Abstract
Objective
To identify the impact of delivery device of inhaled corticosteroids and long-acting β2-agonist (ICS/LABA) on asthma medication compliance, and investigate other factors associated with compliance.
Materials and methods
We conducted a retrospective and multicenter study based on a review of medical registries of asthmatic patients treated with ICS/LABA combinations (n=2,213) whose medical devices were either dry powder inhalers (DPIs, such as Accuhaler®, Turbuhaler®, and NEXThaler®) or pressurized metered-dose inhalers (pMDI). Medication compliance included persistence outcomes through 18 months and medication possession ratios. Data on potential confounders of treatment compliance such as asthma exacerbations, comorbidities, demographic characteristics, and health care resource utilization were also explored.
Results
The probability of asthma medication compliance in case of DPIs was lower compared to pMDIs, which suggests that inhaler devices influence inhalation therapies. There were additional confounding factors that were considered as explanatory variables of compliance. A worse measure of airflow obstruction (forced expiration volume in 1 second), comorbidities and general practitioner (GP) consultations more than once per month decreased the probability of compliance. Within comorbidities, alcoholism was positively associated with compliance. Patients of 29–39, 40–50, and 51–61 age groups or suffering from more than two exacerbations during the study period were more likely to comply with their medication regime. The effects of DPIs toward compliance varied with the different DPIs. For instance, Accuhaler® had a greater negative effect on compliance compared to Turbuhaler® and Nexthaler® in cases of patients who suffered exacerbations. We found that GP consultations reduced the probability of medication compliance for patients treated with formoterol/budesonide combination. For retired patients, visiting the GP increased the probability of medication compliance.
Conclusion
We concluded that inhaler devices influence patients’ compliance for long-term asthma medication. The impact of Accuhaler®, Turbuhaler®, and NEXThaler® on medication compliance was negative. We also identified some confounders of medication compliance such as patient’s age, severity of asthma, comorbidities, and health care costs.
Keywords: adherence, inhaler devices, medication possession ratio, dry powder inhalers, pressurized metered-dose inhalers, persistence
Introduction
Asthma is characterized by the inflammation of respiratory airway, hypersensitivity of airway path, and the variable airflow limitation during short periods of time. A controller medication is daily medication that is used to prevent or improve asthma symptoms in patients who experience them frequently. The medical decision to use a controller medication for a patient with asthma is based on the frequency and type of daytime or nighttime symptoms, frequency of medical visits for asthma, frequency of requiring asthma rescue medications, frequency of oral steroid use, impact of asthma symptoms on daily life, and breathing tests for asthma. Inhalation therapy presents advantages in respect to oral or parenteral treatment such as easy access of the medication to the bronchoalveolar system and lower dosage.1 However, these attributes are diminished due to a poor compliance associated with deficient inhaler technique.2–5 Although inhaled corticosteroids and long-acting β2-agonist (ICS/LABA) fixed-dose combinations have shown to relieve asthma symptoms, similarly,6,7 there is increasing research that claims that inhaler technique might affect compliance and hence, efficacy of pharmacological treatment.8,9
Several studies reported a critical problem of patients’ compliance in chronic airway conditions.2,10,11 Noncompliance with drug treatment continues to be a significant barrier to asthma control, which contributes to costly exacerbations and worsening of the disease over time.12 Medication compliance implies that the patient follows doctor’s orders and patient’s medication-taking behavior corresponds with doctor’s recommendations with respect to timing, dosage, and frequency.13–15 Over the past few years, researchers from several disciplines raised that poor compliance is an important problem for the national health care system around the world and should be treated from a multidisciplinary perspective.9,12,13
The aim of this study was to examine medication compliance in asthmatic patients, focusing on the associations between compliance with asthma medication and inhaler devices such as dry powder inhaler (DPI) or pressurized metered-dose inhaler (pMDI). Moreover, some other confounders of compliance were analyzed, which were beyond clinical aspects.
Materials and methods
Study sample
We conducted a retrospective and multicenter study based on review of medical registries of asthmatic patients treated with ICS/LABA combinations, whose medical devices were either DPIs (such as Accuhaler®, Turbuhaler®, or NEXThaler®) or pMDI. The study population included patients attending primary care centers, whose population was mostly urban, with a low-to-medium socioeconomic level.
The study sample was comprised of all asthmatic patients who started taking ICS/LABA delivered by a DPI or pMDI between 2007 and 2014. Moreover, patients needed to fulfill the following characteristics: 1) aged 18 and over; 2) time of diagnosis >3 years; 3) patients were required to have registries with regular monitoring for 18 months (ie, those who were part of the long-term prescription program to obtain drugs with a confirmed record of the daily dosage, time interval, and duration of each treatment). We excluded patients who were transferred outside the area and patients permanently institutionalized.
Data source
Patient compliance for each medication was tracked for 1.5 years using persistence and adherence information. We obtained information on asthma treatment with ICS/LABA combination accordingly, with the Anatomical Therapeutic Chemical Classification System. Moreover, we linked each ICS/LABA combination to its medical device: Accuhaler®, Turbuhaler®, NEXThaler®, or pMDI. Data on diagnoses for a given individual were linked to medication associated with asthma using pharmacy administrative database and clinical visit data from electronic patient records. These databases are managed by Badalona Serveis Assitencials, which provides services to ten primary care centers, one hospital, and one socio-health center. Ethics approval for the database to be used for research was granted by the Hospital Germans Trías i Pujol Ethics Committee. No consent form from patients or caregivers was required for this study because data included in this database went through an anonymization process for patients’ information, guaranteed by the Badalona Serveis Assistencials S.A. organization that had access to the data.
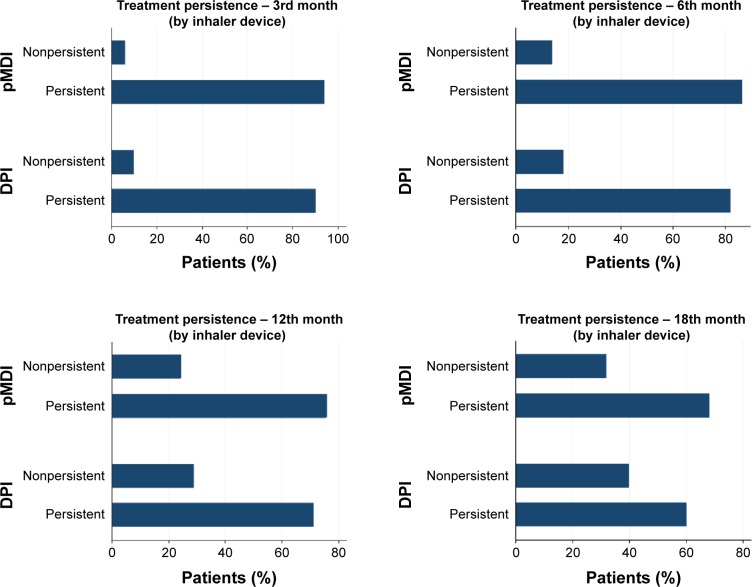
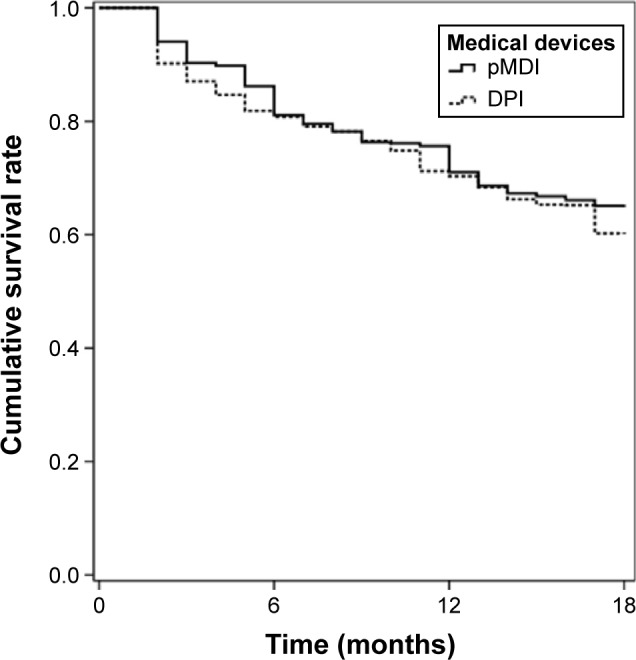
Data on persistence of each patient at the 3rd, 6th, 12th, and 18th month were obtained (Figures 1 and 2) as well as information on medication possession, by patient’s refill count, and duration, by the number of days the patient should be consuming the medication. Other potential explanatory variables of compliance were considered after literature review and most of them were available in electronic patient reports. Number of exacerbations per patient during the study period was collected and more specifically data on number of mild/moderate exacerbations and acute events. These acute exacerbations implied hospitalizations patients could not control the exacerbation at home. We also gathered data on additional medication. However, we did not analyze compliance toward this medication since we were interested on compliance patterns in patients attempting to take ICS/LABA fixed dose combinations chronically.
Figure 1.
Percentage of persistent patients at the 3rd, 6th, 12th, and 18th month.
Abbreviations: DPI, dry powder inhaler; pMDI, pressurized metered dose inhaler.
Figure 2.
Medication compliance.
Abbreviations: DPI, dry powder inhaler; pMDI, pressurized metered dose inhaler.
Clinical data on comorbidities were included.16 It was indicated whether the patient suffered simultaneous condition such as hypertension, diabetes, dyslipidemia, depression, and dementia. We also decided to indicate severe events including organ failures, ischemic heart disease, stroke, cerebrovascular disease, and neoplasm. Behavior attitudes that could result in higher risk of for instance obesity, smoking habit, and alcoholism were also included as comorbidities.17
Moreover, we considered the number of visits in primary care centers for general practitioner (GP) consultation, hospital emergency visits, and we had access to pharmacy registries that gather the gross amount of pharmaceutical expenditure. Amounts were adapted into our model by obtaining patient’s average cost per month, and because Spanish patients have low co-payment levels, this proxy might be more accurate than the gross amount. Sick leave days were also gathered.18 Demographics of this sample were age, sex, and whether the patient was retired or not.19,20
Calculation of patient’s medication compliance
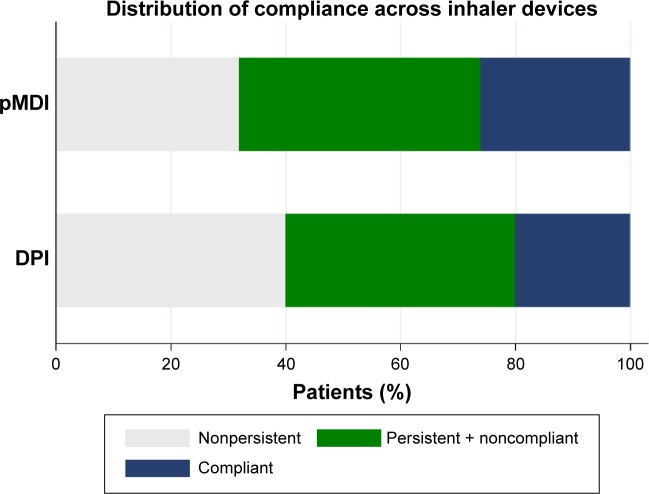
To calculate compliance, we utilized the medication possession ratio (MPR), which is calculated by dividing the number of days supplied for a given medication by the number of days in the study, and persistence data.21 First, we identified persistent patients as those who used their medication for 18 months. These patients were analyzed to obtain compliance by using the MPR. Therefore, within these persistent patients we applied a cut-point of 95% to the MPR, which tends to be overestimated,9,22 thus we also increased the limit to where patients were considered actively compliant. In the end, we had an ordered categorical variable, which showed to be very useful to identify persistence and compliance patterns, since patients first need to be persistent with their treatment and then they need to comply with their prescriptions.9,13 This variable reflects patients who are not either persistent or compliant, patients who are persistent but noncompliant, and patients who are persistent and showed compliance to their medication (Figure 3). Finally, to check the robustness of our findings, we decided to use the most common cut-point that is 80% to the MPR.14,23
Figure 3.
Percentage of patients’ compliance for each inhaler device (n=2,213).
Abbreviations: DPI, dry powder inhaler; pMDI, pressurized metered dose inhaler.
Analysis
To describe the distribution of patient’s compliance within each medical device (DPI or pMDI), we tabulated sample characteristics for patients using either a DPI or a pMDI. Univariate associations between drug compliance, medical device, and several confounders such as age, comorbidities, exacerbations, severity of asthma, concomitant and rescue medication, and drug cost were analyzed.
In order to determine a specification that provides a better fit of the data for explaining compliance outcomes, interaction terms between the proxy of inhaler technique and the confounders of interest were also included. The impact of exacerbations relative to each inhaler device toward adherence was captured by computing the probability of having an exacerbation for each device of interest. The effect of severity of asthma for each type of DPIs was also examined.
With reference to health care utilization, visits to the GP were categorized in order to explore the association of having, on average: none or one visit per month, one to two visits per month, and more than three visits per month. Further, patients’ visits to their GP were computed per active substance. The effect of GP consultation for retired patients was also obtained. We considered a variable for comorbidity status, which was categorized as having 1, 2, 3, or more than 4 conditions.
Additionally, having an acute event (ischemic heart disease, organ failures, stroke, or cerebrovascular diseases) was added into the regression. Moreover, to consider the potential heterogeneity sources across age groups, the continuous variable age was split into seven groups. Finally, the effect of cost toward adherence for each age group was introduced in order to approximate the effect of socioeconomic status.20
Results
Clinical and demographic characteristics
Characteristics of underlying populations of DPI users (73.2%) and pMDI users (26.8%) are presented in Table 1. Asthmatic patients (n=2,213) were predominantly female (61.46%), working-age (59.69%), and age distribution was very similar among the sample except for the oldest age group (5.60%) in which patients were 83–96 years old. Time since asthma was diagnosed (in years) was similar in both groups, however, its distribution varies widely and it goes from 3 years to 76.13 years. The most frequent comorbidity observed in this sample is dyslipidemia (37.7%), followed by obesity (27.9%), and diabetes (24.9%), which were very similar in both groups. Nearly half of the patients had moderate persistent asthma. It was interesting to note that 79.35% of the sample did not experience any exacerbation event. Across clusters, DPI users had higher rate of exacerbations compared to pMDI users (P<0.001) and severe exacerbations were more prevalent for DPI users (P<0.001).
Table 1.
Characteristics of COPD patients according to type of inhaler device used
| Characteristics | pMDI n=593 (26.8%) |
DPI n=1,620 (73.2%) |
Total N=2,213 |
P-value |
|---|---|---|---|---|
| Female, % | 60.9% | 61.7% | 61.5% | 0.735 |
| Age mean (SD), years | 52.3 (19.6) | 53.2 (18.8) | 52.9 (18.5) | 0.326 |
| 18–28 | 15.85% | 7.78% | 9.94% | NA |
| 29–39 | 15.17% | 18.14% | 17.35% | NA |
| 40–50 | 14.84% | 21.19% | 20.02% | NA |
| 51–61 | 17.03% | 19.19% | 18.62% | NA |
| 62–72 | 17.53% | 16.29% | 16.63% | NA |
| 73–83 | 16.02% | 10.31% | 11.84% | NA |
| 83–96 | 3.54% | 6.36% | 5.60% | NA |
| Retirement status | 40.5% | 40.6% | 40.6% | 0.966 |
| Time since diagnosis (SD), years | 13.0 (5.3) | 13.0 (4.2) | 13.0 (4.5) | 0.994 |
| Asthma classification | ||||
| Mild intermittent | 19.7% | 19.4% | 19.5% | NA |
| Mild persistent | 31.0% | 30.5% | 30.6% | NA |
| Moderate persistent | 46.5% | 47.3% | 47.1% | NA |
| Severe persistent | 2.7% | 2.7% | 2.7% | 0.990 |
| FEV1 (SD) | 76.6 (9.9) | 79.1 (9.9) | 79.2 (9.9) | 0.256 |
| Allergy | 43.84% | 49.13% | 47.72% | 0.892 |
| ICS/LABA combination | ||||
| Formoterol/beclomethasone | 31.2% | 14.4% | 18.9% | NA |
| Formoterol/budesonide | 0.0% | 60.6% | 44.4% | NA |
| Salmeterol/fluticasone | 68.8% | 25.0% | 36.7% | NA |
| Exacerbations | 14.7% | 22.8% | 20.7% | <0.001 |
| Moderate | 11.6% | 22.0% | 19.2% | <0.001 |
| Acute | 5.2% | 6.7% | 6.3% | 0.199 |
| Comorbid conditions | ||||
| Obesity | 27.7% | 28.0% | 27.9% | 0.650 |
| Smoking | 21.8% | 22.4% | 22.2% | 0.743 |
| Alcoholism | 2.7% | 2.6% | 2.6% | 0.891 |
| Cardiovascular event | 9.8% | 9.7% | 9.7% | 0.948 |
| Hypertension | 25.6% | 24.7% | 24.9% | 0.650 |
| Diabetes | 14.3% | 13.8% | 13.9% | 0.732 |
| Dyslipidemia | 38.8% | 37.3% | 37.7% | 0.518 |
| Cerebrovascular events | 7.4% | 6.7% | 6.9% | 0.570 |
| Ischemic heart disease | 4.4% | 4.3% | 4.3% | 0.948 |
| Organ failures | 12.3% | 12.3% | 12.3% | 0.987 |
| Dementia | 2.2% | 2.2% | 2.2% | 0.964 |
| Depression | 18.5% | 18.2% | 18.3% | 0.855 |
| Neoplasm | 9.8% | 9.5% | 9.6% | 0.846 |
| Additional medication | ||||
| Oral corticosteroids | 14.7% | 22.8% | 20.7% | <0.001 |
| Systemic antibiotics | 2.7% | 7.0% | 5.9% | <0.001 |
| SABA | 80.3% | 80.4% | 80.3% | 0.958 |
| Number of visits to GP, (SD) | 12.5 (11.3) | 17.0 (14.0) | 15.8 (13.5) | <0.001 |
| Mean of number of visits to the emergency room, (SD) | 0.1 (0.3) | 0.2 (0.5) | 0.2 (0.5) | <0.001 |
| Mean of cost of pharmacological treatment (SD), Euros | 502.0 (510.4) | 407.0 (407.8) | 432.4 (439.5) | <0.001 |
| Days off from work (SD) | 2.5 (18.4) | 3.2 (22.2) | 3.1 (21.2) | 0.444 |
| Mean of total health care cost per patient (SD), Euros | 1,261 (2,093.8) | 1,520 (2,562.9) | 1,449.9 (2,448.4) | 0.024 |
Note: Patients’ characteristics of those who visited their primary care center between January 2007 and June 2014.
Abbreviations: COPD, chronic obstructive pulmonary disease; DPI, dry powder inhaler; FEV1, forced expiratory volume in the first second; GP, general practitioner; ICS/LABA, inhaled corticosteroid and long-acting β2-agonist; NA, not applicable; pMDI, pressurized metered dose inhaler; SABA, short-acting β2-agonist; SD, standard deviation.
Table 2 contains estimates of the univariate analysis and significance levels. At a first glance, having DPIs was negatively associated with compliance. Regarding active substances, patients taking salmeterol/fluticasone combination tend to have higher compliance compared to patients taking formoterol/beclomethasone combinations; in contrast the coefficient for the variable of salmeterol/fluticasone combination was not statistically significant. Most of the comorbidities were not statistically significant and only hypertension and organ failures correlated positively with compliance. Number of exacerbations and most specifically moderate exacerbations were positively correlated with better compliance outcomes. Mild, moderate, and severe persistent asthma were correlated with better compliance compared to intermittent asthma. Regarding utilization of health care resources, an extra visit to the GP was positively linked to compliance, while cost was negatively correlated with it. Patient characteristics such as age, retirement status, and allergy were positively correlated with compliance.
Table 2.
Univariate analysis of potential confounders of compliance
| Category | Compliance
|
||
|---|---|---|---|
| Percentage points | Odds ratio | 95% CI | |
| Explanatory variables | |||
| DPI compared to pMDI | −5.89 | 0.706 | (0.593–0.841) |
| ICS/LABA (baseline formoterol/beclomethasone) | |||
| Formoterol/budesonide | −0.37 | 0.976 | (0.789–1.207) |
| Salmeterol/fluticasone | 5.99** | 1.409** | (1.131–1.755) |
| Therapy line (baseline first line) | |||
| Second line | 17.47* | 2.441* | (1.848–3.224) |
| Third line | 68.98* | 31.384* | (11.00–89.52) |
| FEV1 measure | −0.41 | 0.97 | (0.968–0.983) |
| Age | 0.11** | 1.007** | (1.002–1.011) |
| Male | 1.50 | 1.092 | (0.932–1.281) |
| Retired | 4.17** | 1.278** | (1.090–1.498) |
| Obesity | 1.73 | 1.108 | (0.932–1.317) |
| Smoking | −4.15** | 0.783** | (0.648–0.945) |
| Stroke | 2.52 | 1.160 | (0.839–1.602) |
| Cerebrovascular disease | 3.23 | 1.210 | (0.923–1.584) |
| Hypertension | 3.64* | 1.239* | (1.035–1.484) |
| Diabetes | −2.53 | 0.861 | (0.685–1.082) |
| Dyslipidemia | −0.57 | 0.994 | (0.847–1.167) |
| Alcoholism | 6.18 | 1.439 | (0.905–2.290) |
| Ischemic heart disease | 2.77 | 1.177 | (0.801–1.731) |
| Organ failures | 6.29** | 1.449** | (1.139–1.843) |
| Dementia | −4.29 | 0.777 | (0.450–1.341) |
| Depression | −1.75 | 0.902 | (0.737–1.035) |
| Neoplasm | 0.36 | 1.021 | (0.781–1.335) |
| Allergy | 3.84** | 1.255** | (1.074–1.465) |
| Number of exacerbations | 2.01** | 1.126** | (1.018–1.245) |
| Number of acute exacerbations | 0.76 | 1.046 | (0.761–1.438) |
| Number of mild/moderate exacerbations | 2.77** | 1.178** | (1.047–1.325) |
| Severity of asthma (baseline intermittent) | |||
| Mild persistent | 8.40* | 1.857* | (1.480–2.327) |
| Moderate persistent | 13.52* | 2.473* | (2.000–3.057) |
| Severe persistent | 17.85* | 3.064* | (1.865–5.032) |
| Time from diagnosis | 0.26 | 1.01 | (0.999–1.033) |
| Oral corticosteroids | 2.91 | 1.187 | (0.982–1.435) |
| Systemic antibiotics | 8.40** | 1.642** | (1.187–2.274) |
| SABA | 6.96* | 1.509* | (1.240–1.836) |
| Visits to GP | 0.21* | 1.012* | (1.006–1.018) |
| Emergency visits | 2.05 | 1.12 | (0.962–1.323) |
| Sick leave | −0.04 | 0.99 | (0.993–1.001) |
| Cost | −0.11* | 0.994* | (0.991–0.996) |
Notes:
P<0.001,
P<0.05.
Abbreviations: CI, confidence interval; DPI, dry powder inhaler; FEV1, forced expiratory volume in the first second; GP, general practitioner; ICS/LABA, inhaled corticosteroid and long-acting β2-agonist; pMDI, pressurized metered dose inhaler; SABA, short-acting β2-agonist; SD, standard deviation.
Inhaler technique and other confounders affecting patient’s compliance to the asthma treatment
The patterns of the relationship from the multivariate ordered logistic regression model matched some of those of the univariate results (Table 3). Indeed, the probability of having more negative compliance is larger for DPI users compared to patients using pMDI. These patterns also fit for severity of asthma, allergy, acute exacerbations, and cost. In contrast, age, comorbidities, moderate exacerbations, and retirement were not statistically correlated with compliance. After controlling for the ICS/LABA combination, which became statistically significant, the magnitude of DPIs’ effect on compliance in comparison with pMDI outcomes decreased.
Table 3.
Multivariate results of potential confounders of compliance
| McFadden’s R2=0.029 | Compliance
|
|||||
|---|---|---|---|---|---|---|
| Without active substance
|
Controlling for active substances
|
|||||
| Percentage points | Odds ratio | 95% CI | Percentage points | Odds ratio | 95% CI | |
| Explanatory variables | ||||||
| Medical device DPI | −6.46* | 0.69* | (0.575–0.841) | −4.48** | 0.77** | (0.617–0.972) |
| ICS/LABA | ||||||
| Formoterol/budesonide | – | – | – | 1.02 | 1.065 | (0.830–1.368) |
| Salmeterol/fluticasone | – | – | – | 5.78** | 1.398** | (1.104–1.770) |
| Severity | ||||||
| Persistent mild | 11.02* | 2.22* | (1.737–2.834) | 10.81* | 2.188* | (1.712–2.796) |
| Persistent moderate | 15.42* | 2.82* | (2.203–3.610) | 15.26* | 2.791* | (2.180–3.575) |
| Persistent severe | 24.08* | 4.26* | (1.994–9.124) | 24.41* | 4.322* | (2.017–9.260) |
| Therapy line | ||||||
| Second | 14.04* | 2.10* | (1.558–2.831) | 13.69* | 2.07* | (1.535–2.791) |
| Third | 65.53* | 29.0* | (9.794–85.92) | 65.20* | 28.43* | (9.575–84.45) |
| Age | 0.13 | 1.008 | (0.999–1.016) | 0.12 | 1.007 | (0.999–1.015) |
| Retired | 2.96 | 1.188 | (0.843–1.597) | 2.97 | 1.190 | (0.885–1.601) |
| Smoker | −1.74 | 0.902 | (0.733–1.109) | −1.87 | 0.894 | (0.727–1.100) |
| Hypertension | −0.95 | 0.946 | (0.748–1.194) | −0.95 | 0.945 | (0.748–1.194) |
| Organ failures | 3.06 | 1.195 | (0.896–1.595) | 2.93 | 1.187 | (0.889–1.585) |
| Allergy | 3.70** | 1.24** | (1.045–1.474) | 3.96** | 1.261** | (1.061–1.497) |
| Acute exacerbations | −8.02* | 0.626* | (0.417–0.938) | −8.61** | 0.604** | (0.402–0.907) |
| Mild/moderate exacerbations | 0.67 | 1.040 | (0.868–1.245) | 0.746 | 1.04 | (0.871–1.252) |
| Pharmacological cost | −0.170* | 0.988* | (0.986–0.992) | −0.179* | 0.989* | (0.986–0.992) |
| Visit to GP | 0.04 | 1.002 | (0.995–1.009) | 0.04* | 1.002* | (0.995–1.009) |
Notes:
P<0.001,
P<0.05.
Abbreviations: CI, confidence interval; DPI, dry powder inhaler; GP, general practitioner; ICS/LABA, inhaled corticosteroid and long-acting β2-agonist; pMDI, pressurized metered dose inhaler; –, not considered within the regression.
The final specification showed to fit the data better for explaining compliance patterns (Table 4). Having a DPI would decrease the probability of asthmatic patients to comply with their treatment compared to pMDI. Moreover, deterioration of forced expiratory volume in the first second measure by 1 unit and having ≥3 comorbidities would decrease the probability of medication compliance. More than one doctor’s consultation per month was associated with worse adherence. Patients who belonged to 29–39, 40–50, and 51–61 age groups were more likely to be adherent compared to the oldest group, as well as those who suffered more than two moderate exacerbations compared to those who did not experience any exacerbation. An additional doctor visit was associated with better adherence. Alcoholism, which was the only comorbidity statistically significant, was positively associated with compliance. Again, active substance categorical variable was not statistically significant.
Table 4.
Final specification: ordered logistic regression analysis: predicting compliance for asthmatic patients
| McFadden’s R2=0.239 | Compliance
|
||
|---|---|---|---|
| Percentage points | Odds ratio | 95% CI | |
| Explanatory variables | |||
| DPI compared to pMDI | −10.67** | 0.457** | (0.229–0.909) |
| ICS/LABA compared to formoterol/beclomethasone | |||
| Formoterol/budesonide | 7.75 | 1.761 | (0.837–3.706) |
| Salmeterol/fluticasone | 3.37 | 1.309 | |
| FEV1 | 9.78* | 0.977* | (0.959–0.996) |
| Therapy line | |||
| Second line | 0.75 | 1.056 | (0.755–1.475) |
| Third line | 52.7* | 27.09* | (8.657–84.78) |
| Age groups compared to the oldest group | |||
| 18–28 | 6.25 | 2.236 | (0.841–6.427) |
| 29–39 | 15.64** | 3.625** | (1.375–9.547) |
| 40–50 | 12.68** | 2.961** | (1.150–7.634) |
| 51–61 | 13.59** | 3.154** | (1.368–7.727) |
| 62–72 | −4.59 | 1.496 | (1.496–3.141) |
| 73–83 | −9.38 | 1.598 | (0.738–3.460) |
| Retirement | 1.71 | 1.342 | (0.540–2.379) |
| Allergy | 1.76 | 1.138 | (0.937–1.381) |
| Number of comorbidities | |||
| 1 | −1.86 | 0.877 | (0.665–1.157) |
| 2 | −4.82 | 0.704 | (0.488–1.017) |
| 3 | −7.52** | 0.566** | (0.367–0.874) |
| >4 | −7.16** | 0.584** | (0.505–1.557) |
| Acute event/condition | 1.08 | 1.082 | (0.796–1.471) |
| Obesity | 2.69 | 1.218 | (0.933–1.591) |
| Alcoholism | 7.98** | 1.795** | (1.013–3.179) |
| Smoking | 0.58 | 1.043 | (0.788–1.381) |
| Acute exacerbation | −1.60 | 0.886 | (0.505–1.557) |
| Moderate (compared with no exacerbation) | |||
| 1 moderate exacerbation | 15.03 | 2.736 | (1.003–6.463) |
| ≥2 moderate exacerbations | 20.35** | 3.726** | (1.111–10.49) |
| Visit to the emergency room | −8.20 | 0.517 | (0.235–1.134) |
| Additional visit to the GP | 1.98* | 1.156* | (1.137–1.175) |
| Visiting a GP | |||
| Two times per month (on average) | −25.83* | 0.094* | (0.674–0.131) |
| Three times per month (on average) | −39.12* | 0.025* | (0.001–0.005) |
| Average pharmacological cost per month | 0.22 | 1.016 | (0.998–1.035) |
| Day lost due to some event related with asthma | −0.81 | 0.941 | (0.680–1.300) |
| Potential underlying mechanism | |||
| Severity related to DPI | |||
| Severity/Accuhaler® | 4.98** | 1.441** | (1.049–1.978) |
| Severity/Turbuhaler® | 4.98 | 1.037 | (0.812–1.324) |
| Severity/NEXThaler® | 2.64 | 1.214 | (0.877–1.680) |
| Exacerbations related to each inhaler | |||
| Accuhaler® | −21.98* | 0.199* | (0.074–0.532) |
| Turbuhaler® | −9.84 | 0.482 | (0.193–1.203) |
| NEXThaler® | −15.25** | 0.326** | (0.107–0.995) |
| pMDI | −14.24** | 0.351** | (0.139–0.886) |
| Visit to GP related to each ICS/LABA | |||
| Salmeterol/fluticasone | −65.84* | 0.008* | (0.001–0.498) |
| Formoterol/budesonide | −90.17* | 0.0013* | (0.001–0.009) |
| Formoterol/fluticasone | −65.43* | 0.008* | (0.001–0.055) |
| Visit to GP for retired patient | 4.15** | 1.356** | (0.992–0.055) |
| Costs for each age group (baseline 83–97 group) | |||
| 18–28 | −0.37** | 0.973** | (0.948–0.998) |
| 29–39 | −0.29** | 0.978** | (0.958–0.998) |
| 40–50 | −0.22 | 0.983 | (0.964–1.003) |
| 51–61 | 0.29** | 0.978** | (0.962–0.994) |
| 62–72 | 0.007 | 1.005 | (0.987–1.015) |
| 73–83 | 0.003 | 1.002 | (0.987–1.010) |
Notes:
P<0.001,
P<0.05.
Abbreviations: CI, confidence interval; DPI, dry powder inhaler; FEV1, forced expiratory volume in the first second; GP, general practitioner; ICS/LABA, inhaled corticosteroid and long-acting β2-agonist; pMDI, pressurized metered dose inhaler; SD, standard deviation; –, not considered within the regression.
The introduction of interaction terms revealed interesting results. The effect of exacerbations varies across inhaler devices and the probability of being a compliant patient with Accuhaler® who suffered an exacerbation is lower compared to the rest of DPI users. However, as asthma gets worse, Accuhaler® devices have a small but positive effect toward compliance compared to the pMDI. Estimates of the other DPIs were not statistically significant. In the case of healthcare utilization, the patient’s probability of medication compliance would be lower for those who visited their GP more than 3 times and were treated with Formoterol/Budesonide combination compared to patients taking the other ICS/LABA combinations, although the common effect of visiting their GP was observed to be a positive association with compliance (see previous paragraph). The effect of GP visits toward compliance was greater for retired patients compared to working-age patients and retired ones who did not visit the GP during the study period. The negative effect of cost through age affected mostly younger patients and became not statistically significant for older age groups.
Robustness check confirmed our results, which implied that inhaler technique associated with DPIs would decrease the probability of patients to adhere to asthma medication (P<0.001).
Discussion
Our study shows that the inhaler technique associated with inhaler devices can affect compliance of asthmatic patients. In fact, findings are consistent with previous literature that shows patient’s compliance as a real and latent problem within chronic conditions.12 In our case, we explored the problem of compliance toward asthma medication while controlling for potential confounders. We controlled for the effect of active substance toward the probability to stick to the treatment,24 so we could obtain the potential effect of the inhaler device. Interestingly, findings suggest that ICS/LABA combinations would not impact on the probability of being adherent, which is reasonable since medicines are 100% effective but the inhalation technique is the key for better clinical outcomes.1–3,7
Along with inhaler technique, different confounders of patient’s compliance were explored. Univariate results determined that only a few variables were associated with medication compliance. The proxy for inhaler technique was statistically significant; in contrast, patients’ characteristics, comorbidities, and health outcomes that were significant in the univariate analysis became nonstatistically significant in the basic multivariate specification. However, in our last specification we identified confounders of compliance from the interaction between variables. This indicates that a basic analysis was not enough to identify factors affecting compliance outcomes, and as it has been reported, adherence and compliance are a more complex topic.25 According to our results, having more than three conditions simultaneously would impact negatively on the likelihood of medication compliance. Suffering from a disease is a burden for patients, and it is reported that there is a substantial burden on patients having more than three simultaneous conditions, which starts to affect their compliance to asthma treatment (as we have observed in our results), and may require more complex health management strategies.26,27 Remarkably, unhealthy behaviors of patients were not statistically significant in compliance. However, we identified a scope for improvement since we detected that 22% of the sample were smokers despite their asthmatic condition.
Despite the positive effect of GP consultation for compliance, a high number of visits per month to health care professionals was negatively correlated with it. Indeed, greater number of visits was associated with worse compliance outcomes, which is consistent with previous literature that reported reductions in health care utilization due to good compliance with asthma treatment.28 Further, the effect of visits for retired patients is positive for compliance compared to active patients and retired patients who did not visit their GP during the time they were persistent with their medication. This is consistent with previous findings that suggest for elderly, GP consultation should be encouraged in order to maintain patient’s compliance to medication in the long term.29
Accordingly with our results, younger age groups were more likely to be compliant patients.19 However, this effect might be offset due to the effect of costs toward compliance. Our results for the interaction between age and cost suggest pharmacological cost for younger groups would decrease the probability of being compliant. It may be that the effect of bearing some part of the pharmaceutical cost for patients whose salary was not very high represented an economic burden that influences compliance to a treatment. In contrast, cost for older groups was not statistically significant; this is reasonable since elderly Spanish patients have access to pharmaceutical treatment without co-payments.30
There are limitations to these estimates besides the fact that this is a retrospective study, which is vulnerable to bias. First, this study was conducted in a single health system, thus results may not be extrapolated to other populations. However, trends are similar to previous research.22,23,25 Second, the assumption that obtaining a prescription was equivalent to taking the medication might not be completely accurate. Moreover, the study approach to quantify compliance was done by using the MPR, which has been reported to be biased upwards.9,14 Nevertheless, we tried to correct for this by elevating the cut-off point so fewer patients were seen as compliant with their medication. Robustness test of estimates confirmed our findings. Actually, our estimates could be considered accurate, since we used persistence to asthma treatment data. It is also true that tracking compliance patterns may imply having data across time; however, this could be only possible by assessing compliance prospectively.
It is worth mentioning that physicians have been aware that the majority of patients cannot use their prescribed pMDI correctly;31 and DPIs included in this study were seen as an effective alternative to solve problems related to inhaler technique. However, our results suggest that there is still a crucial barrier to avoid the loss of pharmacological efficacy due to the inhaler technique associated with DPIs included in this analysis, which determines compliance of long-term medication in asthmatic patients. Finally, we could not obtain some patients’ characteristics such as race, level of education, type of job, and some clinical confounders such as lung function. This problem of data limitation also restricts our outcomes.
Conclusion
The effect of inhaler technique associated with DPIs included in this anaylsis (Accuhaler®, nexThaler®, and Turubhlaer) was negative towards compliance for long-term asthma medication. In contrast, the active substance was not significant in exploring compliance patterns. Furthermore, we identified some other confounders of patient’s compliance such as severity of asthma, comorbidities, cost, age, and health care utilization. Associations between exacerbations and patient’s compliance have shown to be divergent across inhaler devices.
Treatment noncompliance is a real and damaging problem for patients and for the Spanish National Healthcare System. The quantification of compliance and persistence is always an issue in current literature, which can limit results; identification of potential factors affecting compliance is necessary in order to guide actions to attenuate the problem of poor compliance in asthma patients. This study assessing treatment compliance across asthma inhaler devices for ICS/LABA combinations confirms that inhaler devices play an important role in asthma management by conditioning compliance, which is consistent with prior research.1–9,17,23 The special feature of our findings is that we adjusted for the effects of the active substances in order to explore the effect of inhaler technique toward compliance, which has shown to be influenced also by patients’ demographic characteristics and clinical aspects. Helping patients to properly use their inhaler devices, has shown to be the key for better compliance outcomes, as we observed that inhaler technique might be diminishing the efficacy of pharmacological treatment.
Acknowledgments
This study was sponsored by Teva Pharma, S.L.U. and BCN Health Economics & Outcomes Research S.L. provided statistical analysis and editorial support.
Footnotes
Disclosure
This study was sponsored by Teva Pharma, S.L.U. JD is employed by the University of Barcelona. GR is an employee of BCN Health Economics and Outcomes Research S.L., Barcelona, Spain, an independent contract health economic organization that has received research funding from Teva Pharma, S.L.U. AS is employed by Badalona Serveis Assistencials S.A. LG and RS are employed by Teva Pharmaceutical Industries Ltd and they work in the Medical Department. ST is also employed by Teva Pharmaceuticals Europe BV. The authors report no other conflicts of interest in this work.
References
- 1.Ubeda Sansano MI, Cortés Rico O, Montón Áacute;lvarez JL, Lora Espinosa A, Praena Crespo M. Dispositivos de inhalación. El Pediatra de Atención Primaria y los dispositivos de inhalación. Documentos técnicos del GVR (publicación DT-GVR-X). [Inhalation devices. The primary care pediatrician and inhalation devices. GVR technical documents (publication DT-GVR-X)] [Accessed February 01, 2015]. Available from: http://aepap.org/grupos/grupo-de-vias-respiratorias. Spanish.
- 2.Melani AS, Bonavia M, Cilenti V, on behalf of Gruppo Educazionale Associazione Italiana Pneumologi Ospedalieri (AIPO) Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Roy A, Battle K, Lurslurchachai L, Halm EA, Wisnivesky JP. Inhaler device, administration technique and adherence to inhaled corticosteroids in patients with asthma. Prim Care Respir J. 2011;20(2):148–154. doi: 10.4104/pcrj.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107:1481–1490. doi: 10.1016/j.rmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102:593–604. doi: 10.1016/j.rmed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Murphy KR, Bender BG. Treatment of moderate to severe asthma: patient perspective on combination inhaler therapy and implications of adherence. J Asthma Allergy. 2009;2:63–72. doi: 10.2147/jaa.s4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Woude HJ, Boorsma M, Bergvist PB, Winter TH, Aalbers R. Budesonide/formoterol in a single inhaler rapidly relives metacholine-induced moderate to severe bronchoconstriction. Pulm Pharmacol Ther. 2004;17(2):89–95. doi: 10.1016/j.pupt.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Lavorini F, Fontana GA, Usmani OS. New inhaler devices – the good, the bad and the ugly. Respiration. 2014;88:3–15. doi: 10.1159/000363390. [DOI] [PubMed] [Google Scholar]
- 9.The Inhaler Error Steering Committee. Price D, Bosnic-Anticevich S, Briggs A, et al. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2013;107(1):37–46. doi: 10.1016/j.rmed.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Bender BG. Advancing the science of adherence measurement: implications for the clinician. J Allergy Clin Immunol Pract. 2013;1(1):92–93. doi: 10.1016/j.jaip.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Barnestein-Fonseca P, Leiva-Fernández J, Vidal-España F, García-Ruíz A, Prados-Torres D, Leiva-Fernández F. Is it possible to diagnose the therapeutic adherence of patients with COPD in clinical practise? A cohort study. BMC Pulm Med. 2011;(24):11–16. doi: 10.1186/1471-2466-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams LK, Pladevall M, Xi H, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. J Allergy Clin Immunol. 2004;114(6):1288–1293. doi: 10.1016/j.jaci.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . Adherence to Long-Term Therapies: Evidence for Action. Geneva: World Health Organization; 2003. [Accessed December 2014]. Available from: http://www.who.int/chp/knowledge/publications/adherence_report/en/ [Google Scholar]
- 15.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 16.Boulet LP. Influence of comorbid conditions on asthma. Eur Respir J. 2009;33:897–906. doi: 10.1183/09031936.00121308. [DOI] [PubMed] [Google Scholar]
- 17.Shavelle RM, Paculdo DR, Kush SJ, Mannino DM, Strauss DJ. Life expectancy and years of life lost in chronic obstructive pulmonary disease: findings from NHANES III follow-up study. Int J Chron Obstruct Pulmon Dis. 2009;4:137–148. doi: 10.2147/copd.s5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanc PD, Cisternas M, Smith S, Yelin EH. Asthma, employment status and disability among adults treated by pulmonary and allergy specialists. Chest. 1966;109(3):688–696. doi: 10.1378/chest.109.3.688. [DOI] [PubMed] [Google Scholar]
- 19.Wieshammer S, Dreyhaupt J. Dry powder inhalers: which factors determine the frequency of handling errors? Respiration. 2008;75:18–25. doi: 10.1159/000109374. [DOI] [PubMed] [Google Scholar]
- 20.Wolstein J, Meng YY, Babey SH. Income Disparities in Asthma Burden and Care in California. Los Angeles, CA: UCLA Center for Health Policy Research; 2010. [Google Scholar]
- 21.Stern L, Berman J, Lumry W, et al. Medication compliance and disease exacerbation in patients with asthma: a retrospective study of managed care data. Ann Allergy Asthma Immunol. 2006;97:402–408. doi: 10.1016/S1081-1206(10)60808-3. [DOI] [PubMed] [Google Scholar]
- 22.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 23.Hughes D, Cowell W, Koncz T, Cramer J, International Society for Pharmacoeconomics and Outcomes Research Economics of Medication Compliance Working Group Methods for integrating medication compliance and persistence in pharmacoeconomic evaluations. Value Health. 2007;10(6):498–509. doi: 10.1111/j.1524-4733.2007.00205.x. [DOI] [PubMed] [Google Scholar]
- 24.Tamm M, Richards DH, Beghé B, Fabbri L. Inhaler corticosteroid and long-acting beta 2 agonist pharmacological profiles: effective asthma therapy in practise. Respir Med. 2012;106(Suppl 1):S9–S19. doi: 10.1016/S0954-6111(12)70005-7. [DOI] [PubMed] [Google Scholar]
- 25.Cochrane GM, Horner R, Chanez P. Compliance with asthma. Respir Med. 1999;93:763–769. doi: 10.1016/s0954-6111(99)90260-3. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Carleton BC, Prosser RJ, Smith AM. The added burden of comorbidity in patients with asthma. J Asthma. 2009;45(10):1021–1026. doi: 10.3109/02770900903350473. [DOI] [PubMed] [Google Scholar]
- 27.Gershon AS, Wang C, Guan J, To T. Burden of comorbidity in individuals with asthma. [Accessed February 2015];Thorax. 2010 65:612–618. doi: 10.1136/thx.2009.131078. Available from: thorax.bmj.com/content/65/7/612.full.pdf. [DOI] [PubMed] [Google Scholar]
- 28.Dalcin Pde T, Grutcki DM, Laporte PP, et al. Impact of a short-term educational intervention on adherence to asthma treatment and on asthma control. Impact Bras Pneumol. 2011;37(1):19–27. doi: 10.1590/s1806-37132011000100005. [DOI] [PubMed] [Google Scholar]
- 29.Mansur N, Weiss A, Hoffman A, Gruenewald T, Beloosesky Y. Continuity and adherence to long-term drug treatment by geriatric patients after hospital discharge. Drugs Aging. 2008;25(10):861–870. doi: 10.2165/00002512-200825100-00005. [DOI] [PubMed] [Google Scholar]
- 30.Ley 29/2006, de 26 de Julio, de garantías y uso racional de los medi-camentos y productos sanitarios. BOE num 178: 28122–28165. [Law 29/2006, July 26, due to guarantees and rational utilization of medicines and medical devices. BOE number 178:28122-28165] [Accessed January 31, 2015]. Available from: http://www.boe.es/buscar/doc.php?id=BOE-A-2006-13554. Spanish.
- 31.Hardwell A, Barber V, Hargadon T, McKnight E, Holmes J, Levy ML. Technique training does not improve the ability of most patients to use pressurised metered-dose inhalers (pMDI) Prim Care Respir J. 2001;20(1):92–96. doi: 10.4104/pcrj.2010.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]