Abstract
Numerous studies have suggested that memories “destabilize” and require de novo protein synthesis in order to reconsolidate following retrieval, but very little is known about how this destabilization process is regulated. Recently, ubiquitin-proteasome mediated protein degradation has been identified as a critical regulator of memory trace destabilization following retrieval, though the specific mechanisms controlling retrieval-induced changes in ubiquitin-proteasome activity remain equivocal. Here, we found that proteasome activity is increased in the amygdala in a CaMKII-dependent manner following the retrieval of a contextual fear memory. We show that in vitro inhibition of CaMKII reversed retrieval-induced increases in proteasome activity. Additionally, in vivo pharmacological blockade of CaMKII abolished increases in proteolytic activity and activity related regulatory phosphorylation in the amygdala following retrieval, suggesting that CaMKII was “upstream” of protein degradation during the memory reconsolidation process. Consistent with this, while inhibiting CaMKII in the amygdala did not impair memory following retrieval, it completely attenuated the memory impairments that resulted from post-retrieval protein synthesis blockade. Collectively, these results suggest that CaMKII controls the initiation of the memory reconsolidation process through regulation of the proteasome.
Keywords: Reconsolidation, amygdala, CaMKII, proteasome, ubiquitin
1. Introduction
The formation of long-term fear memories requires de novo gene transcription and protein translation in neurons during memory consolidation (Johansen, Cain, Ostroff, and LeDoux, 2011; McGaugh, 2000). While once thought to be permanent, it is now widely supported that upon retrieval once consolidated memories “destabilize” and require new protein synthesis in order to “restabilize”, a process referred to as memory reconsolidation (Alberini and Ledoux, 2013; Nader, Schafe, and Le Doux, 2000; Tronson and Taylor, 2007). This reconsolidation process is thought to be dynamic, allowing modification of previously formed memories. Consistent with this, numerous studies have shown that reconsolidation can strengthen, weaken or change the specific content of a memory (De Oliveira Alvares et al., 2013; Inda, Muravieva, and Alberini, 2011; Lee, 2008; 2010; Monfils, Cowansage, Klann, and LeDoux, 2009; Schiller et al., 2010; Sierra et al., 2013), which highlights the therapeutic potential of the reconsolidation process in alleviating fear associated with traumatic memories.
While most studies have focused on the mechanisms that regulate the restabilization or protein synthesis-dependent phase of the reconsolidation process, few have examined the mechanisms that regulate memory trace destabilization. NMDA receptor activation appears to initiate the destabilization process as inhibition of NMDA receptor activity in the amygdala prior to retrieval prevents the memory impairments that result from post-retrieval administration of the protein synthesis inhibitor anisomycin (Ben Mamou, Gamache, and Nader, 2006; Lopez, Gamache, Schneider, and Nader, 2015; Wang, de Oliveira Alvares, and Nader, 2009). Downstream of NMDA receptors, ubiquitin-proteasome mediated protein degradation has been consistently implicated as a critical regulator of memory trace destabilization since blocking functional proteasome activity prevents memories from undergoing reconsolidation and can attenuate reconsolidation-dependent memory modification (Jarome, Werner, Kwapis, and Helmstetter, 2011; Lee, 2008; Lee et al., 2008). However, though NMDA receptor activity can result in increased proteasome activity in vitro and in vivo (Bingol and Schuman, 2006; Jarome et al., 2011), it is hypothesized that this occurs through a second messenger and not as a direct result of calcium influx (Jarome and Helmstetter. 2013). To date, the molecule(s) that links NMDA receptor activation to protein degradation during the destabilization process remains equivocal.
One molecule that is directly activated by increased intracellular calcium levels is the calcium-calmodulin dependent protein kinase II (CaMKII), which has well described roles in the memory consolidation process (Bejar, Yasuda, Krugers, Hood, and Mayford, 2002; Mayford et al., 1996; Rodrigues, Farb, Bauer, LeDoux, and Schafe, 2004; Yasuda and Mayford, 2006). Interestingly, the role of CaMKII in the reconsolidation of fear memories has never been examined. Additionally, studies examining the role of CaMKII in the reconsolidation of memory for other behavioral tasks have found mixed results, with some indicating normal memory retention following post-retrieval inhibition of CaMKII signaling (Arguello et al., 2014; Da Silva, Cardoso, Bonini, Benetti, and Izquierdo, 2013; Sakurai, Yu, and Tan, 2007). One intriguing explanation for these mixed results is that CaMKII regulates protein degradation upstream of its potential (but not proven) regulation of protein synthesis during the reconsolidation process (Jarome and Helmstetter, 2013). Consistent with this, CaMKII can regulate proteasome activity and phosphorylation in vitro and in vivo (Bingol et al., 2010; Djakovic et al., 2012; Djakovic, Schwarz, Barylko, DeMartino, and Patrick, 2009; Hamilton et al., 2012; Jarome, Kwapis, Ruenzel, and Helmstetter, 2013), though this relationship has never been examined during memory reconsolidation. Here, using a combination of biochemical, pharmacological and behavioral approaches, we directly tested whether CaMKII controls memory trace destabilization through its regulation of the proteasome.
2. Materials and Methods
2.1. Subjects
Male Long Evans rats weighing between 300–350g at time of arrival were obtained from Harlan (Madison, WI). All animals were housed individually in shoebox cages with free access to water and rat chow. The colony room was maintained under a 14:10-hr light/dark cycle. Experiments took placed during the light portion of the cycle. All procedures were approved by the University of Wisconsin-Milwaukee Institutional Animal Care and Use Committee and conducted within the ethical guidelines of the National Institutes of Health.
2.2. Surgery
All animals were anesthetized with 2%–4% isoflurane in 100% O2 and implanted with bilateral stainless steel 26-gauge cannulae aimed at the basolateral nucleus of the amygdala (AP - 3.0 mm, ML+/−5.0 mm, DV −7.2 mm) using stereotaxic coordinates relative to bregma. Cannulae were secured to the skull with stainless steel screws, superglue, and dental acrylic. Rats were given a recovery period of at least 7 d before behavioral testing.
2.3 Apparatus
Contextual fear conditioning was conducted in a set of four Plexiglas and stainless-steel observation chambers (Context A) housed in sound-attenuating chambers. The floor was comprised of 18 stainless steel bars 5 mm in diameter spaced 12 mm apart and connected to a shock generator. Ventilation fans produced 62–64 dB of background noise. Each chamber was equipped with a speaker centered in the middle of one end of the chamber. Before testing of each animal, Context A was cleaned with a 5% ammonium hydroxide solution.
2.4. Drug Preparation and Infusion Procedure
Rats received bilateral infusions into the amygdala. The total volume of the infusion (0.5 µl/side) was given over 60-s, and the injection cannula remained in place an additional 90-s to ensure diffusion away from the injector tip. The injection cannulae were cut to extend approximately 0.5mm beyond the guide cannula. Rats were returned to their home cages after infusions. The specific CaMKII inhibitor myristoylated autocamtide-2 related inhibitory peptide (myr-AIP, 6ng/µl; Enzo Life Sciences) was dissolved in distilled H2O. The myristolated version of this peptide was used to enhance cell permeability. This dosage was determined based on prior work from our lab (Jarome et al., 2013). Anisomycin (ANI, 125µg/µl; Sigma) was dissolved in HCl and diluted with artificial CSF. A small amount of NaOH was added to bring the pH to ~7.4.
2.5. Behavioral Procedures
Animals underwent context fear conditioning acquisition and retrieval as described previously (Jarome et al., 2011). Briefly, following 3-days of acclimation to the transporting and injection procedures, animals were placed in novel Context A and after a 2-min baseline, received 5 unsignaled footshock (1.0mA, 1-sec) presentations. After a 2-min post-shock period, the animals were returned to their homecages. The following day, the animals were returned to Context A for 90-sec to reactivate the memory and then returned to their homecages. The testing session occurred the day after retrieval and consisted of an 8-min exposure to Context A. In cases where animals received drug infusions, microinfusions were performed immediately after the animals were removed from the chamber. No retrieval (No React) animals were trained with contextual fear conditioning as described above. The following day they received infusions of vehicle bilaterally into the amygdala and were returned to their home cages. These animals were then sacrificed 1.5 hr after the start of the infusion procedure to match sacrifice times of the separate retrieval groups receiving vehicle or drug infusions.
2.6. Tissue Collection and Crude Synaptosomal Membrane Preparation
Animals were overdosed with isoflurane and the brain was rapidly removed (< 1-min) and immediately frozen on dry ice. Amygdala tissue was then dissected out by blocking the brain in a rat brain matrix (Harvard Apparatus, Holliston, MA) incubated with dry ice. Crude synaptosomal membrane fractions were obtained as described previously (Jarome et al., 2012; Jarome et al., 2011). Briefly, following dissection tissue was homogenized in TEVP with 320mM sucrose and Roche protease inhibitor tablet. Samples were then centrifuged at 1000 × g for 10-min, 4°C. The supernatant was collected and centrifuged at 10,000 × g for 10-min, 4°C. The resulting pellet was denatured in Lysis buffer (50mM Tris-HCl, 150mM NaCl, 1mM EDTA, 1mM NaF, 1% SDS, 1 mM sodium orthovanadate and Roche protease inhibitor tablet), supernatant collected and measured using a Bradford protein assay kit (BioRad, Hercules, CA).
2.7. Proteasome activity assay
Proteasome activity assays were performed as described previously with a small scale modification (Jarome, Kwapis, Hallengren, Wilson, and Helmstetter, 2014; Jarome et al., 2013). Samples (10µg) were diluted in DDH2O and mixed with reaction buffer (250mM HEPES, pH 7.5, 5mM EDTA, 0.5% NP-40, 0.01% SDS, 5mM ATP). Fluorogenic peptide Suc-LLVY-AMC (Millipore) was added to the samples to assess proteasome chymotrypsin-like activity (10µM). The reaction was incubated at 37°C for 2-hrs (Suc-LLVY-AMC) and fluorescence monitored at 360 (excitation)/ 460 (emission) on a monochromatic plate reader (Synergy H1; Biotek). Protein free blanks were used and an AMC standard curve was produced. For in vitro manipulation of CaMKII, samples were incubated with the CAMKII inhibitor AIP (10µM) for 30-min at 37°C prior to the addition of the proteasome substrate.
2.8. Antibodies
Mouse monoclonal primary antibodies included Rpt6 (1:500; Enzo Life Sciences). The phosphorylated Rpt6-Serine120 rabbit polyclonal antibody was generated commercially (ProSci) against a synthetic peptide [NH2-CALRND(pS)YTLHK-OH] as described previously (Jarome et al., 2013).
2.9 Western blotting
Samples (10µg) were loaded on 7.5% TGX gels, ran through SDS-PAGE and transferred using a Turbo Transfer System (Biorad). Membranes were incubated in 3% milk in TBS + 0.1% Tween-20 (blocking buffer) for 1-hr at room temperature, followed by overnight incubation in antibody in 3% BSA in TBS + 0.1% Tween-20. Membranes were then washed and incubated in secondary antibody (1:20,000; Millipore for goat anti-rabbit, Santa Cruz for goat anti-mouse) in blocking buffer for 60-min. Following a final wash, membranes were incubated in enhanced chemiluminescence substrate (SuperSignal West Dura, Thermo) for 5-min and images developed using a CCD-camera based system (GBOX Chemi XT-4; Syngene) and analyzed using GeneTools software.
2.10. Conditioned fear responses
The activity of each rat was recorded on digital video and the amount of movement determined by frame-by-frame changes in pixels using FreezeScan 1.0 software (CleverSys, Reston, VA). The automatic scoring parameters are chosen such that the scored activity matches hand-scoring methods previously used in our lab to measure freezing.
2.11. Statistical analyses
For phosphorylated Rpt6 levels, mean pixel density was calculated for each sample and normalized to total Rpt6 levels. All samples were then expressed as a percentage of the control group. For proteasome activity assays, each raw fluorescence unit (RFU) reading was standardized to the generated AMC standard curve for that plate and normalized to total Rpt6 levels to account for differences in proteasome number. All samples were then taken as a percentage of the control group. Statistical outliers were determined as those samples that fell two or more standard deviations above/below the group mean. All data is presented as group average with standard error of the mean (SEM) and was analyzed using t-test, Analysis of Variance (ANOVA) and Fisher Least Significant Difference (LSD) post hoc test using Graphpad Prism 6 software.
3. Results
3.1. Inhibition of CaMKII in vitro reverses retrieval-induced increases in proteasome activity in the amygdala
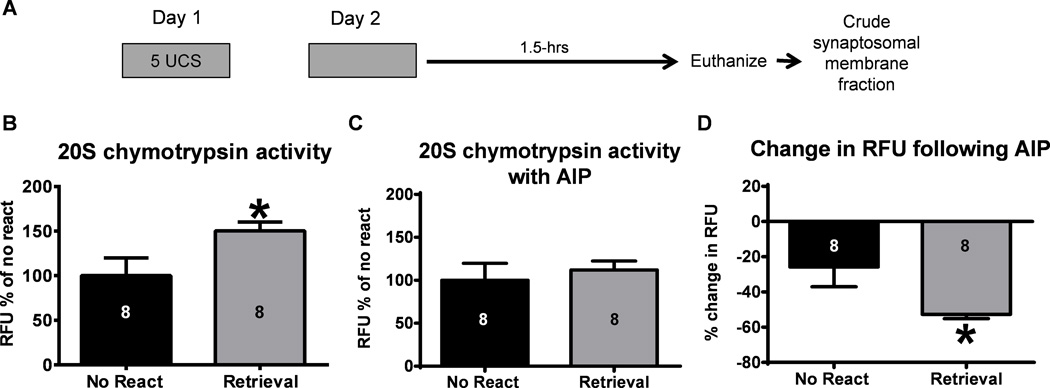
While several studies have reported a role for ubiquitin-proteasome mediated protein degradation in the memory reconsolidation process, it is currently unknown if proteasome catalytic activity is increased as a function of retrieval. To test this, we trained animals to a contextual fear conditioning paradigm and gave them a brief retrieval the following day (Figure 1A). We then measured proteasome activity in amygdala lysates 1.5 hr after retrieval using an in vitro proteasome activity assay (Jarome et al., 2013). As expected, we found an increase in proteasome chymotrypsin activity in the amygdala of animals exposed to the training context during retrieval relative to no retrieval controls (independent samples t-test: t(14) = 2.264, p < 0.05; Figure 1B), suggesting that proteasome activity is increased during the memory reconsolidation process. To test if CaMKII was potentially involved in these increases in proteasome activity, we ran the same samples through the proteasome activity assay again but this time inhibited CaMKII activity in the lysates for 30 min prior to start of the reaction. Interestingly, we found no differences in proteasome activity between groups following CaMKII inhibition (independent samples t-test: t(14) = 0.540, p = 0.59; Figure 1C). To examine this relationship further, we calculated the percent change in fluorescence between the activity assay done with the CaMKII inhibitor and the one done without. Remarkably, we found that while the CaMKII inhibitor reduced fluorescence readings in both groups, the effect was more pronounced in the retrieval group (independent samples t-test: t(14) = 2.338, p < 0.05; Figure 1D). This indicates that the increased proteasome activity in the retrieval group was likely dependent on CaMKII signaling in vivo and that in vitro manipulation of CaMKII can reverse this increase. Collectively, this suggests that CaMKII may control changes in proteasome activity in the amygdala during fear memory reconsolidation.
Figure 1. Inhibiting CaMKII activity in vitro can reverse retrieval-induced changes in proteasome activity in the amygdala.
(A) Animals were trained to contextual fear conditioning and exposed to the training context the following day. Amygdala crude synaptosomal membrane fractions were then collected 1.5-hrs later (n = 8 per group). (B) Amygdala proteasome activity was increased following exposure to the training context during retrieval. (C) There were no differences between groups in the presence of the CaMKII inhibitor AIP indicating that (D) inhibiting CaMKII in vitro reversed the retrieval-induced increases in proteasome activity in the amygdala. Number inside bar denotes group size for that specific assay. * P < 0.05 from No React on independent-samples t-test.
3.2. Inhibition of CaMKII in vivo prevents retrieval-induced increases in proteasome activity and Rpt6 phosphorylation in the amygdala
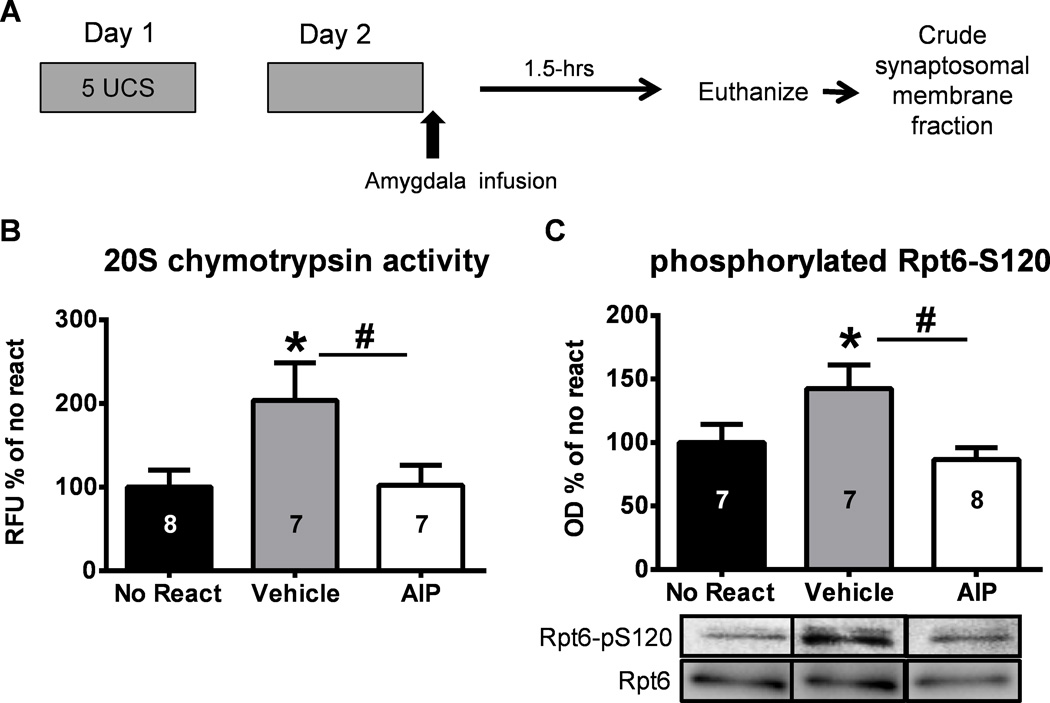
Since we found that inhibition of CaMKII in the amygdala could reverse retrieval-induced increases in proteasome activity, we next tested if CaMKII signaling was critical for increases in proteasome activity in vivo following retrieval. Animals were implanted with chronic cannula aimed at the basolateral amygdala and trained with our context fear conditioning procedure. Following retrieval, they received intra-amygdala infusions of the CaMKII inhibitor myr-AIP or vehicle and amygdala crude synaptosomal membrane fractions were collected 1.5-hrs later (Figure 2A). We found a main effect for drug on proteasome chymotrypsin activity (One-way ANOVA: F(2,19) = 3.616, p < 0.05; Figure 2B). Fisher LSD posthoc tests revealed an increase in proteasome activity in the amygdala of vehicle infused animals exposed to the retrieval context relative to vehicle infused no retrieval controls, which was prevented in the group receiving the CaMKII inhibitor. This suggests that CaMKII activity is necessary for retrieval-induced increases in proteasome activity in the amygdala. Since activity-dependent changes in proteasome activity are thought to be regulated by phosphorylation of proteasome subunit Rpt6 at serine-120 (Rpt6-S120), we next tested if CaMKII inhibition effected phosphorylation of Rpt6-S120 in the amygdala following retrieval using a commercially generated phospho-Rpt6-S120 antibody. We found a main effect for drug on Rpt6 phosphorylation (One-way ANOVA: F(2,19) = 4.172, p < 0.05; Figure 2C). Fisher LSD posthoc tests revealed an increase in phospho-Rpt6-S120 levels in the amygdala of vehicle infused animals exposed to the retrieval context relative to vehicle infused no retrieval controls, which was prevented in the group receiving the CaMKII inhibitor. Collectively, these results suggest that CaMKII activity is necessary for retrieval-induced increases in proteasome activity and phosphorylation in the amygdala.
Figure 2. Inhibiting CaMKII activity in the amygdala in vivo can prevent retrieval-induced changes in proteasome activity and Rpt6-S120 phosphorylation.
(A) Animals were trained to contextual fear conditioning and exposed to the training context the following day. Infusions of the vehicle or a CaMKII inhibitor (myr-AIP) where given into the amygdala immediately after the completion of the retrieval event and amygdala crude synaptosomal membrane fractions were then collected 1.5-hrs later (n = 7–8 per group). A separate group of animals was trained, infused (vehicle) on day 2, and tissue collected 1.5-hrs later. (B) The CaMKII inhibitor prevented retrieval-induced increases in proteasome activity in the amygdala. (C) Retrieval-induced increases in Rpt6-S120 phosphorylation were blocked by the CaMKII inhibitor. Representative western blots are spliced from the same membranes. Number inside bar denotes group size for that specific assay * P < 0.05 from No React, # P < 0.05 from AIP with one-way ANOVA and Fisher LSD posthoc tests.
3.3. CaMKII activity regulates memory trace destabilization following retrieval
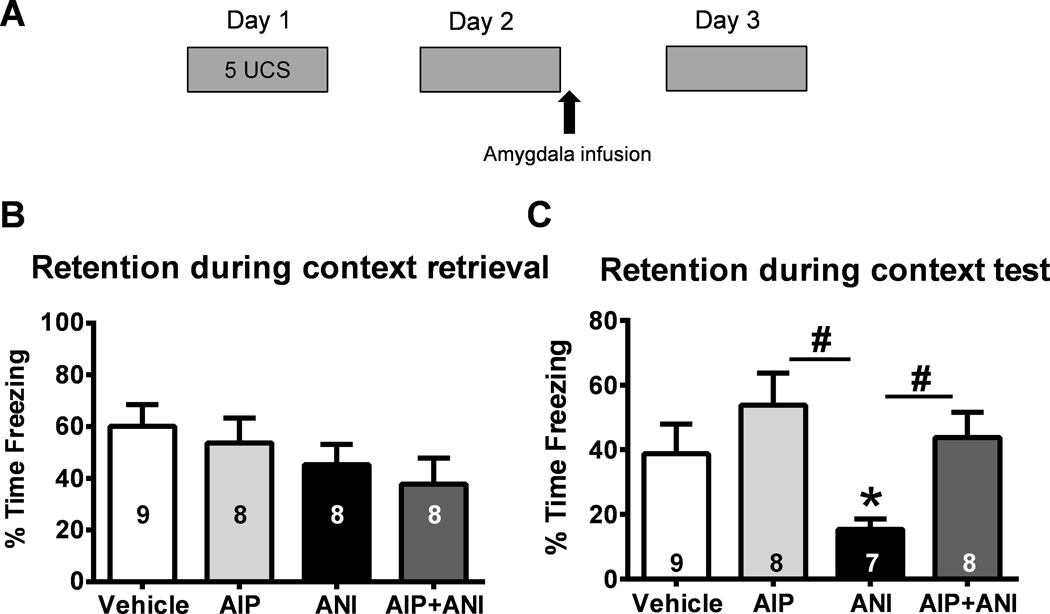
Our current experiments found that inhibiting CaMKII activity in the amygdala prevented changes in proteasome activity and phosphorylation in the amygdala. We have previously demonstrated that proteasome inhibitors applied into the amygdala following retrieval do not impair memory, but rather rescue memory impairments that result from anisomycin-induced protein synthesis blockade (Jarome et al., 2011). This suggests that inhibiting CaMKII following retrieval should prevent memory trace destabilization. To test this directly, we implanted animals with injection cannula aimed at the amygdala and trained them to contextual fear conditioning. The following day, we briefly exposed them to the training context and immediately gave microinfusions of vehicle, the protein synthesis inhibitor anisoymcin (ANI), myr-AIP or a combination of myr-AIP + ANI and tested their retention for the fear conditioning task 24 hrs later (Figure 3A). While there were no differences between groups during the retrieval session (One-way ANOVA: F(3,29) = 1.195, p = 0.329; Figure 3B), we found a main effect for drug during the final test (One-way ANOVA: F(3,28) = 3.572, p < 0.05; Figure 3C). Fisher LSD posthoc tests revealed that while ANI impaired memory relative to vehicle infused controls, the CaMKII inhibitor myr-AIP had no effect. However, simultaneously blocking CaMKII and protein synthesis actually rescued the memory impairments that normally resulted from protein synthesis blockade. Collectively, these results suggest that CaMKII controls destabilization in the amygdala, likely through its regulation of the proteasome.
Figure 3. Inhibiting CaMKII activity in the amygdala prevents memory trace destabilization following retrieval.
(A) Animals were trained to contextual fear conditioning and exposed to the training context the following day. Infusions of vehicle, a CaMKII inhibitor (myr-AIP), the protein synthesis inhibitor anisomycin (ANI) or a combination of myr-AIP and anisomycin (AIP+ANI) where given into the amygdala immediately after retrieval and memory tested the following day (n = 7–9 per group). (B) There were no differences between groups during retrieval. (C) The protein synthesis inhibitor, but not the CaMKII inhibitor, impaired long-term memory. However, simultaneous blockade of protein synthesis and CaMKII rescued the memory impairments that resulted from blocking protein synthesis alone. Number inside bar denotes group size for retrieval or test. * P < 0.05 from Vehicle, # P < 0.05 from ANI with oneway ANOVA and Fisher LSD posthoc tests.
4. Discussion
While numerous studies have identified transcriptional and translational regulators of memory reconsolidation (reviewed in, Alberini and Kandel, 2015; Jarome and Lubin, 2014), very little is known about how the reconsolidation process is initiated following retrieval. One mechanism shown to control memory trace destabilization and the need for de novo protein synthesis following retrieval is ubiquitin-proteasome mediated protein degradation (Jarome et al., 2011; Lee et al., 2008; Lee et al., 2012; Ren et al., 2013; Sol Fustinana, de la Fuente, Federman, Freudenthal, and Romano, 2014). However, how protein degradation is regulated downstream of NMDA receptor activity during the reconsolidation process remains unknown. Here, we found that CaMKII signaling is necessary for increased proteasome activity following retrieval as pharmacological blockade of CaMKII abolished retrieval-induced increases in proteasome activity and phosphorylation in the amygdala. Supporting this, while inhibition of CaMKII signaling did not impair memory following retrieval, it rescued the memory impairments that normally result from post-retrieval blockade of protein synthesis in the amygdala. Collectively, these results identify a critical role for CaMKII signaling in the amygdala during memory reconsolidation through regulation of the proteasome complex.
Several studies have demonstrated that CaMKII is a critical regulator of memory consolidation (e.g., Chen, Bambah-Mukku, Pollonini, and Alberini, 2012; Halt et al., 2012; Naskar, Wan, and Kemenes, 2014; Ota, Monsey, Wu, and Schafe, 2010; Wan, Mackay, Iqbal, Naskar, and Kemenes, 2010), but despite this evidence, little is known about how CaMKII regulates memory reconsolidation following retrieval (Arguello et al., 2014; Da Silva et al., 2013; Sakurai et al., 2007). One theory is that CaMKII is involved in transcriptional and subsequent translational regulation in neurons following retrieval (Tronson and Taylor, 2007), as well as AMPA receptor trafficking (Johansen et al., 2011). However, a recent addition to this theory is that CaMKII signaling could regulate increased protein degradation during the reconsolidation process (Jarome and Helmstetter, 2013). Consistent with this revised theory, in the present study we found that CaMKII activity was critical for retrieval-induced increases in proteasome activity in the amygdala and pharmacological manipulation of CaMKII activity rescued memory impairments that resulted from local infusions of a protein synthesis inhibitor, mirroring the effect we have previously observed with a proteasome inhibitor (Jarome et al., 2011). This result strongly suggests a critical role for CaMKII signaling in the reconsolidation process and may explain findings from other studies where CaMKII inhibitors have not impaired memory when administered following retrieval (Arguello et al., 2014).
Recently, we found that CaMKII signaling regulates proteasome activity during the memory consolidation process (Jarome et al., 2013). However, since CaMKII, proteasome and protein synthesis inhibitors all impair memory when applied into the amygdala following training (Jarome et al., 2011; Rodrigues et al., 2004; Schafe and LeDoux, 2000), it is unknown whether CaMKII regulates protein degradation and synthesis simultaneously during memory storage or if its regulation of one process supersedes the other (reviewed in, Jarome and Helmstetter, 2014). Our present study addresses part of this question, showing that inhibition of CaMKII during retrieval mimics the effects of a proteasome inhibitor by rescuing memory impairments that result from protein synthesis inhibition. This provides the first evidence that CaMKII-dependent regulation of protein degradation may be upstream of CaMKII-dependent regulation of protein synthesis. However, it is unknown if CaMKII simply regulates protein degradation prior to initiating translational mechanisms or if CaMKII regulates protein synthesis by promoting protein degradation, which would in turn remove translational repressor proteins. Furthermore, it is possible that CaMKII regulates protein synthesis and degradation simultaneously, though protein degradation is needed to allow CaMKII to exert its effects on translation. Such would be the case with de novo gene transcription, since it is possible that CREB repressor proteins would need to be degraded before CaMKII-dependent phosphorylation of CREB could positively regulate transcriptional and subsequent translational processes (Upadhya, Smith and Hegde, 2004). If this were the case, the CaMKII-dependent regulation of protein synthesis would actually be dependent on protein degradation, potentially explaining the effects we observed in our study. However, few studies have directly tested if protein synthesis is increased following learning (Hoeffer et al., 2011) and the regulation of protein synthesis through CaMKII signaling during memory storage has never been directly tested, so deciphering the temporal dynamics by which CaMKII regulates protein synthesis and degradation remains complex. As a result, more studies are needed to completely understand how CaMKII simultaneously regulates the protein degradation and synthesis processes during the memory reconsolidation process.
One intriguing finding from our study was that proteasome activity was rapidly increased following retrieval. While this increase is earlier than has previously been reported during initial memory consolidation (Jarome et al., 2013; Lopez-Salon et al., 2001), it was in line with previous studies examining memory reconsolidation as the increase in proteasome activity was slightly delayed from the reported peak increases in protein polyubiquitination (Jarome et al., 2011; Lee et al., 2008). Additionally, the increase in proteasome activity we observed in the present study corresponds with the time-dependent degradation of synaptic scaffolding proteins following retrieval (Lee et al., 2008), further supporting this rapid change in proteasome catalytic activity. While the functional relevance of this rapid degradation of synaptic proteins remains open to interpretation, it is possible that such dynamic changes in overall ubiquitin-proteasome activity are necessary to allow proper modification of the memory trace. Several studies have supported the idea that protein degradation is upstream of protein synthesis during the reconsolidation process (Jarome et al., 2011; Lee et al., 2008; Lee et al., 2012; Ren et al., 2013), however, the temporal dynamics of the retrieval-dependent protein synthesis process remain poorly understood. Future studies will need to address how the rapid protein degradation process interacts with protein synthesis and synaptic remodeling following retrieval.
5. Conclusion
In conclusion, our data identify a novel role for CaMKII signaling in fear memory reconsolidation. Importantly, while we found that CaMKII regulates retrieval-induced increases in protein degradation upstream of protein synthesis, our results do not occlude a potential role for CaMKII in direct translational regulation during the reconsolidation process. These results provide important information about how the reconsolidation process is initiated and identify CaMKII as a critical second messenger that links NMDA receptor activation to increased protein degradation during memory reconsolidation in the amygdala.
Highlights.
Memory retrieval increases proteasome activity and phosphorylation
CaMKII regulates retrieval-induced proteasome activity in vivo and in vitro
Activity driven phosphorylation of RPT6 is significantly reduced after CaMKII inhibition in vivo
Memory trace destabilization is regulated by CaMKII activity
Acknowledgments
This work was supported by National Institute of Health (NIH) grants R01 MH069558 (FJH) and F31-MH088125 (TJJ), and the American Psychological Foundation (TJJ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberini CM, Kandel ER. The regulation of transcription in memory consolidation. Cold Spring Harb Perspect Biol. 2015;7:a021741. doi: 10.1101/cshperspect.a021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberini CM, Ledoux JE. Memory reconsolidation. Curr Biol. 2013;23:R746–R750. doi: 10.1016/j.cub.2013.06.046. [DOI] [PubMed] [Google Scholar]
- Arguello AA, Hodges MA, Wells AM, Lara H, 3rd, Xie X, Fuchs RA. Involvement of amygdalar protein kinase A, but not calcium/calmodulin-dependent protein kinase II, in the reconsolidation of cocaine-related contextual memories in rats. Psychopharmacology (Berl) 2014;231:55–65. doi: 10.1007/s00213-013-3203-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bejar R, Yasuda R, Krugers H, Hood K, Mayford M. Transgenic calmodulin-dependent protein kinase II activation: dose-dependent effects on synaptic plasticity, learning, and memory. J Neurosci. 2002;22:5719–5726. doi: 10.1523/JNEUROSCI.22-13-05719.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mamou C, Gamache K, Nader K. NMDA receptors are critical for unleashing consolidated auditory fear memories. Nat Neurosci. 2006;9:1237–1239. doi: 10.1038/nn1778. [DOI] [PubMed] [Google Scholar]
- Bingol B, Schuman EM. Activity-dependent dynamics and sequestration of proteasomes in dendritic spines. Nature. 2006;441:1144–1148. doi: 10.1038/nature04769. [DOI] [PubMed] [Google Scholar]
- Bingol B, Wang CF, Arnott D, Cheng D, Peng J, Sheng M. Autophosphorylated CaMKIIalpha acts as a scaffold to recruit proteasomes to dendritic spines. Cell. 2010;140:567–578. doi: 10.1016/j.cell.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIalpha-BDNF-CREB pathways to mediate memory consolidation. Nat Neurosci. 2012;15:1707–1714. doi: 10.1038/nn.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva WC, Cardoso G, Bonini JS, Benetti F, Izquierdo I. Memory reconsolidation and its maintenance depend on L-voltage-dependent calcium channels and CaMKII functions regulating protein turnover in the hippocampus. Proc Natl Acad Sci U S A. 2013;110:6566–6570. doi: 10.1073/pnas.1302356110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Oliveira Alvares L, Crestani AP, Cassini LF, Haubrich J, Santana F, Quillfeldt JA. Reactivation enables memory updating, precision-keeping and strengthening: exploring the possible biological roles of reconsolidation. Neuroscience. 2013;244:42–48. doi: 10.1016/j.neuroscience.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Djakovic SN, Marquez-Lona EM, Jakawich SK, Wright R, Chu C, Sutton MA, Patrick GN. Phosphorylation of Rpt6 regulates synaptic strength in hippocampal neurons. J Neurosci. 2012;32:5126–5131. doi: 10.1523/JNEUROSCI.4427-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djakovic SN, Schwarz LA, Barylko B, DeMartino GN, Patrick GN. Regulation of the proteasome by neuronal activity and calcium/calmodulin-dependent protein kinase II. J Biol Chem. 2009;284:26655–26665. doi: 10.1074/jbc.M109.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halt AR, Dallapiazza RF, Zhou Y, Stein IS, Qian H, Juntti S, Wojcik S, Brose N, Silva AJ, Hell JW. CaMKII binding to GluN2B is critical during memory consolidation. EMBO J. 2012;31:1203–1216. doi: 10.1038/emboj.2011.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AM, Oh WC, Vega-Ramirez H, Stein IS, Hell JW, Patrick GN, Zito K. Activity-dependent growth of new dendritic spines is regulated by the proteasome. Neuron. 2012;74:1023–1030. doi: 10.1016/j.neuron.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Cowansage KK, Arnold EC, Banko JL, Moerke NJ, Rodriguez R, Schmidt EK, Klosi E, Chorev M, Lloyd RE, Pierre P, Wagner G, LeDoux JE, Klann E. Inhibition of the interactions between eukaryotic initiation factors 4E and 4G impairs long-term associative memory consolidation but not reconsolidation. Proc Natl Acad Sci U S A. 2011;108:3383–3388. doi: 10.1073/pnas.1013063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, Muravieva EV, Alberini CM. Memory retrieval and the passage of time: from reconsolidation and strengthening to extinction. J Neurosci. 2011;31:1635–1643. doi: 10.1523/JNEUROSCI.4736-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Helmstetter FJ. The ubiquitin-proteasome system as a critical regulator of synaptic plasticity and long-term memory formation. Neurobiol Learn Mem. 2013;105:107–116. doi: 10.1016/j.nlm.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Helmstetter FJ. Protein degradation and protein synthesis in long-term memory formation. Front Mol Neurosci. 2014;7:61. doi: 10.3389/fnmol.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Kwapis JL, Hallengren JJ, Wilson SM, Helmstetter FJ. The ubiquitin-specific protease 14 (USP14) is a critical regulator of long-term memory formation. Learn Mem. 2014;21:9–13. doi: 10.1101/lm.032771.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Kwapis JL, Ruenzel WL, Helmstetter FJ. CaMKII, but not protein kinase A, regulates Rpt6 phosphorylation and proteasome activity during the formation of long-term memories. Front Behav Neurosci. 2013;7:115. doi: 10.3389/fnbeh.2013.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Kwapis JL, Werner CT, Parsons RG, Gafford GM, Helmstetter FJ. The timing of multiple retrieval events can alter GluR1 phosphorylation and the requirement for protein synthesis in fear memory reconsolidation. Learn Mem. 2012;19:300–306. doi: 10.1101/lm.024901.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Lubin FD. Epigenetic mechanisms of memory formation and reconsolidation. Neurobiol Learn Mem. 2014;115:116–127. doi: 10.1016/j.nlm.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarome TJ, Werner CT, Kwapis JL, Helmstetter FJ. Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdala. PLoS One. 2011;6:e24349. doi: 10.1371/journal.pone.0024349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the strengthening of memories by additional learning. Nat Neurosci. 2008;11:1264–1266. doi: 10.1038/nn.2205. [DOI] [PubMed] [Google Scholar]
- Lee JL. Memory reconsolidation mediates the updating of hippocampal memory content. Front Behav Neurosci. 2010;4:168. doi: 10.3389/fnbeh.2010.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Choi JH, Lee N, Lee HR, Kim JI, Yu NK, Choi SL, Lee SH, Kim H, Kaang BK. Synaptic protein degradation underlies destabilization of retrieved fear memory. Science. 2008;319:1253–1256. doi: 10.1126/science.1150541. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kwak C, Shim J, Kim JE, Choi SL, Kim HF, Jang DJ, Lee JA, Lee K, Lee CH, Lee YD, Miniaci MC, Bailey CH, Kandel ER, Kaang BK. A cellular model of memory reconsolidation involves reactivation-induced destabilization and restabilization at the sensorimotor synapse in Aplysia. Proc Natl Acad Sci U S A. 2012;109:14200–14205. doi: 10.1073/pnas.1211997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Salon M, Alonso M, Vianna MR, Viola H, Mello e Souza T, Izquierdo I, Pasquini JM, Medina JH. The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci. 2001;14:1820–1826. doi: 10.1046/j.0953-816x.2001.01806.x. [DOI] [PubMed] [Google Scholar]
- Lopez J, Gamache K, Schneider R, Nader K. Memory retrieval requires ongoing protein synthesis and NMDA receptor activity-mediated AMPA receptor trafficking. J Neurosci. 2015;35:2465–2475. doi: 10.1523/JNEUROSCI.0735-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Naskar S, Wan H, Kemenes G. pT305-CaMKII stabilizes a learning-induced increase in AMPA receptors for ongoing memory consolidation after classical conditioning. Nat Commun. 2014;5:3967. doi: 10.1038/ncomms4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota KT, Monsey MS, Wu MS, Schafe GE. Synaptic plasticity and NO-cGMP-PKG signaling regulate pre- and postsynaptic alterations at rat lateral amygdala synapses following fear conditioning. PLoS One. 2010;5:e11236. doi: 10.1371/journal.pone.0011236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren ZY, Liu MM, Xue YX, Ding ZB, Xue LF, Zhai SD, Lu L. A critical role for protein degradation in the nucleus accumbens core in cocaine reward memory. Neuropsychopharmacology. 2013;38:778–790. doi: 10.1038/npp.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin-dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004;24:3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai S, Yu L, Tan SE. Roles of hippocampal N-methyl-D-aspartate receptors and calcium/calmodulin-dependent protein kinase II in amphetamine-produced conditioned place preference in rats. Behav Pharmacol. 2007;18:497–506. doi: 10.1097/FBP.0b013e3282ee7b62. [DOI] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20:RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra RO, Cassini LF, Santana F, Crestani AP, Duran JM, Haubrich J, de Oliveira Alvares L, Quillfeldt JA. Reconsolidation may incorporate state-dependency into previously consolidated memories. Learn Mem. 2013;20:379–387. doi: 10.1101/lm.030023.112. [DOI] [PubMed] [Google Scholar]
- Sol Fustinana M, de la Fuente V, Federman N, Freudenthal R, Romano A. Protein degradation by ubiquitin-proteasome system in formation and labilization of contextual conditioning memory. Learn Mem. 2014;21:478–487. doi: 10.1101/lm.035998.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Wan H, Mackay B, Iqbal H, Naskar S, Kemenes G. Delayed intrinsic activation of an NMDA-independent CaM-kinase II in a critical time window is necessary for late consolidation of an associative memory. J Neurosci. 2010;30:56–63. doi: 10.1523/JNEUROSCI.2577-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, de Oliveira Alvares L, Nader K. Cellular and systems mechanisms of memory strength as a constraint on auditory fear reconsolidation. Nat Neurosci. 2009;12:905–912. doi: 10.1038/nn.2350. [DOI] [PubMed] [Google Scholar]
- Yasuda M, Mayford MR. CaMKII activation in the entorhinal cortex disrupts previously encoded spatial memory. Neuron. 2006;50:309–318. doi: 10.1016/j.neuron.2006.03.035. [DOI] [PubMed] [Google Scholar]