Abstract
The superior elasticity, excellent resilience at high-frequency, and hydrophilic capacity of natural resilin have motivated investigations of recombinant resilin-based biomaterials as a new class of bio-elastomers in the engineering of mechanically active tissues. Accordingly, we report here the comprehensive characterization of modular resilin-like polypeptide (RLP) hydrogels and introduce their suitability as a novel biomaterial for in vivo applications. Oscillatory rheology confirmed that a full suite of the RLPs can be rapidly crosslinked upon addition of the tris(hydroxymethyl phosphine) (THP) cross-linker, achieving similar in situ shear storage moduli (20k±3.5Pa) across various material compositions. Uniaxial stress relaxation tensile testing of hydrated RLP hydrogels under cyclic loading and unloading showed negligible stress reduction and hysteresis, superior reversible extensibility, and high resilience with Young’s moduli of 30±7.4kPa. RLP hydrogels containing MMP-sensitive domains are susceptible to enzymatic degradation by MMP-1. Cell culture studies revealed that RLP-based hydrogels supported the attachment and spreading (2D) of human mesenchymal stem cells (hMSCs) and did not activate cultured macrophages. Subcutaneous transplantation of RLP hydrogels in a rat model, which to our knowledge is the first such reported in vivo analysis of RLP-based hydrogels, illustrated that these materials do not elicit a significant inflammatory response, suggesting their potential as materials for tissue engineering applications with targets of mechanically demanding tissues such as vocal fold and cardiovascular tissues.
Keywords: bio-elastomer, hydrogel, resilin-like polypeptide, tissue engineering, vocal fold
1. Introduction
Engineering protein-based hydrophilic elastomers with controlled mechanical properties and specified bioactivities continues to offer promising opportunities in regenerative medicine.[1–6] Current interest in engineering mechanically active tissues to treat conditions such as vocal fold disorders and cardiovascular pathologies has continued to motivate the development of novel biomaterials.[7–11] In particular, for applications in treatment of vocal fold disorders, multifunctional materials with well-designed biological function and biomechanical properties intended to mimic those of the native extracellular matrix (ECM) are needed to address underlying injury-induced pathologies and scarring.[8,10–13]
Numerous investigations have highlighted the importance of mechanical stimulation in regulating matrix composition of the vocal fold (VF) lamina propria (LP).[14–17] However, the choice of either natural or synthetic biomaterials for use in vocal fold tissue regeneration has been limited owing to difficulties in meeting necessary mechanical demands (storage moduli: 500-5000Pa over 20-180Hz vibration frequencies; non-linear stress-strain relationship; Young’s modulus of 20-40kPa at low strain)[9,16,18] while also imparting desired cell-tissue interactions.[15,19] Previous attempts toward in vitro vocal fold tissue engineering have utilized reconstituted or chemically modified ECM components (e.g., hyaluronic acid (HA), collagen matrix and fibrin),[10,12,20–22] polymeric scaffolds or hydrogels,[7,15,23] and/or peptide-based materials[11,24,25] as the scaffolding materials. While the mechanical properties of these materials have been possible to control and functional improvements have been reported, the mechanical properties have not reliably matched those required for the vocal folds, with materials showing significant hysteresis and fatigue upon application and release of strain, as well as limited tuning of biological properties.[5,6,26] Thus new biomaterials that exhibit dynamic, instant mechanical response and superior elastomeric properties, with independently tuned biological cues, would offer highly useful alternatives.
There have been numerous reports of the design of recombinantly engineered elastomeric proteins to produce materials with desirable biomechanics and tunable cell-instructive properties that better capture those of the natural extracellular matrix (ECM).[5,6,27–29] Tremendous research effort has been devoted to synthesizing recombinant elastin-like polypeptide-based bio-rubbers that mimic the inherent elasticity of natural elastin with outstanding mechanical properties.[5,6,30,31] These approaches obviate concerns about the potential for disease transmission from native, purified elastins,[32] and generate recombinant tropoelastin-based and elastin-like-polypeptide biomaterials based on the consensus sequence of tropoelastin, VPGVG, for various biomedical applications.[6,11,33–38] The engineered ELP-based biomaterials exhibit tunable mechanical properties (E=0.25MPa~1.2MPa) with resilience values from 50% up to 80%, and support adhesion, proliferation and differentiation of multiple cell lines. Tropoelastin-based biomaterials have significantly overcome solubility issues, demonstrated tunable mechanical stiffness, and exhibited improved resilience values compared to many ELP-based counterparts (single-molecule AFM studies of tropoelastin show its near-perfect resilience).[36,37,39–43] In studies reported to date on bulk hydrogels of these materials, however, the transient mechanical behavior, the resilience, and high-frequency behavior of the materials have only partially met the requirements for the vocal fold.[30,32,39,44–48]
Native resilin, another rubber-like protein found in insects, also exhibits useful elasticity characterized by low stiffness, high extensibility, and efficient energy storage,[49–53] as well as a remarkable fatigue lifetime, surviving repeated contraction/extension cycles (in some cases greater than 400 million cycles) at elevated frequencies from 200 to 4000Hz (which as noted above spans the vibration frequency range of the vocal fold (100-1000Hz)).[1,49,54–56] Biosynthetic approaches have been used to produce recombinant resilins that capture the native protein’s mechanical properties, and these resilin-like polypeptides have demonstrated potential advantages as highly mechanically active scaffolding materials.[56–60] In the first step towards producing such scaffolds for as applications in treating vocal fold pathologies,[61–65] we developed mechanically robust resilin-like polypeptide gels based on the putative resilin consensus sequence from the first exon of the D. melanogaster CG15920 gene, equipped with multiple biological active domains, with results demonstrating mechanical properties comparable to those of native vocal fold tissues. Specifically, these cross-linked RLP hydrogels exhibited excellent resilience and tunable mechanical properties with, at low frequencies, shear moduli ranging from 500Pa to 10kPa and Young’s moduli from 15 to 35kPa, and, at high frequencies (30-150Hz), storage shear moduli between 1000 to 2000Pa.[61,62]
Given these mechanical similarities between RLPs and the properties of vocal fold tissue (mentioned above), and coupled with their increased hydrophilic capacity relative to other elastomers,[52,61–63,65,66] here we present the characterization of an unreported suite of RLP-based hydrogels that exhibit controlled hydrogel degradation, dynamic mechanical properties, and biological responsiveness. These RLP hydrogels not only show similar viscoelastic properties and dynamically reversible elasticity to that of native vocal mucosa tissues, but also promote interactions with hMSCs. Preliminary in vitro and in vivo evaluation of inflammation and biocompatibility of these RLP-hydrogels indicate that these scaffolds show promise as biomaterials for encapsulation of cells for vocal fold regeneration.
2. Results and Discussion
2.1. Tunable Mechanical Properties of Multifunctional RLP-based Hydrogels
The need to independently tailor specific cell-matrix interactions motivated us to expand the versatility of the recombinant RLPs previously produced in our laboratories.[26,61,62,67] We thus designed multiple RLP-based constructs with 12 repetitive consensus motifs (GGRPSDSF/MGAPGGGN) derived from Drosophila melanogaster but bearing different biological modules that can be independently incorporated into a final matrix to impart cell adhesion, MMP-sensitivity, or growth factor sequestration.[63] Here we report the use of these constructs, but with a set of expanded material compositions, in more targeted studies of activities relevant for in vivo tissue engineering applications.
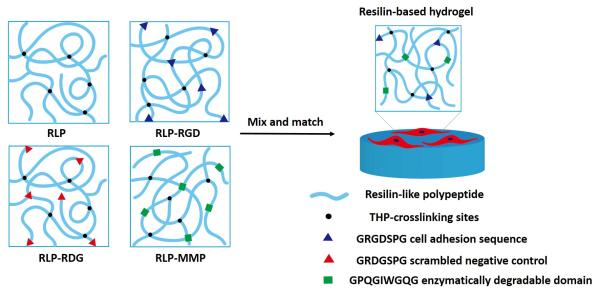
The fibronectin-derived, integrin-binding domain GRGDSPG was employed in these RLP constructs to impart cell adhesion,[68] with GRDGSPG included as a scrambled negative control. The MMP-1-sensitive domain GPQGIWGQG, derived from human α(I) collagen,[69,70] is also included in these constructs owing to not only its successful implementation in cell-mediated remodeling of polymeric biomaterials, but also because its general relevance as a collagenase substrate to mediate cell invasion, migration, proliferation, new tissue growth, and vocal fold scar tissue modulation.[71,72] The MMP-1 sensitive sequence is particularly relevant given that collagens I, II, III are the main proteins in the vocal fold lamina propria.[7,8,18] All RLP constructs were expressed in E coli. M15[pREP4] expression hosts and purified via Ni-NTA affinity chromatography as previously described.[62,63,65] The purified polypeptides were cross-linked with tris(hydroxymethyl phosphine) (THP)-facilitated, Mannich–type condensation chemistry to rapidly form solid hydrogels and support cell encapsulation.[63,73,74] A schematic of the different gel compositions is shown in Scheme 1.
Scheme 1.
Schematic of RLP-based hydrogels with various material compositions.
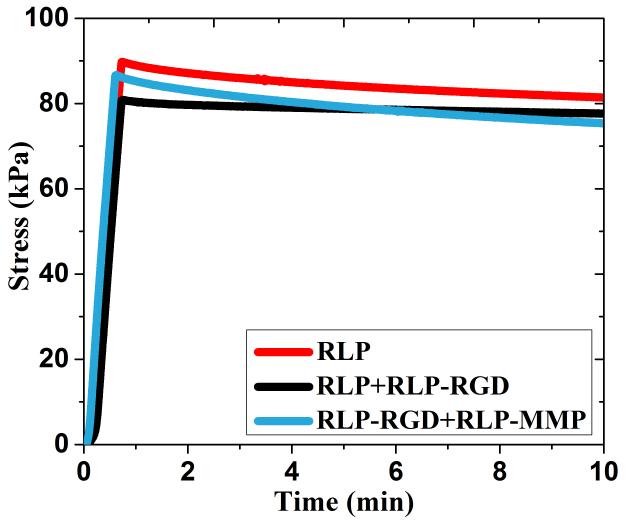
The time-dependent mechanical properties of tissues are important to their function and repair, in particular, the relevance of transient mechanical response of the vocal fold tissue during phonation.[16,65,75,76] Much effort has been focused on matching the shear modulus of materials with tissue intended for their application, however, there are few investigations characterizing how these materials behave under constant mechanical strain. In order to explore energy dissipation behavior, we conducted stress-relaxation experiments on these compositionally diverse RLP-based hydrogels.[65] Oscillatory rheology was employed initially to confirm that RLP hydrogels of three different compositions (100% RLP, 50% RLP+50% RLP-RGD, 50% RLP-RGD+50% RLP-MMP), prepared at a concentration of 20wt%, exhibited similar storage moduli (Figure S1a). Stress relaxation experiments were then conducted on these three 20wt% RLP hydrogels, with the strain set to 60% (in the middle of reported deformation of vocal fold tissues),[7,9,10,76] while the stress was monitored over a 10-minute period; representative data are shown in Figure 1. When the strain was initially applied to the hydrogel, the stress increased rapidly and reached a maximum value (~80kPa); this behavior was observed across all three hydrogel compositions, demonstrating the highly elastic response of the RLP-hydrogels to external energy input despite the differences in final compositions. Importantly, the data illustrate a minimal reduction in stress over 10 minutes, highlighting the efficient energy input and minimal energy dissipation of these RLP-based materials, in sharp contrast to the significant stress relaxation observed for previously reported ELP-based hydrogels and GB1-RLP proteins (which is likely due to the unfolding of those polypeptide chains).[60,76,77] Importantly, the negligible reduction in stress observed in stress relaxation experiments of the RLP (illustrated in Figure 1) are consistent with the lack of stress relaxation that has been previously observed for native canine vocal fold tissues that have been stretched from 12% to 43% strain,[76,78,79] and also to those observed for a swollen cross-linked Rec1-resilin hydrogel, although the strain in the previous RLP studies was limited to only 0.4%.[80]
Figure 1. Representative uniaxial stress relaxation behavior of compositionally different RLP hydrogels at 60% strain.
All hydrogels were formed at a polypeptide concentration of 20wt%, with a 1:1 (amine:hydroxyl) cross-linking ratio. Three repeats of each hydrogel composition were tested and the results are reproducible with less than 10% difference among multiple sample repeats observed.
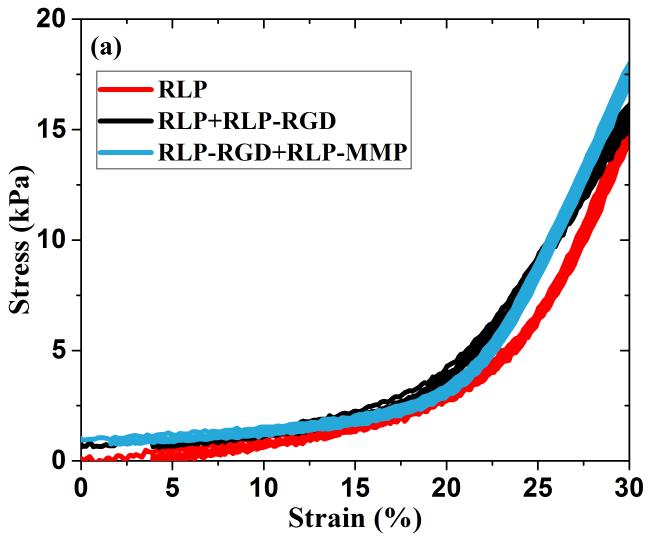
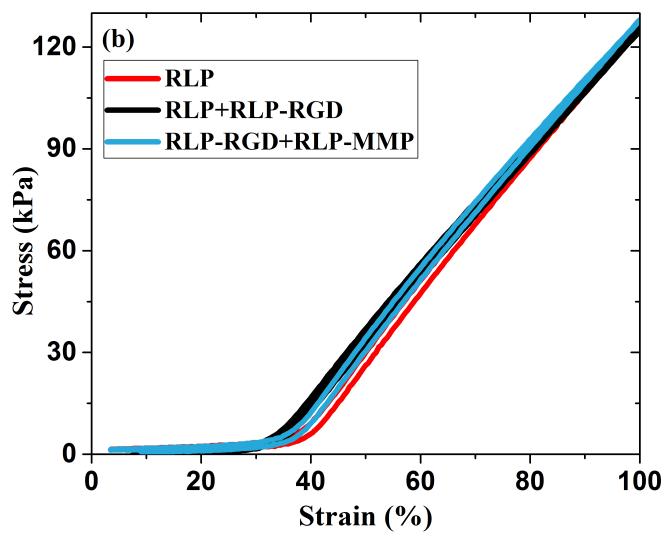
Repetitive loading and unloading in a standard uniaxial tensile testing format, over a range of values relevant to vocal fold deformation, was employed to determine the Young’s modulus and calculate the resilience of the hydrated RLP films (20wt%). For each hydrogel film, three consecutive loading and unloading cycles were applied: first, three cycles to 30%, then three cycles to 60%, and finally three cycles to 100% strain. Figure 2a displays results for three cycles to 30% strain (including the initial loading and unloading cycle), which is the approximate average strain sustained by the lamina propria during phonation.[7,9,16,81] Figure 2b shows the third loading and unloading cycle for 100% strain, of relevance to the longitudinal strain experienced by the vocal fold ligament during the production of high-pitched sound.[7,8,16,81] (Figure S1b shows the third loading and unloading cycle at 60% strain. Three loading and unloading cycles for 60% and 100% strain yielded similar traces compared to those observed in 30% strain and thus only the third stress-strain cycle is presented for simplicity.) The near-perfect overlap of these stress-strain curves during loading and unloading confirms the negligible hysteresis and excellent elasticity of the RLP-based hydrogels over multiple compositions, even up to 100% strain. While the nonlinear generation of stress with applied strain (with very low, but measurable amounts of stress at strains below 30%) is similar to previously reported data for polymer-based elastomers, human vocal ligament tissues, and silk-derived biomaterials,[26,81,82] the observed resilience of these RLP materials is significantly greater than that for other structural protein-based hydrogels, and is consistent with our previously reported results.[26,62,63,65]
Figure 2. Uniaxial tensile testing of RLP-based hydrogels.
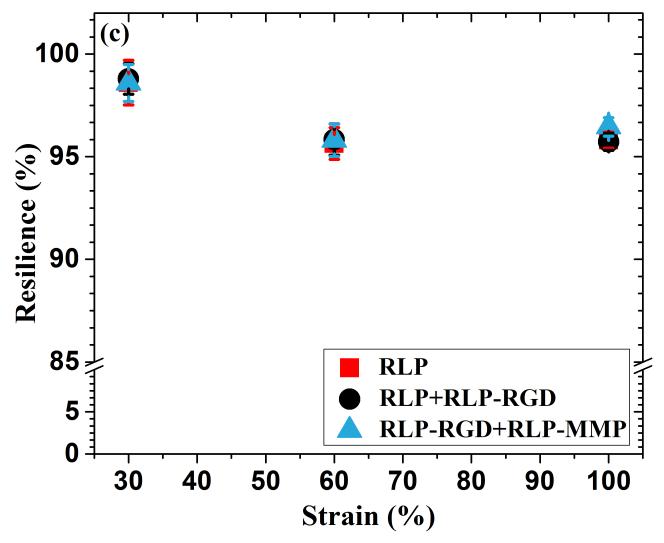
(a) Three repeats of uniaxial cyclic tensile testing up to 30% strain at various RLP hydrogel compositions, (b) third cycle of loading and unloading tensile testing up to 100% strain for 3 different RLP hydrogel compositions, and (c) resilience values of the third cycle of loading and unloading at 30%, 60% and 100% strains for various RLP hydrogel compositions.
The resilience of these hydrated RLP-based gels, calculated by dividing the area under the unloading curve by that under the loading curve for each strain-stress cycle, including the initial cycle that shows a slight hysteresis, is generally in excess of 95% and in some cases as high as 99% (Figure 2c and Table S1), for all three compositions across a full range of strain values relevant to human phonation.[7,9,16,75,81] These values are comparable to those reported for natural resilin (90%)[83,84] and other previously reported resilin-based biomaterials (94-100%),[56,58,60,62,85] and improved over available data reported for bulk hydrogels of other natural elastomeric materials, tropoelastin-based (70-80%)[36,37,40] and ELP-based hydrogels (50-80%).[26,77,86,87] Although select synthetic polymer-based hydrogels (e.g., thiol-norbornene-based PEG-PDMS hydrogels, and poly(acrylic acid)-co-poly(n-butyl acrylate)-based block copolymers) have also achieved such high resilience, the RLP-based hydrogels offer the advantage, in regenerative medicine applications, of exhibiting tunable biological properties.[82,88,89] In general, the hydrogel properties of the three RLP-based composition were also very similar (Table S2). The non-linear stress-strain behavior of RLP hydrogels are consistent with the longitudinal elastic properties reported from the stress-strain measurements of human vocal ligaments in vitro. The Young’s moduli, calculated from the linear region (5-15%) of the stress-strain curve (Figure S2), were approximately 30kPa, which is within the range of those reported from human vocal fold tissues (20-40kPa) and also comparable to other RLPs (~25.5kPa).[56,81,85]
2.2 In vitro Degradation of RLP-based Hydrogels
Synthetic hydrogel scaffolds that are designed as a provisional matrix at a site of injured tissue should have a degradation rate that approximates the kinetics of formation of new cell-secreted extracellular matrix, resulting in optimal tissue integration and mechanical stability comparable to uninjured native tissue.[90,91] Accordingly, an MMP-sensitive domain (GPQGIWGQG) was incorporated into the RLP sequences because of its relevance as a collagenase to mediate cell invasion, proliferation, migration in collagen (type I)-rich connective tissues.[8,72,92] Degradation of the RLP hydrogels was monitored in the presence of 200nM MMP-1 at 37°C over 72 hours via direct measurement of the modulus using oscillatory rheology (Figure 3), to assess the kinetics of degradation of the hydrogels as well as the changes in mechanical properties that occur with degradation.[93] While the high enzyme concentration of MMP-1 (200nM) used in the RLP hydrogel degradation reactions may not be mimetic of MMP-1 concentrations in vivo, it was selected owing to its similarity to concentrations reported in previous studies for ease of comparison.[63,69,70,94] Both axial and radial swelling were negligible; therefore, the reduction in storage moduli was directly related to the scission of MMP-sensitive domain in the RLP-MMP construct. Hydrogels were formed in situ on the Peltier plate; degradation was initiated, by the addition of the MMP-1 enzyme, after the storage modulus of the hydrogels reached an initial plateau (Figure S1d).
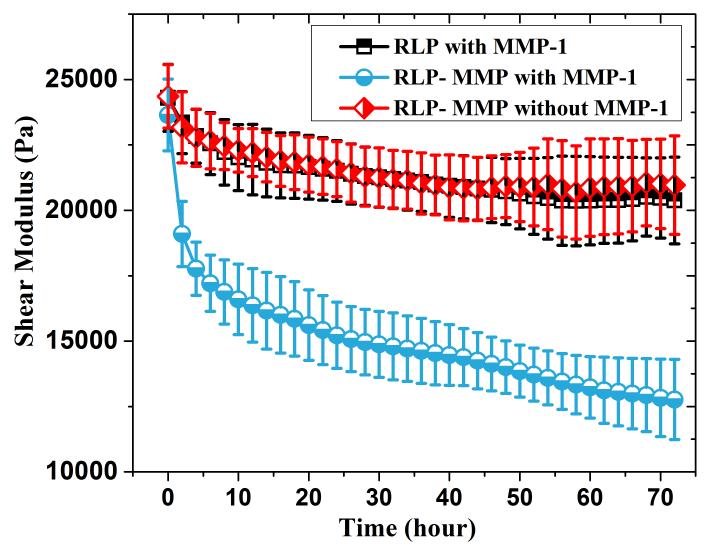
Figure 3. Enzymatic degradation of RLP and RLP-MMP hydrogels monitored via oscillatory rheology.
Time sweep experiments for RLP-MMP (circle) and RLP (square) hydrogels incubated in the presence of 200nM MMP-1, and an RLP-MMP hydrogel incubated without MMP-1 (diamond). All hydrogels were formed at a protein concentration of 20wt%, with a 1:1 (amine : hydroxyl) cross-linking ratio, and incubated at 37°C in 2mL PBS buffer (pH 7.4). A bath volume of 2 mL (50-fold greater than the volume of the hydrogel) provided an adequate sink so that the pH and MMP-1 enzyme concentration were unaffected during the experiment.
As illustrated in Figure 3, an RLP-MMP hydrogel incubated with the MMP-1 enzyme displayed a rapid reduction in storage modulus during the initial 20 hours of incubation followed by a slower decrease for the next two days, with roughly a 50% decrease in modulus (from approximately 24kPa to 12kPa) over 3 days. While this rate of degradation may be more rapid than desired for select tissue regeneration applications, the results clearly indicate the sensitivity of the RLP matrices to degradation by relevant enzymes. No such degradation was observed for two different negative controls (20wt% RLP with 200nM MMP-1 and 20wt% RLP-MMP without MMP-1), only a slight initial decrease that may be due to a small degree of swelling. A rapid (<5hr) initial decrease in modulus of 20wt% RLP-MMP hydrogel incubated with 200nM MMP-1 may be due to heterogeneity derived from loosely cross-linked hydrogel around the periphery. The modulus of the RLP-MMP hydrogels did not decrease to zero, perhaps as a result of limitations in the diffusion of the MMP-1 enzyme (19.9 kDa) to the center of the hydrogel over the time period of the experiment. Maintenance of mechanical integrity over longer timescales, which can also be tuned based on the concentration of MMP-sensitive domains in a given RLP matrix, offers opportunities to facilitate cell proliferation, migration and matrix remodeling as in other ECM-mimetic hydrogel scaffolds.[29,91,94–99]
2.3. Cell Attachment and Spreading
Human mesenchymal stem cells were chosen for cell culture experiments owing to the similarity of cell surface markers, phenotypic characteristics, and differentiation potential with that of human vocal fold fibroblasts (HVFFs) derived from lamina propria.[100] In addition, HVFFs are presently not a clinically useful option for tissue regeneration as they can only be obtained from normal tissue which can be difficult to isolate, and the collection of these cells causes further injury/scarring in patients.[8,10,100,101] The ability of the cross-linked RLP-based hydrogels to support the attachment of hMSCs was investigated via seeding cells on the surface of 20wt% hydrogels with various material compositions (Figures S3 and Figure 4). These experimental conditions were chosen owing to their simplicity in assessing the engagement of the RGD via facile measurement of cell spreading, which is more difficult to assess in three-dimensional culture of the RLP gels. (We have previously demonstrated that encapsulated hMSCs remained highly viable (>90%) for over 14 days in a non-degradable RLP hydrogel system (20wt% 50% RLP+50% RLP-RGD).[63] Cell spreading in these matrices was limited however, and current investigations are focused on promoting spreading in 3D culture via optimization of the concentrations of RGD and MMP domains in RLP hydrogels.) While the ability of hMSCs to adhere to a variety of RGD-modified surfaces has been widely reported,[68,102] the assessment of such behavior on RLP-based matrices of different compositions, but similar mechanical properties, has yet to be confirmed, despite its relevance to applications development. The content of RGD in the gels was systematically varied from 0% RLP-RGD, 50% RLP-RGD to 100% RLP-RGD, while keeping the overall protein concentration constant.
Figure 4. Immunochemical analysis of hMSCs stained, after 72 hours, for nuclei, vinculin, and actin cytoskeleton, on the surface of 20wt% RLP hydrogels.
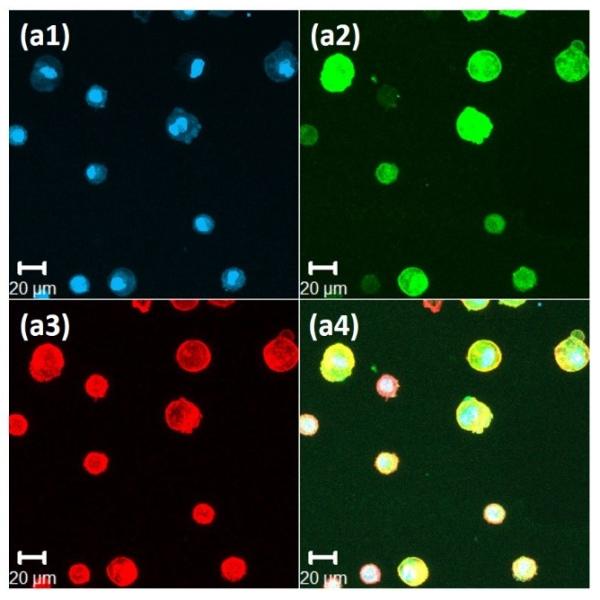
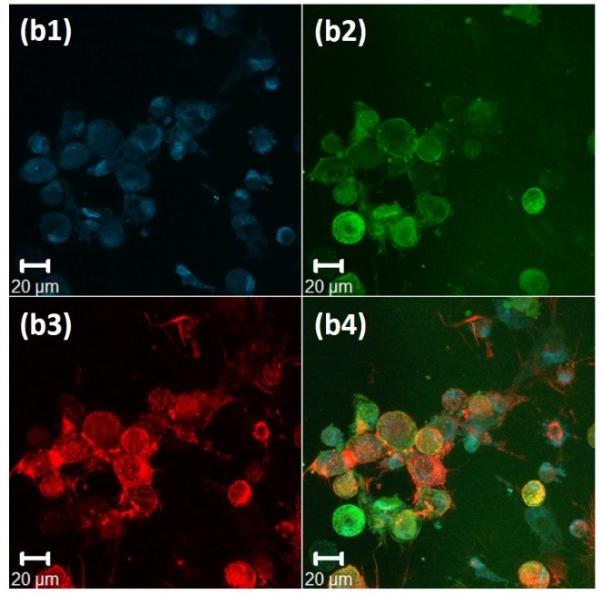
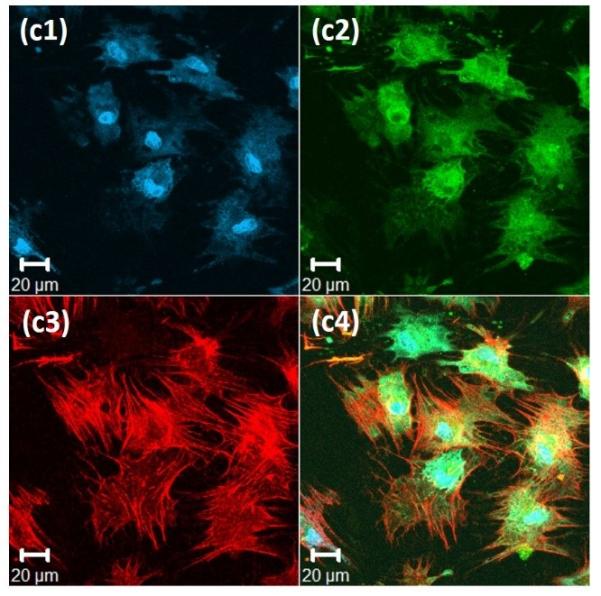
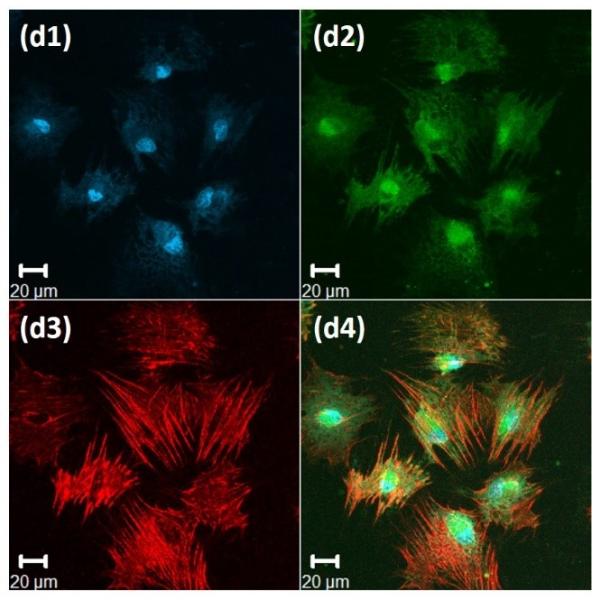
(a) 100% RLP, (b) 100% RLP-RDG, (c) 50% RLP and 50% RLP-RGD and (d) 100% RLP-RGD. All hydrogels were crosslinked at a 1:1 ratio of amine : hydroxyl groups. Data for each hydrogel composition is separated into 4 panels: (1) Cell nuclei counterstained by Draq5 (blue); (2) Focal adhesion sites visualized (green) by treatment with anti-vinculin and a FITC-labeled secondary antibody; (3) F-actin filaments visualized (red) by treatment with TRITC-phalloidin; and (4) the merged image of the triply stained hydrogels (Draq5, vinculin and TRITC-phalloidin).
Immunochemical analysis of hMSCs seeded on various compositions is presented in Figure 4 for 100% RLP (Figure 4a) and 100% RLP-RDG (Figure 4b) as negative controls, as well as 50% RLP+50% RLP-RGD (Figure 4c) and 100% RLP-RGD (Figure 4d). F-actin stress fibers (red) along the edge of the cell body are clearly visualized in Figure 4c3 and Figure 4d3. As shown in Figures 4a and Figure 4b, hMSCs displayed rounded morphologies (with essentially no spreading) after 72 hours when cultured on the surfaces of hydrogels that lack RGD motifs or that contain the scrambled sequence RDG, as expected owing to the lack of RGD integrin-binding motifs.[67,103] hMSCs seeded on the surface of 50% RLP+50% RLP-RGD or 100% RLP-RGD, instead, exhibited stellate morphologies over 3 days of culture. The hMSCs on RGD-containing RLP-hydrogels (shown in Figure 4c and 4d for 10 wt% hydrogels, and observed for 20wt% 50% RLP-MMP+50% RLP-RGD hydrogels (data not shown here)) develop mature transverse stress fibers through the entire cell body, whereas those on RLP-hydrogels without RGD sequences show cortical F-actin and in the case of RDG-containing hydrogels, unattached cells exhibited the tendency to aggregate. hMSCs seeded on all hydrogel compositions exhibited excellent viability (Figure S3); similar assessments of cell viability and phenotype under vibration are underway.
Individual cells (n>35) were manually outlined and cell spreading area and aspect ratio (i.e., elongation) were analyzed using NIH ImageJ software; a histogram of cell area distribution (Figure S4a) and evaluation of cell area and aspect ratios (box charts in Figures S4b and S4c) clearly illustrate the significant increase in cell area (from ca. 500 μm2/cell to ca. 2300 μm2/cell) and increase in aspect ratio (from close to 1.0 to ca. 2.0) when comparing the control hydrogels versus those containing RGD (see Supporting Information for details); greater spreading was observed for the 50% RLP-RGD + 50% RLP hydrogels indicating the opportunity to modulate cell behavior with variations in hydrogel composition. These observations are consistent with previous work demonstrating that primary human skeletal muscle-derived myoblasts with similar cell spreading areas exhibited a bimodal distribution with the maximum elongation (aspect ratio) at an intermediate RGD density, on micropatterned elastin-like polypeptide (ELP)-based biomaterials.[104] Likewise, Schön and coworkers systematically studied the influence of adhesion ligand concentration on cancer cell behavior by using RGD-functionalized gold nanoparticle patterns that were immobilized on glass via block copolymer nanolithography and the results indicated that human melanoma cells spread optimally, formed focal contacts, and reorganized cytoskeletal fibers at an intermediate RGD ligand density of ~350 sites/μm2.[105]
2.5 Inflammatory Responses of RLP-based Hydrogels
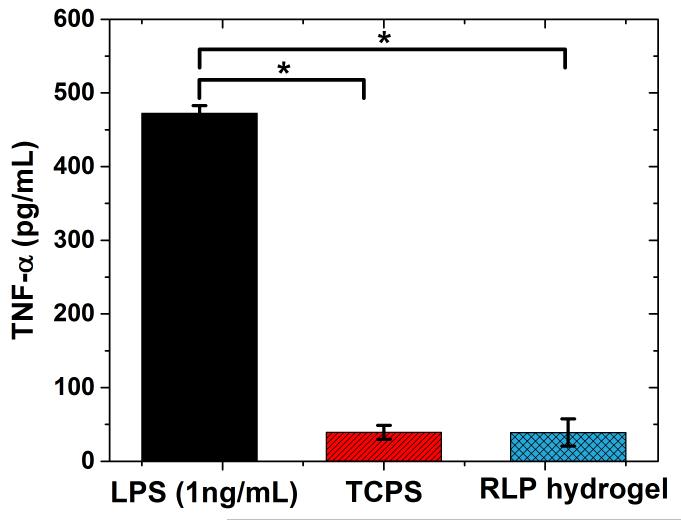
The acute and sub-acute phases of inflammation after hydrogel injection can cause abnormal wound healing and potentially damage host tissue.[106] In particular, pro-inflammatory cytokines (e.g., tumor necrosis factor-alpha: TNF-α) are heavily involved in controlling the orientation of different physiological and pathophysiological processes in the event of wound healing, inflammatory reactions, and foreign body reactions.[8,107,108] Therefore, investigation of the cellular inflammatory responses as measured by production of inflammatory cyotkines is essential to gauge the suitability of the RLP hydrogels for in vivo application. TNF-α was chosen as the inflammatory marker to investigate here based upon its involvement in the attraction of inflammatory cells (e.g., nuetriphils, monocytes), monocyte differentiation into macrophages, as well as in stimulation of MMP secretion from monocytes, macrophages and fibroblasts in vocal fold tissues.[8,10,109,110] A preliminary in vitro investigation of the pro-inflammatory potential of RLP hydrogels was undertaken by culturing murine-derived RAW264.7 macrophage-like cells on the surface of the gels and monitoring the expression of TNF-α, via ELISA, as in biocompatibility/immunogenicity studies of other implantable biomaterials.[111,112] Figure 5 shows that after 8 hours of incubation, the expression of TNF-α by macrophages cultured on the RLP matrices was negligible, similar to the activation level displayed from the negative control, and it was 10-fold lower compared to that of the positive control. Similarly low levels of expression of TNF-α has also been observed for other materials, including a preclinical hydrogel (HyStem®-C: carboxymethyl hyaluronan and thiolated gelatin) cultured with human vocal fibroblasts in 2D and 3D formats,[106,113] as well as for poly(ethylene glycol) (PEG) hydrogels.[114] The lack of significant activation by macrophages by the RLP hydrogels suggests their potential as an elastomeric substrate with minimal inflammatory properties.
Figure 5. Activation of RAW 264.7 macrophages incubated for 8 hours on a 20wt% THP-crosslinked RLP only hydrogel, measured through production of TNF-α.
The same number of RAW264.7 murine macrophages were cultured on the surface of TCPS supplemented with 1ng/mL LPS derived from E coli. as a positive control, on the surface of TCPS without LPS as negative control, and directly on the surface of THP cross-linked RLP hydrogels (20wt%). Macrophages cultured on these surfaces and all hydrogels showed 100% viability (not shown). Error bars correspond to standard deviation from the mean; the TNF-α expression is not statistically different between TCPS and RLP hydrogels; *(p<0.0001) the difference is statistically significant between the indicated groups determined via Student’s t-test analysis.
2.6. In vivo Biocompatibility Studies of Transplanted RLP-based Hydrogel
The in vivo biocompatibility of RLP-based pre-formed hydrogels (20wt% RLP, 1:1 crosslinking ratio (amine: hydroxyl), cast into a 10mm×10mm×1mm thin film) was evaluated histologically after subcutaneous transplantation in a rat model (male Sprague Dawley rats, 7 weeks old). Evaluation of the skin near the site of the implant one week post-transplantation revealed no redness, formation of scabs, or swelling. The tissue surrounding the gel implant (highlighted by the yellow boxed area) was then excised for histological assessment; all replicates showed similar results regarding H&E, CD3 and CD31 immunohistological staining, and representative histological images are presented in Figure 6. Visual observation after sacrifice indicated that the RLP hydrogel showed a less distinct interface between the gel and adjacent tissues than initially, although any degradation of the RLP hydrogels could not be quantified due to the integration of the RLP materials with surrounding subcutaneous tissues at the transplantation site, which is consistent with the initial rapid hydrogel degradation observed in vitro (Figure 3). H&E staining of excised tissues (Figure 6) indicated the presence of a small number of inflammatory cells at the interface of the tissue with RLP hydrogel remnants. Minor traces of cell infiltration were also observed around the transplanted hydrogel, although without any evidence of foreign body giant cells or micro-vessel formation. These data indicate that THP-crosslinked RLP hydrogels did not induce a significant acute inflammatory response at the harvest time points. Likewise, staining for CD3 (cluster of differentiation 3, an immunohistochemical marker for T-cells), and also for CD31 (cluster of differentiation 31, which is a major constituent of endothelial intercellular junctions and present on the surface of monocytes, neutrophils and some types of T-cells),[115–117] showed a lack of positive cells around the transplantation site, further indicating the lack of inflammatory cells (e.g. macrophages, neutrophils, lymphocytes). Given the role of CD31 in the transendothelial migration of neutrophils and the formation of microvasculature, the lack of CD31-positive cells also indicated that the capillary vascularization generally observed with the inflammatory response was absent.
Figure 6. Subcutaneous transplantation of 20wt% RLP hydrogels in Sprague Dawley (SD) rats sacrificed after one week.
Tissue samples were stained and histological images are presented with hematoxylin and eosin (H&E), cluster of differentiation 3 (CD3) and cluster of differentiation 31 (CD31) staining. The yellow box highlights the location of gel transplantation and the remnants of RLP-hydrogel after one week post transplantation.
Together, these results suggest that the RLP hydrogel did not initiate the inflammatory response that is often induced by foreign materials within a few days post-transplantation.[118] The minimal inflammation and cell infiltration observed here is similar to that observed from in situ cross-linkable ELP gels in a goat osteochondral defect model[119] and a pre-formed ELP material in a rabbit osteochondral knee defect.[45] The data here represent a significant improvement, however, over implanted, preformed silk-hydrogels in mouse model (BALBc female mice, 4-6 weeks old) that elicited a typical foreign body response with significant accumulation of immune cells at the interface of the gel and tissue.[26] Although more quantitative analysis of in vivo biocompatibility is needed for the further development of RLPs, the data here comprise the first report of recombinant resilin-based materials in animal models. These results, combined with RLP’s unusual mechanical properties, suggest the promise of RLPs in engineering vocal fold and other mechanically demanding tissues.
3. Conclusions
In summary, the present work characterizes a new class of cytocompatible, biocompatible, and biodegradable elastomeric hydrogels for use in vocal fold and potentially other tissue therapies. These novel injectable RLP-based hydrogels exhibit robust mechanical strength, reversible extensibility, exceptional resilience, and highly tunable properties; they also support cell survival, attachment, and spreading, and elicit minimal inflammatory responses in vitro and in vivo. This unique combination of properties addresses many of the shortcomings of currently employed polymer-based elastomeric biomaterials. The RLP biomaterials have significant potential for supporting the biological and mechanical properties necessary for in vivo application, while maintaining a high level of versatility, thus expanding their potential use in a range of biomedical applications.
4. Experimental Section
Expression and purification of recombinantly synthesized RLPs
All RLP constructs were cloned, transformed, expressed and purified according to previously published protocols.[61–63]
In situ hydrogel degradation via oscillatory shear rheology
The degradation of RLP hydrogels as a function over time was monitored via oscillatory rheology by swelling the perimeter of the hydrogel in PBS buffer with and without the addition of 200nM MMP-1 enzyme. Hydrogels were formed as described in detail in the Supporting Information with slight modification to the previous procedure. Before adding the solutions to the Peltier plate, a polyvinylchloride (PVC) ring with a height of 7mm, inside diameter of 35mm and an outside diameter of 42mm was pre-coated with a thin film of silicone vacuum grease on the bottom and positioned above the geometry by a ring stand and clamp. After the storage modulus reached a steady value, the rheometer bearing was locked to prevent disruption of the hydrogel. The mineral oil was washed away three times with hexane before the PVC tube was firmly pressed onto the Peltier plate to create a water-tight seal with the vacuum grease. Then 2mL buffer (50mM phosphate, 150mM NaCl, at either 0 nM or 200 nM MMP-1 pH7.4) was applied to fill the volume between the gel and cylinder walls, and mineral oil (1.5mL) was gently pipetted on top of the buffer surface to prevent buffer evaporation. The degradation experiment was monitored over 72 hours and data points were collected at 6 rad/s, 1% strain amplitude, and 37 °C. Storage moduli (G’) were approximately 50~100 fold higher than loss moduli (G’’) during the entire degradation experiments; only storage moduli (G’) are presented in the figure for simplicity.
Uniaxial tensile testing
RLP films (width 2mm; length 6mm, n=4) for uniaxial mechanical tensile testing were prepared in contact lens molds (Bausch & Lomb, Rochester, NY) by the addition of desired amounts of THP to 20wt% polypeptide solutions with various RLP compositions in PBS buffer. The uniaxial tensile testing characterization procedure has been previously reported and a detailed description can be found in the Supporting Information.
In vitro cell culture
Human bone marrow-derived mesencymal stem cells (hMSCs) (Lonza, Walkersville, MD, passage 3-6) were sub-cultured at a seeding density of 5000-6000 cells/cm2 on T-150 flasks (Corning, New York, NY) at 37°C with 5% CO2 in MSC maintenance media (Lonza, MD). The cell seeding density was not chosen to mimic the cell density in vivo in vocal fold tissues, but instead was selected based upon previous success of culturing cells on different RLP-based hydrogels. Medium was refreshed every 3 days. Upon reaching ~80% confluence, cells were trypsinized, counted, centrifuged, and resuspended in the same maintenance medium at a desired cell density. Various RLP hydrogels were prepared by adding 50μL of solution into one chamber of an eight-well plate (Electron Microscope Science, Hatfield, PA). The cross-linking reaction was allowed to occur for 30 mins at room temperature followed by sterilization under UV light (254 nm, surface working intensity: 125~500μW/cm2) for 20 mins. The RLP hydrogels were subsequently washed twice with 300uL Dulbecco’s phosphate buffered saline (DPBS) for 15 mins at room temperature before seeding cells on the hydrogel surface at a density of 104 cells per well. Cell attachment and morphology were assessed by Live/Dead assay and F-Actin/Vinculin staining. For Live/Dead assay, RLP hydrogels were stained with propidium iodide (1:2000 in DPBS, Invitrogen, Carlsbad, CA) and Syto-13 (1:1000 in DPBS, Invitrogen, Carlsbad, CA) for 5-10 mins at room temperature and subsequently imaged via multiphoton confocal microscopy (Zeiss 510 NLO, Thornwood, NY). For F-Actin/Vinculin staining, a commercially available actin cytoskeleton and focal adhesion staining kit (Millipore, Bedford, MA) was utilized to assess cell morphology and attachment. RLP hydrogels were rinsed in DPBS, fixed in 4% paraformaldehyde (Electron Microscope Science, Hatfield, PA) for 15 min, and permeabilized in 0.1% Triton X-100 (Fisher, Wilmington, DE) on ice for 5 min. Hydrogels were subsequently blocked with 3% heat inactivated bovine serum albumin (BSA; Jackson ImmunoResearch, West Grove, PA) in DPBS for 30 min, followed by a 1 hour incubation with mouse anti-vinculin monoclonal primary antibody (1:100 in 1% BSA; Millipore, Bedford, MA). RLP hydrogels were subsequently co-stained with AlexaFluor-488 goat anti-mouse IgG (Invitrogen; 1:100 in 1% BSA) and TRITC-conjugated phalloidin (Millipore; 1:200 in 1% BSA) for another hour. Finally, RLP hydrogels were counterstained with Draq-5 (1:1000 in DPBS; Axxora LLC, San Diego, CA) for 5 min before visualization via multiphoton confocal microscopy (Zeiss 510 NLO, Thornwood, NY). In order to quantitatively analyze cell morphologies on the surfaces of different material compositions, images were analyzed using ImageJ (NIH software, Bethesda, NY). Individual cells were manually outlined and the area contained by the perimeter was defined as the spread cell area. Each cell perimeters were computationally approximated to the best-fit with major (X) and minor (Y) axes, and the aspect ratio was defined as X/Y.
In vitro pro-inflammatory assessment
Lyophilized RLP protein, previously filtered through a 0.22μm filter, was dissolved into 40μL of molecular grade H2O at 20wt% concentration. RAW264.7 murine macrophages-like cells were cultured in DMEM supplemented with 10% heat inactivated fetal bovine serum (Denville Scientific), antibiotic/antimycotic (GIBCO), 10mM HEPES, and 55μM β-mercaptoethanol at 37°C to 60-80% confluency and passaged no more than 10 times. Cells were seeded at a concentration of 100,000 cells per well, on a tissue culture treated 96-well plate, in 200μL. TCPS as negative control, LPS (1ng/ml) as positive control, and RLP hydrogels as experimental samples, were then incubated at 37°C for 8 hours before the supernatant was collected and tested. Cytokine secretion was measured via enzyme-linked immunosorbent assay (ELISA) (BD Bioscience) according to the protocols provided by the manufacturer.
In vivo evaluation
In vivo RLP transplantation studies were performed following approval by the National Cerebral and Cardiovascular Center Research Institute of animal experiment guideline (Permit Number: 009017). Male Sprague Dawley rats (SD rats, n=3) (7-week year old; SLC Japan Inc., Shizuoka, Japan) were anesthetized with 50-80 mg/kg pentobarbital. Under general anesthesia, a subcutaneous pocket was created on the back of the rat by a scalpel, and the pre-formed 20wt% RLP hydrogel (10mm×10mm ×1mm) was placed into the pocket. The pocket was sutured with 3-0 silk (Ethicon, Johnson & Johnson, NJ). One week after the surgical operation, the subcutaneous tissues around the implants were extracted, fixed with 10% formalin (Wako Pure Chemical Industries, Ltd., Osaka, Japan), and were embedded in paraffin. Paraffin-embedded tissues were sectioned and stained with hematoxylin-eosin (H&E), CD3, and CD31 by following standard immunohistochemistry staining protocols. Staining was carried out in the Applied Medical Research Laboratory (Osaka, Japan). The images were acquired by digital microscopy (Coolscope II system, Nikon, Tokyo, Japan).
Supplementary Material
Acknowledgements
The authors would like to acknowledge funding support from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (P20-RR017716 (K.L.K.)) and the National Institute on Deafness and Other Communication Disorders (NIDCD, RO1 DC008965 to X.J. and RO1 DC011377A to K.L.K.). This publication was also supported by the Delaware COBRE program (NIGMS; 1 P30 GM110758-01) from the National Institutes of Health.
Footnotes
Supporting information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Linqing Li, Department of Materials Science and Engineering, University of Delaware, Newark, Delaware, 19716, USA
Dr. Atsushi Mahara, Department of Biomedical Engineering, National Cerebral and Cardiovascular Center Research Institute, Fujishiro-dai Suita, Osaka, 565-8565, Japan
Dr. Zhixiang Tong, Department of Materials Science and Engineering, University of Delaware, Newark, Delaware, 19716, USA
Dr. Eric A. Levenson, Department of Materials Science and Engineering, University of Delaware, Newark, Delaware, 19716, USA
Dr. Christopher L. McGann, Department of Materials Science and Engineering, University of Delaware, Newark, Delaware, 19716, USA
Xinqiao Jia, Department of Materials Science and Engineering, Department of Biomedical Engineering, University of Delaware, Newark, DE, 19716, USA
Dr. Tetsuji Yamaoka, Department of Biomedical Engineering, National Cerebral and Cardiovascular Center Research Institute, Fujishiro-dai Suita, Osaka, 565-8565, Japan
Kristi L. Kiick, Department of Materials Science and Engineering, Department of Biomedical Engineering, University of Delaware, Newark, DE, 19716, USA
References
- [1].Li L, Charati MB, Kiick KL. Polym. Chem. 2010;1:1160. doi: 10.1039/b9py00346k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gosline J, Lillie M, Carrington E, Guerette P, Ortlepp C, Savage K. Philos. Trans. R. Soc. London Ser. B-Biological Sci. 2002;357:121. doi: 10.1098/rstb.2001.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chow D, Nunalee ML, Lim DW, Simnick AJ, Chilkoti A. Mater. Sci. Eng. R-Reports. 2008;62:125. doi: 10.1016/j.mser.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Olsen BD. AIChE J. 2013;59:3558. [Google Scholar]
- [5].Cai L, Heilshorn SC. Acta Biomater. 2014;10:1751. doi: 10.1016/j.actbio.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gagner JE, Kim W, Chaikof EL. Acta Biomater. 2014;10:1542. doi: 10.1016/j.actbio.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kutty JK, Webb K. Tissue Eng. Part B Rev. 2009;15:249. doi: 10.1089/ten.TEB.2008.0588. [DOI] [PubMed] [Google Scholar]
- [8].Bartlett RS, Thibeault SL, Prestwich GD. Biomed. Mater. 2012;7:24103. doi: 10.1088/1748-6041/7/2/024103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Teller SS, Farran AJE, Xiao L, Jiao T, Duncan RL, Clifton RJ, Jia X. Tissue Eng. Part A. 2012;18:2008. doi: 10.1089/ten.tea.2012.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gaston J, Thibeault SL. Biomatter. 2013;3:e23799. doi: 10.4161/biom.23799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hughes LA, Gaston J, McAlindon K, Woodhouse KA, Thibeault SL. Acta Biomater. 2015;13:111. doi: 10.1016/j.actbio.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hahn MS, Teply BA, Stevens MM, Zeitels SM, Langer R. Biomaterials. 2006;27:1104. doi: 10.1016/j.biomaterials.2005.07.022. [DOI] [PubMed] [Google Scholar]
- [13].Tse JR, Long JL. Laryngoscope. 2014;124:E326. doi: 10.1002/lary.24605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jiao T, Farran A, Jia X, Clifton RJ. Exp. Mech. 2009;49:235. doi: 10.1007/s11340-008-9126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kutty JK, Webb K. J. Biomater. Sci. Ed. 2009;20:737. doi: 10.1163/156856209X426763. [DOI] [PubMed] [Google Scholar]
- [16].Miri AK. J. Voice. 2014;28:657. doi: 10.1016/j.jvoice.2014.03.001. [DOI] [PubMed] [Google Scholar]
- [17].Tong Z, Duncan RL, Jia X. Tissue Eng. Part A. 2013;19:1862. doi: 10.1089/ten.tea.2012.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Farran AE, Tong Z, Witt R, Jia X. In: In Engineering Biomaterials for Regenerative Medicine SE - 10. Bhatia SK, editor. Springer; New York: 2012. pp. 253–284. [Google Scholar]
- [19].Zeitels SM, Hillman RE, Mauri M, Desloge R, Doyle PB. Ann. Otol. Rhinol. Laryngol. 2002;111:21. doi: 10.1177/0003489402111s1203. [DOI] [PubMed] [Google Scholar]
- [20].Hertegård S, Dahlqvist A, Laurent C, Borzacchiello A, Ambrosio L. Otolaryngol. Head. Neck Surg. 2003;128:401. doi: 10.1067/mhn.2003.96. [DOI] [PubMed] [Google Scholar]
- [21].Thibeault SL, Klemuk SA, Chen X, Quinchia Johnson BH. J. Voice. 2011;25:249. doi: 10.1016/j.jvoice.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia X. Soft Matter. 2012;8:3280. doi: 10.1039/C2SM06463D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lee JH, Kim DW, Kim EN, Park S-W, Kim H-B, Oh SH, Kwon SK. Otolaryngol. -- Head Neck Surg. 2014;151:830. doi: 10.1177/0194599814549527. [DOI] [PubMed] [Google Scholar]
- [24].Grieshaber SE, Farran AJE, Lin-Gibson S, Kiick KL, Jia X. Macromolecules. 2009;42:2532. doi: 10.1021/ma802791z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tong Z, Zerdoum AB, Duncan RL, Jia X. Tissue Eng. Part A. 2014;20:1922. doi: 10.1089/ten.tea.2013.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Partlow BP, Hanna CW, Rnjak-Kovacina J, Moreau JE, Applegate MB, Burke KA, Marelli B, Mitropoulos AN, Omenetto FG, Kaplan DL. Adv. Funct. Mater. 2014;24:4615. doi: 10.1002/adfm.201400526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sengupta D, Heilshorn SC. Tissue Eng. Part B-Reviews. 2009;16:285. doi: 10.1089/ten.teb.2009.0591. [DOI] [PubMed] [Google Scholar]
- [28].Maskarinec SA, Tirrell DA. Curr. Opin. Biotechnol. 2005;16:422. doi: 10.1016/j.copbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [29].Lutolf MP, Hubbell JA. Nat. Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- [30].Nettles DL, Chilkoti A, Setton LA. Adv. Drug Deliv. Rev. 2010;62:1479. doi: 10.1016/j.addr.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kiick KL. Polym. Rev. 2007;47:1. [Google Scholar]
- [32].Daamen WF, Veerkamp JH, van Hest JCM, van Kuppevelt TH. Biomaterials. 2007;28:4378. doi: 10.1016/j.biomaterials.2007.06.025. [DOI] [PubMed] [Google Scholar]
- [33].Simnick AJ, Lim DW, Chow D, Chilkoti A. Polym. Rev. 2007;47:121. [Google Scholar]
- [34].Nagapudi K, Brinkman WT, Thomas BS, Wright ER, Conticello VP, Chaikof EL. Cognizant Communication Corp. 2003:170–171. [Google Scholar]
- [35].Van Eldijk MB, McGann CL, Kiick KL, Van Hest JM. Peptide-Based Materials. Vol. 310. Springer; Berlin Heidelberg: 2012. pp. 71–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yeo GC, Aghaei-Ghareh-Bolagh B, Brackenreg EP, Hiob MA, Lee P, Weiss AS. Adv. Healthc. Mater. 2015 doi: 10.1002/adhm.201400781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Annabi N, Mithieux SM, Zorlutuna P, Camci-Unal G, Weiss AS, Khademhosseini A. Biomaterials. 2013;34:5496. doi: 10.1016/j.biomaterials.2013.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Baldock C, Oberhauser AF, Ma LA, Lammie D, Siegler V, Mithieux SM, Tu YD, Chow JYH, Suleman F, Malfois M, Rogers S, Guo LA, Irving TC, Wess TJ, Weiss AS. Proc. Natl. Acad. Sci. U. S. A. 2011;108:4322. doi: 10.1073/pnas.1014280108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bellingham CM, Lillie MA, Gosline JM, Wright GM, Starcher BC, Bailey AJ, Woodhouse KA, Keeley FW. Biopolymers. 2003;70:445. doi: 10.1002/bip.10512. [DOI] [PubMed] [Google Scholar]
- [40].Srokowski EM, Woodhouse KA. J. Biomater. Sci. Polym. Ed. 2008;19:785. doi: 10.1163/156856208784522038. [DOI] [PubMed] [Google Scholar]
- [41].Wise SG, Weiss AS. Int. J. Biochem. Cell Biol. 2009;41:494. doi: 10.1016/j.biocel.2008.03.017. [DOI] [PubMed] [Google Scholar]
- [42].Mithieux SM, Wise SG, Weiss AS. Adv. Drug Deliv. Rev. 2013;65:421. doi: 10.1016/j.addr.2012.06.009. [DOI] [PubMed] [Google Scholar]
- [43].Ozsvar J, Mithieux SM, Wang R, Weiss AS. Biomater. Sci. 2015;3:800. doi: 10.1039/C5BM00038F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].MacEwan SR, Chilkoti A, Control J. Release. 2014;190:314. doi: 10.1016/j.jconrel.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hrabchak C, Rouleau J, Moss I, Woodhouse K, Akens M, Bellingham C, Keeley F, Dennis M, Yee A. Acta Biomater. 2010;6:2108. doi: 10.1016/j.actbio.2009.12.034. [DOI] [PubMed] [Google Scholar]
- [46].Romano NH, Sengupta D, Chung C, Heilshorn SC. Biochim. Biophys. Acta - Gen. Subj. 2011;1810:339. doi: 10.1016/j.bbagen.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Moore J, Thibeault S. J. Voice. 2012;26:269. doi: 10.1016/j.jvoice.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Smits FCM, Buddingh BC, van Eldijk MB, van Hest JCM. Macromol. Biosci. 2015;15:36. doi: 10.1002/mabi.201400419. [DOI] [PubMed] [Google Scholar]
- [49].Bennet-Clark H. J. Exp. Biol. 2007;210:3879. doi: 10.1242/jeb.001339. [DOI] [PubMed] [Google Scholar]
- [50].Andersen SO, Weis-Fogh T, Beament JETJWL, Wigglesworth VB. Advances in Insect Physiology. Volume 2. Academic Press; 1964. pp. 1–65. [Google Scholar]
- [51].Weis-Fogh T. J. Mol. Biol. 1961;3:648. [Google Scholar]
- [52].Li L, Kiick KL. ACS Macro Lett. 2013;2:635. doi: 10.1021/mz4002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Kappiyoor R, Balasubramanian G, Dudek DM, Puri IK. Soft Matter. 2011;7:11006. [Google Scholar]
- [54].Young D, Bennet-Clark HC. J. Exp. Biol. 1995;198:1001. doi: 10.1242/jeb.198.4.1001. [DOI] [PubMed] [Google Scholar]
- [55].Skals N, Surlykke A. J. Exp. Biol. 1999;202:2937. doi: 10.1242/jeb.202.21.2937. [DOI] [PubMed] [Google Scholar]
- [56].Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DCC, Merritt DJ, Dixon NE. Nature. 2005;437:999. doi: 10.1038/nature04085. [DOI] [PubMed] [Google Scholar]
- [57].Ardell DH, Andersen SO. Insect Biochem. Mol. Biol. 2001;31:965. doi: 10.1016/s0965-1748(01)00044-3. [DOI] [PubMed] [Google Scholar]
- [58].Qin GK, Rivkin A, Lapidot S, Hu X, Preis I, Arinus SB, Dgany O, Shoseyov O, Kaplan DL. Biomaterials. 2011;32:9231. doi: 10.1016/j.biomaterials.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Renner JN, Cherry KM, Su RSC, Liu JC. Biomacromolecules. 2012;13:3678. doi: 10.1021/bm301129b. [DOI] [PubMed] [Google Scholar]
- [60].Lv S, Dudek DM, Cao Y, Balamurali MM, Gosline J, Li HB. Nature. 2010;465:69. doi: 10.1038/nature09024. [DOI] [PubMed] [Google Scholar]
- [61].Charati MB, Ifkovits JL, Burdick JA, Linhardt JG, Kiick KL. Soft Matter. 2009;5:3412. doi: 10.1039/b910980c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li L, Teller S, Clifton RJ, Jia X, Kiick KL. Biomacromolecules. 2011;12:2302. doi: 10.1021/bm200373p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li L, Tong Z, Jia X, Kiick KL. Soft Matter. 2013;9:665. doi: 10.1039/C2SM26812D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].McGann CL, Levenson EA, Kiick KL. Macromol. Chem. Phys. 2013;214:203. doi: 10.1002/macp.201200412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Li L, Kiick K. Front. Chem. 2014;2 doi: 10.3389/fchem.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li L, Luo T, Kiick KL. Macromol. Rapid Commun. 2015;36:90. doi: 10.1002/marc.201400521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Straley KS, Heilshorn SC. Soft Matter. 2009;5:114. [Google Scholar]
- [68].Hersel U, Dahmen C, Kessler H. Biomaterials. 2003;24:4385. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- [69].Nagase H, Fields GB. Biopolymers. 1996;40:399. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C399::AID-BIP5%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- [70].Raeber GP, Lutolf MP, Hubbell JA. Acta Biomater. 2007;3:615. doi: 10.1016/j.actbio.2007.03.013. [DOI] [PubMed] [Google Scholar]
- [71].Werner S, Grose R. Physiol. Rev. 2003;83:835. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- [72].Ferreira DS, Lin Y-A, Cui H, Hubbell JA, Reis RL, Azevedo HS. Adv. Healthc. Mater. 2015;4:602. doi: 10.1002/adhm.201400586. [DOI] [PubMed] [Google Scholar]
- [73].Lim DW, Nettles DL, Setton LA, Chilkoti A. Biomacromolecules. 2007;8:1463. doi: 10.1021/bm061059m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Wang H, Cai L, Paul A, Enejder A, Heilshorn SC. Biomacromolecules. 2014;15:3421. doi: 10.1021/bm500969d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zhang K, Siegmund T, Chan RW. J. Mech. Behav. Biomed. Mater. 2009;2:93. doi: 10.1016/j.jmbbm.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Hunter EJ, Siegmund T, Chan RW. PLoS One. 2014;9:e90762. doi: 10.1371/journal.pone.0090762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wu XY, Sallach RE, Caves JM, Conticello VP, Chaikof EL. Biomacromolecules. 2008;9:1787. doi: 10.1021/bm800012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Alipour-Haghighi F. J. Acoust. Soc. Am. 1985;78:1939. doi: 10.1121/1.392701. [DOI] [PubMed] [Google Scholar]
- [79].Alipour-Haghighi F, Titze IR. Advances in Bioengineering. 1990;17:21–24. [Google Scholar]
- [80].Truong MY, Dutta NK, Choudhury NR, Kim M, Elvin CM, Nairn KM, Hill AJ. Biomaterials. 2011;32:8462. doi: 10.1016/j.biomaterials.2011.07.064. [DOI] [PubMed] [Google Scholar]
- [81].Min YB, Titze IR, Alipour-Haghighi F. Ann. Otol. Rhinol. Laryngol. 1995;104:563. doi: 10.1177/000348949510400711. [DOI] [PubMed] [Google Scholar]
- [82].Xiao L, Liu C, Zhu J, Pochan D. Soft Matter. 2010;6:5293. doi: 10.1039/C0SM00511H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Weis-Fogh T. J. Exp. Biol. 1960;37:889. [Google Scholar]
- [84].Weis-Fogh T, Andersen SO. Nature. 1970;227:718. doi: 10.1038/227718a0. [DOI] [PubMed] [Google Scholar]
- [85].Lyons RE, Nairn KM, Huson MG, Kim M, Dumsday G, Elvin CM. Biomacromolecules. 2009;10:3009. doi: 10.1021/bm900601h. [DOI] [PubMed] [Google Scholar]
- [86].Nagapudi K, Brinkman WT, Thomas BS, Park JO, Srinivasarao M, Wright E, Conticello VP, Chaikof EL. Biomaterials. 2005;26:4695. doi: 10.1016/j.biomaterials.2004.11.027. [DOI] [PubMed] [Google Scholar]
- [87].Hu X, Wang X, Rnjak J, Weiss AS, Kaplan DL. Biomaterials. 2010;31:8121. doi: 10.1016/j.biomaterials.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Cui J, Lackey MA, Madkour AE, Saffer EM, Griffin DM, Bhatia SR, Crosby AJ, Tew GN. Biomacromolecules. 2012;13:584. doi: 10.1021/bm300015s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Cui J, Lackey MA, Tew GN, Crosby AJ. Macromolecules. 2012;45:6104. [Google Scholar]
- [90].Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Adv. Healthc. Mater. 2013;2:57. doi: 10.1002/adhm.201200197. [DOI] [PubMed] [Google Scholar]
- [91].Martino MM, Briquez PS, Güç E, Tortelli F, Kilarski WW, Metzger S, Rice JJ, Kuhn GA, Müller R, Swartz MA, Hubbell JA. Science. 2014;343:885. doi: 10.1126/science.1247663. [DOI] [PubMed] [Google Scholar]
- [92].Lutolf MR, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Nat. Biotechnol. 2003;21:513. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- [93].Baldwin AD, Kiick KL. Polym. Chem. 2013;4:133. doi: 10.1039/C2PY20576A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Mabry KM, Lawrence RL, Anseth KS. Biomaterials. 2015;49:47. doi: 10.1016/j.biomaterials.2015.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kyburz K, Anseth K. Ann. Biomed. Eng. 2015;43:489. doi: 10.1007/s10439-015-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Biomaterials. 2009;30:4318. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Sridhar BV, Brock JL, Silver JS, Leight JL, Randolph MA, Anseth KS. Adv. Healthc. Mater. 2015;4:702. doi: 10.1002/adhm.201400695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kirschner CM, Anseth KS. Acta Mater. 2013;61:931. doi: 10.1016/j.actamat.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hanson SE, Kim J, Johnson BHQ, Bradley B, Breunig MJ, Hematti P, Thibeault SL. Laryngoscope. 2010;120:546. doi: 10.1002/lary.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Chen X, Thibeault SL. J. Tissue Eng. Regen. Med. 2013 doi: 10.1002/term.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Liu B, Liu Y, Riesberg JJ, Shen W. J. Am. Chem. Soc. 2010;132:13630. doi: 10.1021/ja1054669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Benitez PL, Sweet J. a, Fink H, Chennazhi KP, Nair SV, Enejder A, Heilshorn SC. Adv. Healthc. Mater. 2013;2:114. doi: 10.1002/adhm.201200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Sengupta D, Gilbert PM, Johnson KJ, Blau HM, Heilshorn SC. Adv. Healthc. Mater. 2012;1:785. doi: 10.1002/adhm.201200195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Amschler K, Erpenbeck L, Kruss S, Schön MP. ACS Nano. 2014;8:9113. doi: 10.1021/nn502690b. [DOI] [PubMed] [Google Scholar]
- [106].Chen X, Thibeault SL. Acta Biomater. 2010;6:2940. doi: 10.1016/j.actbio.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Brodbeck WG, Voskerician G, Ziats NP, Nakayama Y, Matsuda T, Anderson JM, Biomed J. Mater. Res. Part A. 2003;64A:320. doi: 10.1002/jbm.a.10425. [DOI] [PubMed] [Google Scholar]
- [108].Xia Z, Triffitt JT. Biomed. Mater. 2006;1:R1. doi: 10.1088/1748-6041/1/1/R01. [DOI] [PubMed] [Google Scholar]
- [109].Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE, Badylak SF. Acta Biomater. 2012;8:978. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ullm S, Krüger A, Tondera C, Gebauer TP, Neffe AT, Lendlein A, Jung F, Pietzsch J. Biomaterials. 2014;35:9755. doi: 10.1016/j.biomaterials.2014.08.023. [DOI] [PubMed] [Google Scholar]
- [111].Bhatia S, Arthur S, Chenault HK, Kodokian G. Biotechnol. Lett. 2007;29:1645. doi: 10.1007/s10529-007-9465-8. [DOI] [PubMed] [Google Scholar]
- [112].Haines-Butterick LA, Salick DA, Pochan DJ, Schneider JP. Biomaterials. 2008;29:4164. doi: 10.1016/j.biomaterials.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Thibeault SL, Duflo S. Ann. Otol. Rhinol. Laryngol. 2008;117:221. doi: 10.1177/000348940811700310. [DOI] [PubMed] [Google Scholar]
- [114].Swartzlander MD, Blakney AK, Amer LD, Hankenson KD, Kyriakides TR, Bryant SJ. Biomaterials. 2015;41:79. doi: 10.1016/j.biomaterials.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Newman PJ, Berndt MC, Gorski J, White GC, Lyman S, Paddock C, Muller WA. Science. 1990;247:1219. doi: 10.1126/science.1690453. [DOI] [PubMed] [Google Scholar]
- [116].Pusztaszeri MP, Seelentag W, Bosman FT. J. Histochem. Cytochem. 2006;54:385. doi: 10.1369/jhc.4A6514.2005. [DOI] [PubMed] [Google Scholar]
- [117].Assmann A, Delfs C, Munakata H, Schiffer F, Horstkötter K, Huynh K, Barth M, Stoldt VR, Kamiya H, Boeken U, Lichtenberg A, Akhyari P. Biomaterials. 2013;34:6015. doi: 10.1016/j.biomaterials.2013.04.037. [DOI] [PubMed] [Google Scholar]
- [118].Boehler RM, Graham JG, Shea LD. Biotechniques. 2011;51:239. doi: 10.2144/000113754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Nettles DL, Kitaoka K, Hanson NA, Flahiff CM, Mata BA, Hsu EW, Chilkoti A, Setton LA. Tissue Eng. Part A. 2008;14:1133. doi: 10.1089/ten.tea.2007.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.