Abstract
Background
The present study investigated the effect of dihydromyricetin (DHM) on lipopolysaccharide (LPS)-induced acute kidney injury in a rat model.
Material/Methods
Kidney injury was induced in male Sprague-Dawley rats by injection of LPS through the tail vein. The rats were treated with 5 μg/kg body weight DHM within 12 h of the LPS administration. The urine of the rats was collected over a period of 48 h for determination of calcium and creatinine concentrations. Blood urea nitrogen in the serum was analyzed using a BC-2800 Vet Animal Auto Biochemistry Analyzer. On day 3 after treatment, the rats were sacrificed to extract the kidneys.
Results
Treatment of the endotoxemia rats with DHM caused a significant (P<0.05) decrease in the level of kidney injury molecule-1 and blood urea nitrogen. DHM treatment significantly (P<0.05) decreased the level of calcium in the kidney tissues compared to those of the untreated endotoxemia rats. The level of malonaldehyde (MDA) in the kidney tissues was significantly reduced in the endotoxemia rats by DHM treatment. The results from immunohistochemistry reveled a significant decrease in the expression of osteopontin (OPN) and CD44 levels. The endotoxemia rats showed significantly higher levels of TUNEL-positive stained nuclei compared to the normal controls. However, treatment of the endotoxemia rats with DHM resulted in a significant decrease in the population of TUNEL-positive cells.
Conclusions
DHM may be a promising candidate for the treatment of acute kidney injury.
MeSH Keywords: Abortion, Therapeutic; Creatinine; Technetium Tc 99m Aggregated Albumin
Background
The kidneys are the organs most commonly affected by sepsis and aggregation of calcium oxalate, forming kidney stones [1]. It has been observed that more than half of patients with sepsis develop acute kidney injury and around 75% of acute kidney injury patients die [2,3]. There are reports that generation of reactive oxygen species activates nicotinamide adenine dinucleotide phosphate (NADPH), resulting in kidney injury [4]. This is confirmed by the findings that expression of a kidney injury marker, kidney injury molecular-1 (KIM-1), is inhibited by treatment with an NADPH inhibitor [4]. Aggregation of calcium in the kidney tissues induces formation of stones and kidney injury [5]. The development of kidney injury has been found to be linked with the formation of stones, which require dialysis to avoid immediate problems [6].
Flavonoids isolated from natural sources exhibit a wide range of pharmacological properties. Dihydromyricetin (Figure 1) is a phytochemical isolated from a plant, Ampelopsis grossedentata, and is a flavonoid molecule [7]. Pharmacological analysis of dihydromyricetin revealed its promising antithrombotic, anti-inflammatory, and anti-oxidant activities [7–9]. Further studies demonstrated its potential as an anti-membrane lipid peroxidation agent [10,11]. In the present study we investigated the effect of dihydromyricetin on lipopolysaccharide-induced acute kidney injury in a rat model. The results revealed that dihydromyricetin prevents acute kidney injury in rats by inhibiting the expression of osteopontin and CD44.
Figure 1.
Chemical structure of dihydromyricetin.
Material and Methods
Animals
Fifteen male Sprague-Dawley rats weighing approximately 200 g were obtained from the Laboratory Animal Center of Sun Yat-sen University (Shanghai, China). The animals were maintained in the animal facility house under a 12-h light/dark cycle and had free access to food and drinking water. The experimental procedures were performed according to the criteria of National Institute of Health for the care and use of laboratory animals at Sun Yat-sen University (Guangzhou, China). Approval for the present study was obtained from the Laboratory Animal Care Committee of Sun Yat-sen University (Guangzhou, China) under reference number SYU072/2015.
Chemicals and reagents
Dihydromyricetin was purchased from Sigma-Aldrich (St. Louis, MO, USA) and a 100-mM stock solution was prepared in dimethylsulfoxide (DMSO). The Oxidative Stress kit was purchased from Roche (Basel, Switzerland) and the TUNEL kit was from EarthOx Life Science (Millbrae, CA, USA). Rabbit monoclonal primary and the secondary antibodies against osteopontin were purchased from BD Biosciences (Franklin Lakes, NJ, USA). The mouse monoclonal antibody for CD44 was obtained from Cell Signaling Technology, Inc. (Boston, MA, USA).
Study design and procedure
The 15 animals were assigned randomly into 3 groups of 5 each: normal control, untreated control, and DHM group. Rats in the DHM group received 5 μg/kg DHM 12 h before the injection of LPS. Rats in the untreated and DHM groups were injected with 5 mg/kg body weight LPS (Sigma-Aldrich, St. Louis, MO, USA) through the tail vein.
Biochemical analysis
The urine of the rats was collected over a 48-h period for biochemical analysis. The concentration of calcium and creatinine in the urine samples and that of blood urea nitrogen in the serum of the rats was determined using a BC-2800 Vet Animal Auto Biochemistry Analyzer (Hitachi 7600-020/7170A; Hitachi High-Technologies Corp., Tokyo, Japan). The animals were sacrificed on day 3 after treatment using 3% pentobarbital sodium anesthesia. We extracted the left kidney from each rat and fixed it in 10% formalin for the analysis of pathological alterations. The right kidney was subjected to paraffin embedding before analysis for the presence of kidney injury molecule-1 (KIM-1).
Enzyme-linked immunosorbent assay (ELISA)
For the quantification of KIM-1 in the rats, the anti-KIM-1 antibody was applied to the inner surface of the ELISA plates (USCN Life Science Inc., Wuhan, China). The plates were subjected to incubation with a solution of CaCO3 overnight under anhydrous conditions. Bovine serum albumin was added to each of the wells and incubated for 1 h. The wells were washed with PBS and Tween-20 (PBST) and then treated with the rat urine and serum. After 5-h incubation, the plates were washed with PBS and incubated again with antibody against KIM-1 overnight. After PBS washing, the plates were treated with horseradish peroxidase-conjugated secondary antibody followed by analysis using a microplate reader.
Analysis of calcium aggregation
The paraffin-embedded right kidney was cut into thin 2-μ sections, boiled in xylene, and stained using a von Kossa kit followed by eosin (Beyotime Institute of Biotechnology, Haimen, China) staining. Aggregation of the calcium was analyzed by microscopic examination (Nikon Eclipse 50i; Shanghai Henghao Instruments Co. Ltd., Shanghai, China).
Analysis of OPN and CD44 expression
The right kidney sections were subjected to high-pressure treatment, followed by addition of hydrogen peroxide and then washing with PBS. Incubation of the sections was performed with primary antibodies against OPN and CD44 overnight. The sections were washed with PBS 3 times, followed by incubation with secondary antibodies for 1 h at room temperature. The number of the TUNEL-active cells was calculated by Image-Pro Plus software version 6.0 after sections were counter-stained with methyl green.
Statistical analysis
The data analysis was performed using SPSS software, version 12.0 (SPSS, Inc., Chicago, IL, USA). One-way analysis of variance was used for determination of differences between various groups and comparisons were made by the Tukey post hoc test. Differences were considered statistically significant at a value of P<0.05.
Results
DHM decreases kidney injury molecule-1 level in endotoxemia rats
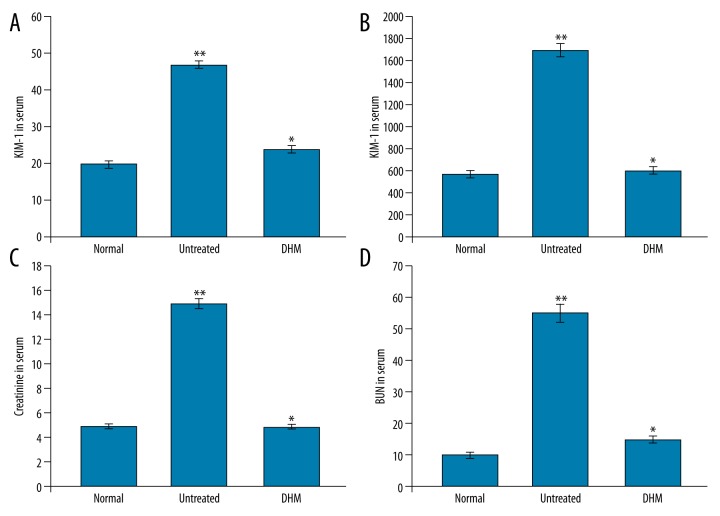
Analysis of the kidney injury molecule-1 level in endotoxemia rats revealed a significantly higher level compared to the normal control group. Treatment of the endotoxemia rats with DHM caused a significant (P<0.05) decrease in the level of kidney injury molecule-1 in the urine (Figure 2A, 1B). In endotoxemia rats, the blood urea nitrogen level was higher than in the normal control group. However, treatment of the endotoxemia rats with DHM exhibited an inhibitory effect on the level of blood urea nitrogen (Figure 2C, 2D). Treatment with DHM exhibited no effect on the serum creatinine content in the endotoxemia rats.
Figure 2.
(A) Effect of DHM on the concentration of KIM-1 in the serum of rats. (B) Effect of DHM on the concentration of KIM-1 in the urine of rats. (C, D) Effect of DHM on the concentration of creatinine and blood urea nitrogen in the serum of rats. The rats in the normal control group were left as such, while those in the untreated received LPS and normal saline and those in and treatment group were given DHM and LPS. * P<0.05 vs. normal control and ** P<0.05 vs. untreated group. The data presented are the mean ± standard deviation.
Effect of DHM on the level of calcium in endotoxemia rats
Examination of the level of calcium in the kidney tissues of endotoxemia rats showed significantly (P<0.05) higher concentrations compared to the normal control group. In the DHM-treated group, the level of calcium was significantly (P<0.05) lower than in the endotoxemia rats (Figure 3).
Figure 3.
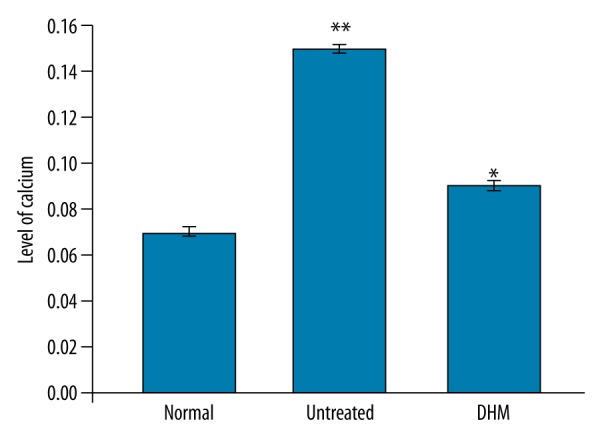
Effect of DHM on calcium aggregation in the kidneys. * P<0.05, compared with the normal control and ** P<0.05, vs. untreated control group.
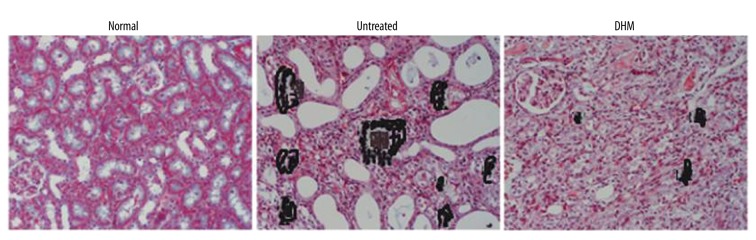
DHM inhibits aggregation of calcium in endotoxemia rats
Analysis of the formation of calcium oxalate crystals in endotoxemia rats by Nikon Eclipse 50i microscopy showed marked accumulation compared to the normal control group. However, DHM treatment inhibited accumulation of calcium oxalate crystals in the kidneys of endotoxemia rats (Figure 4).
Figure 4.
Effect of DHM on the concentration of calcium in the kidney tissues in rats. DHM significantly reduced the concentration of calcium in the kidney tissues.
Effect of DHM on the Ca/creatinine level
In the endotoxemia rats, the concentration of Ca/creatinine in the urine was significantly higher compared to the normal control group. Treatment of the endotoxemia rats with DHM led to a significant (P<0.05) decrease in the level of Ca/creatinine in the urine (Figure 5).
Figure 5.
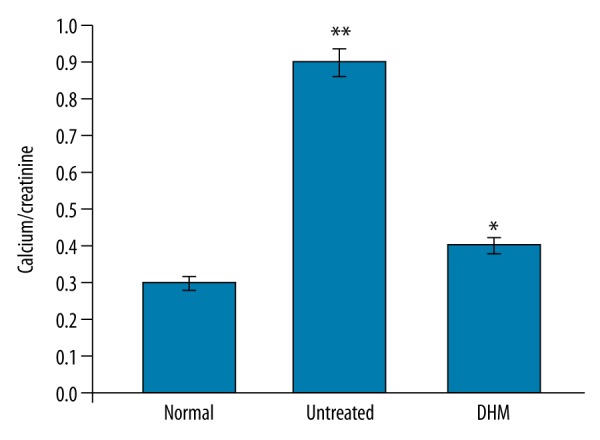
Effect of DHM on the Ca/creatinine ratio in the urine samples of the rats. * P<0.05, vs. normal control and ** P<0.05, vs. untreated control groups.
Effect of DHM on oxidative stress in endotoxemia rats
In the endotoxemia rats, the level of malonaldehyde (MDA) was significantly higher compared to the normal control group. DHM treatment significantly reduced the level of MDA in the kidney tissues of endotoxemia rats (Figure 6).
Figure 6.
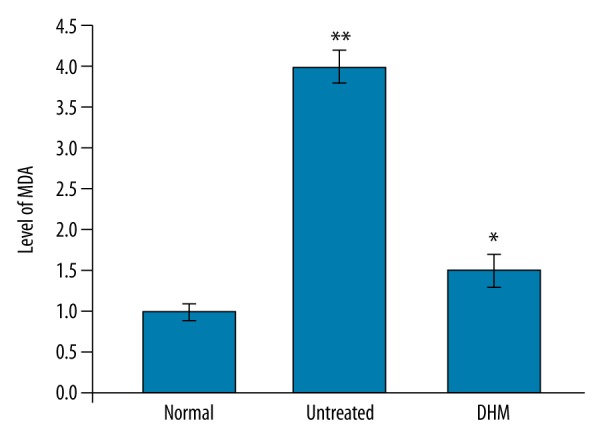
DHM treatment reduced the level of MDA in the right kidney tissues of the rats. * P<0.05, vs. normal control group and ** P<0.05, vs. untreated groups.
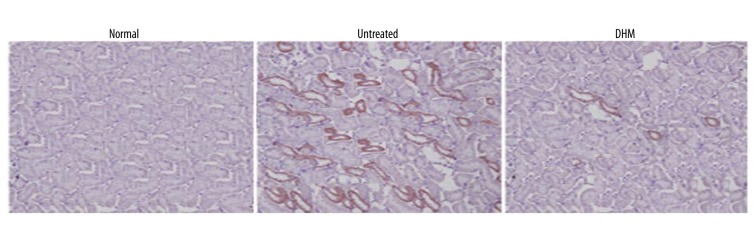
Effect of DHM on the expression of OPN and CD44 in endotoxemia rats
The level of OPN and CD44 in the kidney tissues of endotoxemia rats was significantly higher compared to the normal control group. However, treatment of the endotoxemia rats with DHM significantly decreased the expression of OPN and CD44 (Figure 7).
Figure 7.
Inhibition of OPN and CD44 expression by DHM in the kidney tissues of the rats. Immunostaining revealed a significant decrease in OPN and CD44 after treatment with DHM.
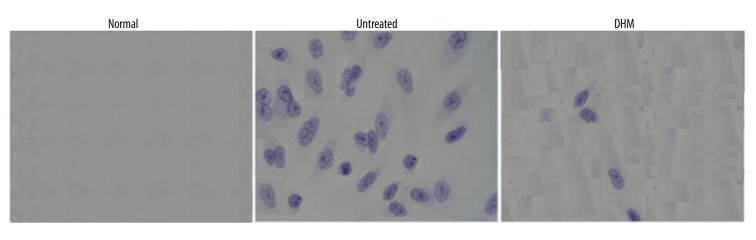
Effect of DHM on TUNEL-positive cells
The endotoxemia rats showed significantly higher levels of TUNEL-positive-stained nuclei compared to the normal controls. However, treatment of the endotoxemia rats with DHM resulted in a significant decrease in the population of TUNEL-positive cells (Figure 8).
Figure 8.
Effect of DHM on the TUNEL-positive cells in the kidney tissues of the rats. The images of the cells were captured at magnification ×400.
Discussion
The present study demonstrates the effect of SAHA treatment of kidney injury in endotoxemia rats. The results revealed a significant decrease in the BUN and Ca/creatinine ratio in the serum and urine of endotoxemia rats treated with SAHA.
The level of kidney injury molecule-1 is higher and is a characteristic feature of kidney injury in the patients [12]. Presence of kidney injury molecule-1 in the kidney tissues is considered to be a diagnostic feature of kidney injury [13]. In the present study, the level of kidney injury molecule-1 was found to be significantly higher in endotoxemia rats. SAHA treatment of endotoxemia rats inhibited the level of kidney injury molecule-1. Higher concentrations of calcium and its subsequent aggregation in the kidney tissues leads to formation of kidney stones [14]. The present study demonstrated that the level of calcium and its aggregation in the kidney tissues of endotoxemia rats was significantly higher. However, SAHA treatment reduced the concentration of calcium, as well as its deposition, in kidney tissues. Reactive oxygen species play an important role in the induction of oxidative stress during kidney injury [15]. It has also been observed that kidney injury induces oxidative stress, leading to aggregation of calcium and formation of crystals [16]. Treatment of mice with antioxidants has been shown to inhibit calcium crystallization [17). In the present study, SAHA treatment reduced the level of MDA in the kidney tissues of endotoxemia rats, which were higher compared to the normal control group. One of the glycoproteins, OPN, is present at higher levels during inflammatory processes [18]. Higher levels of OPN are found in kidney tissues of kidney injury patients and in people with kidney stones [19]. In the present study, endotoxemia rats showed significantly higher levels of OPN compared to the normal rats. SAHA treatment of the endotoxemia rats caused a significant decrease in the level of OPN. Another trans-membrane glycoprotein, CD44, is also present during kidney injury, but not in normal renal tissues [20]. CD44 plays an important role in the activity of OPN [20]. Our results showed increased expression of CD44 in the endotoxemia rats, which was, however, decreased after treatment with SAHA.
Generation of reactive oxygen species leads to induction of oxidative stress and increased concentrations of calcium, and, finally, to kidney injury [21]. The results from the present study showed increased proportions of apoptotic cells. However, SAHA treatment significantly reduced the proportions of apoptotic cells in endotoxemia rats.
Conclusions
SAHA treatment of endotoxemia rats prevented kidney injury by reducing the aggregation of calcium and inhibiting ROS generation. Thus, SAHA may be of therapeutic importance in the treatment of acute kidney injury.
Footnotes
Source of support: Departmental sources
References
- 1.Bagshaw SM, George C, Bellomo R. ANZICS Database Management Committee: Early acute kidney injury and sepsis: A multicentre evaluation. Crit Care. 2008;12:R47. doi: 10.1186/cc6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–69. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 3.Rajapakse S, Rodrigo C, Rajapakse A, et al. Renal replacement therapy in sepsis-induced acute renal failure. Saudi J kidney Dis Transpl. 2009;20:553–59. [PubMed] [Google Scholar]
- 4.Huang HS, Ma MC, Chen J. Low-vitamin E diet exacerbates calcium oxalate crystal formation via enhanced oxidative stress in rat hyperoxaluric kidney. Am J Physiol Renal Physiol. 2009;296:F34–45. doi: 10.1152/ajprenal.90309.2008. [DOI] [PubMed] [Google Scholar]
- 5.Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain and the risk of kidney stones. JAMA. 2005;293:455–62. doi: 10.1001/jama.293.4.455. [DOI] [PubMed] [Google Scholar]
- 6.Shoag J, Halpern J, Goldfarb DS, et al. Risk of chronic and end-stage kidney disease in people with nephrolithiasis. J Urol. 2014;192:1440–45. doi: 10.1016/j.juro.2014.05.117. [DOI] [PubMed] [Google Scholar]
- 7.Chen YQ, Ni DJ, Cheng Q. Study on the hypolipidemic effect of flavones and dihydromyricetin From Tengcha. J Tea Sci. 2007;3:221–25. [Google Scholar]
- 8.Zhong ZX, Zhou GF, Chen XF, Qin JP. Experimental study on the protective effect of dihydromyricetin from Guangxi Ampelopsis grossepentata on liver. Chin J Tradit Med Sci Technol. 2002;9:155–56. [Google Scholar]
- 9.Xu JJ, Yao MJ, Wu MC. Study on biological efficacy of dihydromyricetin. Food Sci. 2008;29:622–25. [Google Scholar]
- 10.Shen Y, Lindemeyer AK, Gonzalez C. Dihydromyricetin as a novel anti-alcohol intoxication medication. J Neurosci. 2012;32:390–401. doi: 10.1523/JNEUROSCI.4639-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He GX, Yang WL, Pei G. Studies on the effect of dihydromyricetin on antilipid-peroxidation. Zhongguo Zhong Yao Za Zhi. 2003;28:1188–90. [in Chinese] [PubMed] [Google Scholar]
- 12.Bonventre JV. Kidney injury molecule-1 (KIM-1): An urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:1–4. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 13.Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–69. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oda E. Medical check-up center, tachikawa medical center: Overweight and high-sensitivity C-reactive protein are weakly associated with kidney stone formation in Japanese men. Int J Urol. 2014;21:1005–11. doi: 10.1111/iju.12499. [DOI] [PubMed] [Google Scholar]
- 15.Moriyama MT, Suga K, Miyazawa K, et al. Inhibitions of urinary oxidative stress and renal calcium level by an extract of Quercus salicinaBlume/Quercus stenophyllaMakino in a rat calcium oxalate urolithiasis model. Int J Urol. 2009;16:397–401. doi: 10.1111/j.1442-2042.2009.02268.x. [DOI] [PubMed] [Google Scholar]
- 16.Hirose M, Yasui T, Okada A. Renal tubular epithelial cell injury and oxidative stress induce calcium oxalate crystal formation in mouse kidney. Int J Urol. 2010;17:83–92. doi: 10.1111/j.1442-2042.2009.02410.x. [DOI] [PubMed] [Google Scholar]
- 17.Khan SR, Joshi S, Wang W, Peck AB. Regulation of macromolecular modulators of urinary formation by reactive oxygen species: Transcriptional study in an animal model of hyperoxaluria. Am J Physiol Renal Physiol. 2014;306:F1285–95. doi: 10.1152/ajprenal.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie Y, Sakatsume M, Nishi S, et al. Expression, roles, receptors and regulation of osteopontin in the kidney. Kidney Int. 2001;60:1645–57. doi: 10.1046/j.1523-1755.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 19.Okada A, Nomura S, Higashibata Y, et al. Successful formation of calcium oxalate crystal deposition in mouse kidney by intraabdominal glyoxylate injection. Urol Res. 2007;35:89–99. doi: 10.1007/s00240-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 20.Asselman M, Verhulst A, De Broe ME, Verkoelen CF. Calcium oxalate crystal adherence to hyaluronan-, osteopontin- and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J Am Soc Nephrol. 2003;14:3155–66. doi: 10.1097/01.asn.0000099380.18995.f7. [DOI] [PubMed] [Google Scholar]
- 21.Khaskhali MH, Byer KJ, Khan SR. The effect of calcium on calcium oxalate monohydrate crystal-induced renal epithelial injury. Urol Res. 2009;37:1–6. doi: 10.1007/s00240-008-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]