Summary
Background
A disappearing or persistent solid pulmonary nodule is a neglected clinical entity that still poses serious interpretative issues to date. Traditional knowledge deriving from previous reports suggests particular features, such as smooth edges or regular shape, to be significantly associated with benignity. A large number of benign nodules are reported among smokers in lung cancer screening programmes.
The aim of this single-center retrospective study was to correlate specific imaging features to verify if traditional knowledge as well as more recent acquisitions regarding benign SPNs can be considered reliable in a current case series of nodules collected in a non-smoker cohort of patients.
Material/Methods
Fifty-three solid SPNs proven as non-growing during follow-up imaging were analyzed with regard to their imaging features at thin-section CT, their predicted malignancy risk according to three major risk assessment models, minimum density analysis and contrast enhanced-CT in the relative subgroups of nodules which underwent such tests.
Results
Eleven nodules disappeared during follow-up, 29 showed volume loss and 16 had a VDT of 1121 days or higher. There were 48 nodules located peripherally (85.71%). Evaluation of the enhancement after contrast media (n=29) showed mean enhancement ±SD of 25.72±35.03 HU, median of 18 HU, ranging from 0 to 190 HU. Minimum density assessment (n=30) showed mean minimum HU ±SD of −28.27±47.86 HU, median of −25 HU, ranging from −144 to 68 HU. Mean malignancy risk ±SD was 15.05±26.69% for the BIMC model, 17.22±19.00% for the Mayo Clinic model and 19.07±33.16% for the Gurney’s model.
Conclusions
Our analysis suggests caution in using traditional knowledge when dealing with current small solid peripheral indeterminate SPNs and highlights how quantitative growth at follow-up should be the cornerstone of characterization.
MeSH Keywords: Lung Neoplasms, Multidetector Computed Tomography, Solitary Pulmonary Nodule
Background
Solitary pulmonary nodule (SPN) characterization is one of the most challenging tasks in thoracic radiology because of the high clinical significance associated with a potential disease. Many factors contribute to confusion, such as a large number of different benign and malignant lesions and the variety of their radiological presentations. For such reasons, an accurate discrimination between benign and malignant forms often requires an interdisciplinary evaluation to pursue an effective management strategy. In this regard a few prediction models have been proposed in literature, which focused on specific features that could suggest malignancy [1–3]. Computed tomography (CT) plays a pivotal role in the workup of an SPN because it can provide basic features such as size, location and density. The advent of submillimetric CT scans and dedicated scanning protocols allowed for deeper understanding of fine nodule properties as detailed information on morphology, spiculation and density measures became available.
In the typical clinical setting, a patient presenting with a new diagnosis of SPN will be invited to undergo additional imaging or functional studies, have the lesion biopsied or undergo surgery on the basis of the perceived risk of malignancy. SPNs that are considered at minimal risk because of their CT appearance will be followed by long-term serial CT scans while high-risk SPNs will get a definitive diagnosis by means of needle or surgical biopsy. To some extent this justifies the vast literature about typical and atypical CT characteristics of malignant SPNs and the limited literature focusing on stable or disappearing SPNs.
The aim of this study was to analyze SPNs which were proven stable or disappeared during follow-up imaging with regard to their imaging features at thin-section CT, their predicted malignancy risk according to three major computational models, minimum density analysis (MinHU) and contrast-enhanced computed tomography (CE-CT) in the relative subgroups of nodules which underwent such tests.
Material and Methods
All CT thoracic scans of patients referred to our center for SPN characterization between March 2003 and April 2013 were jointly reviewed by 2 expert radiologists in thoracic CT (GAS, MM) in this single-centre retrospective study.
Inclusion criteria were as follows:
the presence of one or up to six solid SPNs following the definition “… a rounded or irregular opacity, well or poorly defined, measuring up to 3 cm in diameter” as stated in the Fleischner glossary [4];
an available thin-section multi-detector CT (MDCT) scan encompassing the lungs (0.6–2 mm);
a definitive diagnosis of benign nature by means of serial volume assessments, and in particular once proven stable for at least 2 years or completely disappeared.
Exclusion criteria were as follows:
the presence of promptly recognizable calcifications at usual reporting magnification (magnification ratio between 1.5× and 2×) on medical grade monitors;
status of smoker or former smoker;
the presence of a bioptic or surgical diagnosis.
The nodules were imaged by a 64-row MDCT (Lightspeed, GE Healthcare) or a 256-row MDCT system (Brilliance iCT, Philips Healthcare).
Age, gender, nodule diameter, nodule volume, nodule morphology and affected lobe were collected for each patient in an electronic spreadsheet.
Nodule diameter was computed manually on a professional workstation (CareStream PACS v11.1, Carestream Health Inc.; 2008) by drawing a linear distance on multiplanar images passing through the maximum nodule diameter.
Nodule volume was calculated semi-automatically on a professional workstation (Portal Workstation, Philips Healthcare), occasionally refining by freehand minor segmentation errors.
Nodule morphology was defined based on lung nodule classification suggested by Zwirewich et al. [5] according to the contours of the lesions, dividing them into four classes of increasing edge complexity. In particular SPNs were classified into class 1: sharp and smooth edges; class 2: moderately smooth edges; class 3: undulated borders or minimal spiculation; class 4: gross marginal spiculation.
Location within the lungs was assessed by reviewing the scans on Multi-Planar Reconstruction (MPR) images on a professional workstation (Carestream PACS, Carestream Health, Inc. 2008). Nodules were defined as peripheral if located in the outer third of the lung parenchyma on axial CT images as suggested in literature [6].
Evaluation of the enhancement after contrast media administration was performed following the directions suggested in literature [7,8].
Minimum density assessment was performed on 30 nodules in a semi-automatic fashion, by drawing multiple regions of interests (ROIs) within the solid component of SPN and letting the system detect the minimum density value.
FDG-PET scans were performed in the same institution within 3 months from the first CT exam.
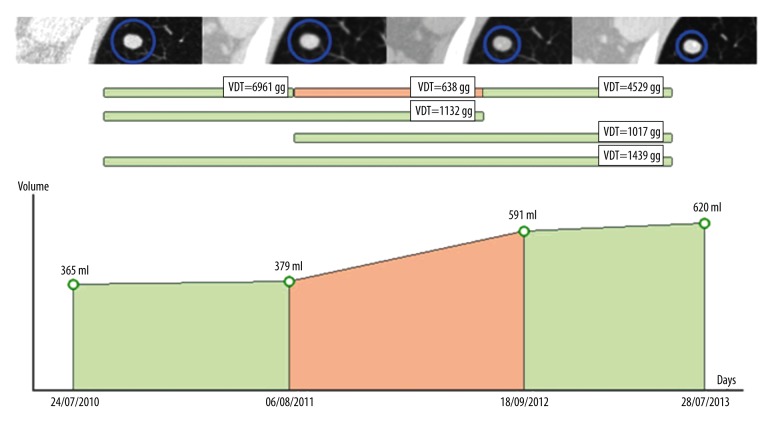
Volume Doubling Time was estimated in a serial fashion, as illustrated in Figure 1, by means of a semi-automated software (BIMC Software, Italy; http://www.simoneperandini.com/bimc/, 2015).
Figure 1.
Serial VDT assessment for the evaluation of nodule growth. Upper row: axial CT images of the nodule under examination at corresponding time points. Middle row: estimated VDTs at different time spans. Graph at the bottom: Volume/time graph for the selected lesion.
Correlation between morphology and peripheral location or nodule diameter was evaluated by means of Pearson’s product-moment correlation coefficient.
The risk of malignancy was assessed by means of three prediction models from literature, in particular using the BIMC [9], Mayo Clinic [10] and Gurney’s [11] models. A nodule was considered at risk of misclassification when the predicted value of malignancy was above 20%. A nodule was considered misclassified when the predicted risk value was above 10%.
Collected data were analyzed with a commercial statistical software (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2013).
Results
The overall study population included 27 males and 23 females. Mean patient age ± standard deviation (SD) was 66±13 years, ranging from 33 to 84 years.
Six patients, 4 males and 2 females, had two SPNs. A total of 56 nodules were analyzed, of which 11 completely disappeared during follow-up, 29 showed volume loss and 16 had a VDT of 900 days or higher.
As concerns those 16 nodules, mean VDT ±SD was 2269.63±976.55 days, median VDT was 2059.50 days, ranging from 1121 to 3721 days.
Mean nodule diameter at discovery ±SD was 10.95±5.35 mm. Median nodule diameter was 10 mm, ranging from 4 to 28 mm.
There were 48 nodules located peripherally (85.71%), while there were 8 located centrally (14.29%). Nine (81.82%) out of 11 nodules which disappeared during follow-up and 39 (86.67%) out of 45 stable nodules were located in the outer third of the lung.
Evaluation of the enhancement after contrast media administration was performed in 29 nodules. Mean enhancement ±SD was 25.72±35.03 HU, median was 18 HU, ranging from 0 to 190 HU.
Minimum density assessment was performed on 30 nodules. Mean minimum HU ±SD was −28.27±47.86 HU, median was −25 HU, ranging from −144 to 68 HU.
An FDG-PET scan was performed in 9 cases, of which 4 yielded negative results while 5 were positive, with a mean ±SD SUV value of 1.76±0.82, median SUV value of 1.50, ranging from 1.5 to 2.5. Nodules that underwent the FDG-PET scan had a mean diameter ±SD of 11.77±3.59 mm.
According to the model proposed by Zwirewich, 34 nodules (60.71%) were classified as class 1, 16 (28.57%) as class 2, 6 (10.71%) as class 3 and none as class 4.
Upper lobe location was observed in 32 nodules (57.14%), middle lobe location in 6 nodules (10.71%), and lower lobe location in 18 nodules (32.14%).
A history of previous extrapulmonary malignancy within 10 years before the first exam was present in 8 patients (14.29%).
Mean malignancy risk ±SD was 15.0±26.69% for the BIMC model, 17.22±19.00% for the Mayo Clinic model and 19.07±33.16% for the Gurney’s model.
There were 11 (19.64%), 14 (25%) and 12 (21.43%) nodules, respectively, at risk of misclassification on the sole basis of prediction algorithm result.
There were 13 (23.21%), 28 (50%) and 14 (25%) misclassified SPNs, respectively.
Distribution of risk values are compared in Figure 2.
Figure 2.
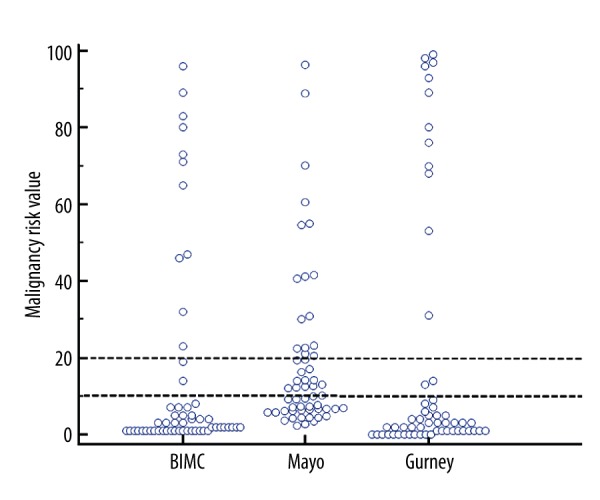
Dot-plot summarizing the distribution of predicted risk values according to the assessed calculators. Two horizontal dotted line highlights the threshold values of 10% and 20%.
No significant correlation was found between peripheral location and morphology or between nodule diameter and morphology.
Discussion
The disappearing or stable pulmonary nodule is a neglected clinical entity that still poses serious interpretative issues to date. There has been extensive research on the possibility to obtain early diagnosis of growing or suspicious-looking nodules by means of cross-sectional imaging, and especially by CT. Peculiar features were described and correlated with specific lesions such as fat density areas in hamartomas, coupled artery and vein in arteriovenous fistulas, “comet tail” appearance in peripheral round atelectasis, “pop-corn”-like calcifications in hamartomas, spiculation in aggressive nodules. However, the mean nodule diameter gets smaller as the use of chest CT scans gets increasingly common even in asymptomatic patients, posing additional issues. Some fine details such as edges or density are more difficult to assess in small nodules, which by contrast get increasingly detected.
Two major assumptions have been stated in literature with regard to small and benign nodules. The former affirms that small nodules are likely to represent benign lesions [12], the latter how benign lesions up to 10 mm are more likely to show a polygonal shape and subpleural location [13]. However, the findings of the current study do not support the previous research. Our case series showed no significant correlation between nodule size and morphology, and no significant correlation between nodule position and its morphology.
This discrepancy could be partly attributed to the smaller mean diameter of the nodules included in this study, since previous studies were carried out when thin-slice CT was not as available as it is today, and when nodules as little as 5 mm would have been easily undetected.
This study confirms how most of the stable or disappearing SPNs are peripheral. Unluckily there is no clear consensus on what is to be considered peripheral in the pulmonary parenchyma, a factor that could limit the comparability of results across studies.
In this study the nodules were defined peripheral if located within the outer third of the lung parenchyma on axial CT images.
With regard to morphology our results are partly in line with those of previous studies. There was a clear majority of stable or disappearing nodules in class 1 category.
One unanticipated finding was that roughly up to 40% of nodules were classified as class 2 or class 3, an unexpected finding that suggests caution when considering nodule edges as a primary concern in risk assessment.
The current study also found that contrast enhancement and minHu analysis were not of significant help in characterizing the selected SPNs. These results are likely to be also related to the small size of the nodules.
These results however need to be interpreted with caution because of the small number of cases which underwent these analyses.
The authors still believe that these two particular studies should be carried out when dealing with complex lesions and that they could be helpful in selected cases.
As concerns the SPN location within the lungs, it was found that stable or disappearing nodules were more common in the upper lobes, a characteristic that they share with malignant nodules [14], posing an additional issue in discrimination between the two.
The VDT threshold adopted as an inclusion criterion in this study, namely 900 days, was found to be sufficiently cautious. As a matter of fact, all nodules had a minimum VDT above 1100 days.
The cut-off value of 900 days was based on the extensive institutional experience on pulmonary nodule analysis, and was already proved to be effective in literature [9]. Optimal VDT thresholds for in-the-wild SPN characterization are however still debated. Probably the most authoritative work is the work of Revel et al. of 2006 [15], in which they described a threshold of 500 days to have a 98% negative predictive value. We personally differ from this opinion, since our experience goes well along with the published work of Soardi [9] in which more than one third of the malignant solid solitary nodules which were VDT-tested had a VDT of 400 days or higher.
One question in this research was the role of risk prediction models in characterization of this particular subset of lesions. SPN risk assessment by means of mathematical models is a promising opportunity, which has gained interest especially in the characterization of lung cancer screening applications. Models dedicated to non-screening scenarios are also available in literature. The three considered prediction models were sufficiently accurate to classify most of the nodules in the lower tail of cancer risk. However, when used as the sole tool for risk assessment, prediction models could have been misleading in up to 50% of cases. In particular the Mayo clinic model performed poorly compared to competitors, despite the fact that recent reports highlighted how this model tends to underestimate SPN malignancy risk [16,17]. One possible implication could be the adoption of Bayesian classifiers like the Gurney or BIMC models as a first-line tool to easily discriminate a large quota of small benign nodules. Further longitudinal studies regarding the role of prediction models in this scenario would be worthwhile and interesting.
The main limitation of this study is the modest number of nodules considered. Unluckily it is uncommon to have a complete and definitive CT follow-up of incidental nodules that are considered at a negligible risk of malignancy. Much higher numbers can be found on lung cancer screening programmes at the cost of the remarkable, if not the most significant, selection bias represented by a strong smoking habit. Our case series reflects a more “in the wild” scenario that we believe to be more faithful to the average working routine.
Conclusions
In conclusion, our case series analysis is consistent with data from literature concerning the location within the peripheral lung parenchyma. However, it differs to some extent as regards nodule morphology, showing that up to 40% of benign nodules may present with class 2 or class 3 morphologies. This study also presents an unprecedented analysis of three major risk prediction models in a cohort of small non-growing non-screening nodules.
Overall the findings suggest caution in using traditional knowledge when dealing with small solid indeterminate SPNs in non-smokers. Single imaging features can be treacherous and unreliable. Growth at follow-up CT, specifically Volume Doubling Time, should be recommended as the cornerstone of characterization.
References
- 1.Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: When is it lung cancer? ACCP Evidence-Based Clinical Practice Guidelines (2nd edition) Chest. 2007;132:108S–30S. doi: 10.1378/chest.07-1353. [DOI] [PubMed] [Google Scholar]
- 2.Gurney JW, Lyddon DM, McKay JA. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part II. Application. Radiology. 1993;186(2):415–22. doi: 10.1148/radiology.186.2.8421744. [DOI] [PubMed] [Google Scholar]
- 3.Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–55. [PubMed] [Google Scholar]
- 4.Hansell DM, Bankier AA, MacMahon H, et al. Fleischner society: glossary of terms for thoracic imaging. Radiology. 2008;246:697–722. doi: 10.1148/radiol.2462070712. [DOI] [PubMed] [Google Scholar]
- 5.Zwirewich CV, Vedal S, Miller RR, Müller NL. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology. 1991;179:469–76. doi: 10.1148/radiology.179.2.2014294. [DOI] [PubMed] [Google Scholar]
- 6.Lee IJ, Gamsu G, Czum J, et al. Lung nodule detection on chest CT: evaluation of a computer-aided detection (CAD) system. Korean J Radiol. 2005;6(2):89–93. doi: 10.3348/kjr.2005.6.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swensen SJ, Brown LR, Colby TV, Weaver AL. Pulmonary nodules: CT evaluation of enhancement with iodinated contrast material. Radiology. 1995;194:393–98. doi: 10.1148/radiology.194.2.7824716. [DOI] [PubMed] [Google Scholar]
- 8.Swensen SJ, Brown LR, Colby TV, et al. Lung nodule enhancement at CT: prospective findings. Radiology. 1996;201:447–55. doi: 10.1148/radiology.201.2.8888239. [DOI] [PubMed] [Google Scholar]
- 9.Soardi GA, Perandini S, Motton M, Montemezzi S. Assessing probability of malignancy in solid solitary pulmonary nodules with a new Bayesian calculator: improving diagnostic accuracy by means of expanded and updated features. Eur Radiol. 2015;25(1):155–62. doi: 10.1007/s00330-014-3396-2. [DOI] [PubMed] [Google Scholar]
- 10.Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med. 1997;157:849–55. [PubMed] [Google Scholar]
- 11.Gurney JW, Lyddon DM, McKay JA. Determining the likelihood of malignancy in solitary pulmonary nodules with Bayesian analysis. Part II. Application. Radiology. 1993;186(2):415–22. doi: 10.1148/radiology.186.2.8421744. [DOI] [PubMed] [Google Scholar]
- 12.Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP Evidence-Based Clinical Practice Guidelines (2nd edition) Chest. 2007;132:94S–107S. doi: 10.1378/chest.07-1352. [DOI] [PubMed] [Google Scholar]
- 13.Takashima S, Sone S, Li F, et al. Small solitary pulmonary nodules (< or =1 cm) detected at population-based CT screening for lung cancer: Reliable high-resolution CT features of benign lesions. Am J Roentgenol. 2003;180(4):955–64. doi: 10.2214/ajr.180.4.1800955. [DOI] [PubMed] [Google Scholar]
- 14.Horeweg N, van der Aalst CM, Thunnissen E, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;187(8):848–54. doi: 10.1164/rccm.201209-1651OC. [DOI] [PubMed] [Google Scholar]
- 15.Revel MP, Merlin A, Peyrard S, et al. Software volumetric evaluation of doubling times for differentiating benign versus malignant pulmonary nodules. Am J Roentgenol. 2006;187(1):135–42. doi: 10.2214/AJR.05.1228. [DOI] [PubMed] [Google Scholar]
- 16.Perandini S, Soardi GA, Motton M, et al. Limited value of logistic regression analysis in solid solitary pulmonary nodules characterization: a single-center experience on 288 consecutive cases. J Surg Oncol. 2014;110(7):883–87. doi: 10.1002/jso.23730. [DOI] [PubMed] [Google Scholar]
- 17.Isbell JM, Deppen S, Putnam JB, Jr, et al. Existing general population models inaccurately predict lung cancer risk in patients referred for surgical evaluation. Ann Thorac Surg. 2011;91(1):227–33. doi: 10.1016/j.athoracsur.2010.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]