Abstract
The retrosplenial cortex (RSC) is known to play a role in the retrieval of context memory, but its involvement in memory formation and consolidation is unclear. To better characterize the role of the RSC, we tested its involvement in the formation and retrieval of memory for trace fear conditioning, a task that requires the association of two cues separated by an empty period of time. We have previously shown that trace fear extinction requires the RSC (Kwapis et al., 2014) and have hypothesized that trace memory may be stored in a distributed cortical network that includes prelimbic and retrosplenial cortices (Kwapis et al., 2015). Whether the RSC participates in acquiring and storing cued trace fear, however, is currently unknown. Here, we demonstrate that blocking protein synthesis in the RSC before, but not after acquisition impairs rats’ memory for trace CS and context fear without affecting memory for the CS in standard delay fear conditioning. We also show that NMDA receptor blockade in the RSC transiently impairs memory retrieval for trace, but not delay memory. The RSC therefore appears to critically contribute to formation of trace and context fear memory in addition to its previously recognized role in context memory retrieval.
Keywords: Retrosplenial cortex, consolidation, retrieval, emotion, trace fear, context fear
1. Introduction
The retrosplenial cortex (RSC) is one of the largest cortical regions in the rat brain (Vogt & Peters, 1981), yet very little is known about its potentially important role in memory formation and storage (Todd & Bucci, In Press). The RSC is well-positioned to coordinate information between higher-order brain regions, as it has direct reciprocal connections to the prefrontal cortex and hippocampus (Vann et al., 2009). Indeed, RSC functional activity usually correlates with autobiographical memory recall in humans (Svoboda et al., 2006), suggesting involvement in explicit memory retrieval. The RSC is therefore particularly well-situated to support retrieval and storage of complex memory.
In rodents, the RSC participates in recent and remote context memory retrieval (Corcoran et al., 2011; Cowansage et al., 2014). Blocking NMDA receptors or damaging the RSC selectively impairs context-shock associations acquired during standard delay fear conditioning, in which an auditory conditional stimulus (CS) is paired with an aversive shock unconditional stimulus (UCS). Neither RSC manipulation prevents the successful acquisition of fear to the auditory CS, however (Corcoran et al., 2011; Keene & Bucci, 2008), suggesting that the RSC is selectively involved in retrieving more complex contextual memory.
The RSC has also been implicated in consolidation of context fear memory. Pre-training protein or mRNA synthesis inhibition in the RSC disrupts long-term memory formation for context-based inhibitory avoidance (Katche et al., 2013). Further, immediate early genes such as Arc and c-Fos are increased in the RSC shortly after context fear conditioning (Robinson et al., 2012), suggesting that RSC neurons are active during the consolidation of context fear memory. In contrast to these results, blocking NMDA receptors reportedly has no effect on the acquisition of context fear (Corcoran et al., 2011). As NMDA receptors are critically important for memory consolidation (Abel & Lattal, 2001), this suggests the RSC is either only involved in certain forms of context memory consolidation or requires NMDAR-independent molecular processes.
The RSC therefore appears to be important for context memory retrieval and possibly memory formation, but its precise role is unknown. The RSC may be selectively involved in context memory or, instead, may play a more general role in relational and composite memories that extends beyond contextual information per se. To better understand the function of the RSC, we tested its involvement in trace fear conditioning, a form of complex, non-contextual memory. In trace conditioning, the CS and UCS are separated by an empty period of time, making the two cues relatively difficult to associate. Trace fear conditioning requires cortical and hippocampal participation (Gilmartin et al., 2014; Gilmartin & Helmstetter, 2010; Gilmartin et al., 2013; Kwapis et al., 2011; Quinn et al., 2002; Reis et al., 2013; Runyan et al., 2004) and contingency awareness (Knight et al., 2006; Weike et al., 2007) for successful acquisition, making it a good candidate for retrosplenial involvement. Recently, we demonstrated that the RSC is involved in trace fear extinction and retrieval (Kwapis et al., 2014), leading us to hypothesize that trace fear memory may be stored in a distributed cortical network that includes the prelimbic and retrosplenial cortices (Kwapis et al., 2015). No one has yet tested whether the RSC participates in trace fear acquisition or consolidation, however.
Here, we show that protein synthesis in the RSC is required for acquisition or early consolidation of contextual and trace CS fear, but is not necessary for delay CS fear. Further, we found that NMDA receptors in the RSC are required to retrieve trace, but not delay fear memory. The RSC therefore participates in trace fear retrieval and consolidation in addition to its known role in context memory retrieval. This is consistent with our hypothesis that a distributed cortical network may participate in the consolidation of trace fear memory.
2. Materials and Methods
2.1. Subjects and Surgery
The subjects were 89 male Long-Evans rats (300–375g) obtained from Harlan (Madison, WI). Rats were individually housed, given free access to food and water, and maintained on a 14:10-h light/dark cycle. All procedures were in accordance with the National Institutes of Health Guidelines and approved by the Institutional Animal Care and Use Committee at the University of Wisconsin-Milwaukee.
All animals were adapted to handling for 3 days before surgery. During surgery, rats were anesthetized with isoflurane (induction, 4%; maintenance, 2%) and placed in a stereotaxic frame. Animals were prepared with bilateral stainless steel 26-gauge cannulae aimed at the retrosplenial cortex (RSC) as previously described (Kwapis et al., 2014). The coordinates used were: 3.5mm posterior, ±0.5mm lateral, and 1.8mm ventral relative to bregma (Paxinos & Watson, 2007).
2.2. Apparatus
Fear conditioning acquisition was conducted in a set of 4 identical chambers housed within sound-dampening boxes (Context A). The floor of each chamber was composed of stainless steel rods through which footshocks were delivered. Each chamber was illuminated by an overhead 7.5 W bulb and ventilation fans provided background noise (~60–62dB). During training, the white noise CS was delivered through a speaker housed in the side of each chamber. Context A was cleaned with a solution of 5% ammonium hydroxide between animals.
A second set of chambers (Context B) was used to measure freezing to the auditory CS independent of the training context. Context B differed from Context A in a number of ways, including infrared illumination, a solid and opaque textured floor panel, and a different cleaning solution (5% acetic acid). Ventilation fans in Context B provided approximately 58–60dB of background noise.
2.3. Infusion Procedure and Drugs
All rats received bilateral infusions of 0.5µl/side into the RSC over a 60s period. After each infusion was complete, the injectors (33-gauge, extending 0.8mm beyond the guide) were kept in place for an additional 90s to ensure proper diffusion. The protein synthesis inhibitor ANI (Tocris; 10 mg) was fully dissolved in 36µl of HCl and diluted to its final concentration of 125µg/µl with 44µl of ACSF. The NMDA receptor antagonist D-APV (Tocris, 10mg) was diluted with 1000µl of ACSF to a final concentration of 10µg/µl (Kwapis et al., 2015; Kwapis et al., 2014).
2.4. Behavior
All rats were exposed to the restraint procedure for three days before training. Each rat was transported to the laboratory, wrapped in a towel, and gently restrained by hand for several minutes while the infusion pump was activated to allow animals to acclimate to its noise.
Fear conditioning and context tests were conducted in Context A while CS tests were conducted in Context B. Animals were trained on day 1 with strength-matched delay (n = 42) or trace (n = 45) conditioning. Previous work from our lab (Kwapis et al., 2015; Kwapis et al., 2014; Kwapis et al., 2011) has demonstrated that a 6-trial trace fear conditioning protocol with a variable intertrial interval (ITI) of 240 ±20s produces approximately the same level of freezing as 4 trials of delay fear conditioning with a shorter ITI of 110 ±20s. For both conditioning types, the CS was a white noise cue (10s; 72dB) and the UCS was a footshock (1s; 1mA). For delay fear conditioning, the UCS was presented at the moment of CS offset. For trace fear conditioning, the CS and UCS were separated by an empty 20s trace interval. Both protocols began with a 6-min baseline period and finished with a 4-minute postshock period.
On day 2, animals were tested to both the CS and context in a counterbalanced manner, with at least 4h between tests. For the context test, animals were returned to the conditioning chamber for 12 minutes. For the CS test, animals were placed in Context B, given a 1-min baseline period, and then given 8 discrete CS presentations (30s; 72dB) with a 60s ITI.
Experiment 2 was a direct follow-up to the first experiment. After completion of the initial CS and context tests, the animals from Experiment 1 were regrouped and, 4 days later, given 2 additional CS tests separated by 24h. These tests were identical to the CS test described above.
2.5. Histology
After behavioral testing was complete, animals were killed with an overdose of isoflurane and transcardially perfused. For detailed procedures, see Kwapis et al. (2009). Briefly, the brains were cryoprotected, frozen, and sectioned into 40µm slices, which were mounted and stained with cresyl violet. Only rats with acceptable cannulae placements in the RSC were included in the analyses.
In order to better visualize the region targeted by our infusions, two untrained animals were implanted with RSC cannulae and injected with a fluorescent antibody (anti-rabbit Alexa 594) at the same volume as our drugs (0.5µl/side). Approximately 10 minutes after infusion, these animals were perfused and the brains were placed in sucrose formalin for 3 days in a dark container. The brains were sliced at 40µm in the dark, mounted on slides, and imaged with a fluorescence microscope (Nikon Eclipse) running NIS-Elements software.
2.6. Analyses
Freezing behavior was used as the measure of conditional fear during all sessions. The average percent time freezing was calculated in real-time with the FreezeScan 1.0 software (Clever Sys, Inc., Reston, VA). Context tests were analyzed on a minute-by-minute basis and the average freezing throughout the session was used for statistical analysis. For CS tests, the percent time freezing during each 30-sec CS presentation was calculated and the average freezing during the first 4 discrete CS presentations was used for statistical analysis. We chose to use the first 4 CS presentations because extinction appeared to begin after the 4th trial for both training types, consistent with results we have previously observed (Kwapis et al., 2015; Kwapis et al., 2014). Each training group was analyzed separately and drug differences in freezing were analyzed using t-tests (context tests) or mixed-model ANOVAs (CS tests) with a repeated measure of Period (baseline vs. CS period of the test session) and a between factor of Drug (vehicle or ANI/APV). Bonferroni post-hoc tests were used to test group difference within each period. In all analyses, an α value of 0.05 was required for significance.
3. Results
3.1. Experiment 1: Post-training blockade of protein synthesis in the RSC has no effect on trace, delay, or context fear
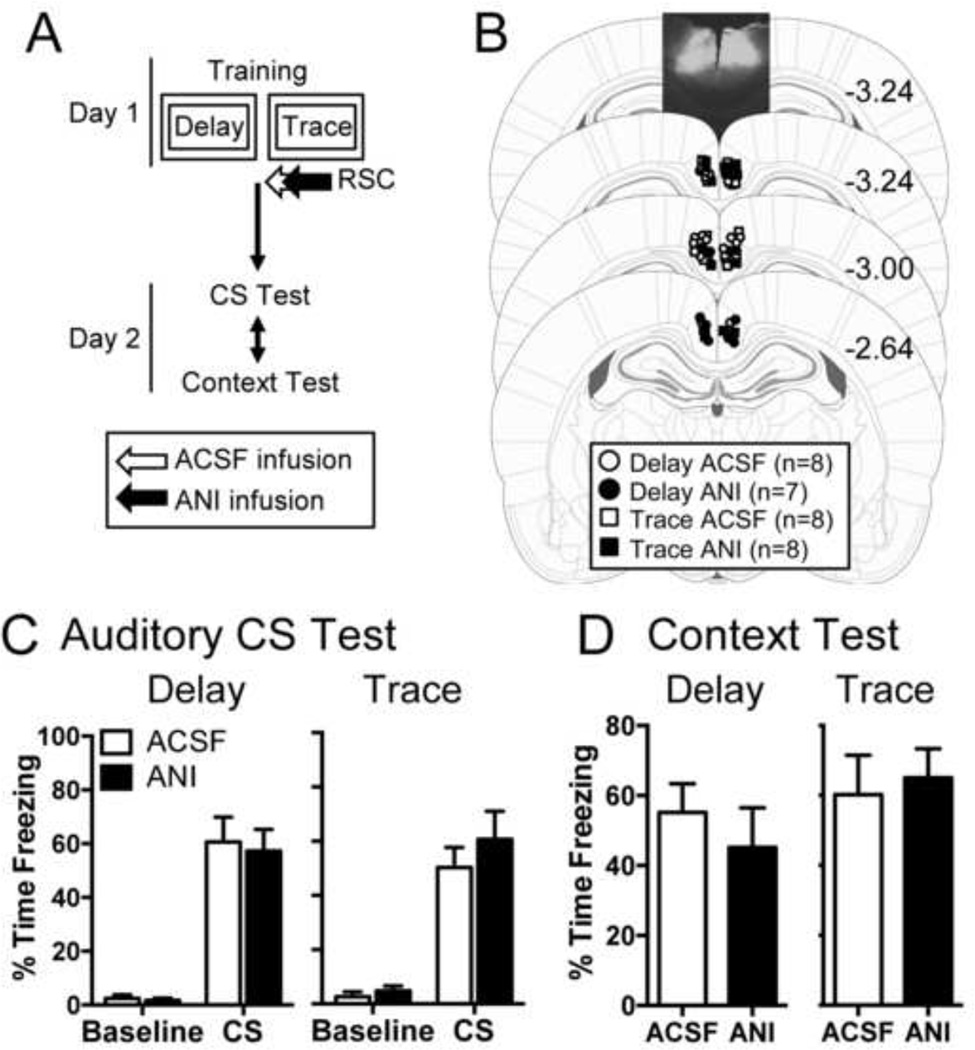
To test whether the RSC is involved in the time-dependent consolidation of trace, delay, or context fear memory, we infused the protein synthesis inhibitor anisomycin into the RSC immediately after training animals with strength-matched delay or trace fear conditioning (Fig. 1A). As de novo translation is a crucial step in the consolidation of long-term memory (Abel & Lattal, 2001), local blockade of protein synthesis is an effective method of identifying whether plasticity in a particular brain region is required for memory consolidation. On day 2, animals were tested to both the CS and context to determine whether post-training ANI infusions affected memory consolidation. Acceptable placements are shown in Figure 1B. All animals showed normal acquisition on day 1 (data not shown). No group differences were observed in the levels of postshock freezing for either delay (t(13) = −0.873, p = 0.398) or trace (t(14) = 0.293, p = 0.774) animals, suggesting that the groups showed similar acquisition levels before drug infusion.
Figure 1.
Blocking protein synthesis in the retrosplenial cortex immediately after training does not affect consolidation of either trace or delay fear conditioning. (A) Experimental timeline. (B) Infusion site locations. Overlayed on the top slice is a representative image following intra-RSC infusion of Alexa 594 antibody. (C) Average freezing during the baseline and first 4 CS presentations of the CS test session for delay (left) and trace (right) animals. (D) Mean freezing during the context test for delay and trace rats.
The following day, animals were tested to both the context and the auditory CS (Fig. 1C–D). We observed similar levels of freezing between drug conditions in both delay and trace animals. During the CS test (Fig. 1C), mixed-model ANOVAs revealed a significant effect of Period in both delay (F(1,13) = 85.4, p<0.0001) and trace (F(1,14) = 88.2, p < 0.0001) groups, indicating that both delay and trace rats froze more during the CS presentation than during baseline. There was no significant main effect of drug in either delay (F(1,13) = 0.1, p = 0.75) or trace (F(1,14) = 0.73, p = 0.41) animals, nor was there a significant interaction between Period and Drug for either delay (F(1,13) = 0.04, p = 0.84) or trace (F(1,14) = 0.56, p = 0.47) animals during the CS test. Similarly, during the context test (Fig. 1D), there were no significant difference between drug conditions for either delay (t(13) = 0.727, p = 0.480) or trace (t(14) = −0.342, p = 0.737) animals. This suggests that post-training protein synthesis in the RSC may not be required for delay, trace, or context fear consolidation.
3.2. Experiment 2: NMDA receptors in the RSC are required for the retrieval of trace, but not delay fear memory
The results from Experiment 1 suggest that protein synthesis in the RSC may not be required for trace or delay consolidation. An alternate possibility, however, is that our infusion procedure was ineffective or incorrectly timed. As the RSC is a large structure in the rat brain, it is possible that our infusion site was too small or too anterior to be effective. To test the efficacy of our infusion location, we attempted to replicate a known effect in the animals from Experiment 1. We have previously shown that APV infusion into the RSC impairs trace, but not delay memory retrieval (Kwapis et al., 2014). Thus, we can test whether our infusions were effective by infusing APV into the RSC of animals from Experiment 1 and testing whether this impairs trace memory retrieval. To this end, we regrouped the animals into new drug conditions and infused them with either APV or vehicle before a CS test (Fig. 2A). We re-tested the animals the following day to determine whether any memory impairments were permanent or transient.
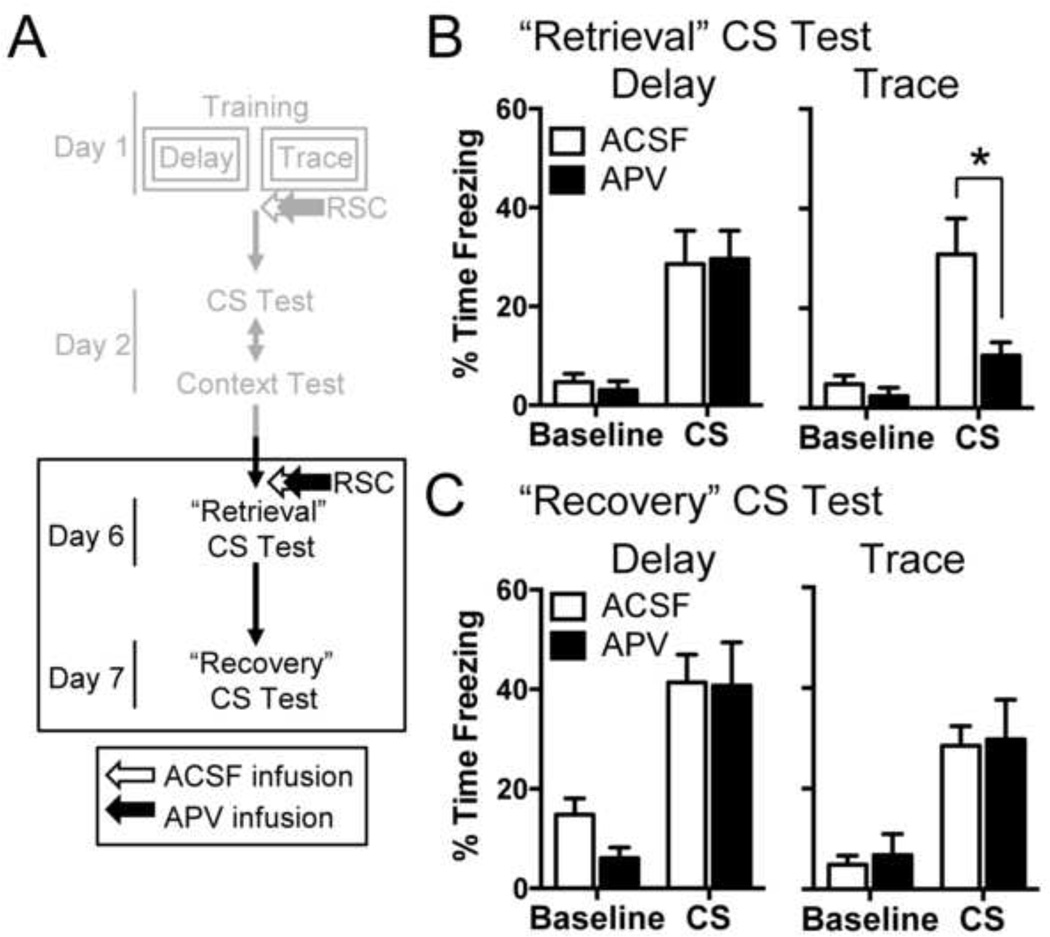
Figure 2.
Blocking NMDA receptors in the retrosplenial cortex transiently disrupts the retrieval of trace, but not delay fear. (A) Experimental timeline. (B) Average freezing during the baseline and first 4 CS presentations of the retrieval test. (C) Mean freezing during the baseline and first 4 CS presentations of the recovery test. *p < 0.05 relative to vehicle
We first ensured that our new ACSF and APV groups showed similar levels of CS freezing before beginning Experiment 2. We observed no significant differences in freezing during the initial CS test for either delay (t(13) = −0.861, p = 0.405) or trace (t(14) = 0.937, p = 0.364) animals before drug injection (data not shown). We then infused either ACSF (Delay, n = 7; Trace, n = 8) or APV (Delay, n = 8; Trace, n = 8) into the RSC 5-min before a CS test (the “Retrieval” test; Fig. 2B). For delay rats, we observed no significant effect of drug on CS freezing during the retrieval test. A mixed-model ANOVA revealed a significant effect of Period (F(1,13) = 37.17, p < 0.0001) but no main effect of Drug (F(1,13) = 0.003, p = 0.96) or Drug×Period interaction (F(1,13) = 0.1, p = 0.75). Thus, APV did not affect retrieval of CS fear in delay rats. For trace rats, on the other hand, APV infusion disrupted freezing to the CS. Specifically, we observed a significant effect of Period (F(1,14) = 24.17, p < 0.001), Drug (F(1,14) = 5.4, p < 0.025), and a significant Drug×Period interaction (F(1,14) = 6.62, p < 0.025) for trace rats. Follow-up Bonferroni post-hoc tests revealed that APV-infused rats froze significantly less than ACSF-infused rats during the CS (p < 0.01). Thus, consistent with our previous report, blocking NMDA receptors in the RSC disrupts retrieval of trace, but not delay CS fear (Kwapis et al., 2014).
The following day, rats were tested drug-free (i.e. the “Recovery” test). No drug effects were observed for either trace or delay animals (Fig. 2C). For both groups, a mixed-model ANOVA revealed a main effect of Period (delay: F(1,13) = 38.01, p <0.0001; trace: F(1,14) = 38.94, p < 0.001), but no significant main effect of Drug (delay: F(1,13) = 0.55, p = 0.47; trace: F(1,14) = 0.07, p = 0.79) or significant interaction (delay: F(1,13) = 0.67, p = 0.43; trace: F(1,14) = 0.006, p = 0.94). These results confirm that NMDA receptors in the RSC play a key role in memory retrieval for trace, but not delay fear (Kwapis et al., 2014). Further, they indicate that this effect is transient, as memory fully recovers the following day. Importantly, as we replicated a positive effect in these animals, we can rule out the possibility that our null effect in Experiment 1 was due to an ineffective infusion procedure.
3.3. Experiment 3: Blocking protein synthesis in the RSC before training impairs context and TFC fear
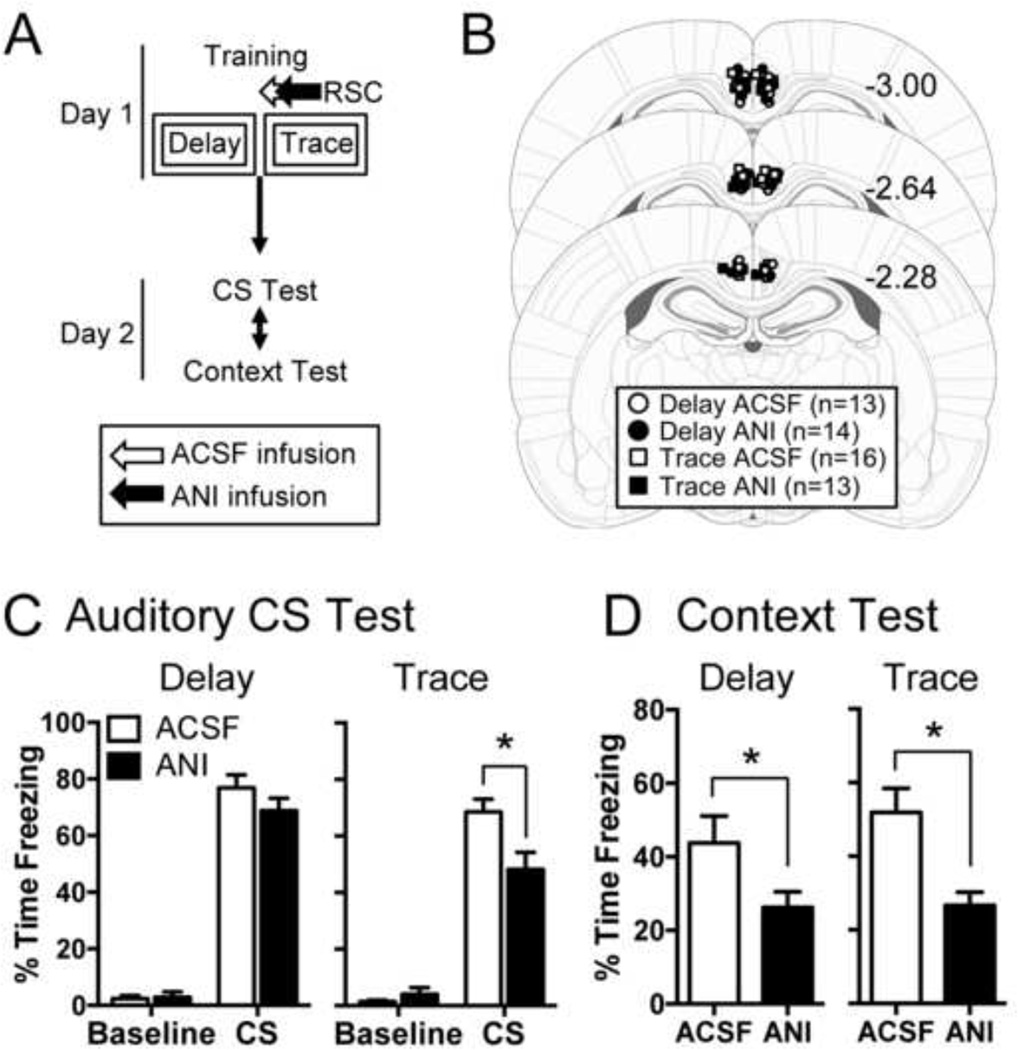
Finally, we tested whether blocking protein synthesis in the RSC before training would impair trace or delay consolidation. We observed no effect when we inhibited protein synthesis immediately after training (Experiment 1), but our infusion may have been administered too late to be effective. If an early wave of protein synthesis in the RSC is required for consolidation, post-training anisomycin infusion may not fully block protein synthesis in time. We tested this possibility in Experiment 3, in which we moved the injection from post-training (Fig. 1A) to 15-min pre-training (Fig. 3A). Acceptable placements are illustrated in Figure 3B. No group differences were observed in the levels of postshock freezing for either delay (t(25) = 0.598, p = 0.555) or trace (t(27) = −0.609, p = 0.548) animals, suggesting normal acquisition occurred in the presence of anisomycin (data not shown).
Figure 3.
Blocking protein synthesis in the retrosplenial cortex before training impairs context and trace CS fear without affecting delay CS fear. (A) Experimental timeline. (B) Infusion site locations. (C) Mean freezing during the baseline and first 4 CS presentations of the CS test session. (D) Mean freezing during the context test. *p < 0.05 relative to vehicle.
On day 2, animals were tested to both the white noise and context. Pre-training ANI reduced freezing to the CS in trace animals and reduced context freezing in both delay and trace animals (Fig. 3C–D). During the CS test (Fig. 3C), a mixed-model ANOVA on the Delay group revealed a significant effect of Period (F(1,25) = 549.0, p < 0.0001) but no effect of Drug (F(1,25) = 1.07, p = 0.31) and no significant interaction (F(1,25) = 2.06, p = 0.16). Thus, APV did not affect acquisition of delay CS fear. For trace animals during the CS test, we observed a significant main effect for Drug (F(1,27) = 4.35, p < 0.05), Period (F(1,27) = 272.5, p < 0.0001), and a significant Drug×Period interaction (F(1,27) = 11.47, p < 0.0025). Follow-up Bonferroni post-hoc tests revealed that ANI-infused rats froze significantly less than ACSF-infused rats during the CS (p < 0.001). This indicates that trace CS fear is impaired by pre-training protein synthesis blockade in the RSC whereas delay CS fear is not affected by this manipulation.
In contrast, context fear for both delay- and trace-trained animals was reduced by intra-RSC anisomycin (Fig. 3D). Delay rats given ANI froze significantly less to the training context than ACSF controls (t(25) = 2.104, p = 0.046). Similarly, trace rats given ANI also showed significantly less context freezing than their ACSF counterparts (t(27) = 3.159, p = 0.004). These results demonstrate that protein synthesis in the RSC is required to consolidate trace CS fear and context fear acquired during both delay and trace conditioning. Together with Experiments 1 and 2, our results indicate that protein synthesis in the RSC is required either during or immediately after conditioning for the proper consolidation of context and trace CS fear. Delay CS fear, on the other hand, was not affected by intra-RSC anisomycin infusion, suggesting that the RSC is not involved in the consolidation of delay CS fear.
4. Discussion
Here, we tested the role of the RSC in the acquisition, consolidation, and retrieval of trace and delay fear memory. We found that blocking protein synthesis in the RSC before, but not after conditioning disrupted memory for trace and context, but not delay CS fear. We also demonstrated that NMDA receptors in the RSC are required to retrieve trace, but not delay fear memories, consistent with at least one previous report (Kwapis et al., 2014). Additionally, we demonstrated that these retrieval impairments are transient; the memory fully recovers after APV is eliminated. Together, our findings suggest that the RSC plays a role in the acquisition or early consolidation of trace CS and context fear memory but is not involved in forming delay fear memory. More broadly, our study indicates that the role of the RSC extends beyond context memory, including other forms of complex, relational associations.
Interestingly, pre- but not post-training anisomycin effectively disrupted context and trace CS fear. We can rule out the possibility that our post-training intra-RSC infusion was too small or incorrectly located, as both APV (Fig. 2) and pre-training anisomycin (Fig. 3) delivered at the same site were effective. This suggests that trace and context memory consolidation may require protein synthesis in the RSC either during or immediately after acquisition. Although it is unclear why this is the case, there is evidence to suggest that the RSC is similarly involved in the early consolidation of context fear in an inhibitory avoidance task, as well (Katche et al., 2013). Further, other research has shown that cortical plasticity occurs immediately after trace conditioning; in the medial prefrontal cortex, ERK phosphorylation was increased immediately (but not at 1 or 4 hours) after trace fear conditioning (Runyan et al., 2004). As the mPFC is also involved in trace fear consolidation (Gilmartin & Helmstetter, 2010; Runyan & Dash, 2004; Runyan et al., 2004), it is likely that ERK phosphorylation occurs along a similar time course in both of these cortical regions. Our post-training infusion of anisomycin, therefore, may not have fully blocked protein synthesis in the RSC until after this early wave of plasticity was complete. Infusing anisomycin 15 minutes before training, on the other hand, would have disrupted protein synthesis during this immediate post-training period of plasticity. Regardless, our results clearly show that pre- but not post-training protein synthesis blockade impairs trace and context fear.
Our study demonstrates for the first time that the trace fear conditioning involves plasticity in the RSC in addition to the medial prefrontal cortex (Gilmartin & Helmstetter, 2010; Runyan & Dash, 2004; Runyan et al., 2004) for successful memory formation. This suggests that a distributed cortical network contributes to the consolidation of trace fear in addition to the hippocampus and amygdala (Kwapis et al., 2011; Quinn et al., 2002). Along with previous work from our lab (Gilmartin & Helmstetter, 2010; Gilmartin et al., 2012; Kwapis et al., 2015; Kwapis et al., 2014) and others (Runyan & Dash, 2004; Runyan et al., 2004), this finding supports the contention that a coordinated distribution of cortical areas may participate in the consolidation of relatively complex trace fear associations (Kwapis et al., 2015). As the RSC is reciprocally connected to hippocampal and cortical regions that participate in trace fear acquisition (Gilmartin & Helmstetter, 2010; Gilmartin et al., 2013; Todd & Bucci, In Press), it is perhaps not surprising that it is involved in trace memory formation. We propose that trace fear memory, which is more complex and relational than standard delay fear, requires a distributed network of cortical structures for successful storage and extinction (Kwapis et al., 2015), including both the prefrontal and retrosplenial cortices. Although it unclear why trace fear conditioning recruits the RSC in addition to the prefrontal cortex and hippocampus, it is possible that the complex temporal relationship between the CS and UCS in trace conditioning is processed by the RSC. Indeed, the RSC is thought to be a key structure in the where/when pathway, delivering information about the physical and temporal context to the hippocampus (Bucci & Robinson, 2014; Todd & Bucci, In Press). It is therefore tempting to speculate that when the demands of the learning task require significant where/when processing, as occurs in trace or context fear conditioning, the RSC is recruited for acquisition.
While our study demonstrates that the RSC plays a role in trace and context fear memory, the results should be interpreted with caution. For example, it cannot be conclusively determined whether our infusions disrupted induction of plasticity or time –dependent consolidation. As pre- but not post-training infusions of anisomycin impaired trace and context memory, it is possible that blocking protein synthesis during acquisition disrupted the formation of the memory, rather than its consolidation into lasting long-term memory. Although this is conceivable, it is unlikely for two reasons. First, animals with pre-training anisomycin infusions showed normal freezing throughout the session relative to vehicle, including during the 4-minute postshock period, which gauges both general acquisition strength and short-term memory for context fear (Fanselow, 1980; Wood & Anagnostaras, 2011). Thus, the animals appeared to learn the task normally even in the presence of intra-RSC anisomycin. Second, protein synthesis is generally considered to be a requirement for consolidation, rather than acquisition. Previous work has shown that anisomycin infusions disrupt the consolidation of long-term memory without affecting short-term memory (e.g. Schafe & LeDoux, 2000), suggesting that acquisition is not affected by anisomycin infusion. Our results therefore suggest that the RSC participates in the early consolidation, rather than the acquisition of trace and context fear.
A second caveat is that in addition to blocking protein synthesis, anisomycin can produce unintended side effects, such as producing cell death (Rudy, 2008) and inducing aberrant gene expression (Radulovic & Tronson, 2008). It is therefore difficult to conclude with certainty that our observed behavioral impairments were produced by translation inhibition per se. It is clear, however, that anisomycin infusion into the RSC impairs memory for trace and context, but not delay fear. Even considering the side effects of anisomycin, it is apparent that disrupting the RSC impairs memory for complex forms of fear conditioning (context and trace) without affecting delay memory. Now that a role for the RSC in trace fear has been established, future studies should test whether more specific translational inhibitors (such as rapamycin) similarly disrupt trace and context memory formation. Further, the specific molecular mechanisms that participate in consolidation in RSC neurons will need to be identified in future work.
In conclusion, we have shown that infusion of anisomycin into the RSC before, but not after conditioning impairs memory for trace and context fear without affecting memory for delay CS fear. Further, we found that NMDA receptors in the RSC are required for successful retrieval of trace, but not delay fear memory. Together, our results indicate that the RSC is involved in the acquisition or early consolidation of trace and context fear memory in addition to its known involvement in context and trace memory retrieval.
Highlights.
Pre-training infusion of anisomycin into the RSC impairs trace and context fear conditioning
Blocking NMDA receptors in the RSC also impairs retrieval of trace CS fear
Delay CS fear is not affected by these RSC manipulations
This suggests that the RSC is involved selectivity in trace and context fear memory formation
Acknowledgements
This research was supported by the National Institute of Mental Health (NIMH) grant R01MH069558 to F.J.H and NIMH grant F31MH090685 to J.L.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11(2):180–187. doi: 10.1016/s0959-4388(00)00194-x. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Robinson S. Toward a conceptualization of retrohippocampal contributions to learning and memory. Neurobiol Learn Mem. 2014;116:197–207. doi: 10.1016/j.nlm.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Donnan MD, Tronson NC, Guzman YF, Gao C, Jovasevic V, Radulovic J. NMDA receptors in retrosplenial cortex are necessary for retrieval of recent and remote context fear memory. J Neurosci. 2011;31(32):11655–11659. doi: 10.1523/JNEUROSCI.2107-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84(2):432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci. 1980;15(4):177–182. doi: 10.1007/BF03001163. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends Neurosci. 2014;37(8):455–464. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Helmstetter FJ. Trace and contextual fear conditioning require neural activity and NMDA receptor-dependent transmission in the medial prefrontal cortex. Learn Mem. 2010;17(6):289–296. doi: 10.1101/lm.1597410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Kwapis JL, Helmstetter FJ. Trace and contextual fear conditioning are impaired following unilateral microinjection of muscimol in the ventral hippocampus or amygdala, but not the medial prefrontal cortex. Neurobiol Learn Mem. 2012;97(4):452–464. doi: 10.1016/j.nlm.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Miyawaki H, Helmstetter FJ, Diba K. Prefrontal activity links nonoverlapping events in memory. J Neurosci. 2013;33(26):10910–10914. doi: 10.1523/JNEUROSCI.0144-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katche C, Dorman G, Slipczuk L, Cammarota M, Medina JH. Functional integrity of the retrosplenial cortex is essential for rapid consolidation and recall of fear memory. Learn Mem. 2013;20(4):170–173. doi: 10.1101/lm.030080.112. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behavioral Neuroscience. 2008;122(1):89–97. doi: 10.1037/0735-7044.122.1.89. [DOI] [PubMed] [Google Scholar]
- Knight DC, Nguyen HT, Bandettini PA. The role of awareness in delay and trace fear conditioning in humans. Cogn Affect Behav Neurosci. 2006;6(2):157–162. doi: 10.3758/cabn.6.2.157. [DOI] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Helmstetter FJ. The role of the medial prefrontal cortex in trace fear extinction. Learn Mem. 2015;22(1):39–46. doi: 10.1101/lm.036517.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lee JL, Gilmartin MR, Helmstetter FJ. Extinguishing trace fear engages the retrosplenial cortex rather than the amygdala. Neurobiol Learn Mem. 2014;113:41–54. doi: 10.1016/j.nlm.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Lonergan ME, Helmstetter FJ. Protein kinase Mzeta maintains fear memory in the amygdala but not in the hippocampus. Behavioral Neuroscience. 2009;123(4):844–850. doi: 10.1037/a0016343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapis JL, Jarome TJ, Schiff JC, Helmstetter FJ. Memory consolidation in both trace and delay fear conditioning is disrupted by intra-amygdala infusion of the protein synthesis inhibitor anisomycin. Learn Mem. 2011;18(11):728–732. doi: 10.1101/lm.023945.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. San Diego, CA: Academic Press; 2007. [Google Scholar]
- Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12(4):495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC. Protein synthesis inhibitors, gene superinduction and memory: too little or too much protein? Neurobiol Learn Mem. 2008;89(3):212–218. doi: 10.1016/j.nlm.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis DS, Jarome TJ, Helmstetter FJ. Memory formation for trace fear conditioning requires ubiquitin-proteasome mediated protein degradation in the prefrontal cortex. Front Behav Neurosci. 2013;7:150. doi: 10.3389/fnbeh.2013.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Poorman C, Marder T, Bucci D. Identification of functional circuitry between retrosplenial and postrhinal cortices during fear conditioning. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(35):12076–12086. doi: 10.1523/JNEUROSCI.2814-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy JW. Is there a baby in the bathwater? Maybe: some methodological issues for the de novo protein synthesis hypothesis. Neurobiol Learn Mem. 2008;89(3):219–224. doi: 10.1016/j.nlm.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiol Learn Mem. 2004;82(2):65–70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J Neurosci. 2004;24(6):1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, LeDoux JE. Memory consolidation of auditory pavlovian fear conditioning requires protein synthesis and protein kinase A in the amygdala. J Neurosci. 2000;20(18):RC96. doi: 10.1523/JNEUROSCI.20-18-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44(12):2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Bucci DJ. Retrosplenial cortex and long-term memory: Molecules to behavior. Neural Plasticity. doi: 10.1155/2015/414173. (In Press). Article ID 414173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10(11):792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Peters A. Form and distribution of neurons in rat cingulate cortex: areas 32, 24, and 29. J Comp Neurol. 1981;195(4):603–625. doi: 10.1002/cne.901950406. [DOI] [PubMed] [Google Scholar]
- Weike AI, Schupp HT, Hamm AO. Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology. 2007;44(1):170–180. doi: 10.1111/j.1469-8986.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- Wood SC, Anagnostaras SG. Interdependence of measures in pavlovian conditioned freezing. Neurosci Lett. 2011;505(2):134–139. doi: 10.1016/j.neulet.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]