Abstract
Purpose
We compared dosimetry and clinical toxicity for 3-dimensional conformal radiotherapy (3D-crt), intensity-modulated radiotherapy (imrt), and RapidArc (Varian Medical Systems, Palo Alto, CA, U.S.A.) in locally advanced pancreatic cancer (lapcc). We hypothesized that the technique with better sparing of organs at risk (oars) and better target dose distributions could lead to decreased clinical toxicity.
Methods
The study analyzed 280 patients with lapcc who had undergone radiotherapy. The dosimetry comparison was performed using 20 of those patients. Dose–volume histograms for the target volume and the oars were compared. The clinical toxicity comparison used the 280 patients who received radiation with 3D-crt, imrt, or RapidArc.
Results
Compared with 3D-crt, RapidArc and imrt both achieved a better conformal index, homogeneity index, V95%, and V110%. Compared with 3D-crt or imrt, RapidArc reduced the V10, V20, and mean dose to duodenum, the V20 of the right kidney, and the liver mean dose. Compared with 3D-crt, RapidArc reduced the V35, and V45 of duodenum, the mean dose to small bowel, and the V15 of right kidney. The incidences of grades 3 and 4 diarrhea (p = 0.037) and anorexia (p = 0.042) were lower with RapidArc than with 3D-crt, and the incidences of grades 3 and 4 diarrhea (p = 0.027) were lower with RapidArc than with imrt.
Conclusions
Compared with 3D-crt or imrt, RapidArc showed better sparing of oars, especially duodenum, small bowel, and right kidney. Also, fewer acute grades 3 and 4 gastrointestinal toxicities were seen with RapidArc than with 3D-crt or imrt. A technique with better sparing of oars and better target dose distributions could result in decreased clinical toxicities during radiation treatment for lapcc.
Keywords: Dosimetric comparisons, 3d-crt, imrt, RapidArc, pancreatic cancer, organ preservation
INTRODUCTION
Pancreatic cancer (pcc) is the 4th leading cause of cancer-related death in developed countries1. Despite considerable advancements in surgical, chemotherapeutic, and radiotherapeutic treatment modalities, the overall 5-year survival in this disease remains approximately 5%1. In locally advanced pcc (lapcc), median survival ranges from 8 months to 12 months2.
Systemic chemotherapy is a standard of care in the management of lapcc, but some controversy surrounds the role of radiation therapy (rt) in the treatment of lapcc3. The radiation dose to the tumour is largely limited by the tolerances of the nearby critical and sensitive organs, especially the duodenum, small bowel, and kidneys. However, in modern rt techniques, the ability to spare adjacent organs at risk (oars) while delivering a therapeutic dose to the target has improved4. The improvements might not only lead to reduced treatment toxicity, but also to improved local control.
Three-dimensional conformal radiotherapy (3D-crt) has superior dose distribution and normal-tissue sparing, which could offer a chance of long survival for some lapcc patients when combined with chemotherapy5. A few studies have shown that, compared with 3D-crt for pcc, intensity-modulated radiotherapy (imrt) might improve tolerability by lowering the dose to critical organs6,7. And compared with imrt8 or 3D-crt9 for pcc, RapidArc (Varian Medical Systems, Palo Alto, CA, U.S.A.) was previously shown to be able to improve normal-tissue sparing while maintaining adequate target coverage.
In the present study, we performed a dosimetric comparison of 3D-crt, imrt, and RapidArc for the treatment of lapcc to identify the technique that would be more effective in maintaining target coverage and minimizing dose to critical structures, with a special focus on preservation of duodenum, small bowel, and kidneys. Furthermore, we compared clinical toxicity with 3D-crt, imrt, and RapidArc to identify the technique that would be more effective in reducing acute gastrointestinal (gi) toxicity. We hypothesized that the technique with better sparing of oars and better target dose distributions might lead to less clinical toxicity.
METHODS
Patients
Between October 2012 and July 2014 at Shandong Cancer Hospital, 280 patients with inoperable lapcc were accrued to our study. The dosimetry comparison was performed for 20 of those patients. The clinical toxicity comparison was performed using all 280 patients. The study protocol was approved by the Medical Ethics Committee of the Shandong Cancer Hospital and Institute.
Dosimetric Comparison
Target and normal-tissue definitions accorded with reports 50 and 62 from the International Commission on Radiation Units and Measurements10,11. The internal gross tumour volume included the gross tumour and any lymph node metastasis, based on 4-dimensional computed tomography (4D-ct). This internal gross tumour volume was uniformly expanded with 5 mm margins and was then manually modified to respect anatomic boundaries, thus creating the clinical target volume (ctv). The ctv was then expanded with 5–10 mm margins to create the planning target volume (ptv)9. The “small-bowel region” contour included the abdominal contents after subtracting the ptv, the duodenum, other oars, the vertebral bodies, and the retroperitoneal space12.
Three sets of plans—3D-crt, imrt, and RapidArc— were all designed on the same Eclipse treatment planning system (version 8.6.23: Varian Medical Systems). Calculations were performed using the analytical anisotropic algorithm (version 8.9.17: Varian Medical Systems) with a grid of 2.5 mm. All gantry angles and radiation fields were confirmed by the relationships of the ptvs and oars in various situations. We applied the 4-field technique in the 3D-crt plans and the 5-field step-and-shoot technique in the imrt plans. The RapidArc plans used two simultaneously optimized volumetric arcs. In the RapidArc plans, rotation of the collimator was 10 degrees for the first arc and 350 degrees for the second arc. No couch rotations or avoidance sectors were used. All three sets of plans used 6 MV photons and were designed by the same experienced physicist. The Compass dosimetric system (version 2.0.7.0: IBA Dosimetry, Beijing, P.R.C.) was used to verify all imrt and RapidArc plans. Once reasonable plans were attained, they were reviewed and approved by an attending radiation oncologist who specializes in gi malignancies.
These target coverage goals were used: minimum dose to the ptv of at least 95% of the prescription dose, and maximum dose to the ptv of 110% of the prescription dose. The oars included duodenum, small bowel, kidneys, liver, spinal cord, and stomach. Table i lists the normal organ constraints13,14. The conformal index was calculated as Vt,ref / Vt × Vt,ref / Vref, where Vt is the volume of the ptv, Vref is the volume enclosed by the prescription dose line, and Vt,ref is the volume of the ptv within the Vref15. The homogeneity index was calculated as D5% / D95%, where Dx% is the minimum dose delivered to x% of the ptv16. The conformal index ranged from 0 to 1. The homogeneity index ranged from infinity to 1. The closer the value approached to 1, the better17. The treatment delivery time is the time recorded from beam-on for the first field to beam-off for the last field. The dose and volume parameters for the ptv and oars were appropriately defined and used for comparison.
TABLE I.
Initial planning constraints
| Structure | Dose |
|---|---|
| Planning target volume | Dmin ≥ 95% prescribed dose |
| Dmax ≤ 110% prescribed dose | |
| Kidneys | V20 < 50% |
| Liver | V30 < 33% |
| Stomach | V45 < 15%, V50 < 5% |
| Small bowel | V45 < 15%, V50 < 5% |
| Duodenum | V45 < 33%, V50 < 5% |
| Spinal cord | Dmax < 45 Gy |
Dmin = minimum dose to the organ; Dmax = maximum dose to the organ; Vx = volume of the organ at risk receiving a dose greater than x Gy.
Clinical Toxicity Comparison
The main endpoint was acute gi toxicity. Information about toxicities was collected from a review of weekly physician undertreatment notes. Data on the incidence and grade of gi toxicities were collected from the start of radiotherapy until 1 month after treatment completion. Toxicities were graded using the U.S. National Cancer Institute’s Common Terminology Criteria for Adverse Events (version 4.0), and the incidences of grades 3 and 4 acute gi toxicities were evaluated with consideration for the risk factors of sex, age, tumour location, tumour size, gemcitabine-based chemotherapy after rt, ptv volume, V45 of bowel, Karnofsky performance status, and radiation technique.
Patients received a median dose of 50.4 Gy (range: 45–54 Gy) to the gross tumour. Most patients received 1–2 cycles of gemcitabine-based chemotherapy before rt. Chemotherapy was never concurrent with rt. At the discretion of the treating medical oncologist, patients received further treatment with gemcitabine after rt completion. Simulation was performed using 3D-ct in 196 patients and 4D-ct in the others. When 4D-ct simulation was used, an internal target volume was created to account for respiratory motion in 3 dimensions. When 3D-ct simulation was used, a 0.5–1 cm expansion of the gross tumour volume was used to set the ctv margin. The ctv or the internal target volume–to–ptv expansion accounted for tumour location, tumour diameter, respiratory movement, penumbra, and so on. The ptv margin was a 5–10 mm expansion of the ctv or internal target volume, but was individualized for each patient.
Statistical Analyses
To appraise the differences between the techniques, the paired two-tailed Student t-test was applied. All variables were entered into the multivariate analyses, which used a forward stepwise logistic regression model. Univariate analyses were performed using the Fisher exact test. A p value less than 0.05 was considered statistically significant. All analyses were performing using the SPSS Statistics software for Windows (version 17.0: SPSS, Chicago, IL, U.S.A.).
RESULTS
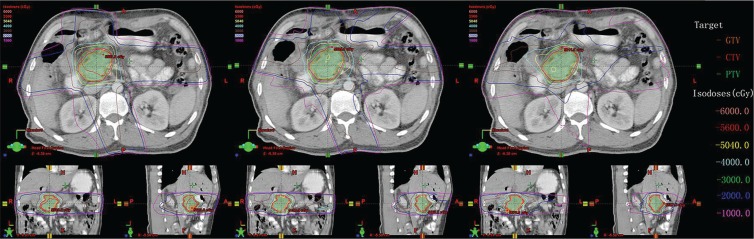
Table ii reports the parameters of dose–volume histograms, with mean ± standard deviation for the ptv, and homogeneity index, conformal index, monitor units (mus), and delivery time. Table iii shows the mean ± standard deviation for each oar parameter considered. Figure 1 shows, for one representative comparison, the dose distributions in the axial, sagittal, and coronal views for the three rt techniques.
TABLE II.
Summary of the dosimetric results for the planning target volumes
| Parameter | (A) 3D-CRT | (B) IMRT | (C) RapidArca | p Valueb | ||
|---|---|---|---|---|---|---|
|
| ||||||
| A vs. B | A vs. C | B vs. C | ||||
| V95% (%) | 99.70±0.41 | 99.96±0.04 | 99.97±0.04 | 0.046 | 0.046 | 0.707 |
| V110% (%) | 8.56±5.24 | 0.46±0.29 | 0.23±0.18 | 0.001 | 0.001 | 0.382 |
| Dmax (Gy) | 56.43±0.45 | 56.08±0.48 | 56.37±0.85 | 0.210 | 0.816 | 0.371 |
| Dmean (Gy) | 53.61±0.29 | 52.99±0.24 | 53.05±0.01 | 0.001 | 0.000 | 0.409 |
| Conformity index | 0.72±0.06 | 0.84±0.03 | 0.88±0.05 | 0.000 | 0.001 | 0.036 |
| Homogeneity index | 1.09±0.01 | 1.07±0.01 | 1.06±0.01 | 0.001 | 0.000 | 0.120 |
| Monitor units | 290.4±18.93 | 630.02±62.14 | 565.07±76.12 | 0.000 | 0.000 | 0.028 |
| Delivery time (min) | 0.97±0.06 | 2.10±0.21 | 1.05±0.10 | 0.000 | 0.095 | 0.000 |
Varian Medical Systems, Palo Alto, CA, U.S.A.
By paired t-test analysis.
3D-CRT = 3-dimensional conformal radiotherapy; IMRT = intensity-modulated radiotherapy; Vx% = volume receiving x% or more of the prescribed dose; Dmax = maximum dose to the organ; Dmean = mean dose to the organ.
TABLE III.
Summary of the dosimetric results for the organs at risk
| Organ | Parameter | (A) 3D-CRT | (B) IMRT | (C) RapidArca | p Valueb | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| A vs. B | A vs. C | B vs. C | |||||
| Duodenum | V10 | 69.13±23.50 | 61.88±23.23 | 58.64±23.30 | 0.012 | 0.003 | 0.281 |
| V20 | 56.03±17.21 | 43.12±13.68 | 33.99±17.00 | 0.001 | 0.002 | 0.050 | |
| V35 | 26.32±18.03 | 19.11±9.81 | 14.83±12.20 | 0.074 | 0.007 | 0.051 | |
| V45 | 15.19±15.93 | 8.94±6.88 | 7.39±9.91 | 0.078 | 0.016 | 0.321 | |
| V50 | 9.56±13.13 | 3.96±4.48 | 2.04±2.54 | 0.100 | 0.069 | 0.155 | |
| Dmean | 23.40±7.24 | 19.48±5.98 | 17.08±6.47 | 0.006 | 0.000 | 0.034 | |
| Small bowel | V30 | 5.17±4.40 | 5.52±2.30 | 3.74±2.43 | 0.794 | 0.185 | 0.004 |
| V45 | 0.20±0.22 | 0.16±0.23 | 0.75±1.38 | 0.377 | 0.203 | 0.158 | |
| Dmean | 9.76±3.97 | 8.76±2.79 | 7.28±2.59 | 0.097 | 0.007 | 0.012 | |
| Left kidney | V15 | 7.11±10.99 | 6.33±8.04 | 5.10±10.39 | 0.721 | 0.430 | 0.679 |
| V20 | 5.74±9.95 | 3.76±5.04 | 2.29±6.39 | 0.482 | 0.158 | 0.553 | |
| Dmean | 4.88±4.01 | 4.15±2.86 | 5.01±2.56 | 0.350 | 0.891 | 0.348 | |
| Right kidney | V15 | 9.38±9.97 | 6.98±7.90 | 4.55±4.43 | 0.260 | 0.043 | 0.169 |
| V20 | 6.88±8.28 | 4.03±4.45 | 1.67±2.57 | 0.192 | 0.047 | 0.035 | |
| Dmean | 6.72±3.10 | 6.37±2.52 | 5.25±2.21 | 0.426 | 0.042 | 0.039 | |
| Stomach | V30 | 10.32±16.26 | 7.33±12.62 | 6.89±10.23 | 0.040 | 0.153 | 0.742 |
| V45 | 3.32±5.96 | 0.86±1.43 | 2.36±3.48 | 0.124 | 0.357 | 0.083 | |
| Dmean | 8.22±8.96 | 6.84±7.21 | 6.81±7.31 | 0.043 | 0.097 | 0.953 | |
| Liver | V30 | 2.58±5.71 | 1.84±4.03 | 1.56±3.34 | 0.222 | 0.222 | 0.419 |
| Dmean | 4.05±4.06 | 3.78±4.02 | 3.45±3.59 | 0.005 | 0.004 | 0.047 | |
| Spinal cord | Dmax | 35.75±2.39 | 31.44±6.45 | 27.86±7.97 | 0.072 | 0.010 | 0.202 |
Varian Medical Systems, Palo Alto, CA, U.S.A.
By paired t-test analysis.
Vx = volume of the organ at risk receiving a dose greater than x Gy; Dmean = mean dose to the organ; Dmax = maximum dose to the organ.
FIGURE 1.
Isodose curves on axial, coronal, and sagittal views for one representative case of pancreatic cancer. Left panel: 3-Dimensional conformal radiotherapy (3D-CRT). Middle panel: Intensity-modulated radiotherapy (IMRT). Right panel: RapidArc (Varian Medical Systems, Palo Alto, CA, U.S.A.). Compared with 3D-CRT and IMRT, RapidArc achieved better conformality. GTV = gross target volume; CTV = clinical target volume; PTV = planning target volume.
Target Coverage, Homogeneity Index, and Conformity Index
All plans had excellent coverage of the ptv, with 99% or more of the ptv receiving 95% or more of the prescribed dose. The V95% of ptv was lower for 3D-crt (99.69% ± 0.41%) than for imrt (99.96% ± 0.04%, p = 0.046) or RapidArc (99.97% ± 0.04%, p = 0.046). We observed a significant difference in V110% between RapidArc and 3D-crt (p = 0.001) and between imrt and 3D-crt (p = 0.001). The conformal index of RapidArc was significantly better than those of 3D-crt (p = 0.001) and imrt (p = 0.036). The homogeneity index was better for imrt (1.07 ± 0.01, p = 0.001) and for RapidArc (1.05 ± 0.01, p < 0.001) than for 3D-crt (1.09 ± 0.01).
MUs and Delivery Time
Mean mus were 290.4 ± 18.9 for 3D-crt, 630.0 ± 62.1 for imrt, and 565.1 ± 76.1 for RapidArc. The mus were thus significantly fewer with 3D-crt than with imrt (p < 0.001) or Rapid Arc (p = 0.028). Treatment delivery time was significant higher with imrt than with 3D-crt (p < 0.001) or RapidArc (p < 0.001). Delivery time was shorter with 3D-crt than RapidArc, but the difference was nonsignificant (p = 0.095).
OARs
For duodenum, the planning constraint of V45 less than 33% was met in 90%, 100%, and 100% of cases with 3D-crt, imrt, and RapidArc respectively. Mean dose was lower with RapidArc than with 3D-crt (p < 0.0001) or imrt (p = 0.034). The mean dose to duodenum was lower with imrt than with 3D-crt (p = 0.006). With respect to V10 and V20, volumes were higher with 3D-crt than with imrt (p = 0.012 and p = 0.001 respectively) or with RapidArc (p = 0.003 and p = 0.002 respectively). For the high-dose region, V35 was highest with 3D-crt and lowest with RapidArc, with the p value of the paired t-test evaluating the difference between the two techniques being 0.007. For the V45, the volume was lower with RapidArc than with 3D-crt, but no difference between RapidArc and imrt (p = 0.078) or between 3D-crt and imrt (p = 0.321) was found. We observed no significant differences between the three techniques for the V50.
For small bowel, the planning constraints of V45 less than 15% and V50 less than 5% were met in all plans. The mean dose to small bowel was significantly lower with RapidArc (7.28 ± 2.59 Gy) than with 3D-crt (9.76 ± 3.97 Gy, p = 0.007) or imrt (8.76 ± 2.79 Gy, p = 0.012). Compared with 3D-crt, RapidArc significantly decreased the volume receiving 30 Gy (p = 0.004). With respect to the V45, the volume was higher with RapidArc than with imrt or 3D-crt, but no statistical significance was observed (p = 0.203 and p = 0.158 respectively).
All plans were able to keep the V20 for the bilateral kidneys to less than 50%. Compared with 3D-crt and imrt, Rapid Arc offered a lower V15 and V20 for the left kidney, although the differences were not statistically significant. The mean dose to left kidney was highest with RapidArc and lowest with imrt, but the difference was nonsignificant (p = 0.348). However, compared with 3D-crt (p = 0.042) and imrt (p = 0.039), RapidArc was superior in terms of mean dose to the right kidney. The V15 and V20 for the right kidney were both significantly lower with RapidArc than with 3D-crt (p =0.043 and p = 0.047). Additionally, compared with imrt, RapidArc allowed for significant reductions in the V20 (p = 0.035).
In only 1 patient could 3D-crt not meet the required constraint for stomach. In the present study, we found no significant differences between the three techniques for V45 of stomach, and yet, compared with 3D-crt, reductions in the V30 and mean dose were achieved with imrt (p = 0.040 and p = 0.043 respectively). All plans were able to keep the V30 for liver to less than 33%, and using the three techniques, we observed no significant differences in the V30. However, the mean doses using each technique were significantly different, being highest for 3D-crt (4.05 ± 4.06 Gy) and lowest for RapidArc (3.45 ± 3.59 Gy); the p values from the paired t-test evaluating the differences were 0.004 for imrt compared with 3D-crt, 0.005 for 3D-crt compared with RapidArc, and 0.047 for imrt compared with RapidArc. For spinal cord, the maximum dose was lower with RapidArc than with 3D-crt (p = 0.01). All plans were able to keep the maximum dose below 45 Gy.
Acute GI Toxicity
The study cohort included 169 men and 111 women with a median age of 58 years (range: 27–76 years). Median ptv in the cohort was 184.83 cm3 (range: 133.79–241.85 cm3), and median V45 of bowel was 41.37 cm3 (range: 20.02–59.41 cm3). Within 1 month after rt, 134 of the patients received gemcitabine- based chemotherapy; the remaining 146 did not.
On univariate analysis, age, performance status, ptv volume, V45 of bowel, and gemcitabine-based chemotherapy at 1 month post-rt were not statistically associated with the incidence of grades 3 and 4 acute gi toxicity. Radiation technique was, however, significantly associated with the incidence of grades 3 and 4 acute gi toxicity (Table iv). The overall incidence of severe (grades 3 and 4) acute gi toxicity was low in patients treated by the RapidArc technique (Table v). Within the entire cohort of patients, the incidences of grades 3 and 4 diarrhea (p = 0.037) and anorexia (p = 0.042) were significantly reduced with the use of RapidArc compared with 3D-crt. The incidence of grades 3 and 4 diarrhea were also significantly reduced (p = 0.027) with the use of RapidArc compared with imrt. No significant differences between 3D-crt and imrt were observed.
TABLE IV.
Multivariate analysis of factors related to the development of grades 3 and 4 acute gastrointestinal toxicity
| Characteristic | Value | OR | 95% CI | p Valuea |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 58 | 0.996 | 0.675 | 0.979 to 1.014 |
| Range | 27–76 | |||
| Sex (n) | ||||
| Men | 169 | 0.938 | 0.557 to 1.582 | 0.811 |
| Women | 111 | |||
| Tumour location (n) | ||||
| Head | 187 | 1.267 | 0.74 to 2.17 | 0.388 |
| Body or tail | 93 | |||
| Tumour size (cm) | ||||
| Median | 4.1 | 1.115 | 0.944 to 1.318 | 0.200 |
| Range | 5.1–2.0 | |||
| Gemcitabine chemotherapy after RT (n) | ||||
| <1 Month | 134 | 0.806 | 0.485 to 1.338 | 0.404 |
| ≥1 Month | 146 | |||
| Planning target volume (cm3) | ||||
| Median | 184.83 | 0.997 | 0.988 to 1.006 | 0.516 |
| Range | 133.79–241.85 | |||
| Bowel V45 (cm3) | ||||
| Median | 41.37 | 0.995 | 0.973 to 1.018 | 0.693 |
| Range | 20.02–59.41 | |||
| Karnofsky PS (n) | ||||
| <80 | 194 | 0.787 | 0.456 to 1.358 | 0.389 |
| ≥80 | 86 | |||
| RT technique (n) | ||||
| 3D-CRT | 137 | |||
| IMRT | 97 | 3.384 | 1.469 to 7.798 | 0.004 |
| RapidArcb | 46 | 2.563 | 1.075 to 6.113 | 0.034 |
By logistic regression analysis.
Varian Medical Systems, Palo Alto, CA, U.S.A.
OR = odds ratio; CI = confidence interval; RT = radiotherapy; V45 = volume receiving 45% or more of the prescribed dose; PS = performance status; 3D-CRT = 3-dimensional conformal radiotherapy; IMRT = intensity-modulated radiotherapy.
TABLE V.
Incidence of acute gastrointestinal toxicity in the study patients
| Toxicity | (A) 3D-CRT | (B) IMRT | (C) RapidArca | p Valueb | ||
|---|---|---|---|---|---|---|
|
| ||||||
| A vs. B | A vs. C | B vs. C | ||||
| Nausea [n (%)] | ||||||
| Grades 0–2 | 116 (85) | 85 (88) | 41 (89) | 0.276 | 0.275 | 0.476 |
| Grades 3–4 | 21 (15) | 12 (12) | 5 (11) | |||
| Vomiting [n (%)] | ||||||
| Grades 0–2 | 128 (93) | 91 (94) | 44 (96) | 0.566 | 0.446 | 0.495 |
| Grades 3–4 | 9 (7) | 6 (6) | 2 (4) | |||
| Diarrhea [n (%)] | ||||||
| Grades 0–2 | 126 (92) | 88 (91) | 46 (100) | 0.456 | 0.037 | 0.027 |
| Grades 3–4 | 11 (8) | 9 (9) | 0 (0) | |||
| Anorexia [n (%)] | ||||||
| Grades 0–2 | 121 (88) | 90 (93) | 45 (98) | 0.183 | 0.042 | 0.207 |
| Grades 3–4 | 16 (12) | 7 (7) | 1 (2) | |||
Varian Medical Systems, Palo Alto, CA, U.S.A.
By Fisher exact test.
3D-CRT = 3-dimensional conformal radiotherapy; IMRT = intensity-modulated radiotherapy.
DISCUSSION
Our study investigated dosimetry differences in three plans—3D-crt, imrt, and RapidArc—for lapcc. In the dosimetric analysis, particular emphasis was placed on improving the sparing of duodenum, small bowel, and kidneys. Additionally, we compared the clinical toxicities of the three plans to identify the technique that was more effective in lowering acute gi toxicity.
It is difficult to deliver higher doses to the pancreas without exceeding bowel tolerance—and especially that of duodenum, which is adjacent to the pancreatic head. An increase in the volume of duodenum receiving a low dose (10–20 Gy) was associated with a trend toward increased important late gi toxicity; moreover, acute toxicity seemed to be correlated with the volume of duodenum receiving 35 Gy or more when pcc patients received concurrent full-dose gemcitabine and rt18. In the present study, dose to duodenum was evaluated as V10, V20, V35, V45, V50, and mean dose. Compared with imrt or RapidArc, 3D-crt resulted in significantly higher V10 and V20 for duodenum. In the study conducted by Warren et al.12, V45 and V50 for duodenum were less with RapidArc than with 3D-crt. In our study, the V45 was significantly higher with 3D-crt than with RapidArc (p = 0.016). In addition, the V50 was lower with RapidArc than with the other two techniques, but the differences were nonsignificant. By contrast, the V35 was significantly higher with 3D-crt than with RapidArc (p = 0.007). In a comparative planning study of RapidArc and imrt conducted by Eppinga et al.19 in pcc, RapidArc was observed to modestly lower the mean dose to duodenum (p = 0.004). In our analysis, the mean dose to duodenum was similarly lower with RapidArc than with imrt (p = 0.034) or 3D-crt (p < 0.001).
The V30 and V45 for small bowel are thought to correlate with acute toxicity20,21. A dosimetric comparison6 in pcc found that, compared with 3D-crt, imrt significantly reduced the V30 for small bowel and predicted a 9.36% probability of small-bowel complication (compared with the 18.9% with 3D-crt, p = 0.021). Another study19 showed that, compared with imrt, RapidArc lowered the V30 for small bowel. In our study, the V30 for small bowel was significantly lower with RapidArc than with imrt (p = 0.004), but a difference between imrt and 3D-crt was not observed (p = 0.794). Poppe et al.22 reported that imrt offered obvious improvement over 3D-crt in lowering the V45 for small bowel. However, in our study, the V45 was not significantly improved with either RapidArc or imrt than with 3D-crt. Clinical investigations in pcc observed that the mean dose to small bowel was lower with RapidArc than with imrt19 or 3D-crt9, which is in line with our results. It must be highlighted that the separate bowel loops were not identified; instead, a surrogate “small-bowel region” was defined to represent the possible positions of the small bowel20,23,24. Our defined small-bowel volume accorded with the definitions in those studies.
Kidney-sparing was also a focus in our study. The V20 is commonly considered to be a measure of renal tolerance. Renal scintigrams have revealed a decline in renal function within 6 months to 1 year after exposure to 20 Gy, with a reduction in function to nearly 52% of baseline at 18 months’ follow-up25. The same study also showed a significant correlation between mean dose to kidney and progressive decline in renal function25. Cassady26 proved that the V15 is another useful threshold of renal tolerance.
Sparing of the left kidney was similar in all three techniques that we evaluated. The V15 for the right kidney was significantly less with RapidArc than with 3D-crt (p = 0.043). The V20 and the mean dose to the right kidney were both significantly reduced with RapidArc (in comparison with 3D-crt or imrt). Those results are similar to findings reported by Vieillot et al.9, in which three radiotherapy modalities could offer good preservation of the left kidney, which was away from the target. While maintaining target coverage, 3D-crt and imrt allow for total preservation of the left kidney, but have a detrimental effect on the right kidney. RapidArc, a new form of imrt optimization, combines a single gantry rotation with the capability to vary dose rate, gantry speed, and dynamic multileaf collimation27. It can minimize the dose to both kidneys as much as possible for bilateral preservation.
Based on dosimetric comparisons in prostate, liver, and stomach tumours28–30, RapidArc demonstrated both a high conformal index and the advantages of ptv coverage, results that accord with our analysis. Compared with 3D-crt, RapidArc and imrt showed significant improvements in the conformal and homogeneity indices. Of the three techniques, RapidArc and imrt achieved higher V95% and lower V110% values than did 3D-crt. In our study, “hot spots” were almost always located at the tumour, and so the influence of hot spots was small between the three plans. Compared with the other two techniques, 3D-crt achieved the highest mean dose to the ptv, but the difference was clinically irrelevant. That finding indicates that, compared with 3D-crt, RapidArc and imrt can achieve better ptv coverage and decrease hot spots in exchange for a slightly lower mean dose to the ptv.
The 3D-crt technique had the fewest mus and the shortest delivery time. Reduction of delivery time might improve comfort on the couch for the patient, reduce the risk of interfraction movements, and minimize organ displacement.
The main goal of our study was to investigate whether a technique with better oar sparing and target dose distribution could lead to less clinical toxicity. Acute toxicity involving the bowel is related to radiation-induced epithelial damage and typically manifests primarily as diarrhea; acute toxicity involving the stomach typically presents as nausea or vomiting. Yovino et al.31 published a series of 46 patients whose pcc or ampullary cancer was treated with concurrent chemoradiation using inverse-planned imrt. In their report (Radiation Therapy Oncology Group 97-04), the incidence of grades 3 and 4 nausea and vomiting (0% vs. 11%, p = 0.024) and diarrhea (3% vs. 18%, p = 0.017) were significantly different when treatment was planned with imrt rather than with a 3D technique. The present study is one of the largest to comparatively evaluate acute gi toxicity with use of RapidArc, imrt, and 3D-crt. We confirmed that, compared with 3D-crt or imrt, RapidArc reduces the rate of grades 3 and 4 acute gi toxicity.
Radiotherapy for pcc is technically challenging. Recent technical advances have spurred an increase in the number of new treatments and marked improvements in existing treatments5. Advances in treatment planning techniques, such as the use of 4D-ct simulation24, functional imaging, and magnetic resonance imaging32, can also help to optimize the definition of target volumes in patients with pcc. Compared with 3D-crt and imrt, helical tomotherapy results in better sparing of most oars (mainly stomach and duodenum)14,33. Stereotactic body radiation therapy has been actively investigated as an option for the delivery of radiation in pcc34.
The limitations of our study should also be noted. The number of cases used for the dosimetric analysis was relatively small. Also, the reported data were collected in a retrospective chart review and lack follow-up for overall survival duration.
CONCLUSIONS
RapidArc might be superior to 3D-crt or imrt for sparing of duodenum, small bowel, and right kidney. Better preservation of oars might be achieved with imrt than with 3D-crt. In lapcc, the RapidArc and imrt techniques achieve similar target dose distributions, which are both better than those achieved with the 3D-crt technique. Compared with 3D-crt or imrt, RapidArc might reduce the rate of grades 3 and 4 gi acute toxicity. A technique with better oar sparing and target dose distributions could lead to less clinical toxicity in the treatment of lapcc, but proving an optimal technique requires further prospective studies.
ACKNOWLEDGMENTS
This work was supported by the Shandong Cancer Prevention and Control Institute ethics committee and by the Promotive Research Fund for Excellent Young and Middle-Aged Scientists of Shandong Province (BS2013YY040).
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Johung K, Saif MW, Chang BW. Treatment of locally advanced pancreatic cancer: the role of radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82:508–18. doi: 10.1016/j.ijrobp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Malik NK, May KS, Chandrasekhar R, et al. Treatment of locally advanced unresectable pancreatic cancer: a 10-year experience. J Gastrointest Oncol. 2012;3:326. doi: 10.3978/j.issn.2078-6891.2012.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loehrer PJ, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–12. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trakul N, Koong AC, Maxim PG, Chang DT. Modern radiation therapy techniques for pancreatic cancer. Gastroenterol Clin North Am. 2012;41:223–35. doi: 10.1016/j.gtc.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Landry JC, Yang GY, Ting JY, et al. Treatment of pancreatic cancer tumors with intensity-modulated radiation therapy (imrt) using the volume at risk approach (vara): employing dose-volume histogram (dvh) and normal tissue complication probability (ntcp) to evaluate small bowel toxicity. Med Dosim. 2002;27:121–9. doi: 10.1016/S0958-3947(02)00094-8. [DOI] [PubMed] [Google Scholar]
- 7.Brown MW, Ning H, Arora B, et al. A dosimetric analysis of dose escalation using two intensity-modulated radiation therapy techniques in locally advanced pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2006;65:274–83. doi: 10.1016/j.ijrobp.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Ali AN, Dhabaan AH, Jarrio CS, Siddiqi K, Landry JC. Dosimetric comparison of volumetric modulated arc therapy and intensity-modulated radiation therapy for pancreatic malignancies. Med Dosim. 2012;37:271–5. doi: 10.1016/j.meddos.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Vieillot S, Azria D, Riou O, et al. Bilateral kidney preservation by volumetric-modulated arc therapy (RapidArc) compared to conventional radiation therapy (3D-crt) in pancreatic and bile duct malignancies. Radiat Oncol. 2011;6:147. doi: 10.1186/1748-717X-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Commission on Radiation Units and Measurements (icru) Prescribing, Recording, and Reporting Photon Beam Therapy. Bethesda, MD: ICRU; 1993. icru report 50. [Google Scholar]
- 11.International Commission on Radiation Units and Measurements (icru) Prescribing, Recording, and Reporting Photon Beam Therapy. Bethesda, MD: ICRU; 1999. icru report 62 [supplement to report 50] [Google Scholar]
- 12.Warren S, Partridge M, Fokas E, Eccles CL, Brunner TB. Comparing dose–volume histogram and radiobiological endpoints for ranking intensity-modulated arc therapy and 3D-radiotherapy treatment plans for locally-advanced pancreatic cancer. Acta Oncol. 2013;52:1573–8. doi: 10.3109/0284186X.2013.813072. [DOI] [PubMed] [Google Scholar]
- 13.Ding X, Dionisi F, Tang S, et al. A comprehensive dosimetric study of pancreatic cancer treatment using three-dimensional conformal radiation therapy (3D-crt), intensity-modulated radiation therapy (imrt), volumetric-modulated radiation therapy (vmat), and passive-scattering and modulated-scanning proton therapy (pt) Med Dosim. 2014;39:139–45. doi: 10.1016/j.meddos.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Taylor R, Opfermann K, Jones BD, et al. Comparison of radiation treatment delivery for pancreatic cancer: linac intensity-modulated radiotherapy versus helical tomotherapy. J Med Imaging Radiat Oncol. 2012;56:332–7. doi: 10.1111/j.1754-9485.2012.02373.x. [DOI] [PubMed] [Google Scholar]
- 15.van’t Riet A, Mak AC, Moerland MA, Elders LH, van der Zee W. A conformation number to quantify the degree of conformality in brachytherapy and external beam irradiation: application to the prostate. Int J Radiat Oncol Biol Phys. 1997;37:731–6. doi: 10.1016/S0360-3016(96)00601-3. [DOI] [PubMed] [Google Scholar]
- 16.Yoo S, Wu QJ, Lee WR, Yin FF. Radiotherapy treatment plans with RapidArc for prostate cancer involving seminal vesicles and lymph nodes. Int J Radiat Oncol Biol Phys. 2010;76:935–42. doi: 10.1016/j.ijrobp.2009.07.1677. [DOI] [PubMed] [Google Scholar]
- 17.Cilla S, Macchia G, Digesù C, et al. 3D-Conformal versus intensity-modulated postoperative radiotherapy of vaginal vault: a dosimetric comparison. Med Dosim. 2010;35:135–42. doi: 10.1016/j.meddos.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Robertson JM, Ye H, Margolis J, Nadeau L, Yan D. Dose–volume analysis of predictors for gastrointestinal toxicity after concurrent full-dose gemcitabine and radio-therapy for locally advanced pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2012;83:1120–5. doi: 10.1016/j.ijrobp.2011.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Eppinga W, Lagerwaard F, Verbakel W, Slotman B, Senan S. Volumetric modulated arc therapy for advanced pancreatic cancer. Strahlenther Onkol. 2010;186:382–7. doi: 10.1007/s00066-010-2094-5. [DOI] [PubMed] [Google Scholar]
- 20.Devisetty K, Mell LK, Salama JK, et al. A multi-institutional acute gastrointestinal toxicity analysis of anal cancer patients treated with concurrent intensity-modulated radiation therapy (imrt) and chemotherapy. Radiother Oncol. 2009;93:298–301. doi: 10.1016/j.radonc.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose–volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys. 2010;76(suppl):S101–7. doi: 10.1016/j.ijrobp.2009.05.071. [DOI] [PubMed] [Google Scholar]
- 22.Poppe MM, Narra V, Yue NJ, Zhou J, Nelson C, Jabbour SK. A comparison of helical intensity-modulated radiotherapy, intensity-modulated radiotherapy, and 3D-conformal radiation therapy for pancreatic cancer. Med Dosim. 2012;36:351–7. doi: 10.1016/j.meddos.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Roeske JC, Lujan A, Rotmensch J, Waggoner SE, Yamada D, Mundt AJ. Intensity-modulated whole pelvic radiation therapy in patients with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2000;48:1613–21. doi: 10.1016/S0360-3016(00)00771-9. [DOI] [PubMed] [Google Scholar]
- 24.van der Geld YG, van Triest B, Verbakel WF, et al. Evaluation of four-dimensional computed tomography-based intensity-modulated and respiratory-gated radiotherapy techniques for pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1215–20. doi: 10.1016/j.ijrobp.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Jansen EP, Saunders MP, Boot H, et al. Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys. 2007;67:781–5. doi: 10.1016/j.ijrobp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Cassady JR. Clinical radiation nephropathy. Int J Radiat Oncol Biol Phys. 1995;31:1249–56. doi: 10.1016/0360-3016(94)00428-N. [DOI] [PubMed] [Google Scholar]
- 27.Otto K. Volumetric modulated arc therapy: imrt in a single gantry arc. Med Phys. 2008;35:310–17. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 28.Palma D, Vollans E, James K, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 29.Chen D, Wang R, Meng X, et al. A comparison of liver protection among 3-D conformal radiotherapy, intensity-modulated radiotherapy and RapidArc for hepatocellular carcinoma. Radiat Oncol. 2014;9:48. doi: 10.1186/1748-717X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Li G, Zhang Y, et al. Single-arc volumetric-modulated arc therapy (svmat) as adjuvant treatment for gastric cancer: dosimetric comparisons with three-dimensional conformal radiotherapy (3D-crt) and intensity-modulated radiotherapy (imrt) Med Dosim. 2013;38:395–400. doi: 10.1016/j.meddos.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 31.Yovino S, Poppe M, Jabbour S, et al. Intensity-modulated radiation therapy significantly improves acute gastrointestinal toxicity in pancreatic and ampullary cancers. Int J Radiat Oncol Biol Phys. 2011;79:158–62. doi: 10.1016/j.ijrobp.2009.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng M, Balter JM, Normolle D, et al. Characterization of pancreatic tumor motion using cine MRI: surrogates for tumor position should be used with caution. Int J Radiat Oncol Biol Phys. 2009;74:884–91. doi: 10.1016/j.ijrobp.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sangalli G, Passoni P, Cattaneo GM, et al. Planning design of locally advanced pancreatic carcinoma using 4DCT and imrt/igrt technologies. Acta Oncol. 2011;50:72–80. doi: 10.3109/0284186X.2010.484425. [DOI] [PubMed] [Google Scholar]
- 34.Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys. 2013;86:516–22. doi: 10.1016/j.ijrobp.2013.02.022. [DOI] [PubMed] [Google Scholar]