Abstract
Climbing fiber inputs to Purkinje cells are thought to play a teaching role by generating the instructive signals that drive cerebellar learning. To investigate how these instructive signals are encoded, we recorded the activity of individual climbing fibers during cerebellar-dependent eyeblink conditioning in mice. Our findings show that climbing fibers signal both the unexpected delivery and the unexpected omission of the periocular airpuff that serves as the instructive signal for eyeblink conditioning. In addition, we report the surprising discovery that climbing fibers activated by periocular airpuffs also respond to stimuli from other sensory modalities, if those stimuli are novel or if they predict that the periocular airpuff is about to be presented. This pattern of climbing fiber activity is strikingly similar to the responses of dopamine neurons during reinforcement learning, which have been shown to encode a particular type of instructive signal known as a temporal difference prediction error.
Keywords: prediction error, Rescorla-Wagner, temporal difference model, reinforcement learning, eyeblink conditioning, cerebellum, Purkinje cell, complex spike
INTRODUCTION
Climbing fibers originating in the inferior olive project to the cerebellar cortex1, where they are thought to play a teaching role by providing the instructive signals necessary for cerebellar learning2-4. Some of the strongest support for this hypothesis comes from studies of Pavlovian eyeblink conditioning5-8, a cerebellar task in which animals learn to close the eyelid in response to a conditioned stimulus (CS) like an LED light, if it is repeatedly paired with a blink-eliciting unconditioned stimulus (US) like a periocular airpuff. Consistent with their presumed role as “teachers”, climbing fibers carry signals about the instructive US in this associative learning task9-11. Furthermore, direct electrical stimulation of climbing fibers can serve as the US during conditioning, providing a teaching signal that is as effective as periocular stimulation12.
It has been suggested that the teaching signals transmitted by climbing fibers are encoded as prediction errors in cerebellar learning tasks4,13-15. During eyeblink conditioning, for example, climbing fibers fire if the US is presented unexpectedly9-11, i.e. positive prediction error, and they are inhibited if an expected US is omitted11, i.e. negative prediction error. This type of error coding can be used to generate an effective teaching signal16,17, by alerting the brain that current expectations about the likelihood of the instructive US are incorrect and need to be updated. Indeed, climbing fiber signals about positive and negative US prediction errors feature prominently in many computational models of cerebellar-dependent conditioning15,18,19.
Prediction error signals about the US are well suited for driving simple forms of associative learning such as first-order acquisition and extinction of the conditioned eyelid response15-19. However, for higher-order learning in which animals must learn from non-primary reinforcers like the CS, teaching signals related to the US are not enough20. Theories based on the influential temporal difference (TD) model20,21 have proposed that higher-order instructive signals must also alert the brain about the CS events that reliably predict the occurrence of the US. Such CS-triggered signals have been found in midbrain dopamine neurons during reinforcement learning tasks14,22,23. Our goal was to examine if climbing fibers may encode the same type of predictive TD signals during cerebellar-dependent associative learning.
We have taken advantage of a new system for eyeblink conditioning in head-fixed mice24 to examine the neural coding of prediction errors in climbing fibers. Based on the predictions of the TD model, we hypothesized that in addition to their well-known activation by an unexpected US, climbing fibers should also fire in response to presentations of the CS at the end of conditioning, i.e. after the primary association between the CS and the US has been established.
RESULTS
Monitoring climbing fibers during eyeblink conditioning
The goal of our experiments was to examine the signals that climbing fibers send to Purkinje cells during cerebellar learning, and to evaluate if these signals conform to the predictions of the temporal difference (TD) model20,21. Figure 1a,b shows our experimental set-up. We used a head-fixed apparatus to train mice in a simple delay eyeblink conditioning task that is critically dependent on the cerebellum24-26. Daily conditioning sessions comprised 100-200 trials in which a conditioned stimulus (CS) like a tone or an LED light was followed after 220 ms by an aversive airpuff directed at the eye, which served as the instructive blink-eliciting unconditioned stimulus (US). All mice (n=7) learned to make well-timed conditioned responses (CR) over the course of 10-15 conditioning sessions, i.e. they learned to blink in response to the CS, closing their eyelids in anticipation of the aversive periocular airpuff.
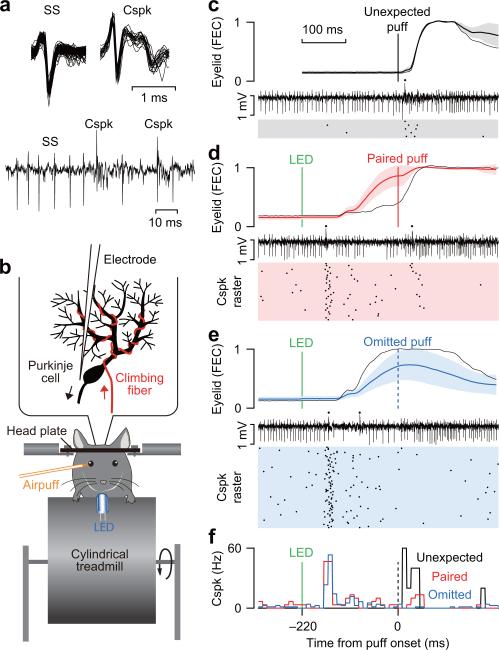
Figure 1. Experimental design and approach.
(a) Examples of waveforms (top) and raw extracellular signal (bottom) for simple spikes (SS) and complex spikes (Cspk) fired by a representative Purkinje cell during eyeblink conditioning in a treadmill apparatus for head-fixed mice (b). (c-e) Eyelid movements (mean ± s.d.) and simultaneously recorded Cspk's of the Purkinje cell in (a) in trials with unexpected periocular airpuff (c), paired LED and periocular airpuff (d), and LED without periocular airpuff (e). Raw extracellular traces in c-e show Cspk's (dots) in an example trial (thin black eyelid trace). (f) Peristimulus time histograms (bin size = 10 ms) for the Cspk's fired in the trials corresponding to the 3 raster plots of c-e.
To measure climbing fiber signals on any given conditioning session, we lowered an electrode into an identified eyeblink region of cerebellar cortex27,28 and recorded the extracellular activity of individual Purkinje cells (Fig. 1b). Each activation of the powerful climbing fiber input results in a massive depolarization of the post-synaptic Purkinje cell2,4, which can be detected in the raw extracellular record as a characteristic complex spike29 (Cspk, Fig. 1a). The waveform of the climbing fiber-driven Cspk can be distinguished from normal Purkinje cell action potentials known as simple spikes29 (SS, Fig. 1a). Because each Purkinje cell receives input from a single climbing fiber1,4 (red, Fig. 1b), the Cspk's fired by a well-isolated Purkinje cell provide a straightforward way to measure the activity of an individual climbing fiber.
To evaluate the coding of prediction error signals, we first trained the mice and then examined the climbing fiber-driven Cspk's of individual Purkinje cells in daily sessions with three types of trials: (1) unexpected presentations of the periocular airpuff US (unexpected, Fig. 1c,f), (2) paired trials in which the CS (tone or LED) was presented 220 ms before the US, as was the case for normal conditioning trials during training (paired, Fig. 1d,f), and (3) trials in which the CS was presented by itself, without the US (omitted, Fig. 1e,f). Note that even in well-trained mice, there is considerable trial-by-trial variation in performance (Fig. 1d,e, cloud around average eyelid traces represents standard deviation), and that the size of the eyeblink CR before the US is presented can range from very small in some individual trials (Fig. 1d, black eyelid trace) to very large in others (Fig. 1e, black eyelid trace).
Climbing fibers encode US-related prediction errors
Our first analysis was designed to examine climbing fiber-driven Cspk's in the 120 ms time window after the periocular airpuff (US period, Fig. 2a). We will refer to Cspk's in this US period as CspkUS. Because climbing fibers do not modulate their firing rate much and typically fire just once in response to each stimulus presentation2, we computed the activity of each climbing fiber by constructing a peristimulus time histogram (PSTH) that averages the number of Cspk's fired by the same Purkinje cell across multiple trials (Fig. 1f shows some example PSTH's for a representative Purkinje cell, for details see online Methods).
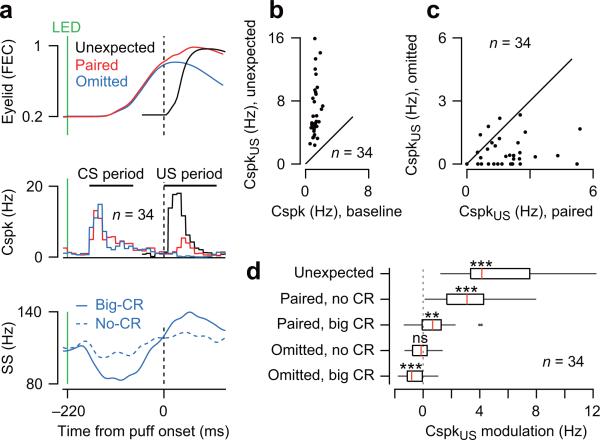
Figure 2. Climbing fiber responses in the US period.
(a) Population averages of eyelid movements and simultaneously recorded Cspk's and SS's in trials with unexpected periocular airpuff (black), paired LED and periocular airpuff (red), and LED without periocular airpuff (blue). For SS, data in LED trials without airpuff are plotted separately for trials with a conditioned eyelid response (big-CR) and trials without (no-CR). Time windows for CS and US analysis are indicated. (b-d) The probability of a Cspk during baseline and in the US period of different types of trials is plotted for all individual Purkinje cells (b-c), and summarized for the population (d). In (d), modulation of Cspk activity in the US period is relative to baseline. Median (red line), interquartile range (box), data range (whisker), and outliers (dots). An outlier [14.7] was omitted from the unexpected-puff condition (first row). Statistical significance indicated after correcting for multiple comparisons (ns is not significant, ** is P<0.01/5; *** is P<0.001/5).
Consistent with previous reports10,11,28, we found that the climbing fiber input of many Purkinje cells (n=34) in the eyeblink region provides a bidirectional signal that encodes both positive and negative prediction errors about the US14: the probability of a CspkUS was higher than baseline when the periocular airpuff was presented unexpectedly (unexpected, Figs. 1c,f and 2a,b,d; Wilcoxon signed rank test with Bonferroni correction, P<0.001/5), and also in paired conditioning trials in which the mouse failed to make a CR (example trial in Fig. 1d; paired, no CR Fig. 2d; Wilcoxon signed rank test with Bonferroni correction, P<0.001/5). In contrast, the probability of a CspkUS was lower than baseline in trials in which the mouse made a CR and the periocular airpuff was omitted (omitted, Figs. 1e,f and 2a,c). Note that the reduction of climbing fiber activity in the omitted trials was very reliable (omitted big CR Fig. 2d; Wilcoxon signed rank test with Bonferroni correction, P<0.001/5), but that the size of the modulation was small because climbing fibers fire at very low rates around 1Hz during baseline2.
We examined the simple spike (SS) activity of the same group of Purkinje cells (SS, Fig. 2a) to gain some insight about the mechanisms underlying the coding of prediction errors in climbing fibers. Purkinje cells reduced their SS firing rate prior to the US period, but only in trials with a CR (big CR, Fig. 2a), not when the mouse failed to make a CR (no CR, Fig. 2a). It has been suggested that such a reduction in SS firing rate could be used to inhibit climbing fiber activity at the time of the US18,30, via a double inhibitory pathway from Purkinje cells to neurons in the deep cerebellar nuclei and then to the inferior olive where climbing fibers originate31. In this model, climbing fiber activity during the US period is modulated by two inputs converging in the inferior olive: (1) excitation from US-driven trigeminal neurons32,33, and (2) inhibition from CR-related neurons in the deep cerebellar nucleus10,30,34. This anatomical organization could help explain why climbing fiber activity is highest in positive prediction error trials when there is no CR-related inhibition of the inferior olive at the time of the US (unexpected and paired no CR, Fig. 2d), reduced when US-related excitation is counterbalanced by CR-related inhibition (paired big CR, Fig. 2d), near baseline in the absence of US-related and CR-related inputs (omitted no CR, Fig. 2d), and suppressed below baseline in negative prediction trials when CR-related inhibition of the inferior olive occurs without the US (omitted big CR, Fig. 2d).
Climbing fibers responses to the CS in well-trained mice
Previous electrophysiology studies9-11,30, including the analysis presented above (Fig. 2b-d), have focused exclusively on climbing fiber signals around the time of the instructive US. However, to comply with the requirements of the TD model20,21, climbing fibers must fire in response to stimuli like the CS that reliably predict the occurrence of the US. To assess if climbing fibers meet this criterion, we examined Cspk's in a 100 ms time window starting 0-50 ms after the LED or tone stimulus used as the CS (CS period, Fig. 2a). We will refer to Cspk's in this CS period as CspkCS.
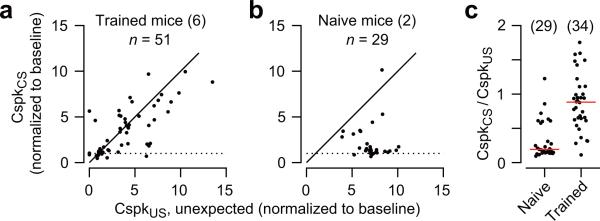
Presentation of the CS elicited Cspk's reliably in well-trained mice (Fig. 2a). The probability of a CspkCS was higher in Purkinje cells that also had a reliable Cspk response to unexpected periocular airpuffs (Fig. 3a; R=0.72). In contrast, the probability of a CspkCS was near baseline in the majority of Purkinje cells recorded in naïve mice, even those that fired Cspk's reliably in response to unexpected presentation of the periocular airpuff (Fig. 3b). Thus, Purkinje cells in this region of cerebellar cortex fired Cspk's much more robustly to the unexpected US than to the CS in naïve mice (naïve, Fig. 3c), and equally well to both stimuli after training (trained, Fig. 3c). These results are consistent with a hypothesis in which the majority of climbing fibers are initially unresponsive to the CS, and gradually acquire a response during conditioning, as the CS becomes predictive of the instructive US. However, it is clear that the CS can activate some climbing fibers even in naïve mice (Fig. 3b,c). We will return to this observation below.
Figure 3. Climbing fiber responses in the CS period.
Probability of Cspk activity in the CS period relative to the Cspk probability after unexpected delivery of periocular airpuff, plotted for all individual Purkinje cells in well-trained mice (a), and in naïve mice (b). All Cspk responses have been normalized to the Cspk activity of each Purkinje cell during baseline. (c) The responsiveness of climbing fibers to the CS in well-trained and naïve mice is plotted as a ratio of Cspk response probabilities to the CS and to the unexpected presentation of the periocular airpuff stimulus. Data for individual Purkinje cells (dots) and median (red line) are shown.
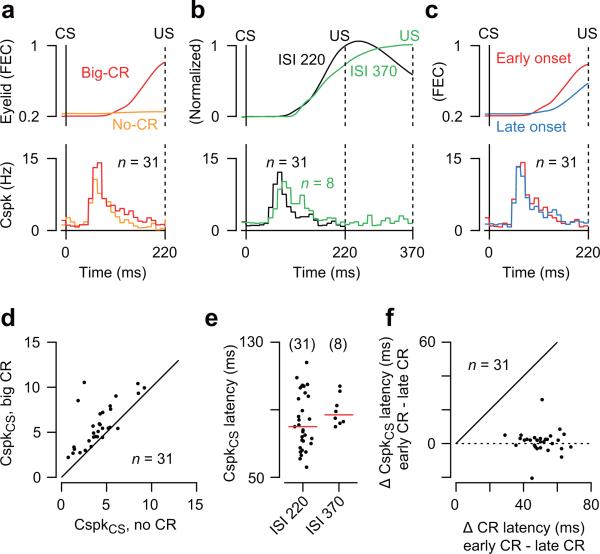
In addition to providing the teaching signals necessary for cerebellar learning, it has been suggested that climbing fibers also contribute to the ongoing control of movement timing35. We performed three analyses to assess if there is a relationship between CspkCS activity and the CR-related movement of the eyelid (Fig. 4). First, we found that in most Purkinje cells, the probability of a CspkCS was only marginally higher in trials with a big CR than in trials without a CR (Fig. 4a,d; Wilcoxon signed rank test, P<0.001). Second, we recorded an additional 8 Purkinje cells in a mouse trained with a 370 ms CS-US interstimulus interval (ISI, Fig. 4b,e), and found that the latency of the CspkCS fell within the range observed for Purkinje cells in mice trained with the 220 ms interval (Fig. 4e; Wilcoxon rank sum test, P=0.22), even though the temporal profile of the CR was different24,36 (compare eyelid traces in Fig. 4c). Third, we confirmed that the latency of the CspkCS did not depend on the latency of the CR and was essentially the same for trials in which the eyelid started closing early or late (Fig. 4c), i.e. the difference in CspkCS latency between trials with early CR's and late CR's was near zero for most of the Purkinje cells (Fig. 4f; Wilcoxon signed rank test, P=0.14). Thus, as expected for a TD prediction error signal20,21, climbing fibers fired at a fixed latency after the CS in well-trained mice regardless of the expected time of the US or the timing of the CR. Furthermore, we can rule out the possibility that CspkCS's are driven by CR-related movement of the eyelid because they were clearly present both in trials with and without a CR (Fig. 4a,d).
Figure 4. Complex spikes in CS period are not driven by eyelid movement.
(a) Comparison of population-averaged Cspk activity in trials with (big-CR) and without (no-CR) a conditioned eyelid response, and (d) the corresponding Cspk response in the CS period for all individual Purkinje cells. (b) Comparison of population-averaged Cspk activity in mice trained with a 220 ms ISI (ISI 220) and a mouse trained with a 370 ms ISI (ISI 370), and (e) the corresponding median Cspk latency in the CS period for all individual Purkinje cells (dots) and population median (red line). Eyelid traces in (b) have been normalized to provide direct comparison of movement time course. (c) Comparison of population-averaged Cspk activity in trials with early-onset and late-onset CR movements (f) The difference between the latency of the Cspk in trials with early-onset and late-onset CR's is plotted for all individual Purkinje cells.
Climbing fiber responses to novel stimuli
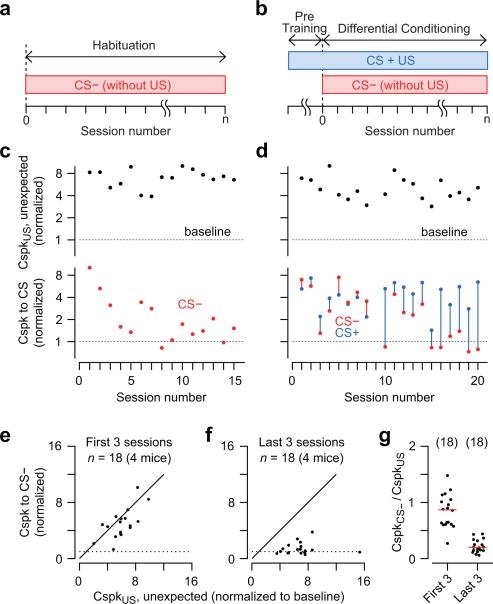
In recent computational studies based on the TD model, instructive signals incorporate a component related to the novelty of the CS37,38. We performed two additional experiments to assess if there is a relationship between climbing fiber activity and stimulus novelty (n= 4 mice): (1) Habituation training, in which naïve mice received daily sessions comprising 20-50 repeated presentations of a tone or LED stimulus (CS−) without the periocular puff (Fig. 5a), and (2) Differential conditioning, in which mice were first trained by pairing one stimulus (CS+) with the periocular puff, and after learning they received daily sessions in which the same CS+ continued to be paired with the airpuff in 75% of all trials, and a different CS− was presented without the airpuff in 25% of all trials (Fig. 5b). None of the mice made eyelid movements in response to the CS− in any of our experiments (Supplementary Fig. 1).
Figure 5. Climbing fiber responses to novel stimuli.
(a-b) Experimental design for mice in habituation (a), and differential conditioning (b) groups. (c,d) Probability of Cspk response to the unexpected airpuff (black) and to the CS+ (blue) and CS– (red) over the course of multiple sessions of habituation (c) or differential conditioning (d). Each session shows data for a different Purkinje cell. All Cspk responses have been normalized to the Cspk activity of each Purkinje cell during baseline. (e-f) Probability of Cspk response to the CS− in the first three (e), and last three (f) recording sessions. The data for tone and LED stimuli and for habituation and differential conditioning sessions were combined. (g) The responsiveness of climbing fibers to the CS− in the first three and the last three sessions is plotted as a ratio of the Cspk response probabilities to the CS− and to the unexpected presentation of the periocular airpuff stimulus. Data for individual Purkinje cells (dots) and median (red line) are shown.
Figure 5c,d summarizes the Cspk responses of Purkinje cells recorded in two representative mice during habituation and differential conditioning (one Purkinje cell per session). Purkinje cells recorded in the first few sessions of habituation (Fig. 5c) and differential conditioning (Fig. 5d) fired Cspk's reliably in response to the CS−. In contrast, Purkinje cells recorded in subsequent sessions were much less likely to fire Cspk's to the CS− than to the CS+ or to unexpected presentations of the periocular airpuff (Fig. 5c,d and Supplementary Fig. 1 c,f,j). Pooling the data for all the mice revealed a relationship between climbing fiber activity and the novelty of the CS− (Fig. 5e-g): the CS− triggered a Cspk with high probability when it was relatively novel, i.e. in the first 3 sessions of habituation or differential conditioning (Fig. 5e,g; Wilcoxon signed rank test, P<0.001), but not in the last 3 sessions when it had been presented many times and was more familiar (Fig. 5d,g; Wilcoxon signed rank test, P=0.09). The same result was obtained by comparing the Cspk responses to the CS– in the first 2 and the last 2 sessions, or in the first 5 and the last 5 sessions (Supplementary Fig. 2). This finding explains our previous observation that the CS can trigger climbing fiber-driven Cspk's in some Purkinje cells even in naïve mice (Fig. 3b,c). Indeed, the 8 responsive Purkinje cells in Figure 3b,c were all recorded in the first two days of experiments, during initial exposure to the CS.
DISCUSSSION
We have examined the activity of climbing fibers during eyeblink conditioning in mice, by recording the complex spikes (Cspks) of Purkinje cells in an eyelid region of cerebellar cortex. Our results confirm previous reports9-11 indicating that many climbing fibers in this region signal both the unexpected delivery and the unexpected omission of the periocular airpuff that serves as the instructive unconditioned stimulus (US). In addition, we have made the surprising discovery that under certain conditions the same climbing fibers can also fire in response to a conditioned stimulus (CS) from a different sensory modality, like a tone or an LED light. Below, we discuss the implications of these results for understanding the neural representation of instructive signals during cerebellar learning.
We have shown that climbing fibers encode a prediction error signal that satisfies three basic principles of temporal difference (TD) models20,21: (1) climbing fibers fire in response to unexpected presentation of the US, i.e. positive prediction error, (2) climbing fibers are inhibited if an expected US is omitted, i.e. negative prediction error, and (3) climbing fibers fire in response to the predictive CS in well-trained mice, i.e. after the relationship between the CS and the US has been learned. According to the TD model20,21, the response to the CS should develop gradually during conditioning, progressively increasing in strength as learning proceeds and the association between the CS and US is established. We were unable to test this hypothesis directly because cerebellar-dependent eyeblink conditioning in mice is a slow learning process24, and it is currently unfeasible to maintain good isolation and track the activity of the same climbing fiber over long periods of time. Until the right tools become available, we can at least note that in our experiments climbing fibers with similar responses to the unexpected presentation of the US fired much more reliably to the CS in well-trained mice than in naïve mice. This finding is consistent with the hypothesis that climbing fiber responses to the CS start out relatively weak and get progressively stronger during learning, as predicted by TD models20,21.
Our experiments also revealed that climbing fibers often fire in response to the initial presentations of the CS in naïve mice. Because this response habituates if the CS continues to be repeatedly presented without the US, we interpret it as a signal related to stimulus novelty. Novelty signals were not part of the original TD model20,21, but they have been recently incorporated into the general TD framework where they play a key role in triggering exploratory and orienting actions that help determine the meaning of stimuli with high potential importance37,38. Thus, in our working hypothesis climbing fibers have the capacity to multiplex: they generate novelty signals about stimuli whose meaning is yet to be determined, as well as prediction error signals about stimuli whose meaning is known or has been learned. An alternative hypothesis that can account for many of our observations is that climbing fibers encode a signal about stimulus salience, which is thought to be important for associative learning39. However, our results indicate that climbing fibers do more than provide saliency information. For example, we have shown that climbing fiber activity is suppressed below baseline when an expected US is omitted, which is a hallmark of a negative prediction error signal but it's difficult to explain with a pure saliency code.
Climbing fiber responses to visual and auditory CS's were obvious and highly prevalent in our experiments, but they are conspicuously absent in previous studies of eyeblink conditioning9-11. In fact, we could only find one preliminary report (S.A. Edgley, A. Mostofi & T. Holtzman, Society for Neuroscience, 2010) and three publications that briefly mention in passing what may have been occasional CS-related responses10,40,41. There are two important features of our experimental approach that could help explain this apparent discrepancy: First, we only examined the activity of climbing fibers that project to an identified eyeblink region of cerebellar cortex27,28 and that responded reliably to unexpected presentations of the periocular airpuff stimulus. This selection is likely to be important because the response properties of climbing fibers can vary widely depending on their exact location within the anatomical microzonal organization of the cerebellar cortex28. Second, we performed all our experiments in head-fixed mice that were free to locomote on top of a treadmill24, while others before us have often immobilized their subjects9-11 or used a decerebrate preparation to increase recording stability11. Whether any of these methodological considerations can account for the lack of CS-related climbing fiber responses in previous studies remains an open question. However, we note that climbing fiber responses to peripheral stimulation are strongly modulated by behavioral state42 and differ greatly between resting and walking conditions43,44.
Our experiments provide some clues about the origin of the multimodal responses of climbing fibers to somatosensory, visual and auditory stimuli. All our recordings targeted Purkinje cells in a small eyeblink region of cerebellar cortex that receives climbing fiber inputs from neurons located in three subdivisions of the IO28: the dorsal accessory olive (DOA), the medial accessory olive (MAO) and the medial dorsal olive (MDO). Neurons in these three subdivisions of the IO appear to be well positioned for multisensory processing because they receive converging inputs from proprioceptive and cutaneous receptors via ascending spino-olivary pathways and from multiple sensorimotor areas of the cortex via descending cerebro-olivary pathways3,45,46. In addition, some cells in the MAO respond to flashes of light and tapping sounds47, possibly via activation of inputs from the superior colliculus48. Importantly, we found that climbing fibers of well-trained mice fired in response to auditory or visual CS's even in trials in which the mouse failed to make a conditioned eyelid movement. This observation rules out the possibility that what may have looked like a sensory response to the CS was really a proprioceptive response or a reafference response driven by the neurons or muscles that control the eyelid. However, a non-eyelid motor source related to orienting or startle movements to the CS is still possible41.
Our results are disruptive and call for a major revision of existing theories about the function of climbing fibers during cerebellar learning tasks like eyeblink conditioning. In the prevailing view, climbing fibers carry a prediction error signal about the instructive US5-8,15,18,19. The anatomy supports such a view because it is well-known that the neurons of the inferior olive (IO) that send their climbing fiber axons to eyeblink regions of the cerebellar cortex receive a hardwired excitatory input from US-responsive areas in the trigeminal nucleus32,33. In addition, the same IO neurons also receive an inhibitory input from cells of the deep cerebellar nucleus that fire during the conditioned response (CR)10,30,34, thus establishing an anatomical basis for computing positive- and negative-prediction error signals about the US, i.e. IO cells will be excited by the US when it is not predicted, and they will be inhibited by CR-related activity if a predicted US is omitted. This anatomical arrangement of excitatory and inhibitory synaptic inputs in the IO is the foundation for current models of cerebellar learning based on the “comparator” hypothesis15,18,19. However, none of the existing theories take into account our surprising discovery that the same climbing fibers that carry error-related information about the instructive US also respond to visual and auditory CS's if these stimuli are novel or if they have been previously conditioned.
The pattern of climbing fiber responses that we have recorded during eyeblink conditioning bear a striking resemblance to the responses of many dopamine neurons during reinforcement learning tasks22,49,50. We already knew that in both cases these responses encode positive and negative prediction error signals about the instructive US, i.e. about the periocular airpuff used for eyeblink conditioning in the case of climbing fibers, and about the reward used for reinforcement learning in the case of dopamine neurons. In this study, we have demonstrated that the similarities go beyond coding of US-related instructive signals. Just like the climbing fibers recorded in our experiments, dopamine neurons in reinforcement learning tasks fire in response to auditory or visual stimuli if these stimuli are novel22,49, or if they predict that the US is about to be presented22,49,50. In theories of reinforcement learning based on the TD model, these dopamine responses are important for driving higher-order acquisition of approach behavior to potential reward23,37,38,49,50. Future experiments will help determine if the novelty and CS-related signals of climbing fibers may play a similar teaching role during cerebellar learning.
ONLINE METHODS
Animal preparation
All procedures were performed in C57BL/6 male mice approximately 11-14 weeks of age (Jackson Laboratories; n=10), following the guidelines of the National Institutes of Health and a protocol approved by the University of Pennsylvania Animal Care and Use Committee. Before starting our experiments, we implanted a head plate and a recording chamber above the right cerebellar cortex following surgical procedures described previously24.
Stimulus control and behavioral procedures
In all experiments, mice were head-fixed on top of a cylindrical treadmill and allowed to walk freely24. Facial and eyelid movements ipsilateral to the recording site were monitored with a high-speed monochrome camera (GE680, Allied Vision) under infrared illumination. Video frames (200 fps) were triggered by a Sys3 processor (RZ5, TDT) and stored with the MATLAB Video Acquisition Toolbox. A Sys3 processor (RZ5, TDT) was used to control the timing of stimuli during conditioning. The unconditioned stimulus (US) was an airpuff of nitrogen (40 psi, 10-20 ms) delivered via a 23-gauge flat-ended needle placed ~4 mm in front of the right cornea of the mouse. The conditioned stimuli (CS) were either a 500 ms blue light LED positioned 8 cm in front of the mouse's face, or a 500 ms tone of white noise delivered via a speaker (4-Ω magnetic speaker, FF1, TDT) positioned 20 cm in front of the mouse. The volume of the white noise was set before the first conditioning session to just below the threshold for eliciting short-latency startle movement of the eyelid.
After 3 days of acclimatization to the cylindrical treadmill, mice were submitted to one of three behavioral procedures: (i) Habituation: daily sessions comprising 20-50 unpaired presentations of the CS− (LED or tone without the US), (ii) Eyeblink conditioning: daily sessions comprising 21-179 paired trials in which the CS+ (LED or tone) was presented for 500 ms and the US was delivered 220 ms after the CS+ onset (220 ms interstimulus interval; ISI). For one mouse, the ISI was 370 ms, In ~25% of the trials the US was omitted, but this fraction was increased in a few recording sessions to collect enough data for analysis, (iii) Differential conditioning: mice first received 15-30 sessions of eyeblink conditioning comprising paired presentations of the CS+ (LED or tone) and the US (220 ms ISI; see above). After the mice learned to make conditioned eyelid responses to the CS+, they began receiving daily sessions comprising a mixture of trials in which the same CS+ continued to be paired with the US (approximately 75% of all trials), and CS− trials in which a different stimulus (e.g. when the CS+ was a tone, the CS− was an LED, and vice versa) was presented without the US (approximately 25% of all trials). In all recording sessions, the US was delivered unexpectedly to the ipsi- and contralateral corneas in occasional trials randomly interleaved during each session. The minimum interval between trials (intertrial interval; ITI) was 7-16 seconds, but trials could only start if the eyelid position was stable for at least 0.6 seconds.
Behavioral analysis
Movement of the eyelid was calculated frame by frame by counting the number of white pixels in a thresholded binary image of the eye and surrounding fur, according to a procedure described previously24. Eyelid closure was measured in units of “fraction eyelid closure” (FEC), ranging from 0 (fully open) to 1 (fully closed). Trials in which FEC did not reach at least 0.1 in the ISI period were defined as “no-CR” trials (Figs. 2d and 4a,d). For Figure 4b, the eyelid traces were normalized for each session so that the average eyelid position was 0 at the onset of the CS and 1 at the onset of the US. For Figure 4c, the trials of each recording session were divided into 3 equal-sized groups according to the onset latency of the CR: early-onset, middle-onset, and late-onset CRs. CR onset was defined for each trial in the session as the time when the low-pass filtered eyelid velocity trace reached a threshold of 0.002 FEC/ms. No-CR trials were excluded from this analysis. For Figure 4f, the median CR latencies for the early-onset and late-onset groups were subtracted from each other for each recording session and plotted on the x-axis.
Single unit recording
Extracellular recording of simple spikes and Cspk's in Purkinje cells was performed with 1-5 MΩ tungsten microelectrodes (75 μm of shaft diameter, FHC) or glass capillary electrodes (BF150-86-10, Sutter instrument) with 2-7 μm tip and 3-6 MΩ impedance (P-1000, Sutter instrument). The electrodes were controlled with a hydraulic microdrive (MMO-220A, Narishige) mounted on a three-axis manual micromanipulator (M325, WPI). The voltage signal was acquired at a 24,414 Hz sampling rate, and band-pass filtered between 0.1-10kHz using a digital processor (RZ5, TDT).
To target the eyeblink microzone located near the floor of the primary fissure27,28 (2000-2400 μm below the surface of dura matter), electrodes were directed along a 15-deg angled axis from posterior dorsal to anterior ventral, relative to the vertical plane. The eyeblink microzone could be identified by the presence of a large negative LFP signal (400-800 μV) in the molecular layer of the cerebellar cortex in response to ipsilateral periocular airpuffs (10-20 ms), and by the corresponding Cspk recorded in individual Purkinje cells. As reported previously for the eyeblink microzone in rabbits28, contralateral periocular airpuffs did not evoke Cspk's as reliably.
Electrophysiology analysis
We recorded a total of 151 Purkinje cells (29 from mice in habituation group, 51 from mice trained with the 220 ms ISI, 8 cells from a mouse trained with the 370 ms ISI, and 63 from mice in the differential conditioning group). Cspk's could be isolated in all 151 Purkinje cells, and simple spikes in 122 of them. Cspk's and simple spikes were sorted off-line using the threshold-crossing and template-matching algorithm of Spike2 software (Cambridge Electronic Design). For Cspk's we also examined the voltage waveform traces trial-by-trial and performed additional manual sorting, blind to task condition and behavior. Finally, to confirm that the Cspk's and the simple spikes originated from the same Purkinje cell, we checked that there was a 10-40 ms pause in simple spike activity after each Cspk4. Although climbing fibers typically fired just once in response to the CS+ and to the unexpected US, we observed occasional doublets of Cspk's in quick succession (raw record in Fig. 1a and raster in Fig. 1c). Similar doublets have been observed before51. Also note that after firing to the CS+, the refractory period of climbing fibers (approximately 100 ms)4 is too brief to affect how the climbing fiber will fire in response to the US (which is delivered 220-370 ms after the CS, after the refractory period is over).
A Cspk peristimulus time histogram (PSTH) was constructed for each climbing fiber and for each trial type (see example PSTH's for “unexpected”, “paired” and “omitted” trials types of a representative climbing fiber in Fig. 1f). Climbing fiber activity in the PSTH was expressed as frequency in Hz, by adding all the Cspk's fired in each time bin and dividing by the number of trials multiplied by the bin size (0.01 seconds). We took three measurements from each PSTH: (i) The spontaneous (baseline) frequency of the climbing fiber in the 500 ms time window before the trials started, (ii) the frequency of the climbing fiber in the 120 ms window after delivery (or omission) of the US, and (iii) the frequency of the climbing fiber in the 100 ms time window after delivery of the tone CS, or 50 ms after delivery of the LED CS to take into account longer delays related to visual processing. The Cspk response to the CS or the unexpected US was considered to be statistically significant if it was at least two standard deviations higher than the baseline frequency. Of the 51 cells in mice trained with a 220 ms ISI, 34 had a statistically significant Cspk response to the unexpected presentation of the US (analyzed in Fig. 2 and 3c), and 31 had a statistically significant Cspk response to the CS (analyzed in Fig. 4). In addition, statistically significant responses to the unexpected US were found in 29 cells from mice in the naïve/habituation group (analyzed in Fig. 3b and 5e-g, note that in Fig. 5e-g only a fraction of the 29 cells were recorded in the first 3 or the last 3 sessions), 8 cells from a mouse trained with the 370 ms ISI (analyzed in Fig. 4b,e), and 45 cells from mice in the differential conditioning group (analyzed in Fig. 5e-g, note that only a fraction of these 45 cells were recorded in the first 3 or the last 3 sessions). The frequency of each climbing fiber was normalized by its baseline frequency for Figures 3a-b, 4d, and 5e-f.
Statistical analysis
Mean and within-session variability (s.d.) are displayed for eyelid traces in Figure 1d-e. Average of all sessions is displayed for eyelid traces and Cspk histograms in Figure 2a and 4a-c. All statistical analyses were performed using the Statistics toolbox in MATLAB. We applied the Bonferroni correction for multiple comparisons In Figure 2d, to test the difference of Cspk modulation (firing frequency above baseline) from zero. We used nonparametric statistical tests without assuming normality, except for data in Figure 3a, where we used Pearson's correlation coefficient. All tests were two-sided. No randomization was used, but mice were assigned to specific experimental group without bias and no animals were excluded. The experimenter was blind to task condition and behavior during spike sorting and further analyses. A Supplementary Method Checklist is available.
Supplementary Material
ACKNOWLEDGMENTS
Supported by a grant to JM from the National Institutes of Health (NIH R01 MH093727), and a grant to SO from Japan Society for the Promotion of Science (Grant-in-Aid for JSPS Fellows) and from the Uehara Memorial Foundation. We thank Keiko Ohmae for technical support and Shane Heiney for help with analysis and neurophysiological approach.
Footnotes
AUTHOR CONTRIBUTIONS
SO and JM designed the research plan. SO performed all experiments and analyzed data. JM and SO prepared figures and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare no competing financial interests.
REFERENCES
- 1.Eccles JC, Llinás R, Sasaki K. The excitatory synaptic action of climbing fibres on the Purkinje cells of the cerebellum. J Physiol. 1966;182:268–96. doi: 10.1113/jphysiol.1966.sp007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Najafi F, Medina JF. Beyond “all-or-nothing” climbing fibers: graded representation of teaching signals in Purkinje cells. Front Neural Circuits. 2013;7:115. doi: 10.3389/fncir.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Zeeuw CI, et al. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- 4.Simpson JI, Wylie DR, De Zeeuw CI. On climbing fiber signals and their consequence(s). Behav Brain Sci. 1996;19:384–398. [Google Scholar]
- 5.Medina JF, Nores WL, Ohyama T, Mauk MD. Mechanisms of cerebellar learning suggested by eyelid conditioning. Curr Opin Neurobiol. 2000;10:717–724. doi: 10.1016/s0959-4388(00)00154-9. [DOI] [PubMed] [Google Scholar]
- 6.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–55. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- 7.Freeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learn Mem. 2011;18:666–77. doi: 10.1101/lm.2023011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson RF, Thompson JK, Kim JJ, Krupa DJ, Shinkman PG. The Nature of Reinforcement in Cerebellar Learning. Neurobiol Learn Mem. 1998;70:150–176. doi: 10.1006/nlme.1998.3845. [DOI] [PubMed] [Google Scholar]
- 9.Sears LL, Steinmetz JE. Dorsal accessory inferior olive activity diminishes during acquisition of the rabbit classically conditioned eyelid response. Brain Res. 1991;545:114–122. doi: 10.1016/0006-8993(91)91276-7. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson DA, Freeman JH., Jr. Addition of inhibition in the olivocerebellar system and the ontogeny of a motor memory. Nat Neurosci. 2003;6:532–7. doi: 10.1038/nn1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen A, Jirenhed DA, Hesslow G. Simple and complex spike firing patterns in Purkinje cells during classical conditioning. Cerebellum. 2008;7:563–6. doi: 10.1007/s12311-008-0068-2. [DOI] [PubMed] [Google Scholar]
- 12.Mauk MD, Steinmetz JE, Thompson RF. Classical conditioning using stimulation of the inferior olive as the unconditioned stimulus. Proc Natl Acad Sci USA. 1986;83:5349–5353. doi: 10.1073/pnas.83.14.5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito M. Error detection and representation in the olivo-cerebellar system. Front Neural Circuits. 2013;7:1. doi: 10.3389/fncir.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu Rev Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- 15.Dean P, Porrill J. Decorrelation learning in the cerebellum: computational analysis and experimental questions. Prog Brain Res. 2014;210:157–92. doi: 10.1016/B978-0-444-63356-9.00007-8. [DOI] [PubMed] [Google Scholar]
- 16.Rescorla RA, Wagner AR. In: Classical Conditioning: II. Current Research and Theory. Black AH, Prokasy WF, editors. Appleton-Century-Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- 17.Widrow B, Hoff ME. Adaptive switching circuits. IRE WESCON Convention Rec. 1960:96–104. [Google Scholar]
- 18.Mauk MD, Donegan NH. A model of Pavlovian eyelid conditioning based on the synaptic organization of the cerebellum. Learn Mem. 1997;3:130–158. doi: 10.1101/lm.4.1.130. [DOI] [PubMed] [Google Scholar]
- 19.Medina JF, Mauk MD. Computer simulation of cerebellar information processing. Nat Neurosci. 2000;3(Suppl):1205–1211. doi: 10.1038/81486. [DOI] [PubMed] [Google Scholar]
- 20.Sutton RS, Barto AG. In: Learning and computational neuroscience: foundations of adaptive networks. Gabriel M, Moore JW, editors. MIT Press; Cambridge, MA: 1990. pp. 497–538. [Google Scholar]
- 21.Sutton RS. Learning to predict by the methods of temporal differences. Machine Learning. 1988;3:9–44. [Google Scholar]
- 22.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 23.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 24.Heiney SA, Wohl MP, Chettih SN, Ruffolo LI, Medina JF. Cerebellar-dependent expression of motor learning during eyeblink conditioning in head-fixed mice. J Neurosci. 2014;34:14845–53. doi: 10.1523/JNEUROSCI.2820-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y, Lei C, Feng H, Sui JF. The neural circuitry and molecular mechanisms underlying delay and trace eyeblink conditioning in mice. Behav Brain Res. 2014;278C:307–314. doi: 10.1016/j.bbr.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Boele HJ, Koekkoek SK, De Zeeuw CI. Cerebellar and extracerebellar involvement in mouse eyeblink conditioning: the ACDC model. Front Cell Neurosci. 2010;3:19. doi: 10.3389/neuro.03.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heiney SA, Kim J, Augustine GJ, Medina JF. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J Neurosci. 2014;34:2321–30. doi: 10.1523/JNEUROSCI.4547-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mostofi A, Holtzman T, Grout AS, Yeo CH, Edgley SA. Electrophysiological localization of eyeblink-related microzones in rabbit cerebellar cortex. J Neurosci. 2010;30:8920–34. doi: 10.1523/JNEUROSCI.6117-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thach WT., Jr. Somatosensory receptive fields of single units in cat cerebellar cortex. J Neurophysiol. 1967;30:675–696. doi: 10.1152/jn.1967.30.4.675. [DOI] [PubMed] [Google Scholar]
- 30.Hesslow G, Ivarsson M. Inhibition of the inferior olive during conditioned responses in the decerebrate ferret. Exp Brain Res. 1996;110:36–46. doi: 10.1007/BF00241372. [DOI] [PubMed] [Google Scholar]
- 31.Andersson G, Garwicz M, Hesslow G. Evidence for a GABA-mediated cerebellar inhibition of the inferior olive in the cat. Exp Brain Res. 1988;72:450–456. doi: 10.1007/BF00250590. [DOI] [PubMed] [Google Scholar]
- 32.Van Ham JJ, Yeo CH. Somatosensory Trigeminal Projections to the Inferior Olive, Cerebellum and other Precerebellar Nuclei in Rabbits. Eur J Neurosci. 1992;4:302–317. doi: 10.1111/j.1460-9568.1992.tb00878.x. [DOI] [PubMed] [Google Scholar]
- 33.Swenson RS, Castro AJ. The afferent connections of the inferior olivary complex in rats. An anterograde study using autoradiographic and axonal degeneration techniques. Neuroscience. 1983;8:259–75. doi: 10.1016/0306-4522(83)90064-7. [DOI] [PubMed] [Google Scholar]
- 34.Svensson P, Bengtsson F, Hesslow G. Cerebellar inhibition of inferior olivary transmission in the decerebrate ferret. Exp Brain Res. 2006;168:241–53. doi: 10.1007/s00221-005-0086-y. [DOI] [PubMed] [Google Scholar]
- 35.Llinás RR. Cerebellar motor learning versus cerebellar motor timing: the climbing fibre story. J Physiol. 2011;589:3423–32. doi: 10.1113/jphysiol.2011.207464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chettih SN, McDougle SD, Ruffolo LI, Medina JF. Adaptive timing of motor output in the mouse: the role of movement oscillations in eyelid conditioning. Front Integr Neurosci. 2011;5:72. doi: 10.3389/fnint.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kakade S, Dayan P. Dopamine: generalization and bonuses. Neural Netw. 2002;15:549–59. doi: 10.1016/s0893-6080(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 38.Laurent PA. The emergence of saliency and novelty responses from Reinforcement Learning principles. Neural Netw. 2008;21:1493–9. doi: 10.1016/j.neunet.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–52. [PubMed] [Google Scholar]
- 40.Berthier NE, Moore JW. Cerebellar Purkinje cell activity related to the classically conditioned nictitating membrane response. Exp Brain Res. 1986;63:341–350. doi: 10.1007/BF00236851. [DOI] [PubMed] [Google Scholar]
- 41.Nicholson DA, Freeman JH., Jr. Developmental changes in eye-blink conditioning and neuronal activity in the inferior olive. J Neurosci. 2000;20:8218–26. doi: 10.1523/JNEUROSCI.20-21-08218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bengtsson F, Jorntell H. Ketamine and xylazine depress sensory-evoked parallel fiber and climbing fiber responses. J Neurophysiol. 2007;98:1697–705. doi: 10.1152/jn.00057.2007. [DOI] [PubMed] [Google Scholar]
- 43.Ozden I, Dombeck DA, Hoogland TM, Tank DW, Wang SS-H. Widespread state-dependent shifts in cerebellar activity in locomoting mice. PLoS One. 2012;7:e42650. doi: 10.1371/journal.pone.0042650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apps R. Gating of climbing fibre input to cerebellar cortical zones. Prog Brain Res. 2000;124:201–11. doi: 10.1016/S0079-6123(00)24017-X. [DOI] [PubMed] [Google Scholar]
- 45.Jorntell H, Garwicz M, Ekerot CF. Relation between cutaneous receptive fields and muscle afferent input to climbing fibres projecting to the cerebellar C3 zone in the cat. Eur J Neurosci. 1996;8:1769–79. doi: 10.1111/j.1460-9568.1996.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 46.Pardoe J, Edgley SA, Drew T, Apps R. Changes in excitability of ascending and descending inputs to cerebellar climbing fibers during locomotion. J Neurosci. 2004;24:2656–66. doi: 10.1523/JNEUROSCI.1659-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gellman R, Houk JC, Gibson AR. Somatosensory properties of the inferior olive of the cat. J Comp Neurol. 1983;215:228–43. doi: 10.1002/cne.902150210. [DOI] [PubMed] [Google Scholar]
- 48.Saint-Cyr JA, Courville J. Descending projections to the inferior olive from the mesencephalon and superior colliculus in the cat. An autoradiographic study. Exp Brain Res. 1982;45:333–48. doi: 10.1007/BF01208593. [DOI] [PubMed] [Google Scholar]
- 49.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daw ND, Doya K. The computational neurobiology of learning and reward. Curr Opin Neurobiol. 2006;16:199–204. doi: 10.1016/j.conb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Eccles JC, Sabah NH, Schmidt RF, Taborikova H. Cutaneous mechanoreceptors influencing impulse discharges in cerebellar cortex. 3. In Purkyne cells by climbing fiber input. Exp Brain Res. 1972;15:484–97. doi: 10.1007/BF00236404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.