Abstract
The two biological mechanisms that determine types of malignancy are infiltration and metastasis, for which tumour microenvironment plays a key role in developing and establishing the morphology, growth and invasiveness of a malignancy. The microenvironment is formed by complex tissue containing the extracellular matrix, tumour and non-tumour cells, a signalling network of cytokines, chemokines, growth factors, and proteases that control autocrine and paracrine communication among individual cells, facilitating tumour progression. During the development of the primary tumour, the tumour stroma and continuous genetic changes within the cells makes it possible for them to migrate, having to count on a pre-metastatic niche receptor that allows the tumour’s survival and distant growth. These niches are induced by factors produced by the primary tumour; if it is eradicated, the active niches become responsible for activating the latent disseminated cells. Due to the importance of these mechanisms, the strategies that develop tumour cells during tumour progression and the way in which the microenvironment influences the formation of metastasis are reviewed. It also suggests that the metastatic niche can be an ideal target for new treatments that make controlling metastasis possible.
Keywords: cancer, infiltration, metastasis, microenvironment, metastatic niche, latency, epithelial–mesenchymal transition
Introduction
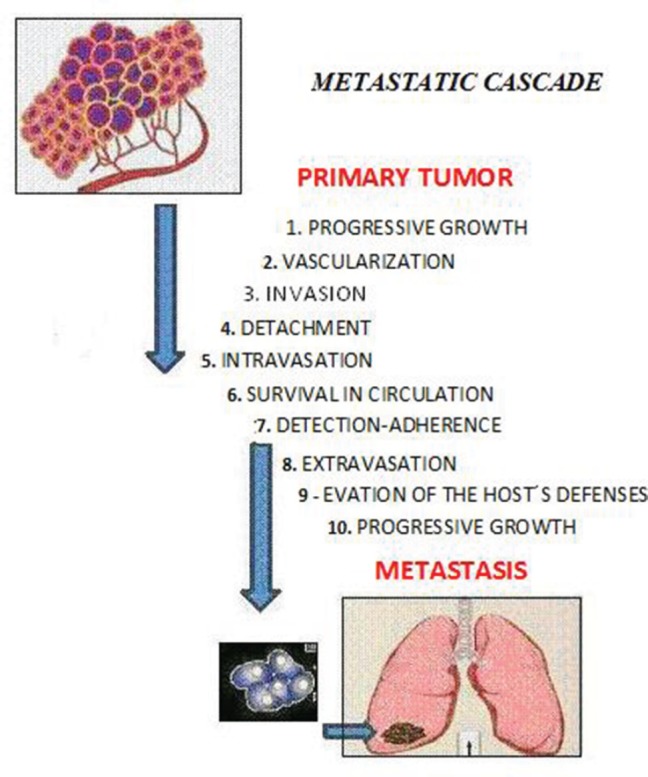
Metastasis is defined as ‘the process of dissemination of cancer cells from their origin to a distant organ’ [1], a complex process involving several stages, which are as follows: (a) the activation of epithelial–mesenchymal transition (EMT), during which cancer cells lose all cell–cell contact, such as substrate adhesion, acquiring ownership of movement; (b) local invasion, whereby malignant cells degrade the basal lamina, the special extracellular matrix that organises and separates epithelial tissues from the stroma, which plays an important role in both cell signalling and being a reservoir of growth factors released by tumour cells; (c) intravasation, during which tumour cells pass through the walls of blood vessels and enter the bloodstream; (d) the ability to survive in the bloodstream; (e) extravasation, whereby tumour cells exit the bloodstream, passing through the walls of blood vessels into the tissue of a particular organ; (f) establishment of tumour cells in the tissues of the organ where metastasis will form; in other words, the establishment of a pre-metastatic niche to create a favourable environment for the growth of cancer cells (Figure 1).
Figure 1. The sequential steps in the pathogenesis of metastasis are shown in the figure above, where each step is regulated by transitory and permanent changes in the DNA, the RNA, or by proteins. Also, most tumor cells fail to complete all of the steps, and the ‘few’ cells with metastatic ability ‘defeat’ the multiple mechanisms which impedes the formation of metastasis.
Each of the steps necessary in producing metastasis, from the arrival of malignant cells to their growth and proliferation in the host organ, is led by the genetic and/or epigenetic alterations acquired and accumulated during the course of tumour progression [2, 3]. Despite recent advances made in surgical techniques, radiotherapy and the development of molecularly targeted therapies, the majority of cancer-related deaths (more than 90%) are the result of progressive growth of therapy-resistant metastasis [4]. Metastatic cells come from a heterogeneous cell population within the primary tumour which, over time, are selected, experience a high spontaneous mutation rate, are more prone to suffering from rapid phenotypic diversification and are resistant to therapeutic treatments [5].
The survival of the malignant cells that form micrometastasis in the receptor organ is not ensured, as there may be differences between the primary tumour’s microenvironment and the location where the cancer cells will be established [6]. Therefore, the pre-metastatic niche model has been proposed, which can be described as ‘the location with the necessary microenvironmental conditions for the disseminated tumour cells’ survival’ [7]. For adaption to occur, they deploy mechanisms to modify the new microenvironment. To do so, along with the stroma cells, they establish a signalling network to promote their growth, to satisfy the metabolic demands to synthesise proangiogenic proteins in order to form new vascular networks and to facilitate their initial survival in the new ectopic location [8].
Metastasis is a largely ineffective process, leading it to be called ‘metastatic inefficiency’, since only 0.01% of the tumour cells that enter the bloodstream in animal models successfully form a secondary tumour [9]. With these models, it has been possible to choose cell populations with metastatic phenotypes and these cells in particular show increasing selectivity after each selection cycle for a specific organ [10]. Furthermore, the genome instability of neoplasms increases the likelihood of other cells developing skills that allow for the development of metastasis. Several studies have shown that an increase in metastatic ability is not the result of the adaption mechanisms of the tumour cells that allows for growth in a specific organ; rather, it is the gradual selection of a clone with different mutations to those observed in the primary tumour. The genome instability and heterogeneity of cancer cells is shown in the gains, losses, and chromosomal rearrangement of the tumours [11]. New technologies in parallel sequencing have allowed high-resolution genome analysis of cancer patients, making it possible to compare the primary tumour with regard to metastasis.
These studies have proven that there are inactive genes in the primary tumour, while they are active in metastasis, allowing for the activation of other oncogenes, confirming the concept of tumour heterogeneity [12]. Additionally, it has been shown that mutation patterns are shared in the metastasis of an organ, whereas metastasis in the different organs of a patient is different, establishing the hypothesis that metastasis results from clonal expansion where the subclones that have colonised an organ already have genetic alterations that allow specific adaption to the environment [13]. The period of time that passes between infiltrating the organ and colonising it is known as the latency period, where several tumour cells remain outside the cell cycle of secondary organs, while others are incapable of provoking the angiogenic changes necessary for tumour expansion [14].
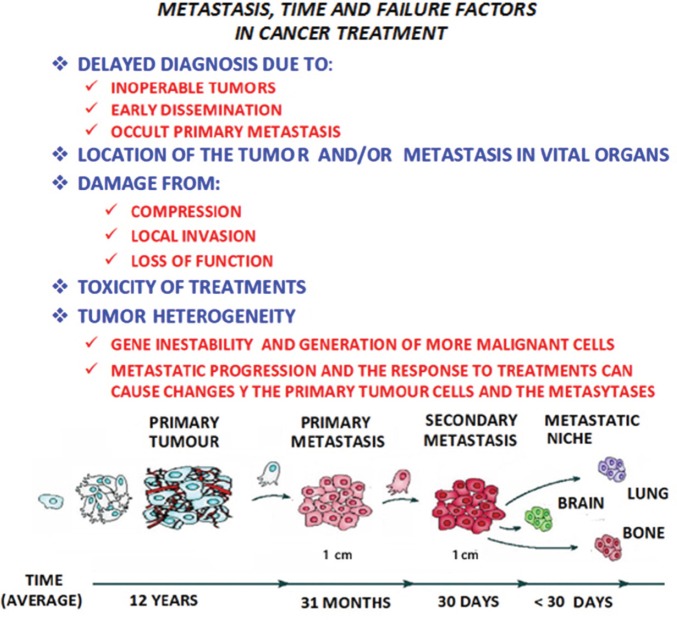
It would appear that metastasis is a process determined by a complex network of interactions between metastatic cells and their microenvironment in the affected organs, making it necessary to update the contributions made for the knowledge of the different microenvironment elements involved in the formation of metastasis. It is necessary to focus on the main interactions established between the tumour cells and the microenvironment, from the primary tumour to reach the location where metastasis originates and develops. In all these phenomena, time has to also be considered as a factor of singular and paramount importance (Figure 2).
Figure 2. In metastatic progression, time and the failure factors indicated in the figure above, play a crucial role in the prognosis and treatment of the patient with cancer, as shown in the model of the linear progression of a tumor. In time, the cells increase in malignancy. Thus the concept of ‘metastasis of metastasis’ is used in reference to the metastasis becoming in the course of time more malignant, resulting in the death of the patient, as the illness cannot be controlled.
Components of the pre-metastastic niche
The metastatic niche plays an important role within the crucial factors that determine the success or failure of metastasis. A series of events take place in its formation, including: the modification of the extracellular matrix [ECM]; the restructuring of the vascular network; the participation of bone marrow cells; hypoxia and the expression of a wide variety of signalling molecules. Added to this is the participation of untransformed cells, such as fibroblasts and endothelial cells, as well as the deposition of molecules such as fibronectin, tenascin-c, and periostin [15]. Regarding particular tissue receptors, fibulin-5 reduces its levels so that matrix metallopeptidase 9 [MMP-9] restructures the matrix in metastasis in the liver and lungs, thus contributing to the formation of the metastatic niche [16]. Lysyl oxidase [LOX] actively participates in restructuring the extracellular matrix and the formation of the niche, so it has the ability to be linked to collagen and elastin. The expression of LOX is increased in tumour cells exposed to hypoxia conditions [17]. The development and consolidation of these investigations originated from the pioneering work made by Kaplan and co. [18], who used as many human tissue samples from cancer patients as Lewis lung carcinoma (LLC) and B16 cell lines from an animal model. They show that haematopoietic bone marrow-derived progenitor cells, which express vascular endothelial growth factor receptor 1 (VEGFR-1), form aggregated cells in pre-metastatic niches. The use of specific antibodies against VEGFR-1, as well as the elimination of these aggregated pre-metastatic cells, impedes the formation of metastasis. They also studied the adhesion and formation of aggregated cells after the implantation of the tumour cells, observing a growth in the expression of fibronectin and an increase in the dissemination of fibroblasts found in the primary tumour.
Furthermore, matrix metallopeptidases – particularly MMP-9 produced by bone marrow-derived progenitor cells – degrade the basal membrane, accelerating the extravasation of cells with VEGFR-1+ phenotype in the niche. Along with fibronectin, associated with stroma cells, VEGFR-1+ alters the local environment, activating integrin and chymosin to promote adhesion, survival, and growth in tumour cells. The primary tumour induces the production of MMP-9 in endothelial cells and macrophages in the lungs via a mechanism dependant on VEGFR-1/FLT-1 tyrosine kinase (TK) that promote metastasis. Additionally, they studied the expression of MMP-9 in healthy areas of the lungs in samples extracted from patients with tumours located in organs besides the lungs. In the lungs of patients with oesophageal cancer, melanoma skin cancer, ovarian cancer etcetera, a high expression of MMP-9 was found which suggests that primary tumours can stimulate the production of MMP-9 in pre-metastatic areas [19].
Types of cells
There are several types of cells that are important components of the metastatic niche, including heterogeneous populations in immune cells, endothelial cells, fibroblasts, macrophages, pericytes, and bone marrow-derived progenitor cells, which are described below:
Immune cells
They are represented by cells that actively participate in immunity processes, including macrophages, neutrophils, mast cells, myeloid-derived suppressor cells, dendritic cells, natural killer cells (NK) and more adaptive immune cells, such as T- and B-type lymphocytes. Immune cells that infiltrate the tumour, excluding NK cells, produce tumour cytokine promoters, such as tumour necrosis factor alpha [TNF-α] and interleukins IL-1β, IL-6, and IL-8, which increase signalling in pre-malignant cells. Likewise, this signalling not only stimulates tumour progression but also induces the production of cytokines by the tumour cells themselves [20].
Neutrophils
They are the most abundant human leukocytes as they are the first cells that gather at the infection site. Their degranulation releases lytic enzymes, as well as reactive oxygen species (ROS), hydrogen peroxide (H2O2), and hypochlorite (HOCL) with microbial potential [21]. Cytokines are also produced, such as TNF-α, IL-1β, IL-1Rα, IL-12, and VEGF; chemokines CXCL1, CXCL8, CXCL9, CXCL10, CCL3, and
CCL4 are involved in angiogenesis [22]. CXCL8 is produced in abundance by tumour cells, which once released into the microenvironment represent a potent chemo-attractant of neutrophils within the tumour. Furthermore, CXCL8 and other chemokines are associated with angiogenesis by the direct extracellular activation of CXCR2 [23], in particular, MMP-9 [24]. TNF-α, released in the tumour microenvironment, is linked with tumour progression inducing degranulation of the neutrophils, releasing VEGF and favouring angiogenesis with the production of CXCL8 and CXCL1 [25].
Dendritic cells
They represent a heterogeneous population made up of two types of cells: myeloids CD11c+ and CD123lo, and plasmacytoids CD11c− and CD123hi [26]. It has been shown that in various types of tumours, they present specific modifications in its stimulating ability with the consequent abnormal development of the differentiation of myeloid cells [27]. One of the mechanisms by which there is an abnormal differentiation of myeloid cells is the constitutive activation of the signal transducer and activator of transcription 3 [STAT3], which promotes continuing proliferation and the accumulation of immature myeloid cells, contributing to the suppression of the immune response before tumour angiogenesis [28]. Two pro-inflammatory molecules released by dendritic cells, TNF-α and osteopontin for instance, are associated with angiogenesis [29, 30]. Dendritic cells can secrete pro-angiogenic chemokines, such as CXCL1, CXCL2, CXCL3, CXCL5, CXCL8, and CCL2 [31]. In contrast, mature dendritic cells can inhibit angiogenesis by releasing cytokine IL-12 and chemokines CXCL9, CXCL10, and CCL21 [32].
Myeloid-derived suppressor cells (MDSC)
They represent an active role in the promotion of tumours and the evasion of the immune response. When they are immature cells, they seem to have monocyte/macrophage and granulocyte characteristics [33]. High levels of pro-inflammatory factors in the microenvironment of the tumour, such as GM-CSF, IL-1b, IL-6, and S-100, induce the recruitment and expansion of myeloid-derived suppressor cells, increasing pro-tumour activity [34]. Myeloid-derived suppressor cells also participate in the promotion of angiogenesis in the tumour through the release of soluble factors like MMP9 and VEGF. Experimental data suggest that these cells are also capable of being different from endothelial cells [35].
Natural killer cells
Natural killer (NK) cells are lymphocyte effectors of the innate immunity that could potentially control tumours by their cytotoxic activity. Like other types of cells, NK cells can infiltrate the mass, whose microenvironment is capable of affecting the functionality of these cells by a broad range of cytokines and soluble factors, either by inhibiting the cytotoxic function or promoting angiogenic phenotype. CD56bright and CD16- NK cells predominant in lung cancer exert lower cytotoxicity in K562 cells [36]. It has been reported that the invasion of NK cells in lung cancer (non-small-cell lung cancer [NSCLC]) produces high levels of VEGF, PIGF, and IL-8, inducing ‘ex vivo’ angiogenic activity [37]. The reduction in activity by NK cells is associated with the generation of the pre-metastatic niche and the efficiency of metastasis in murine models [38].
T cells
The inhibition of the flow of T-lymphocytes during angiogenesis and stroma restructuring represents a characteristic of the tumour microenvironment, giving way to alterations to its functionality. This is due to the activation and expansion of myeloid cells and soluble factors secreted by the tumour and inflammatory cells. The typical immunosuppressive tumour environment is characterised by a strong induction by CD4+, CD25+, FOXP3, and tumour-infiltrating regulatory T cells, and the activation of Th2 and Th17 [39, 40]. In ovarian cancer, hypoxia induces angiogenesis in humans and mice, where CD4+, CD25+ and tumour-infiltrating regulatory T cells secrete high quantities of VEGFA and promote the dissemination of endothelial cells, both ‘in vitro’ and ‘in vivo’. In an ovarian tumour transplanted in a mouse, the depletion of tumour-infiltrating regulatory T cells correlates with a reduction in VEGFA, supporting the role of tumour-infiltrating regulatory T cells in angiogenesis in this type of tumour [41].
B cells
The stimulation of B cells culminates in the production of immunoglobulins [Ig] that participate in humoural immunity. They segregate a variety of cytokines such as interleukins IL-6 and IL-10, TNF-α, granulocyte-macrophage colony-stimulating factor (GM-CSF), and lymphotoxin (LT) that participate in humoural immunity [42]. In the development of solid tumours with similar characteristics to damaged tissues with immune dysfunction, such as chronic immune cell infiltration, tissue restructuring or angiogenesis, it is unsurprising that individuals with autoimmune diseases are at increased risk of developing cancer [43]. Complement proteins are associated with immunoglobulins and form circulating immune complexes (CIC), whose deposition in the parenchyma is due to defects in the vascular network, either by the tumour or by pathological angiogenesis, which starts a chain reaction [44]. In certain types of cancer, CIC levels in the tumour parenchyma are linked to an increase in tumour burden, indicating a poor prognosis [45]. In the K14-HPV16 mouse model, a carrier of squamous cell carcinoma (SCC), the suppression of B- and T-lymphocytes produced a decrease in angiogenesis and epithelial hyperproliferation. The transfer of B cells (B220+ and CD19+) in K14-HPV16 mice to K14-HPV16 mice (sic), deficient in T- and B-lymphocytes, restored malignant characteristics, such as hyperproliferation and angiogenesis. These data indicate that the activation of B cells is essential for the development of an epithelial neoplasm and that soluble mediators secreted by B cells are necessary to establish an inflammatory process that boosts tumour progression [46].
Mast cells
They represent a peculiar subtype of granulocytes that play a central role in the inflammatory process, participating in vascularisation during arthritis [47]. They have also been found to participate in the vascularisation of haematological malignancies, where they can integrate in the blood vessel wall by vascular mimicry [48]. The participation of mast cells in angiogenesis is associated with the production of various cytokines and chemokines [49]. Furthermore, proteases produced by mast cells promote angiogenesis [50]. B-triptase is a neutral protease that represents an abundant mediator found next to mast cell granules and plays an important role in inflammation, activating the release of protease by type 2 receptors that are directly involved in vascularisation [51].
Tumour-associated macrophages (TAM)
They act as regulators of tumourigenesis, either as residents or as derived from the spleen or bone marrow. Macrophages are typically considered as effector cells during immune defence but numerous studies have shown their role in tumour progression [52]. They are an important source of proteases, such as cysteine and cathepsin, that participate in tumour progression [53]. Tumour-associated macrophages have antagonistic functions between the homeostasis of normal tissues and tumorigenesis, which is why macrophages are functionally plastic and can alter their phenotype to suit different physiological conditions [54]. They can present a ‘M1’ phenotype that produces a type 1 pro-inflammatory cytokine that participates in antigen presentation, playing an antitumorigenic role, as well as a ‘M2’ phenotype that produces a type 2 cytokine that promotes the anti-inflammatory response and pro-tumorigenic function [55]. It has been suggested that in several environmental conditions, such as tumour hypoxia in a ‘M1’ to ‘M2’ transition, tumour-associated macrophages accumulate in hypoxic areas within the tumour, as well as endotheline-2 and VEGF. It should be noted that the accumulation of tumour-associated macrophages is linked with angiogenesis and the subsequent acquisition of invasive phenotypes [56]. Furthermore, a population of metastasis-associated macrophages (MAM) has been identified in a mouse model that promotes extravasation, dissemination and growth of breast cancer cells in the lungs. The inhibition of signalling by CCL2–CCR2 inhibits the accumulation of metastasis-associated macrophages and reduces metastasis [57]. Blood clotting plays an important role during metastasis, due to the blood-clotting proteins and tissue factor (TF) being linked to a poor prognosis for patients as they interfere with NK cells through the lysis of micrometastases. TF induces the formation of blood clots with platelets that stimulate the recruitment of bone marrow-derived macrophages, resulting in the survival of melanoma cells in the lungs. These blood clots recruit myeloid-derived suppressor cells in secondary niches, impeding the immunological rejection of the tumour [58].
Cancer-associated fibroblasts (CAF)
They are predominant cells in the connective tissue responsible for the elaboration of the extracellular matrix components and basal membrane, associated with the differentiation of epithelial cells and mediators of the immune response [59]. There are numerous cancer-associated fibroblasts in the tumour microenvironment, differing from normal fibroblasts. In mice, normal prostrate epithelial cells originate in intraepithelial tumours when coinjected with cancer-associated fibroblasts, but not when they are injected with normal fibroblasts [60]. Likewise, in breast cancer, cancer-associated fibroblasts stimulate the metastasis of malignant cells, while normal fibroblasts suppress metastasis [61]. This shows that cancer-associated fibroblasts make up a cell different to its normal counterpart. Moreover, their origin during the progression of the disease is unclear [62]; several studies suggest that they generate through the epithelial–mesenchymal transition of endothelial cells from blood vessels associated with tumours [63]. The mesenchymal–epithelial transition (MET) promotes cancer-associated fibroblast generation in tumours of epithelial origins; for instance, in breast cancer and prostate cancer, epithelial cells dedifferentiate to generate mesenchymal cells that express cancer-associated fibroblast markers [64]. Cancer-associated fibroblasts interact with tumour cells and additional components of the stroma through the production and secretion of various growth factors, cytokines, and chemokines. Once activated by the infiltration of immune cells, these fibroblasts produce pro-inflammatory chemokines such as CXCL1 and CXCL2 through the recruitment of tumour-associated macrophages in primary tumours [65], while CCL5 being secreted by these fibroblasts recruits tumour-infiltrating regulatory T cells by signalling through the CCR1 receptor expressed in these cells [66]. CCL5 is secreted by mesenchymal stem cells (MSC) that also act through the CCR5 receptor expressed by breast cancer cells, increasing the invasion and metastasis [67]. Moreover, CXCL12 and fibroblast growth factor receptor 2 (FGF-2), released by cancer-associated fibroblasts, stimulate neoangiogenesis by recruiting endothelial progenitor cells and vascular endothelial cells [68]. In mesenchymal–epithelial transition, tumour-associated fibroblasts are activated by TGF-β, PDGF, FGF, and proteases [69]. Once activated, cancer-associated fibroblasts secrete growth factors, including VEGF that induces vascular permeability and angiogenesis [70, 71].
Pericytes
They are specialised mesenchymal cells that are linked to smooth muscle, which act as support to endothelial cells and contribute both towards homoeostasis and the stabilisation, maturation and restructuring of capilliaries [72]. The intimate anatomical relationship between endothelial cells and pericytes suggests a stretched interaction between cell contacts by paracrine signalling. Platelet-derived growth factor B (PDGFB) is a family member of PDGF secreted by endothelial cells that joins with the tyrosine kinase receptor, PDGFR, expressed on the surface of pericytes. When PDGFB joins with PDGFR, dimerisation occurs and an intracellular signalling cascade that promotes cell proliferation and migration begins [73]. Angiopoietin-1 (Ang-1) is a soluble ligand produced by pericytes that joins with the tyrosine kinase receptor Tie-2, expressed by endothelial cells [74]. The interaction between Ang-1 and Tie-2 is fundamental for the maturation and stabilisation of the endothelium [75]. Transforming growth factor β (TGF-β) is a growth factor expressed by endothelial cells and pericytes during angiogenesis [76]. Vascularisation in tumours is chaotic and irregular, an instability that has been frequently attributed to a reduction in the number of pericytes [77]. The presence of pericytes can vary according to the type of tumour, considering that they increase in pancreatic cancer for example and decrease in glioblastoma, a notable fact when compared with the respective normal tissues. In reality, they are found in the majority of tumours, even though their association with the endothelium is abnormal [78]. Different studies have shown that they are essential in maintaining the tumour vascular network, as well as normal blood vessels, while the VEGF produced by the pericyte is necessary for the survival of endothelial cells in both contexts [79]. A hypothesis considers that the reduction of the number of pericytes in tumour vessels can increase intravasation of cancer cells, promoting its haematogenous dissemination [78]. In fact, it has shown the existence of an inverted link between the contents in pericytes of tumour vessels and the number of metastasis in colorectal cancer patients [80].
The composition of an inflammatory environment
An inflammatory environment may be created with the establishment of a tumour [81], as it has been observed that in many cases a pro-inflammatory environment is created which is composed of cytokines, chemokines, growth factors, activated stroma, metalloproteinases which degrade the extracellular matrix, and agents that cause damage to the DNA [82]. The inflammatory microenvironment introduced by the tumour cells affects the immune effector functions by means of immunosuppressor cells of the tumour-associated macrophage (TAM) type, immature Grl+ and Mac1+ myeloid cells, T regulator lymphocytes, T reg, CD4+ CD25+, natural killer T, NKT, etc. It is also possible that this occurs through the reduction of the number of dendritic cells, which are essential to initiating and maintaining an antitumour immune response [83]. The acute phase C-reactive protein or CRP and the A-amyloid or SAA proteins play a very important role in the induction of this inflammatory medium [84]. The SAA proteins are also chemotactic for other inflammatory cells such as the mastocytes and T lymphocytes [85], as well as in the induction of the expression of enzymes for the remodelling of the extracellular matrix [86] and in the production of inflammatory cytokines, such as TNF-α, that promote tumour growth [87]. The CD11b+ Gr1+ myeloid suppressor cells inhibit the T and NK cells, protecting the tumour cells from immunological destruction [88]. The T regulator cells (Treg) are found in the tumour microenvironment and have several immunomodulator activities in cancer [89]. In normal physiological conditions, the Treg cells regulate the expansion and activation of the T and B lymphocytes, playing a critical role in the cytotoxic homoeostasis of the lymphocytes [90]. Based on the response to different environmental stimuli, the Treg cells have different effects on tumorigenesis, for example in breast tumours, which show that an increase in Treg cells is correlated with a lower survival [91], while in colorectal cancer, the Treg cells are associated with a higher survival [92]. Similar to the myeloid-derived suppressor cells or MDSCs, the Treg cells suppress the introduction of tumour-associated antigen and also interfere with the function of the cytotoxic T cells by means of inhibition through the release of cytolytic granules [93]. The S100A8 and S100A9 proteins, produced by primary tumours, induce the accumulation of haematopoietic progenitor cells and macrophages in pre-metastatic regions of the lung. In these regions, the serum amyloid A (SAA)3, induced by S100A8 and S100A9, acts as a regulating agent in the accumulation of the myeloid cells. In the lung, in the endothelial cells and macrophages, the toll-like4 (TLR4) receptor acts as a receptor for A(SAA)3 in the pre-metastatic phase, which also stimulates the signalling of NFKappa B, facilitating metastasis. This pro-inflammatory condition accelerates the migration of the primary tumour cells to the pre-metastatic niche in the lung [94]. In an animal model with metastasis in the lung, significant changes were observed in the vascular permeability that contributes to the establishment of the metastasis. The MD2 receptor represents a TLR4 coreceptor that creates regions of hyperpermeability by means of the overregulation of the CCR2 chemokine receptor. The CCR2-CCL2 system induces the secretion of permeability factors such as serum amyloid A3 and S100A8. This result suggests the possibility that the overregulation of CCR2 represents a marker for the regions of greater susceptibility to metastasis in lung cancer [95].
The epithelial–mesenchymal transition
Local invasion implies profound changes in the adhesion and the proteolytic and migratory properties of the tumour cells, which favours cellular disassociation, degradation of the extracellular matrix, and migration to adjacent tissues. In addition, the excessive proliferation of epithelial cells and angiogenesis are the markers of initiation and growth, which can be observed in a primary carcinoma [96]. During the progression of a carcinoma, the already differentiated tumour cells alter their genome, which confers an advantage on the cell in terms of growth. In later stages, the cells continue to change their genome and exhibit a non-differentiated phenotype frequently accompanied by a low expression of epithelial markers, which leads to a loss of intercellular junctions and epithelial polarity. These changes are often accompanied by an increase in the expression of mesenchymal markers, as well as the mobility of the cells, which gives them greater invasive capacity. The process by which the cells change from an epithelial phenotype to a mesenchymal phenotype is known as the epithelial–mesenchymal transition or EMT. This process can be defined as a cellular programme that permits the phenotype transition of the cell from epithelial to mesenchymal [97, 98].
The marginal zone of the tumour is an active and interactive region of great importance to the tumour microenvironment, where immune cells and stromal cells accumulate. In the case of the immature myeloid cells that accumulate in this region, they impede the differentiation of the antigen initiator cells, thus favouring the evasion of the tumour cells [99]. The macrophages are another type of principal cells that are found in the marginal zone and are recruited by the products secreted by the tumour cells [100]. Studies have demonstrated the importance of the stroma during the epithelial–mesenchymal transition in cancer by means of the presence or absence of TGF-β [101]. In teratocarcinomas, the accumulation of macrophages induces the epithelial–mesenchymal transition due to the TGF-β factor produced by the macrophages associated with the tumours [102].
The epithelial–mesenchymal transition can also be induced by TGF-β secreted by the platelets [103]. The macrophages also promote the invasion of tumour cells by means of the supply of migratory factors, such as EGF, which through the regulation of the production of fibrillar collagen, accelerate cellular motility and induces proteolytic activity for the remodelling of the extracellular matrix [104]. During the EMT transition, alterations occur in cell–cell adhesion, cell–substrate interaction, degradation of the extracellular matrix, and the reorganisation of the cytoskeleton [105]. EMT is fully achieved when degradation of the basal membrane occurs and a mesenchymatic cell can migrate, this acquiring invasive capacity, which permits metastatic dissemination. The activation of the EMT programme has been proposed as a critical mechanism for the acquisition of the malignant phenotype by the epithelial cells [106].
The cells that initiate the EMT transition are found on the invasive front of the primary tumours expressing mesenchymal markers that are capable of effecting intravasation, as they are transported through the circulation, into micro or macrometastatic forms [6]. In addition, the metastasis and the primary tumours are histologically similar, which can be interpreted as a reversible EMT that would permit migration and dissemination to different organs in the first place. Once the cells that have undergone EMT are in place, they would activate the opposite programme, the MET transition, which would permit them to establish secondary colonies by returning to epithelial morphology and re-acquiring the ability to grow and proliferate [107].
Transcriptional regulation of the epithelial–mesenchymal transition
A large number of molecular processes cooperate in the initiation and completion of the EMT transition, including the activation of transcription factors, the expression of specific surface proteins, the reorganisation and expression of proteins in the cytoskeleton, the production of enzymes that degrade the extracellular matrix, and the changes in the expression of miRNAs [108]. A key step in the EMT is the reduction in cell–cell adhesion by means of the transcriptional repression of the cadherins, components of the aherens junctions, such as occludin and claudin, components of tight junctions, to which is added the desmoplaquins, components of the desmosomes [109]. β-Catenin forms part of the adherens junctions, such that on breaking it migrates to the nucleus, where it functions as a cofactor for the Tcf or T-cell factor/lef transcription factors [110]. These then activate the transcription of genes such as c-myc, which increases cellular proliferation [111].
The expression of the intermediate filaments changes during the EMT with the substitution of keratin by vimentin [112], while the metalloproteinases increase during the EMT, participating in the loss of cell–cell junctions and the degradation of the basal membrane [113]. Several transcription factors that regulate the EMT inhibit apoptosis through activation of the MAPK and PI3K pathways [114]. During tumour progression, E-cadherin can be inactivated through the repression by means of the hypermethylation and deacetylation of the promoter through joining with the transcription receptors [115]. E-boxes, or short sequences of six nucleotides, -CACCTG or CAGGTG-, which determine specific expression in epithelial cells, were identified through analysis of the proximal promotor of E-cadherin in rats. The inactivation of these sequences activates the transcription of the E-cadherin in mesenchymal cells, which indicates the presence of repressors that silence the expression of this protein in non-epithelial cells [116]. The first repressors identified were the transcription factors with zinc fingers, Snail 1, and Snail 2 (slug) domains [117, 118], and the Zeb1 and Zeb2 transcription factors [119, 120], all of which are able to join with the E boxes of the cadherin promoter. Other repressors include the basic helix-loop-helix E12/E47 (TCF3) and Twist transcription factors [121, 122]. These repressors repress E-cadherin by means of the recruitment of some corepressors, such as for example, in the case of twist, activating the expression of other E-cadherin repressors. Furthermore, the overexpression of these factors in the epithelial cells not only produces the repression of E-cadherin, but also the reprogramming of the cell to a mesenchymal state. During the EMT, these repressors also repress other molecules from the adherens junctions and induce mesenchymal characteristics in a coordinated manner [123]. Also, the expression of Snail 1 induces the expression of fibronectin or vitronectin [124]. In the case of Twist, this regulator of the EMT induces the expression of the Akt kinase, a P13K effector and an important regulator in the survival pathways during the EMT [125].
The small non-coding RNa or microRNAs also act as regulators of the EMT transition, inhibiting the gene expression at the post-transcriptional level with the consequent reduction of the stability of the mRNAs, which are their target [126]. The constituents of the metastatic niche, as well as the remodelling of the ECM, have been associated with the induction of the EMT. Periostin or osteoblast-specific factor OSF-2 promotes its induction [127]. The EMT is induced by the metalloproteinases, MMPs that are activated in the metastatice niche [128]. Hypoxia also induces the activation of the EMT transition [129]. Other studies have demonstrated that the induction of EMT in the MCF10A cells with a high expression of SNAIL contributes to (a) resistance to antitumour drugs, (b) the acquisition of the mother cell phenotype through an increase in the expression of the CD44+/CD24− surface markers, (c) the capacity to form mammospheres [130]. In the case of the resistance to drugs developed by the mother cells, this represents one of the greatest challenges for chemotherapy against cancer [131]. This mainly affects tumour cells with rapid proliferation, while the mother cells grow slowly and have an effective resistance mechanism. This resistance to treatment leads to an increase in the rate of proliferation of the mother cells, which results in a recurrence of the cancer and metastasis [132].
Signalling pathways that activate the epithelial transition
The interaction of the tumour cells with the local microenvironment induces the autocrine or paracrine secretion of the growth factors, cytokines, and components of the extracellular matrix that can trigger the molecular programme for the EMT transition [133]. A large number of signalling pathways and growth factors have been associated with the EMT transition, such as the epidermal growth factor EGF, the fibroblastic growth factor FGF [134], and the hepatic growth factor or HGF [135]. The Wnt/β-catenin pathway is related to the EMT transition [136], while the TGF-β factor is another EMT inducer, as the signals activated by this factor inhibit some epithelial proteins, such as E-cadherin and keratin. They also activate the expression of mesenchymal proteins, such as fibronectin and vimentin, and in the case of TGF-β, it acts in the activation of the EMT through the Smad proteins [137]. Another pathway implicated in the induction of the EMT is that activated by NOTCH, which in turn is induced by hypoxia [138]. The NF-kβ factor acts as a regulator of the EMT, which is important for protection against apoptosis and in the induction of metastasis [139].
The metalloproteinases
The metalloproteinases or MMPs belong to a family of zinc-dependent endopeptidases that intervene both in the physiological processes of organogenesis and in the scar formation and in various pathological conditions, particularly cancer [140]. Although the first well-studied function of the metalloproteinases is the degradation of the ECM, it is currently believed that they play an important role in the processing of bioactive molecules such as growth factors, cytokines and chemokines, as well as their respective receptors [141]. The MMPs modulate the mediators of inflammation, such as the cytokines and chemokines, establishing the necessary gradients for chemotaxis of the inflammatory cells. They facilitate the migration of epithelial cells by interacting with these and the ECM proteins through proteolysis of the same matrix or the tissue adhesion proteins, such as E-cadherins [142]. In the vascular system, they influence the migration of tumour cells, the liberation of cytokines and growth factors linked to the cellular membrane, such as the transforming growth factor alpha TGF-α and the epidermal growth factor EGF [143]. In many malignant tumours, there is overexpression of ADAMs, desintegrin, and metalloproteinase, where their role in tumour growth and dissemination is related to their proteolytic activity [144]. Several MMPs and MT-MMPs have an important activating action on other pro-MMPs, while they are activated by other proteases. These processes usually take place in the extracellular space, but there is a group of proteases, the MT-MMPs, MMP-11, MMP-23, and MMP-28, which activate within the cell by means of a furin-type proprotein convertase [145]. The MMPs also act as signalling molecules and can then modulate other cell signalling molecules. The platelet-derived growth factor PDGF produces an increase in the expression of MMP-1, which acts in conjunction with TGF-β, producing an overexpression of MMP-3 and TIMP-1 [146]. The epidermal growth factor EGF induces the expression of MMP-1 [147], and the vascular endothelial growth factor VEGF and fibroblast growth factor FGF-2 are angiogenic factors that can induce the expression of the MMP proteases, facilitating metastatic dissemination [148]. The tumour necrosis factor alpha TGF-α is a pro-inflammatory cytokine liberated by macrophages, T lypmhocytes, and mastocytes that induce the overexpression of some MMPs such as MMP-2, MMP-3, MMP-7, and MMP-9 in the tumour microenvironment, which increases the invasive capacity of the malignant cells [149].
The metalloproteinases and the progression of cancer
The MMPs are the principal mediators in the changes observed in the tumour microenvironment during the progression of cancer [150], which play a fundamental role in tumour growth through degradation of the connective tissue and the components of the basal membrane, in addition to activating growth factors, surface receptors for adhesion molecules and for chemokines [151]. This interaction with the components of the ECM alters the cellular response to the microenvironment, making the tumour cells less adherent so they have more potential to migrate and produce metastasis [152]. During carcinogenesis, the tumour cells interact with the growth factors, the cytokines, and other cells, such as endothelial cells, fibroblasts, macrophages, mastocytes, and pericites, that are present in the tumour microenvironment [153]. All of this demonstrates that the remodelling of the ECM is an active event during tumour progression that leads to the formation of a niche for the survival and proliferation of the tumour cells [154].
The MMPs can play different roles during the progression of the cancer, depending on the stage of the tumour. In early stages, the proteolysis of MMPs 3 and 7, which link growth factors, contribute to cellular proliferation, but later, the cleavage of E-cadherin and CD44 activates the motility of the tumour cells facilitating metastasis [155]. In contrast, MMP-8 has a protective effect as it diminishes the metastatic potential of breast cancer cells [156], but in the case of the overexpression of MMPs 2 and 9, which indicates an unfavourable prognosis as it degrades type 4 collagen, located in the basal membranes, and induces the expression of angiogenic factors [157].
The local invasion of the tumours depends on the degradation of the proteins of the basal membranes, such as type 4 or 5 collagen, and the proteolysis of type 1, 2, or 3 interstitial collagen present in the connective tissue that surrounds the tumour cells [158]. In addition, the MMPs intervene in angiogenesis, promoting the migration of the endothelial cells, liberating VEGF and other proangiogenic factors of the ECM, such as FGF-2 and TGFβ, which also favour the proliferation and migration of these cells [159, 154]. In animal models, it has been demonstrated that the MMPs regulate the formation and maturation of new blood vessels through the control they exercise on the growth factors and cytokines, which act on the recruitment of pericytes [160]. Some MMPs can have an inhibiting effect on angiogenesis; for example, the hydrolysis of plasminogen creates fragments of angiostatin and the proteolysis of XVIII collagen produces endostatin [161]. The transcription of the MMPs is induced by inflammatory cytokines, such as IL-1, IL-6, TNF-α, and growth factors such as EGF, HGF, and TGF-β, giving them a preponderant role in the chronic inflammation which is present in the tumour microenvironment. Other factors, such as TGF-α and IL-4 inhibit their expression, and can be considered as therapeutic targets for cancer [162].
Angiogenesis
Angiogenesis is a crucial mechanism in the development of cancer due to the growing need for oxygen and nutrients, as the lack of these will lead to the latency and death of the tumour cells [163]. Tumour angiogenesis depends on both the angiogenic growth factors, with stimulants such as angiogenin, angiopoietin-1, cyclo-oxygenase, hepatocyte growth factor, and tumour growth factor produced by the tumour cells and those of the host, and the antiangiogenic factors, which are inhibitors and include angiopoietin-2, angiostatin, interferon-α/β endostatin, and vasostatin, which keep the existing blood vessels in a state of quiescence [164].
The vascularisation of the tumour requires the participation of many types of cells in the tumour microenvironment, including the vascular endothelial cells, the pericytes, and the bone marrow precursor cells, whose participation is regulated by hypoxia [165, 166]. Added to these are the tumour-associated macrophages, the tumour-associated fibroblasts, and the mesenchymal mother cells, which also contribute to the vascularisation of the tumour through the liberation of pro-angiogenic signals in the tumour microenvironment. In patients with advanced, vascularised breast cancer, a high mobilisation of mesenchymal mother cells was found in the circulation, which indicates that these cells have an active participation in tumour progression [167, 168]. Lymphangiogenesis is another route for tumour vascularisation, as the cancer cells can also be disseminated through the lymph vessels [169]. In human cervical cancer, the activated macrophages can produce the VEGF-C and VEGF-D factors, which correlate with lymphangiogenesis [170].
In order to initiate neo-vascularisation, an avascular tumour must acquire an angiogenic phenotype that permits it to ‘turn on’ the angiogenic interruptor [171]. Obviously, the mechanism through which the angiogenic interruptor is ‘connected’ must involve partial oxygen pressure sensors that are sufficiently sensitive to rapidly detect the hypoxia produced in the interior of the solid avascular tumour [172]. The response to hypoxia is the induction of the gene that codes the VEGF protein, considered the most potent angiogenic inducer, while it is also very likely that other factors exist, which can play a role in the response of the tumour to hypoxia [173]. It is accepted that the angiogenic interruptor is ‘turned off’, while the effect of the pro-angiogenic molecules is suspended by the antiangiogenic molecules, and the interruptor turns on when the net balance shifts in favour of angiogenesis. The net angiogenic activity of a tumour is the result of the disequilibrium between stimulating and inhibiting signals [174].
Tumour latency and progression
Tumours can remain in a latent state for years when there is an equilibrium between proliferation and apoptosis, a phenomenon that can be defined ‘as a state of temporal latency of the arrest of tumour growth’ [175]. This condition can be divided into three categories:
(a) Cellular latency
Structural components of the niche can promote the survival of the tumour cells while keeping them in a state of latency [176]. Such is the case of the cytoskeleton, which can reactivate these cells, which suggests that the rigidity of the extracellular matrix presents the property of promoting the exit from latency. In an in vitro model using cells from a hepatocellular carcinoma, the increase in rigidity of the microenvironment was linked to the TGF-ß factor through induction of the D1 and D3 cyclins [177], showing that less rigid environments favour the latency of tumour cells. In a breast cancer model, it was shown that TGF-ß1 induced fibrosis through deposition of type 1 collagen in the lung, which favoured the escape from latency [178]. In breast cancer, the fibroblasts associated with the cancer can interact with proteins in the extracellular matrix to modulate the intracellular adhesions, cellular contractibility, and forces within the tumour microenvironment. It has been demonstrated that caveolin-1, or Cav-1, produced by the fibroblasts promotes the rigidity of the tumour microenvironment through the activation of GTPasa, thus stimulating tumour progression [179]. The induction of the EMT within the niche causes the tumour cells to enter into a state of latency due to the increase in the levels of p16INK4a [180], the repression of cyclin D [181] and a sustained expression of twist [182]. The latency confers resistance to tumour cells against the action of antitumour agents, whether due to arrest of the cell cycle or a slow proliferation which makes the action of the antitumour agents ineffective [183]. On the other hand, the latent cells must be reactivated in order to grow and form a metastasis. Once the niche has matured, some of its components may act to liberate the cells from latency. Changes in the cytokines can liberate the latent cells, induced by the TCD4+ cells [184]. Also, the remodelling of the extracellular matrix by type 1 collagen has been implicated in the liberation from latency through integrin signalling mediated by FAK [185]. The uPAR urokinase receptor activates the β1 integrin, which on interacting with fibronectin, permits the liberation of the tumour cell from latency [186].
(b) Angiogenic latency
As with normal tissues, the tumours provide nutrients to the cells, such that an inefficient vascularisation causes the tumour mass to remain constant due to an equilibrium between the cells that are dividing and those which are dying. This is the reason why tumour cells, when forming a micrometastasis, must vascularise it in order to survive, as if this does not occur, it can disappear or enter into a state of latency [187], a condition in which it will remain until genetic, epigenetic, and microenvironmental signals can activate angiogenesis. In a study of immunodeficient mice who carried a liposarcoma that was able to remain latent for more than 90 days, high levels of thrombospondin (TSP) and angiomotin were demonstrated [188]. Thrombospondin or TSP is a glycoprotein of the cellular matrix which in physiological conditions is segregated by the fibroblasts and other cells, such as the endothelial cells [189]. On the other hand, TSP was found in latent breast cancer tumour cells that were in contact with the microvasculature of the organs in which they would have metastasised. In a three-dimensional in vitro model, the endothelial cells that are part of the vascular system produce the TSP, which acts as a suppressor of angiogenesis and causes the tumour cells to remain in a state of latency. This can explain the effect of TSP on the latency of tumour cells [190]. TSP is a suppressor protein of angiogenesis, however, with the formation of new vessels the endothelial cells produce TGF-ß1 and periostin (POSTN), which will permit the latent cells to initiate their proliferation [191]. The overexpression of Notch-delta 4 (DLL4) in endothelial cells can promote the exit of T-ALL cells from a latent state through joining with the Notch 3 receptor. This overexpression of DLL4 can be induced by the vascular endothelial growth factor VEGF, with the resulting exit from the latent state [192].
(c) Immunologic latency
A critical step in tumour progression is the evasion and suppression of the immune system of the host [193], which can be achieved through the inhibition of immune effector cells or through the stimulation of immunosuppressor cells. One of the most common mechanisms of immune evasion in patients is due to the activity of the myeloid-derived suppressor cells or MDSCs, which are defined as immature immunosuppressant myeloid cells that maintain the homoeostasis of normal tissue in response to adverse situations such as infections, post-traumatic stress, etc [194]. During tumorigenesis, the MDSC cells infiltrate the tumour promoting its vascularisation [195] and disturbing the immune mechanisms such as the production of antigens by dendritic cells or DCs [196], the activation of T cells [197], the polarisation of M1 macrophages [198] and the inhibition of the cytotoxic NK cells [199]. The MDSC cells promote tumour progression, which has been demonstrated in animal models, and is supported by the elevated number of these cells which is found in patients with cancer, which correlates with an advanced stage of the disease and treatment failure [200].
Stroma and growth of the tumour cells: pre-clinical studies
As we have seen, in a tumour the different cells of the stroma can interact directly with each other through direct contact or through paracrine signalling. In particular case of the tumour cells, these can induce changes in the cells of the stroma and the extracellular matrix through several mechanisms. They can also alter the stroma through cell–cell contact, modify the extracellular matrix, or liberate soluble factors. They often secrete cytokines, chemocytokines, growth factors, metalloproteinases, and inflammatory mediators that can promote invasivity. However, the mechanisms through which the stroma cells facilitate the growth of tumour cells are not known, and further studies are needed to understand the mechanisms implicated between the cells and the tumour microenvironment in order to design new strategies against cancer and improve existing treatments. Preclinical studies have tested treatments blocking the mechanisms that the tumour cells develop to evade the immune response. Ipilimumab, an antibody that activates the T cells, promoting antitumour activity, was tested on patients with metastatic melanoma, and increased their survival in comparison to patients not treated with this antibody [201]. The CD40 antibody reverses immune suppression activating the antigen-producing cells and promoting the antitumour response of the T cells [202]. An elevated expression of programmed cell death protein 1, PD-1, is produced with cancer.
It has been demonstrated that Pembrolizumab or MK-3475 blocks PD-1 and its ligand PD-L1 expressed in tumour cells in the tumour microenvironment, both in melanomas and in other types of tumours [203]. Dasatinib, an Src gene inhibitor, makes it difficult for melanoma tumour cells to survive [204] as CSF-1R, a regulator or the macrophages associated with tumours, inhibits apoptosis, migration, and invasion in the breast cells of canines [205]. Nintedanib is a simultaneous inhibitor of VEGFR, PDGFR, and FGR, which shows antiangiogenic and antineoplasic inhibitory activity in lung cancer, impeding the growth of tumour cells [206].
In addition, products have been used, which impede the degradation of bone to avoid its colonisation by tumour cells, as is the case with prostate cancer. Micro RNA, miR-34a, acts as a suppressor of osteoclastogenesis, bone resorption, and the formation of a metastatic niche, impeding the establishment of metastatic cells [207]. Resveratrol inhibits the EMT transition in the cellular line of LoVo colon cancer through the inhibition of the TGF-β1/Smads pathway mediated by the expression of Snail/E-cadherin, impeding invasion and metastasis [208]. Curcumin inhibits the EMT transition and induces apoptosis in PANC-1 pancreatic cells through the Shh-GLI1 pathway [209].
Conclusion
Cancer is not only the transformation of individual cells into a state of cellular proliferation, but a disruption of the forms in which the tissues regulate their processes and affect the systemic interactions with the affected organism. Currently, the fundamental treatments against cancer continue to be surgery, radiation therapy, and chemotherapy, which usually destroys the primary tumour, but whose action is very limited against metastasis. This is why it is necessary to continue investigating to find new prognostic markers and new therapeutic targets for metastasis before it occurs, as the early detection of these markers could determine which cases require treatment and avoid it in those patients without a risk of metastasis. Thus, for example, the monitoring of growth factors and cytokines in the blood which may induce the formation of the premetastatic niche would be fundamental. At the same time, determining the blood levels of components of the metastatic niche such as the VEGFR1 protein circulating or interfering with the formation of inflammatory components such as type CD11b+ myeloid cells is indispensable for this purpose.
Genetic, cellular biology, and molecular studies, as well as those of the internal and external environmental contexts, indicate that tumour growth is not only determined by its cells, but also by the tumour microenvironment and the entire context in which the organism functions. In this way, the progression of cancer is the result of a very complex relationship between the different malignant and non-malignant cell types, components of the stroma, and the entire body of the organism.
Due to the implication of metastasis in mortality due to cancer, it is also necessary to search for new ways to integrate the two focuses which dominate the current science: the reductionist vision and the systemic vision sustained by the science of complexity.
References
- 1.Lugassy C, Escande JP. The haematogenous theory of metastasis: récamier did not propose it. Virchows Arch. 1997;431(5):371. doi: 10.1007/s004280050113. [DOI] [PubMed] [Google Scholar]
- 2.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–92. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvelo F, Poupon MF. Aspectos moleculares y celulares de la metástasis cancerosa. Acta Cient Venez. 2001;52:304–12. [PubMed] [Google Scholar]
- 4.Langley RR, Fidler IJ 2007. Tumor cell-organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr Rev. 28:297–321. doi: 10.1210/er.2006-0027. [DOI] [PubMed] [Google Scholar]
- 5.Talmadge JE, Benedict K, Madsen J, et al. Development of biological diversity and susceptibility to chemotherapy in murine cancer metastases. Cancer Res. 1984;44:3801–5. [PubMed] [Google Scholar]
- 6.Arvelo F. Micrometástasis: estrategias para su detección. Invest Clínic. 2013;54:206–25. [PubMed] [Google Scholar]
- 7.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nat Rev Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 9.Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970;45:773–82. [PubMed] [Google Scholar]
- 10.Hill RP, Young SD, Cillo C, et al. Metastatic cell phenotypes: quantitative studies using the expèrimental metástasis assay. Cancer Rev. 1986;5:118–51. [Google Scholar]
- 11.Ruddon RW. New York: Oxford University Press; 1995. Cancer biology. [Google Scholar]
- 12.Vizoso M, Ferreira HJ, Lopez-Serra P, et al. Epigenetic activation of a cryptic TBC1D16 transcript enhances melanoma progression by targeting EGFR. Nat Med. 2015. [DOI] [PMC free article] [PubMed]
- 13.De Vita VT, Hellman S, Rosemberg SA. New York: Lippincott & Wilkins; 2004. Cancer, principles & practice of oncology. [Google Scholar]
- 14.Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15:807–17. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oskarsson T, Acharyya S, Zhang XH, et al. Breast cancer cells produce tenascin C as a metastatic niche component to colonize the lungs. Nature Med. 2011;17:867–74. doi: 10.1038/nm.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moller HD, Ralfkjaer U, Cremers N, et al. Role of fibulin-5 in metastatic organ colonization. Mol Cancer Res. 2011;9:553–63. doi: 10.1158/1541-7786.MCR-11-0093. [DOI] [PubMed] [Google Scholar]
- 17.Wong CC, Gilkes DM, Zhang H, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 2011;108:16369–74. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan RN, Riba R, Zacharoulis S, et al. VEGFR-1 positive haemapoietec bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan RN, Rafiis S, Lyden D. Preparing the “soil” the premetastatic niche. Cancer Res. 2006;66:11089–93. doi: 10.1158/0008-5472.CAN-06-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borregaard N, Cowland JB. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood. 1997;89:3503–21. [PubMed] [Google Scholar]
- 22.Scapini P, Lapinet-Vera JA, Gasperini S, et al. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065X.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 23.Strieter RM, Burdick MD, Gomperts BN, et al. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Balkwill F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006;25:409–16. doi: 10.1007/s10555-006-9005-3. [DOI] [PubMed] [Google Scholar]
- 26.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 27.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–52. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 28.Yu H, Pardoll D, Jove R. STATs in cáncer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braumuller H, Wieder T, Brenner E, et al. T-helper-1-cell cytokines drive cancer in to senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 30.Shinohara ML, Lu L, Bu J, et al. Osteopontin expression is essential for interferon-alpha production by plasmacytoid dendritic cells. Nat Immunol. 2006;7:498–506. doi: 10.1038/ni1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scimone ML, Lutzky VP, Zittermann SI, et al. Migration of polymorphonuclear leucocytes is influenced by dendritic cells. Immunol. 2005;114:375–85. doi: 10.1111/j.1365-2567.2005.02104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albini A, Brigati C, Ventura A, et al. Angiostatin anti-angiogenesis requires IL-12: the innate immune system as a key target. J Transl Med. 2009;7:5–13. doi: 10.1186/1479-5876-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–44. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Bunt SK, Sinha P, Clements VK, et al. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 35.Murdoch C, Muthana M, Coffelt SB, et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 36.Platonova S, Cherfils-Vicini J, Damotte D, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–22. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- 37.Bruno A, Focaccetti C, Pagani A, et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia. 2013;15:133–42. doi: 10.1593/neo.121758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sceneay J, Chow MT, Chen A, et al. Primary tumor hipoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Res. 2012;72(16):3906–11. doi: 10.1158/0008-5472.CAN-11-3873. [DOI] [PubMed] [Google Scholar]
- 39.Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cáncer and other tales. Nat Rev Immunol. 2011;11:702–11. doi: 10.1038/nri3064. [DOI] [PubMed] [Google Scholar]
- 40.Hoechst B, Gamrekelashvili J, Manns MP, et al. Plasticity of human Th17 cells and iTregs is orchestrated by different subsets of myeloid cells. Blood. 2011;117:6532–41. doi: 10.1182/blood-2010-11-317321. [DOI] [PubMed] [Google Scholar]
- 41.Facciabene A, Peng X, Hagemann IS, et al. Tumour hipoxia promotes tolerance and angiogenesis via CCL28 and T (reg) cells. Nature. 2011;475:226–30. doi: 10.1038/nature10169. [DOI] [PubMed] [Google Scholar]
- 42.Pillai S, Mattoo H, Cariappa A. B cells and autoimmunity. Curr Opin Immunol. 2011;23:721–731. doi: 10.1016/j.coi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernatsky S, Ramsey-Goldman R, Clarke A. Malignancy and autoimmunity. Curr Opin Rheumatol. 2006;18:129–34. doi: 10.1097/01.bor.0000209423.39033.94. [DOI] [PubMed] [Google Scholar]
- 44.Gunderson AJ, Coussens LM. B cells and their mediators as targets for therapy in solid tumors. Exp Cell Res. 2013;319:1644–9. doi: 10.1016/j.yexcr.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu H, Goodell V, Disis ML. Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res. 2008;7:1388–94. doi: 10.1021/pr700818f. [DOI] [PubMed] [Google Scholar]
- 46.De Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–23. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 47.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–41. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 48.Nico B, Mangieri D, Crivellato E, et al. Mast cells contribute to vasculogenic mimicry in multiple myeloma. Stem Cells Dev. 2008;17:19–22. doi: 10.1089/scd.2007.0132. [DOI] [PubMed] [Google Scholar]
- 49.Marichal T, Tsai M, Galli SJ. Mast cells: potential positive and negative roles in tumor biology. Cancer Immunol Res. 2013;1:269–79. doi: 10.1158/2326-6066.CIR-13-0119. [DOI] [PubMed] [Google Scholar]
- 50.de Souza DA, Toso VD, Campos MR, et al. Expression of mast cell proteases correlates with mast cell maturation and angiogenesis during tumor progression. PLoS One. 2012;7(7):e40790. doi: 10.1371/journal.pone.0040790. Epub 2012 Jul 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ammendola M, Sacco R, Sammarco G, et al. Correlation between serum tryptase, mast cells positive to tryptase and microvascular density in colorectal cancer patients: possible biological-clinical significance. PLoS One. 2014;9(6):e99512. doi: 10.1371/journal.pone.0099512. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shree T, Olson OC, Elie BT, et al. Macrophages and cathepsin proteases blunt chemotherapeutic response in breast cancer. Genes & dev. 2011;25:2465–79. doi: 10.1101/gad.180331.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cáncer as a paradigm. Nat Immunol. 2010;11:889–96. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 56.Escribese MM, Casas M, Corbi AL. Influence of low oxygen tensions on macrophage polarization. Immunobiol. 2012;217:1233–40. doi: 10.1016/j.imbio.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gil-Bernabe AM, Ferjancic S, Tlalka M, et al. Recruitment of monocytes/macrophages by tissue factor-mediated coagulation is essential for metastatic cell survival and premetastatic niche establishment in mice. Blood. 2012;119:3164–75. doi: 10.1182/blood-2011-08-376426. [DOI] [PubMed] [Google Scholar]
- 59.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 60.Olumi AF, Grossfeld GD, Hayward SW, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dumont N, Liu B, Defilippis RA, et al. Breast fibroblasts modulate early dissemination, tumorigenesis, and metastasis through alteration of extracellular matrix characteristics. Neoplasia. 2013;15:249–62. doi: 10.1593/neo.121950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochimica et Biophysica Acta. 2013;1832:1070–8. doi: 10.1016/j.bbadis.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Potenta S, Zeisberg E, Kalluri R. The role of endothelial-to-mesenchymal transition in cancer progression. Br J Cancer. 2008;99:1375–9. doi: 10.1038/sj.bjc.6604662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orr B, Riddick AC, Stewart GD, et al. Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate. Oncogene. 2012;31:1130–1142. doi: 10.1038/onc.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erez N, Truitt M, Olson P, et al. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 66.Tan W, Zhang W, Strasner A, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature. 2011;470:548–53. doi: 10.1038/nature09707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 68.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–48. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 69.Marsh T, Pietras K, McAllister SS. Fibroblasts as architects of cancer pathogenesis. Biochimica et Biophysica Acta. 2013;1832:1070–8. doi: 10.1016/j.bbadis.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fukumura D, Xavier R, Sugiura T, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–25. doi: 10.1016/S0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 71.Chowdhury R, Webber JP, Gurney M, et al. Cancer exosomes trigger mesenchymal stem cell differentiation into pro-angiogenic and pro-invasive myofibroblasts. Oncotarget. 2015;6(2):715–31. doi: 10.18632/oncotarget.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dore-Duffy P, Cleary K. Morphology and properties of pericytes. Methods Mol Biol. 2011;686:49–68. doi: 10.1007/978-1-60761-938-3_2. [DOI] [PubMed] [Google Scholar]
- 73.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:12831316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 74.Sundberg C, Kowanetz M, Brown LF, et al. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82:387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 75.Falcón BL, Hashizume H, Koumoutsakos P, et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am J Pathol. 2009;175:2159–70. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goumans MJ, Valdimarsdottir G, Itoh S, et al. Balancing the activation state of the endothelium via two distinct TGF-beta type I receptors. EMBO J. 2002;21:1743–53. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barlow KD, Sanders AM, Soker S, et al. Pericytes on the tumor vasculature: jekyll or hyde? Cancer Micro environ. 2013;6:1–17. doi: 10.1007/s12307-012-0102-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raza A, Franklin MJ, Dudek AZ. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am J Hematol. 2010;85:593–8. doi: 10.1002/ajh.21745. [DOI] [PubMed] [Google Scholar]
- 79.Darland DC, Massingham LJ, Smith SR, et al. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev Biol. 2003;264:275–88. doi: 10.1016/j.ydbio.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 80.Yonenaga Y, Mori A, Onodera H, et al. Absence of smooth muscle actin-positive pericyte coverage of tumor vessels correlates with hematogenous metastasis and prognosis of colorectal cancer patients. Oncol. 2005;69:159–66. doi: 10.1159/000087840. [DOI] [PubMed] [Google Scholar]
- 81.Muller AJ, Scherle P. Targeting the mechanisms of tumoral immune tolerance with small-molecules inhibitors. Nature Rev. 2006;6:611–25. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 82.Le NT, Xue M, Castelnoble LA, et al. The dual personalities of matrix metalloproteinases in inflammation. Front Biosci. 2007;12:1475–87. doi: 10.2741/2161. [DOI] [PubMed] [Google Scholar]
- 83.Nelson D, Ganss R. Tumor growth or regression: powered by inflammation. J Leukoc Biol. 2006;80:685–690. doi: 10.1189/jlb.1105646. [DOI] [PubMed] [Google Scholar]
- 84.Lukanidin E, Sleeman JP. Building the niche: the role of the S100 proteins in metastatic growth. Sem Cancer Biol. 2012;22:216–25. doi: 10.1016/j.semcancer.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 85.Badolato R, Wang JM, Murphy WJ, et al. Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J Exp Med. 1994;180:203–9. doi: 10.1084/jem.180.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Malle E, Sodin-Semrl S, Kovacevic A. Serum amyloid A: an acute-phase protein involved in tumour pathogenesis. Cell Mol Life Sci. 2009;66:9–26. doi: 10.1007/s00018-008-8321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheumat Dis. 2011;70(Suppl 1):104–8. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 88.Yan HH, Pickup M, Pang Y, et al. Gr-1+ CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Res. 2010;70:6139–49. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Whiteside TL, Schuler P, Schilling B. Induced and natural regulatory T cells in human cancer. Expert Opin Biol Ther. 2012;12:1383–97. doi: 10.1517/14712598.2012.707184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gasteiger G, Hemmers S, Firth MA, et al. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med. 2013;210:1179–87. doi: 10.1084/jem.20122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bates GJ, Fox SB, Han C, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24:5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 92.Frey DM, Droeser RA, Viehl CT, et al. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–43. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 93.von Boehmer H, Daniel C. Therapeutic opportunities for manipulating T (Reg) cells in autoimmunity and cancer. Nature Rev Drug Discov. 2013;12:51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- 94.Hiratsuka S, Watanabe A, Sakura Y, et al. The S100A8 serum amyloid A3-TLR4 paracrine cascade establishes premetastatic phase. Nat Cell Biol. 2013;10:1349–55. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 95.Hiratsuka S, Ishibashi S, Tomita T, et al. Primary tumours modulate innate immune signalling to create pre-metastatic vascular hyperpermeability foci. Nat Commu. 2013;4:1853. doi: 10.1038/ncomms2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 97.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 98.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 99.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–6. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 101.Thiery JP, Acloque H, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 102.Bonde AK, Tischler V, Kumar S, et al. Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors. BMC Cancer. 2012;12:35. doi: 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gocheva V, Wang HW, Gadea BB, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev. 2010;24:241–55. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–58. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 106.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 107.Tsai JH, Yang J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013;27:2192–206. doi: 10.1101/gad.225334.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bullock MD, Sayan AE, Packham GK, et al. MicroRNAs: critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biol Cell. 2012;104:3–12. doi: 10.1111/boc.201100115. [DOI] [PubMed] [Google Scholar]
- 109.De Wever O, Pauwels P, De Craene B, et al. Molecular and pathological signatures of epithelial-mesenchymal transitions at the cancer invasion front. Histochem Cell Biol. 2008;130:481–94. doi: 10.1007/s00418-008-0464-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–76. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- 111.He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 112.Omary MB, Coulombe PA, McLean WH. Intermediate filament proteins and their associated diseases. N Engl J Med. 2004;351:2087–100. doi: 10.1056/NEJMra040319. [DOI] [PubMed] [Google Scholar]
- 113.Tania M, Khan MA, Fu J. Epithelial to mesenchymal transition inducing transcription factors and metastatic cancer. Tumour Biol. 2014;35:7335–42. doi: 10.1007/s13277-014-2163-y. [DOI] [PubMed] [Google Scholar]
- 114.Kajita M, McClinic KN, Wade PA. Aberrant expression of the transcription factors snail and slug alters the response to genotoxic stress. Mol Cell Biol. 2004;24:7559–66. doi: 10.1128/MCB.24.17.7559-7566.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Iwatsuki M, Mimori K, Yokobori T, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Cancer Sci. 2010;101:293–9. doi: 10.1111/j.1349-7006.2009.01419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Batlle E, Sancho E, Francí C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–9. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 117.Cano A, Pérez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 118.Eger A, Aigner K, Sonderegger S, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene. 2005;24:2375–85. doi: 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- 119.Perez-Moreno MA, Locascio A, Rodrigo I, et al. A new role for E12/E47 in the repression of E-cadherin expression and epithelial-mesenchymal transitions. J Biol Chem. 2001;276:27424–31. doi: 10.1074/jbc.M100827200. [DOI] [PubMed] [Google Scholar]
- 120.Comijn J, Berx G, Vermassen P, et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell. 2001;7:1267–78. doi: 10.1016/S1097-2765(01)00260-X. [DOI] [PubMed] [Google Scholar]
- 121.Yang J, Mani SA, et al. RATwist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–39. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 122.Oda H, Tsukita S, Takeichi M. Dynamic behavior of the cadherin-based cell-cell adhesion system during Drosophila gastrulation. Dev Biol. 1998;203:435–50. doi: 10.1006/dbio.1998.9047. [DOI] [PubMed] [Google Scholar]
- 123.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 124.Cheng GZ, Chan J, Wang Q, et al. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–87. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 125.Park SM, Gaur AB, Lengyel E, et al. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Creighton CJ, Gibbons DL, Kurie JM. The role of epithelial mesenchymal transition programming in invasion and metastasis: a clinical perspective. Cancer Manag Res. 2013;5:187–95. doi: 10.2147/CMAR.S35171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ruan K, Bao S, Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell Mol Life Sci. 2009;66:2219–30. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cichon MA, Nelson CM, Radisky DC. Regulation of epithelial-mesenchymal transition in breast cancer cells by cell contact and adhesion. Cancer Inform. 2015;9(Suppl 3):1–13. doi: 10.4137/CIN.S18965. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu ZJ, Semenza GL, Zhang HF. Hypoxia-inducible factor 1 and breast cancer metastasis. J Zhejiang Univ Sci B. 2015;16:32–43. doi: 10.1631/jzus.B1400221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lim S, Becker A, Zimmar A, et al. SNAIL1 mediated epithelial mesenchymal transition confers chemoresistance. PLos One. 2013;8:e66558. doi: 10.1371/journal.pone.0066558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li L, Bathia R. Stem cell quiescence. Clin Cancer Res. 2011;17:4936–41. doi: 10.1158/1078-0432.CCR-10-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mallini P, Lennard T, Kirby J, et al. Epithelial-to-mesenchymal transition: what is the impact breast cáncer stem cell and drug resistance. Cancer Treat Rev. 2014;40:341–8. doi: 10.1016/j.ctrv.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 133.Ribeiro AS, Paredes JP. Cadherin linking breast cancer stem cells and invasion: a promising marker to identify an "intermediate/metastable" EMT state. Front Oncol. 2015;4:371. doi: 10.3389/fonc.2014.00371. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shirakihara T, Horiguchi K, Miyazawa K, et al. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J. 2011;30(4):783–95. doi: 10.1038/emboj.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee JK, Joo KM, Lee J, et al. Targeting the epithelial to mesenchymal transition in glioblastoma: the emerging role of MET signaling. Onco Targets Ther. 2014;7:1933–44. doi: 10.2147/OTT.S36582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jing Y, Han Z, Zhang S, et al. Epithelial-mesenchymal transition in tumor microenvironment. Cell Biosci. 2011;1:29. doi: 10.1186/2045-3701-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 138.Sahlgren C, Gustafsson MV, Jin S, et al. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci U S A. 2008;105:6392–7. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lindsey S, Langhans SA. Crosstalk of oncogenic signaling pathways during epithelial-mesenchymal transition. Front Oncol. 2014;4:358. doi: 10.3389/fonc.2014.00358. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Felibert P, Quintana J, Arvelo F. Metaloproteasas Propiedades, funciones y sus usos como blancos terapéuticos en el tratamiento antineoplásico. Acta Científica Venezolana. 2009;60:11–21. [Google Scholar]
- 141.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–47. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Manicone AM, Mc Guire JK. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol. 2008;19:34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xiao LJ, Lin P, Lin F, et al. ADAM17 targets MMP-2 and MMP-9 via EGFR-MEK-ERK pathway activation to promote prostate cancer cell invasion. Int J Oncol. 2012;40:1714–24. doi: 10.3892/ijo.2011.1320. [DOI] [PubMed] [Google Scholar]
- 145.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function and biochemistry. Circ Res. 92:827–39. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 146.Ito I, Fixman ED, Asai K, et al. Platelet-derived growth factor and transforming growth factor-beta modulate the expression of matrix metalloproteinases and migratory function of human airway smooth muscle cells. Clin Exp Allergy. 2009;39:1370–80. doi: 10.1111/j.1365-2222.2009.03293.x. [DOI] [PubMed] [Google Scholar]
- 147.Park CH, Chung JH. Epidermal growth factor-induced matrix metalloproteinase-1 expression is negatively regulated by p38 MAPK in human skin fibroblasts. J Dermatol Sci. 2011;64:134–41. doi: 10.1016/j.jdermsci.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 148.Stetler-Stevenson WG. The tumor microenvironment: regulation by MMP-independent effects of tissue inhibitor of metalloproteinases-2. Cancer Metastasis Rev. 2008;27:57–66. doi: 10.1007/s10555-007-9105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hagemann T, Robinson SC, Schulz M, et al. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–9. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- 150.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011;278:16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 151.Van Damme J, Struyf S, Opdenakker G. Chemokineprotease interaccions in cancer. Semin Cancer Biol. 2004;14:201–8. doi: 10.1016/j.semcancer.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 152.Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:1618. doi: 10.1016/j.semcancer.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Noël A, Jost M, Maquoi E. Matrix metalloproteinases at cancer tumor-host interface. Semin Cell Dev Biol. 2008;19:52–60. doi: 10.1016/j.semcdb.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 154.Hua H, Li M, Luo T, et al. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci. 2011;68:3853–68. doi: 10.1007/s00018-011-0763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chabottaux V, Noel A. Breast cancer progression: insights into multifaceted matrix metalloproteinases. Clin Exp Metastasis. 2007;24:647–56. doi: 10.1007/s10585-007-9113-7. [DOI] [PubMed] [Google Scholar]
- 156.Decock J, Hendrickx W, Vanleeuw U, et al. Plasma MMP1 and MMP8 expression in breast cancer: protective role of MMP8 against lymph node metastasis. BMC Cancer. 2008;8:77. doi: 10.1186/1471-2407-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gonzalez LO, Corte MD, Vazquez J, et al. Study of matrix metalloproteinases and their tissue inhibitors in ductal in situ carcinomas of the breast. Histopathology. 2008;53:403–415. doi: 10.1111/j.1365-2559.2008.03136.x. [DOI] [PubMed] [Google Scholar]
- 158.Rucci N, Sanită P, Angelucci A. Expanding view of the role of matrix metalloproteases in metastatic growth. Curr Mol Med. 2011;11:609–22. doi: 10.2174/156652411797536705. [DOI] [PubMed] [Google Scholar]
- 159.Hua H, Li M, Luo T, et al. Matrix metalloproteinases in tumorigenesis: an evolving paradigm. Cell Mol Life Sci. 2011;68:3853–68. doi: 10.1007/s00018-011-0763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sounni NE, Paye A, Host L, et al. MT-MMPS as regulators of vessel stability associated with angiogenesis. Front Pharmacol. 2011;2:111. doi: 10.3389/fphar.2011.00111. eCollection 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lin HC, Chang JH, Jain S, et al. Matrilysin cleavage of corneal collagen type XVIII NC1 domain and generation of a 28-kDa fragment. Invest Ophthalmol Vis Sci. 2001;42:2517–24. [PubMed] [Google Scholar]
- 162.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 164.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005;94:209–31. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 165.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature Med. 2011;17:1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 166.Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene. 2013;32:4057–63. doi: 10.1038/onc.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cuiffo BG, Karnoub AE. Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh Migr. 2012;6:220–30. doi: 10.4161/cam.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Alitalo A, Detmar M. Interaction of tumor cells and lymphatic vessels in cancer progression. Oncogene. 2012;31:4499–508. doi: 10.1038/onc.2011.602. [DOI] [PubMed] [Google Scholar]
- 169.Arvelo F. Células Madre y Cáncer. Invest Clín. 2014;55:371–91. [PubMed] [Google Scholar]
- 170.Schoppmann SF, Birner P, Stöckl J, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–56. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/S0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 172.Arvelo F. Hipoxia en la malignidad del cáncer. Invest Clin. 2009;50:529–46. [PubMed] [Google Scholar]
- 173.Goodsell D. The molecular perspective: VEGF and angiogenesis. Stem Cells. 2003;21:118–9. doi: 10.1634/stemcells.21-1-118. [DOI] [PubMed] [Google Scholar]
- 174.North S, Moenner M, Bikfalvi A. Recent developments in the regulation of the angiogenic switch by cellular stress factors in tumors. Cancer Lett. 2005;218:1–14. doi: 10.1016/j.canlet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 175.Klein CA. Framework models of tumor dormancy from patient-derived observations. Curr Opin Genet Dev. 2010;21:42–49. doi: 10.1016/j.gde.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 176.Kienast Y, Baumgarten L, Fuhrmann M, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nature Med. 2010;16(1):116–22. doi: 10.1038/nm.2072. [DOI] [PubMed] [Google Scholar]
- 177.Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011;53:1192–205. doi: 10.1002/hep.24108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Barkan D, El Touny LH, Michalowski AM, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–16. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Goetz JG, Minguet S, Navarro-Lerida I, et al. Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. 2011;146:148–63. doi: 10.1016/j.cell.2011.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fischer AN, Fuchs E, Mikula M, et al. PDGF essentially links TGF-beta signaling to nuclear beta-catenin accumulation in hepatocellular carcinoma progression. Oncogene. 2007;26:3395–405. doi: 10.1038/sj.onc.1210121. [DOI] [PubMed] [Google Scholar]
- 181.Barkan D, El Touny LH, Michalowski AM, et al. Metastatic growth from dormant cells induced by a col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–16. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Barkan D, Green JE, Chambers A F. Extracellular matrix: a gatekeeper in the transition from dormancy to metastatic growth. Eur J Cancer. 2010;46:1181–8. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mejlvang J, Kriajevska M, Vandewalle C, et al. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell. 2007;18:4615–624. doi: 10.1091/mbc.E07-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Muller-Hermelink N, Braumuller H, Pichler B, et al. TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell. 2008;13(6):507–18. doi: 10.1016/j.ccr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 185.Tran DD, Corsa CA, Biswas H, et al. Temporal and spatial cooperation of Snail1 and Twist1 during epithelial–mesenchymal transition predicts for human breast cancer recurrence. Mol Cancer Res. 2011;9:1644–57. doi: 10.1158/1541-7786.MCR-11-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vega S, Morales AV, Ocana OH, et al. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18(10):1131–43. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Holmgren L, O’Reilly MS, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nature Med. 1995;1:149–53. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 188.Almog N, Henke V, Flores L, et al. Prolonged dormancy of human liposarcoma is associated with impaired tumor angiogenesis. FASEB J. 2006;20:947–9. doi: 10.1096/fj.05-3946fje. [DOI] [PubMed] [Google Scholar]
- 189.Dameron K, Volpert O, Tainsky M, et al. Control of angiogenesis in fibroblast by p53 regulation of trombospondin-1. Science. 1994;265:1582–4. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 190.Weinstat-Saslow DL, Zabrenetzky VS, VanHoutte K, et al. Transfection of thrombospondin 1 complementary DNA into a human breast carcinoma cell line reduces primary tumor growth, metastatic potential, and angiogenesis. Cancer Res. 1994;54:6504–11. [PubMed] [Google Scholar]
- 191.Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nature Cell Biol. 2013;15:807–17. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Indraccolo S, Minuzzo S, Masiero M, et al. Cross-talk between tumor and endothelial cells involving the Notch3-Dll4 interaction marks escape from tumor dormancy. Cancer Res. 2009;69:1314–23. doi: 10.1158/0008-5472.CAN-08-2791. [DOI] [PubMed] [Google Scholar]
- 193.Lindau D, Gielen P, Kroesen M, et al. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunol. 2013;138:105–15. doi: 10.1111/imm.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 195.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nature Rev Cancer. 2013;13:739–52. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gabrilovich DI, Velders MP, Sotomayor EM, et al. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2005;166:5398–406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 198.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 199.Liu C, Yu S, Kappes J, et al. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor bearing host. Blood. 2007;109:4336–42. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Diaz-Montero CM, Salem ML, Nishimura MI, et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hwu P. Treating cáncer by targeting the immune system. N Engl J Med. 2010;363:779–81. doi: 10.1056/NEJMe1006416. [DOI] [PubMed] [Google Scholar]
- 202.Vonderheide RH, Glennie MJ. CD40 antibodies and cáncer therapy. Clin Cancer Res. 2013;19:1035–43. doi: 10.1158/1078-0432.CCR-12-2064. Agonistic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.McDermott J, Jimeno A. Pembrolizumab: PD-1 inhibition as a therapeutic strategy in cancer. Drugs Today (Barc) 2015;51:7–20. doi: 10.1358/dot.2015.51.1.2250387. [DOI] [PubMed] [Google Scholar]
- 204.Eustace AJ, Kennedy S, Larkin AM, et al. Predictive biomarkers for dasatinib treatment in melanoma. Oncoscience. 2014;1:158–66. doi: 10.18632/oncoscience.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Król M, Majchrzak K, Mucha J, et al. CSF-1R as an inhibitor of apoptosis and promoter of proliferation, migration and invasion of canine mammary cancer cells. BMC Vet Res. 2013;5(9):65. doi: 10.1186/1746-6148-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rashdan S, Hanna N. Nintedanib for the treatment of non-small-cell lung cancer. Expert Opin Pharmacother. 2014;15(5):729–739. doi: 10.1517/14656566.2014.897695. Epub 2014 Mar 5. [DOI] [PubMed] [Google Scholar]
- 207.Krzeszinski JY, Wei W, Huynh H, et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. 2014;28(7515):431–5. doi: 10.1038/nature13375. 512 Epub 2014 Jun 25. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 208.Ji Q, Liu X, Han Z, et al. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated Snail/E-cadherin expression. BMC Cancer. 2015;15:97. doi: 10.1186/s12885-015-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol Rep. 2013;29:2401–7. doi: 10.3892/or.2013.2385. [DOI] [PubMed] [Google Scholar]