covidien, CSL Behring, Dyax, Genzyme, Leo Pharmaceuticals, Novartis, Novo Nordisk, Octapharma, Pharmacyclics, Pfizer, Sanofi, Shire, Talecris, and Wyeth. BCR has also received funding for investigator-initiated studies from Baxter, CSL Behring, Novartis, Novo Nordisk, Pfizer, and Wyeth. LHS, DAS, and SL have no other conflicts of interest to declare.
REFERENCES
- 1.Hallek M. Chronic lymphocytic leukemia: 2013 update on diagnosis, risk stratification and treatment. Am J Hematol. 2013;88:803–16. doi: 10.1002/ajh.23491. [DOI] [PubMed] [Google Scholar]
- 2.Statistics Canada . Table 103-0550: New cases of primary cancer (based on the February 2014 CCR tabulation file), by cancer type, age group and sex, Canada, provinces and territories [Web resource] Ottawa, ON: Statistics Canada; 2015. [Available at: http://www5.statcan.gc.ca/cansim/a26?lang=eng&retrLang=eng&id=1030550&tabMode=dataTable&srchLan=-1&p1=-1&p2=9; cited 25 June 2015] [Google Scholar]
- 3.Seftel MD, Demers AA, Banerji V, et al. High incidence of chronic lymphocytic leukemia (cll) diagnosed by immunophenotyping: a population-based Canadian cohort. Leuk Res. 2009;33:1463–8. doi: 10.1016/j.leukres.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Foa R, Del Giudice I, Guarini A, Rossi D, Gaidano G. Clinical implications of the molecular genetics of chronic lymphocytic leukemia. Haematologica. 2013;98:675–85. doi: 10.3324/haematol.2012.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wintrobe MM, Hasenbush LL. Chronic leukemia: the early phase of chronic leukemia, the results of treatment and the effects of complicating infections; a study of eighty-six adults. Arch Intern Med. 1939;64:701–18. doi: 10.1001/archinte.1939.00190040042003. [DOI] [Google Scholar]
- 6.Morra E, Nosari A, Montillo M. Infectious complications in chronic lymphocytic leukaemia. Hematol Cell Ther. 1999;41:145–51. doi: 10.1007/s00282-999-0145-0. [DOI] [PubMed] [Google Scholar]
- 7.Dhalla F, Lucas M, Schuh A, et al. Antibody deficiency secondary to chronic lymphocytic leukemia: should patients be treated with prophylactic replacement immunoglobulin? J Clin Immunol. 2014;34:277–82. doi: 10.1007/s10875-014-9995-5. [DOI] [PubMed] [Google Scholar]
- 8.Hamblin AD, Hamblin TJ. The immunodeficiency of chronic lymphocytic leukaemia. Br Med Bull. 2008;87:49–62. doi: 10.1093/bmb/ldn034. [DOI] [PubMed] [Google Scholar]
- 9.BC Cancer Agency (bcca) Home > Health Professionals > Professional Resources > Cancer Management Guidelines > Lymphoma, Chronic Leukemia, Myeloma > Chronic Leukemia [Web page] Vancouver, BC: BCCA; 2012. [Available at: http://www.bccancer.bc.ca/health-professionals/professional-resources/cancer-management-guidelines/lymphoma-chronic-leukemia-myeloma/chronic-leukemia; cited 25 June 2015] [Google Scholar]
- 10.Stewart DA, Chen C, Sehn H, Shustik C. A Canadian perspective on the management of chronic lymphocytic leukemia [Web article] New Evidence Oncology. 2010 Aug; [Available online at: http://www.newevidence.com/oncology/a-canadian-perspective-on-the-management-of-chronic-lymphocytic-leukemia-2/; cited 25 June 2015] [Google Scholar]
- 11.Hallek M, Cheson BD, Catovsky D, et al. on behalf of the International Workshop on Chronic Lymphocytic Leukemia Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sehn LH. BCCA Protocol Summary for Treatment of Chronic Lymphocytic Leukemia or Prolymphocytic Leukemia and Relapsed Indolent Lymphoma with Fludarabine and Rituximab. Vancouver, BC: BC Cancer Agency; 2014. [Available online at: http://www.bccancer.bc.ca/NR/rdonlyres/30FDD508-96AC-4555-B682-294EA3635B06/70449/LYFLUDR_Protocol_1Jun2014.pdf; cited 14 April 2015] [Google Scholar]
- 13.BC Cancer Agency (bcca) BCCA Cancer Drug Manual: Rituximab. Vancouver, BC: BCCA; 2014. [Available online at: http://www.bccancer.bc.ca/drug-database-site/Drug%20Index/Rituximab_monograph_1Sep2015.pdf; cited 14 April 2015] [Google Scholar]
- 14.Sehn L. BCCA Protocol Summary for the Treatment with Subcutaneous or Intravenous Alemtuzumab for Fludarabine-Refractory B-Chronic Lymphocytic Leukemia (B-CLL) or with Intravenous Alemtuzumab for Previously Untreated T-Prolymphocytic Leukemia (T-PLL) Vancouver, BC: BC Cancer Agency; 2014. [Available online at: http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Lymphoma-Myeloma/LYALEM_Protocol_1Jun2014.pdf; cited 1 April 2015] [Google Scholar]
- 15.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Non-Hodgkin’s LymphomasVer 1.2011.Fort Washington, PA: NCCN; 2011[Current version available online at: http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf (free registration required); cited 25 June 2015] [Google Scholar]
- 16.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naive and previously treated patients with cll and sll receiving single-agent ibrutinib. Blood. 2015;125:2497–506. doi: 10.1182/blood-2014-10-606038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aue G, Farooqui M, Jones J, et al. In patients with chronic lymphocytic leukemia (cll) ibrutinib effectively reduces clonal IgM paraproteins and serum free light chains while increasing normal IgM, IgA serum levels, suggesting a nascent recovery of humoral immunity [abstract] Blood. 2013;122:4182. doi: 10.1182/blood-2013-07-453241. [Available online at: http://www.bloodjournal.org/content/122/21/4182; cited 21 January 2016] [DOI] [PubMed] [Google Scholar]
- 18.Hersey P, Wotherspoon J, Reid G, Gunz FW. Hypogammaglobulinaemia associated with abnormalities of both B and T lymphocytes in patients with chronic lymphatic leukaemia. Clin Exp Immunol. 1980;39:698–707. [PMC free article] [PubMed] [Google Scholar]
- 19.Sampalo A, Navas G, Medina F, Segundo C, Cámara C, Brieva JA. Chronic lymphocytic leukemia B cells inhibit spontaneous Ig production by autologous bone marrow cells: role of CD95–CD95L interaction. Blood. 2000;96:3168–74. [PubMed] [Google Scholar]
- 20.Boughton BJ, Jackson N, Lim S, Smith N. Randomized trial of intravenous immunoglobulin prophylaxis for patients with chronic lymphocytic leukaemia and secondary hypogammaglobulinaemia. Clin Lab Haematol. 1995;17:75–80. doi: 10.1111/j.1365-2257.1995.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 21.Gamm H, Huber C, Chapel H, Lee M, Ries F, Dicato MA. Intravenous immune globulin in chronic lymphocytic leukaemia. Clin Exp Immunol. 1994;97(suppl 1):17–20. [PMC free article] [PubMed] [Google Scholar]
- 22.Martin W, Abraham R, Shanafelt T, et al. Serum-free light chain—a new biomarker for patients with B-cell non-Hodgkin lymphoma and chronic lymphocytic leukemia. Transl Res. 2007;149:231–5. doi: 10.1016/j.trsl.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Bernstein ZP, Fitzpatrick JE, O’Donnell A, Han T, Foon KA, Bhargava A. Clinical significance of monoclonal proteins in chronic lymphocytic leukemia. Leukemia. 1992;6:1243–5. [PubMed] [Google Scholar]
- 24.Deegan MJ, Abraham JP, Sawdyk M, Van Slyck EJ. High incidence of monoclonal proteins in the serum and urine of chronic lymphocytic leukemia patients. Blood. 1984;64:1207–11. [PubMed] [Google Scholar]
- 25.Besa EC. Recent advances in the treatment of chronic lymphocytic leukemia: defining the role of intravenous immunoglobulin. Semin Hematol. 1992;29(suppl 2):14–23. [PubMed] [Google Scholar]
- 26.Mouthon L, Fermand JP, Gottenberg JE. Management of secondary immune deficiencies: what is the role of immunoglobulins? Curr Opin Allergy Clin Immunol. 2013;13(suppl 2):S56–67. doi: 10.1097/01.all.0000433132.16436.b5. [DOI] [PubMed] [Google Scholar]
- 27.Haddad É, Barnes D, Kafal A. Home therapy with subcutaneous immunoglobulins for patients with primary immunodeficiency diseases. Transfus Apher Sci. 2012;46:315–21. doi: 10.1016/j.transci.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan B, Kopyltsova Y, Khokhar A, Lam F, Bonagura V. Rituximab and immune deficiency: case series and review of the literature. J Allergy Clin Immunol Pract. 2014;2:594–600. doi: 10.1016/j.jaip.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Grant C, Wilson WH, Dunleavy K. Neutropenia associated with rituximab therapy. Curr Opin Hematol. 2011;18:49–54. doi: 10.1097/MOH.0b013e3283414edf. [DOI] [PubMed] [Google Scholar]
- 30.Orange JS, Ballow M, Stiehm ER, et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2012;130:S1–24. doi: 10.1016/j.jaci.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Sinisalo M, Aittoniemi J, Käyhty H, Vilpo J. Vaccination against infections in chronic lymphocytic leukemia. Leuk Lymphoma. 2003;44:649–52. doi: 10.1080/1042819031000063408. [DOI] [PubMed] [Google Scholar]
- 32.Kaveri S. Advances in the treatment of primary and secondary immune deficiences. Curr Opin Allergy Clin Immunol. 2013;13(suppl 2):S51–2. doi: 10.1097/ACI.0b013e328360c98d. [DOI] [PubMed] [Google Scholar]
- 33.CSL Behring Canada Inc . Privigen Product Monograph. Ottawa, ON: CSL Behring Canada Inc.; 2014. [Google Scholar]
- 34.CSL Behring Canada Inc . Hizentra Product Monograph. Ottawa, ON: CSL Behring Canada Inc.; 2014. [Google Scholar]
- 35.Grifols Therapeutics Inc. IGIVnex Product Monograph. Mississauga, ON: Grifols Therapeutics Inc.; 2014. [Google Scholar]
- 36.Grifols Therapeutics Inc. Gamunex Product Monograph. Mississauga, ON: Grifols Therapeutics Inc.; 2014. [Google Scholar]
- 37.Baxter Corporation . Gammagard S/D Product Monograph. Mississauga, ON: Baxter Corporation; 2014. [Google Scholar]
- 38.Fairley GH, Scott RB. Hypogammaglobulinaemia in chronic lymphatic leukaemia. Br Med J. 1961;2:920–4. doi: 10.1136/bmj.2.5257.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Intravenous immunoglobulin for the prevention of infection in chronic lymphocytic leukemia A randomized, controlled clinical trial. Cooperative Group for the Study of Immunoglobulin in Chronic Lymphocytic Leukemia. N Engl J Med. 1988;319:902–7. doi: 10.1056/NEJM198810063191403. [DOI] [PubMed] [Google Scholar]
- 40.Griffiths H, Brennan V, Lea J, Bunch C, Lee M, Chapel H. Crossover study of immunoglobulin replacement therapy in patients with low-grade B-cell tumors. Blood. 1989;73:366–8. [PubMed] [Google Scholar]
- 41.Sklenar I, Schiffman G, Jønsson V, et al. Effect of various doses of intravenous polyclonal IgG on in vivo levels of 12 pneumococcal antibodies in patients with chronic lymphocytic leukaemia and multiple myeloma. Oncology. 1993;50:466–77. doi: 10.1159/000227231. [DOI] [PubMed] [Google Scholar]
- 42.Chapel H, Dicato M, Gamm H, et al. Immunoglobulin replacement in patients with chronic lymphocytic leukaemia: a comparison of two dose regimes. Br J Haematol. 1994;88:209–12. doi: 10.1111/j.1365-2141.1994.tb05002.x. [DOI] [PubMed] [Google Scholar]
- 43.Jurlander J, Geisler CH, Hansen MM. Treatment of hypogammaglobulinaemia in chronic lymphocytic leukaemia by low-dose intravenous gammaglobulin. Eur J Haematol. 1994;53:114–18. doi: 10.1111/j.1600-0609.1994.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 44.Molica S, Musto P, Chiurazzi F, et al. Prophylaxis against infections with low-dose intravenous immunoglobulins (ivig) in chronic lymphocytic leukemia. Results of a crossover study. Haematologica. 1996;81:121–6. [PubMed] [Google Scholar]
- 45.Compagno N, Malipiero G, Cinetto F, Agostini C. Immunoglobulin replacement therapy in secondary hypogammaglobulinemia. Front Immunol. 2014;5:626. doi: 10.3389/fimmu.2014.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137:21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 47.Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125:1354–60.e4. doi: 10.1016/j.jaci.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 48.Weeks JC, Tierney MR, Weinstein MC. Cost effectiveness of prophylactic intravenous immune globulin in chronic lymphocytic leukemia. N Engl J Med. 1991;325:81–6. doi: 10.1056/NEJM199107113250202. [DOI] [PubMed] [Google Scholar]
- 49.Molica S, Levato D, Levato L. Infections in chronic lymphocytic leukemia. Analysis of incidence as a function of length of follow-up. Haematologica. 1993;78:374–7. [PubMed] [Google Scholar]
- 50.Menzin J, Sussman M, Munsell M, Zbrozek A. Economic impact of infections among patients with primary immunodeficiency disease receiving ivig therapy. Clinicoecon Outcomes Res. 2014;6:297–302. doi: 10.2147/CEOR.S63200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haddad E, Berger M, Wang EC, Jones CA, Bexon M, Baggish JS. Higher doses of subcutaneous IgG reduce resource utilization in patients with primary immunodeficiency. J Clin Immunol. 2012;32:281–9. doi: 10.1007/s10875-011-9631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ducruet T, Levasseur MC, Des Roches A, Kafal A, Dicaire R, Haddad E. Pharmacoeconomic advantages of subcutaneous versus intravenous immunoglobulin treatment in a Canadian pediatric center. J Allergy Clin Immunol. 2013;131:585–7.e1–3. doi: 10.1016/j.jaci.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 53.Martin A, Lavoie L, Goetghebeur M, Schellenberg R. Economic benefits of subcutaneous rapid push versus intravenous immunoglobulin infusion therapy in adult patients with primary immune deficiency. Transfus Med. 2013;23:55–60. doi: 10.1111/j.1365-3148.2012.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerth WC, Betschel SD, Zbrozek AS. Implications to payers of switch from hospital-based intravenous immunoglobulin to home-based subcutaneous immunoglobulin therapy in patients with primary and secondary immunodeficiencies in Canada. Allergy Asthma Clin Immunol. 2014;10:23. doi: 10.1186/1710-1492-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rai KR, Sawitsky A. Diagnosis and treatment of chronic lymphocytic leukaemia. In: Wiernik PA, Canellos GP, Kyle RA, Schifkr CA, editors. Neoplastic Diseases of the Blood. 2nd ed. London, UK: Churchill Livingstone; 1991. pp. 97–108. [Google Scholar]
- 56.Shaw R, Szwed C, Boggs D, Fahey J. Infection and immunity in chronic lymphocytic leukemia. Arch Intern Med. 1960;106:467–78. doi: 10.1001/archinte.1960.03820040005002. [DOI] [Google Scholar]
- 57.Shehata N, Palda V, Bowen T, et al. The use of immunoglobulin therapy for patients with primary immune deficiency: an evidence-based practice guideline. Transfus Med Rev. 2010;24(suppl 1):S28–50. doi: 10.1016/j.tmrv.2009.09.011. [DOI] [PubMed] [Google Scholar]