Abstract
Levels of hepcidin, a key modulator of iron metabolism, are influenced by erythropoiesis, iron, and inflammation, all of which may be increased in patients with sickle cell disease (SCD). The objectives of this study were to determine: 1) the variation in hepcidin level, and 2) the relative contribution of erythropoietic drive, iron, and inflammation to differences in hepcidin level in an adult cohort with SCD. In a prospective study, cross-sectional measurements of hepcidin, reticulocyte percentage, erythropoietin, ferritin, and high-sensitivity CRP were obtained. A regression tree analysis was used to measure the association between these interacting factors and hepcidin level. The cohort was comprised of 40 adults with SCD. Median age was 26 years, 68% were female, and all had HbSS. Hepcidin values ranged from 30 ng/ml to 326 ng/ml, with a median of 87 ng/ml. Regression tree analysis demonstrated that reticulocyte percentage, erythropoietin, ferritin and hs-CRP all were associated with hepcidin. The highest hepcidin values were found in subjects with low reticulocyte percentage and erythropoietin. In conclusion, erythropoietic drive, iron status, and inflammation all contribute to variation in hepcidin level. The strongest contributor is erythropoietic drive. Future studies could determine whether suppression of erythropoiesis with chronic transfusion influences hepcidin level.
Keywords: Sickle cell disease, hepcidin, cross-sectional study, erythropoietin
INTRODUCTION
In patients with sickle cell disease (SCD), frequent red cell transfusion often leads to iron overload [1–3]. Despite the risks of excess iron, including liver and heart failure, patients with SCD and iron overload have less end-organ damage than other patient populations with similar iron burden [4–10]. This difference suggests that patients with SCD may manage excess iron in a more organ-protective manner than other transfused populations, such as those with β-thalassemia [4].
Hepcidin, the key regulator of iron metabolism, may modulate the risk of end-organ damage from transfusion-related iron toxicity. A negative regulator of iron homeostasis, hepcidin decreases intestinal absorption and cellular release of iron [11–13]. Higher levels of hepcidin, therefore, may limit tissue injury through a reduction of iron in circulation and sequestration of iron within cells, including toxic non-transferrin bound iron (NTBI) [4]. One potential explanation for the lower incidence of iron-related end-organ disease in patients with SCD compared to other transfused populations would be higher levels of hepcidin and lower levels of NTBI. Although lower levels of NTBI have been reported in patients with SCD compared to other transfused populations [6,14], studies of hepcidin level have yielded inconsistent results [15–18].
Selection bias may have limited the results of prior studies. Iron and inflammation are positive regulators of hepcidin, whereas erythropoietic drive (as defined by markers such as erythropoietin) is a negative regulator [11–13,19]. Since iron, inflammation and erythropoietic drive can all be increased in patients with SCD to varying degrees, hepcidin levels likely differ significantly from patient-to-patient based on their levels of positive and negative regulators. Prior studies largely reported lower levels of hepcidin in patients than controls [17,18]. These studies were limited, however, by narrow patient selection, mostly children without iron excess. In a cohort with more iron excess or inflammation, or less erythropoietic drive, hepcidin levels may be higher. To address this limitation, we examined hepcidin levels in a cohort of adults with SCD with a significant history of transfusion and iron overload. Our objectives were two-fold: 1) to determine variation in hepcidin levels, and 2) to elucidate the contribution of erythropoietic drive, iron burden, and inflammation to the observed variation. Further insight into the regulation of hepcidin may lead to strategies to modulate hepcidin levels in order to maximize organ-protective effects.
METHODS
Patients and data collection
Patients with HbSS who were greater than 18 years of age were eligible for this study. The Medical College of Wisconsin institutional review board approved this study. All patients provided written consent prior to participation.
Blood was collected from adult patients with SCD at steady state, defined as patient report of baseline symptoms and without admission to the emergency department (ED) or hospital in the previous 4 weeks. Patients on a chronic transfusion regimen had samples collected up to 72 hours prior to the transfusion. Samples were tested for erythropoietin (EPO) by immunoassay (Dynacare Laboratories, Milwaukee, WI), high sensitivity C-reactive protein (hs-CRP) (Dynacare Laboratories, Milwaukee, WI), and plasma hepcidin (Intrinsic LifeSciences, La Jolla, CA). Patient demographics were obtained from the electronic medical record. Chronic transfusions, simple or exchange, were administered on a 4–8 week schedule as part of their routine, non-acute care. The most recent available hemoglobin, alanine aminotransferase (ALT), glomerular filtration rate (GFR), hemoglobin S percent (%), reticulocyte percent (%), and ferritin were also obtained. When possible, the result was obtained at the same time as the hepcidin sample.
Statistical analysis
The primary outcome of interest in this study was plasma hepcidin (ng/ml) and its dependence on other patient characteristics. Descriptive statistics were used to summarize participant characteristics. Patient-related factors were compared using the Kruskal Wallis test for continuous variables and the Fisher’s Exact test for categorical variables. Potential factors to predict hepcidin included age, gender, days from last transfusion, number of transfusions in the last 12 months, EPO, hs-CRP, ALT, GFR, hemoglobin, ferritin, hemoglobin S %, and reticulocyte %, each of which were examined with a Spearman correlation.
Since measures of iron, inflammation, and erythropoietic drive (reticulocyte % and erythropoietin) all interact with each other to influence hepcidin level, we performed a regression tree analysis. A regression tree or recursive partitioning analysis is a nonparametric regression methodology, where the recorded dependent variables noted previously are evaluated and selected to best explain the differences between hepcidin values. The advantages of a tree analysis over, for example, a traditional linear regression, is that highly related variables (reticulocyte % and erythropoietin) can be included as independents. Further, by specifying an optimization function such as least median absolute deviation (least MAD), rather than least squares (used by linear regression), outliers will affect the model less. The tree initially divides the dataset into two groups based on optimizing, by our choice, the MAD, since the data had outliers. It then continues to partition each branch into two sets and so on. Once a cohort of 5 subjects is reached, the partitioning process is halted for that group. A statistical significance (alpha) level of 0.05 was used throughout. SPSS 21 for Windows and Salford Systems CART for the tree analysis were used.
Sample size calculation
The sample size was chosen to enable investigation of the interrelationships between hepcidin levels and known mediators: EPO, ferritin and CRP. In general, 5 cases per covariate, including any interaction variables, in a model is the accepted minimum needed for appropriate fitting of the model. A sample size of 40 subjects would therefore allow us to consider 3 variables and their 3 interactions. Further, with a sample size of 40 subjects, we would have at least 80% power to detect a significant correlation of ≥ 0.45 at a Bonferroni corrected alpha of 0.017 (for 3 tests).
RESULTS
Demographics and laboratory values
There were 40 patients evaluated in this study (Table 1). The median age of study participants was 26 years (range: 20–57), all were HbSS, and most were female (68%). These adult patients were all heavily transfused, and no patients were transfusion naïve at the start of the study. Nineteen subjects (48%) were receiving chronic red cell transfusions at the time of the study, 11 (58%) via red cell exchange. Only 5 (12.5%) subjects received no red blood cell transfusions in the previous 12 months. An elevated reticulocyte % (median 8%, range 0.6–23%; reference range 0.5–2%) and EPO (median 57 mIU/ml, range 19–2962 mIU/ml; reference range 3–19 mIU/ml) was observed, consistent with increased erythropoietic drive. The cohort demonstrated iron overload (median ferritin 2,969 ng/ml, range 20–12,300 ng/ml; reference range 13–400 ng/ml), consistent with their transfusion history, and increased inflammation (median hs-CRP 5.6 mg/L, range 0.4–60 mg/L; reference range <1.0 mg/L). Median hepcidin level for the cohort was 87 ng/ml (range: 30–326 ng/ml, reported reference range 17–286 ng/ml female/ 29–254 ng/ml male).
Table 1.
Patient characteristics and lab values at steady state and their relationship to hepcidin.
| Patient Characteristics | N=40 | r | P-value |
|---|---|---|---|
| Age (median years), (range) | 26 (20–57) | −0.001 | 1.0 |
|
| |||
| BMI (median kg/m2), (range) | 24 (17–35) | 0.082 | 0.61 |
|
| |||
| Female gender, (%) | 68 | NA | 0.1** |
|
| |||
| Therapy | |||
| Hydroxyurea (%) | 40 | NA | 1.0** |
| Iron chelation (%) | 40 | NA | 0.2** |
| RBCs in last year | 15 (0–78) | −0.1 | 0.7 |
| Days since last RBC | 45 (4–5990) | −0.2 | 0.2 |
|
| |||
| Laboratory Values | |||
| Erythropoietin, ng/dl, median (range) | 57 (19–2962) | −0.3 | 0.03* |
| Hemoglobin, g/dl, median (range) | 8.3 (5.2–10.9) | −0.1 | 0.7 |
| Hemoglobin S percent, median (range) | 50.5 (3.3–89.6) | −0.3 | 0.1 |
| Reticulocyte percent, median (range) | 7.9 (0.6–23.0) | −0.5 | 0.002* |
| Ferritin, ng/dl, median (range) | 2969 (20–12300) | 0.6 | 0.0002* |
| Hs-CRP, mIU/ml, median (range) | 5.6 (0.4–59.5) | 0.3 | 0.1 |
| ALT, mIU/dl, median (range) | 27 (8–147) | 0.02 | 1.0 |
| GFR, % in normal range (>60) | 95 | NA | 0.5 |
| Hepcidin, ng/dl, median (range) | 87 (30–326) | NA | NA |
Significant relationships were defined as p ≤ 0.05 in all cases.
Spearman’s correlations were evaluated in all cases except for gender, hydroxyurea, iron chelation use, and GFR, where a Kruskal Wallis test was applied.
Univariate associations with hepcidin
In univariate analyses, hepcidin level had a negative correlation with erythropoietin (r= −0.34, p=0.03), and reticulocyte % (r=−0.47, p=0.002) (Table 1). A positive correlation was noted between hepcidin and ferritin (r=0.56, p=0.0002). No significant association was found between hepcidin and any other variable, including hs-CRP (r=0.25, p=0.11) or any treatment protocol, including red cell transfusion history (P>0.1).
Regression tree analysis to predict hepcidin
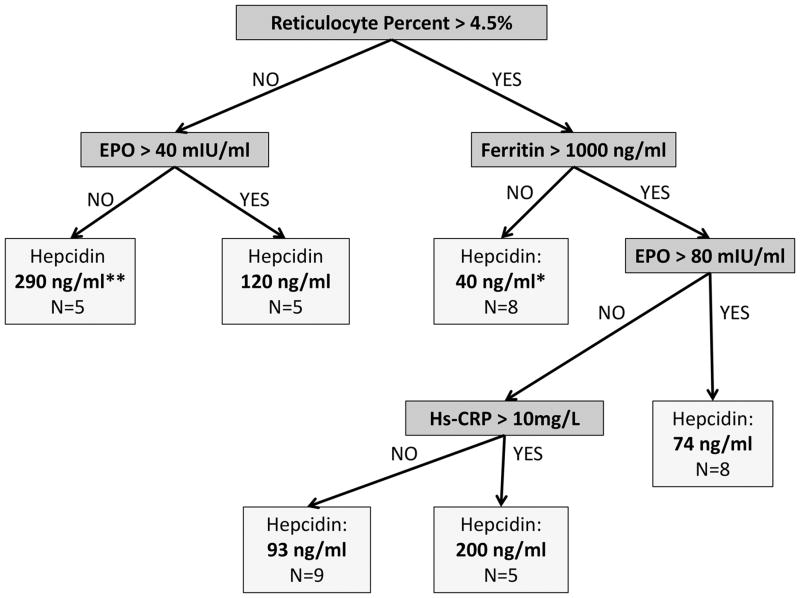
Significant interactions exist between reticulocyte %, EPO, ferritin, and hs-CRP when univariate analyses are performed to test their association with hepcidin (Supplementary figure). To delineate the relationship between these interacting factors and hepcidin, a regression tree analysis was performed. In the best fit model, markers of erythropoietic drive (reticulocyte % and EPO), iron load (ferritin), and inflammation (hs-CRP) were all significantly associated with hepcidin level (Figure 1). Of these, erythropoietic drive (reticulocyte % and EPO) was the strongest predictor of hepcidin level. Patients with a low reticulocyte % and EPO concentration had the highest hepcidin levels (median=289.6 ng/ml). Again, no markers of transfusion history, including number of red cells in the past 12 months, number of days from a previous transfusion, or chronic transfusion protocol type significantly impacted the observed hepcidin values in this model.
Figure 1. Regression tree analysis representing the significant predictors of median hepcidin concentrations (IQR) in adult patients with SCD (N=40).
Reticulocyte percent, erythropoietin, ferritin, and hs-CRP are the key variables that predict hepcidin concentration in adult patients with SCD, with reticulocyte percent representing the primary predictor. Higher reticulocyte percent (>4.5%) and erythropoietin (>40 and >80 mIU/ml) predicts lower median hepcidin concentrations, and higher ferritin (>1000 ng/ml) and hs-CRP (>10 mg/L) predicts higher hepcidin median concentrations in this population.
* The lowest median hepcidin concentrations were best predicted by a reticulocyte percent >4.5% and a ferritin <1000 ng/ml (N=8).
** The highest median hepcidin concentrations were best predicted by a reticulocyte percent <4.5% and EPO < 40 mIU/ml (N=5).
DISCUSSION
For the first time, we reveal the relationship between erythropoietic drive, iron, inflammation, and hepcidin level among a heavily transfused cohort of adults with SCD. In contrast to prior studies of patients with SCD, which mostly found hepcidin levels to be low [17,18], our cohort demonstrated significant variation. To explain differences in hepcidin level, we used a regression tree analysis, because the influence of hepcidin’s regulatory factors (erythropoietic drive, iron stores, and inflammation) were not independent of one another. The regression tree analysis demonstrated a regulatory hierarchy for hepcidin in SCD whereby erythropoietic drive (reticulocyte % and EPO) was the strongest predictor of hepcidin level, followed to a lesser extent by iron (ferritin) and inflammation (hs-CRP). These significant relationships were found independent of the patient’s recent treatment history, including previous red cell transfusion use.
Hepcidin values were highly variable in our cohort. The current study examined an adult cohort with significant iron burden from intermittent or chronic red cell transfusion therapy, which may explain why hepcidin levels were more variable than previous studies. Prior studies included children who uniformly had less iron burden compared to our cohort [17,18]. Low hepcidin values would be anticipated in a younger cohort with less iron burden due to a high erythropoietic drive without modulation from excess iron [20–25]. These low hepcidin values, expressed over a narrow range, may also have impacted the ability of prior studies to identify associations with clinical characteristics. The significant variation in hepcidin levels among subjects in our study, probably related to the heterogeneity of the cohort, likely allowed us to identify associations with reticulocyte %, EPO, and ferritin, even in univariate analyses. Additional analyses beyond univariate correlations were still necessary, however, because well-established interactions between the regulatory factors, especially EPO/reticulocyte percent and ferritin/hs-CRP, were present in our data, potentially affecting our ability to detect other associations.
To manage the interactions and determine the contribution of erythropoietic drive, iron, and inflammation to hepcidin level, we used a regression tree analysis. Based on this analysis, we found reticulocyte %, a marker of erythropoietic drive, to have the single largest effect on hepcidin concentration. Although the effect of erythropoietic drive on hepcidin has been well described in animal models and other patient populations [12,20–22,24–29], the precise mechanism by which this occurs is not known [30]. EPO decreases hepcidin expression in humans and animal models, but EPO is not a direct regulator of hepcidin [12]. Instead, EPO is thought to stimulate erythroid precursors to produce proteins (growth differentiation factor 15 (GDF15), twisted gastrulation protein (TWSG1), and erythroferrone) which act to suppress hepcidin in the context of ineffective erythropoiesis [24,29,31,32]. Because the regulatory mechanism of erythropoietic drive on hepcidin in humans has not been fully elucidated, we decided to include both reticulocyte % and EPO in the decision tree analysis. Potentially, reticulocyte % is more closely related to hepcidin level than EPO, because reticulocyte % more directly reflects erythoid activity in the bone marrow and thus GDF15, TWSG1, and erythroferrone production.
Since erythropoietic drive is the strongest regulator of hepcidin level, suppression of erythropoiesis may increase hepcidin and lower NTBI to produce an organ-protective effect, similar to studies in β-thalassemia [18,22–24]. The current study was not designed to answer this question. Although modulation of hepcidin could be a successful strategy to limit tissue injury, important differences between the pathogenesis of SCD and β-thalassemia may influence NTBI levels besides hepcidin. In β-thalassemia, hemolysis is mostly intramedullary due to ineffective hematopoiesis, whereas intravascular hemolysis occurs in SCD [33]. Compared to intravascular hemolysis, ineffective hematopoiesis may be associated with higher amounts of iron in the plasma compartment, which may saturate transferrin and generate more NTBI [6,14]. Given the complexities of iron metabolism in SCD, only a prospective, longitudinal study would be able to determine if suppression of erythropoiesis would decrease NTBI and limit end-organ damage from excess iron.
Our study has limitations. There was no control group with which to compare levels of hepcidin. The main objective of the study, however, was not to determine whether hepcidin levels were higher than controls, but to examine hepcidin within a complex cohort of adult patients with SCD and explore the relationship between hepcidin and regulatory factors. Another limitation was the cross-sectional design of the study. A one-time measurement of hepcidin may not represent the trend of values over time within an individual. Even with a single measure, however, the study was able to identify significant associations with markers of the hepcidin regulatory pathway. Finally, our study had a small sample size. Individual nodes in the regression analysis only had 5 subjects, which increase the possibility of type 1 and type 2 errors. Still, our study was adequately powered to determine medium and large effect sizes, and the main result of the study was very consistent with the known relationship between erythropoietic drive and hepcidin level as established for patients with β-thalassemia [22–24].
In summary, hepcidin levels are variable in previously transfused adults with SCD. Erythropoietic drive is the largest contributor to this variation. Our data suggests that the subset of patients with lower reticulocyte % and EPO have the highest hepcidin levels. Future studies could explore the modulation of hepcidin in patients with SCD and the effects on NTBI and tissue injury from excess iron.
Supplementary Material
Panel A shows hepcidin levels in patients with lower and higher reticulocyte percentages. Panels B–C then further examines hepcidin levels in the subgroup of patients with higher reticulocyte percentages. (A) Median hepcidin was higher in patients with erythropoietin ≤40 mlU/ml compared to those with erythropoietin > 40 mlU/ml, but only in the subgroup whose reticulocyte percentage ≤ 4.5% (N=10, 290 ng/ml vs. 120 ng/ml, p=0.03). (B) In the subgroup of patients with a reticulocyte percent > 4.5%, hepcidin was greater in those with erythropoietin > 80 mIU/ml compared to erythropoietin ≤ 80 mIU/ml, but only in the subgroup whose ferritin was > 1000 ng/ml (N=22, 118 ng/ml vs. 74 ng/ml, p=0.004). (C) In the subgroup of patients with a reticulocyte percent > 4.5%, hepcidin was greater in those with hs-CRP >10 mg/L compared to those with hs-CRP ≤ 10 mg/L, but only in the subgroup whose ferritin > 1000 ng/ml (N=22, 155 ng/ml vs. 75 ng/ml, p=0.04).
Acknowledgments
Funding Sources: None
We would like to thank the Clinical and Translational Sciences Institute (CTSI) and the Translational Research Unit (TRU) of the Medical College of Wisconsin for supporting this original research.
Footnotes
Author contributions: MSK designed the study, analyzed the data, and prepared the manuscript. KLK, ABR, and DN participated in study implementation, study recruitment, and manuscript revision. PS participated in data analysis and manuscript revision. JJF directly assisted in all phases of the study.
Conflicts of Interest: JJF is a consultant for NKT Therapeutics and receives research funding from NKT Therapeutics and Astellas.
References
- 1.Fung EB, Harmatz P, Milet M, Ballas SK, De Castro L, et al. Morbidity and mortality in chronically transfused subjects with thalassemia and sickle cell disease: A report from the multi-center study of iron overload. Am J Hematol. 2007;82:255–265. doi: 10.1002/ajh.20809. [DOI] [PubMed] [Google Scholar]
- 2.Darbari DS, Kple-Faget P, Kwagyan J, Rana S, Gordeuk VR, et al. Circumstances of death in adult sickle cell disease patients. Am J Hematol. 2006;81:858–863. doi: 10.1002/ajh.20685. [DOI] [PubMed] [Google Scholar]
- 3.Ballas SK. Iron overload is a determinant of morbidity and mortality in adult patients with sickle cell disease. Seminars in Hematology. 2001;38:30–36. doi: 10.1016/s0037-1963(01)90058-7. [DOI] [PubMed] [Google Scholar]
- 4.Porter J, Garbowski M. Consequences and management of iron overload in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2013:447–456. doi: 10.1182/asheducation-2013.1.447. [DOI] [PubMed] [Google Scholar]
- 5.Walter PB, Fung EB, Killilea DW, Jiang Q, Hudes M, et al. Oxidative stress and inflammation in iron-overloaded patients with beta-thalassaemia or sickle cell disease. Br J Haematol. 2006;135:254–263. doi: 10.1111/j.1365-2141.2006.06277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koren A, Fink D, Admoni O, Tennenbaum-Rakover Y, Levin C. Non-transferrin -bound labile plasma iron and iron overload in sickle-cell disease: a comparative study between sickle-cell disease and beta-thalassemic patients. Eur J Haematol. 2010;84:72–78. doi: 10.1111/j.1600-0609.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 7.Voskaridou E, Douskou M, Terpos E, Papassotiriou I, Stamoulakatou A, et al. Magnetic resonance imaging in the evaluation of iron overload in patients with beta thalassaemia and sickle cell disease. Br J Haematol. 2004;126:736–742. doi: 10.1111/j.1365-2141.2004.05104.x. [DOI] [PubMed] [Google Scholar]
- 8.Wood JC, Tyszka JM, Carson S, Nelson MD, Coates TD. Myocardial iron loading in transfusion-dependent thalassemia and sickle cell disease. Blood. 2004;103:1934–1936. doi: 10.1182/blood-2003-06-1919. [DOI] [PubMed] [Google Scholar]
- 9.Vichinsky E, Butensky E, Fung E, Hudes M, Theil E, et al. Comparison of organ dysfunction in transfused patients with SCD or beta thalassemia. Am J Hematol. 2005;80:70–74. doi: 10.1002/ajh.20402. [DOI] [PubMed] [Google Scholar]
- 10.Ghoti H, Goitein O, Koren A, Levin C, Kushnir T, et al. No evidence for myocardial iron overload and free iron species in multitransfused patients with sickle/beta -thalassaemia. Eur J Haematol. 2010;84:59–63. doi: 10.1111/j.1600-0609.2009.01355.x. [DOI] [PubMed] [Google Scholar]
- 11.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108:3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramos E, Kautz L, Rodriguez R, Hansen M, Gabayan V, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53:1333–1341. doi: 10.1002/hep.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piga A, Longo F, Duca L, Roggero S, Vinciguerra T, et al. High nontransferrin bound iron levels and heart disease in thalassemia major. Am J Hematol. 2009;84:29–33. doi: 10.1002/ajh.21317. [DOI] [PubMed] [Google Scholar]
- 15.Ghoti H, Fibach E, Westerman M, Gordana O, Ganz T, et al. Increased serum hepcidin levels during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndrome. Br J Haematol. 2011;153:118–120. doi: 10.1111/j.1365-2141.2011.08587.x. [DOI] [PubMed] [Google Scholar]
- 16.Ezeh C, Ugochukwu CC, Weinstein J, Okpala I. Hepcidin, haemoglobin and ferritin levels in sickle cell anaemia. Eur J Haematol. 2005;74:86–88. doi: 10.1111/j.1600-0609.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- 17.Kroot JJ, Laarakkers CM, Kemna EH, Biemond BJ, Swinkels DW. Regulation of serum hepcidin levels in sickle cell disease. Haematologica. 2009;94:885–887. doi: 10.3324/haematol.2008.003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kearney SL, Nemeth E, Neufeld EJ, Thapa D, Ganz T, et al. Urinary hepcidin in congenital chronic anemias. Pediatr Blood Cancer. 2007;48:57–63. doi: 10.1002/pbc.20616. [DOI] [PubMed] [Google Scholar]
- 19.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 20.Ginzburg Y, Rivella S. beta-thalassemia: a model for elucidating the dynamic regulation of ineffective erythropoiesis and iron metabolism. Blood. 2011;118:4321–330. doi: 10.1182/blood-2011-03-283614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gardenghi S, Marongiu MF, Ramos P, Guy E, Breda L, et al. Ineffective erythropoiesis in beta-thalassemia is characterized by increased iron absorption mediated by down-regulation of hepcidin and up-regulation of ferroportin. Blood. 2007;109:5027–5035. doi: 10.1182/blood-2006-09-048868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kattamis A, Papassotiriou I, Palaiologou D, Apostolakou F, Galani A, et al. The effects of erythropoetic activity and iron burden on hepcidin expression in patients with thalassemia major. Haematologica. 2006;91:809–812. [PubMed] [Google Scholar]
- 23.Origa R, Galanello R, Ganz T, Giagu N, Maccioni L, et al. Liver iron concentrations and urinary hepcidin in b-thalassemia. Haematologica. 2007;92:583–588. doi: 10.3324/haematol.10842. [DOI] [PubMed] [Google Scholar]
- 24.Pasricha SR, Frazer DM, Bowden DK, Anderson GJ. Transfusion suppresses erythropoiesis and increases hepcidin in adult patients with beta-thalassemia major: a longitudinal study. Blood. 2013;122:124–133. doi: 10.1182/blood-2012-12-471441. [DOI] [PubMed] [Google Scholar]
- 25.Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, et al. Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica. 2010;95:505–508. doi: 10.3324/haematol.2009.013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vokurka M, Krijt J, Sulc K, Necas E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol Res. 2006;55:667–674. doi: 10.33549/physiolres.930841. [DOI] [PubMed] [Google Scholar]
- 27.Theurl I, Aigner E, Theurl M, Nairz M, Seifert M, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113:5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 28.Kemna EH, Kartikasari AE, van Tits LJ, Pickkers P, Tjalsma H, et al. Regulation of hepcidin: insights from biochemical analyses on human serum samples. Blood Cells Mol Dis. 2008;40:339–346. doi: 10.1016/j.bcmd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Constante M, Layoun A, Santos MM. Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood. 2009;113:3593–3599. doi: 10.1182/blood-2008-08-173641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganz T, Nemeth E. Hepcidin and iron homeostasis. Biochim Biophys Acta. 2012;1823:1434–1443. doi: 10.1016/j.bbamcr.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, et al. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tanno T, Bhanu NV, Oneal PA, Goh SH, Staker P, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13:1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 33.Mariani R, Trombini P, Pozzi M, Piperno A. Iron metabolism in thalassemia and sickle cell disease. Mediterr J Hematol Infect Dis. 2009;1:e2009006. doi: 10.4084/MJHID.2009.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Panel A shows hepcidin levels in patients with lower and higher reticulocyte percentages. Panels B–C then further examines hepcidin levels in the subgroup of patients with higher reticulocyte percentages. (A) Median hepcidin was higher in patients with erythropoietin ≤40 mlU/ml compared to those with erythropoietin > 40 mlU/ml, but only in the subgroup whose reticulocyte percentage ≤ 4.5% (N=10, 290 ng/ml vs. 120 ng/ml, p=0.03). (B) In the subgroup of patients with a reticulocyte percent > 4.5%, hepcidin was greater in those with erythropoietin > 80 mIU/ml compared to erythropoietin ≤ 80 mIU/ml, but only in the subgroup whose ferritin was > 1000 ng/ml (N=22, 118 ng/ml vs. 74 ng/ml, p=0.004). (C) In the subgroup of patients with a reticulocyte percent > 4.5%, hepcidin was greater in those with hs-CRP >10 mg/L compared to those with hs-CRP ≤ 10 mg/L, but only in the subgroup whose ferritin > 1000 ng/ml (N=22, 155 ng/ml vs. 75 ng/ml, p=0.04).