Abstract
Objectives
Highly active antiretroviral therapy (HAART) provision to eligible HIV-infected pregnant and post-partum women is critical for optimizing maternal health. We assessed the impact of maternal HAART on HIV-free survival of breastfed infants in Malawi.
Methods
The Post-Exposure Prophylaxis of Infants (PEPI)-Malawi trial (2004-2009) enrolled mothers/infants during labor or immediately post-partum to evaluate 14-week extended infant antiretroviral prophylaxis for preventing HIV transmission through breastfeeding. Mothers meeting national HAART guidelines were referred for therapy. Child HIV-free survival--survival without HIV infection --was compared by maternal HAART status.
Results
Overall, 3,022 mother-infant pairs contributed 4,214 infant/person-years (PY) at-risk for HIV infection or death, with 532 events (incidence 12.6/100 PY, 95% confidence interval [CI] 11.6-13.7). During follow-up, 349 mothers were HAART initiated; 581 remained HAART naïve with CD4 cell counts <250 cells/mm3, and 2,092 were never HAART-eligible. By three months, 11% of infants with HAART naïve mothers (CD4<250) were infected with HIV or died versus 7% of infants of HAART-initiated mothers and 4% of infants of HAART-ineligible mothers. Maternal HAART was associated with a 46% reduction in infant HIV infection or death as compared to infants with HAART naïve mothers (CD4<250) (adjusted Hazards Ratio: 0.54,95% CI 0.36-0.81). Among HIV-exposed, uninfected infants, breastfeeding, but not HAART, was significantly associated with decreased child mortality.
Conclusions
HIV infection and mortality are high during the first 3 months post-partum in infants of mothers with advanced HIV, and rapid maternal HAART initiation can significantly improve HIV-related infant outcomes.
Keywords: HIV-1, prevention of mother-to-child transmission, highly active antiretroviral therapy, child survival, breastfeeding
INTRODUCTION
Breastfeeding is the cornerstone of infant survival in many resource-constrained settings, but is also associated with a significant risk of mother-to-child HIV transmission (MTCT) (1). Clinical trials have demonstrated that antiretroviral drugs given to infants during breastfeeding or to lactating mothers significantly reduce postnatal HIV transmission (2-8). The World Health Organization's (WHO) HIV treatment guidelines continue to change in light of new evidence and now recommend starting adult highly active antiretroviral therapy (HAART) at ≤500 cells/mm3 or WHO Stage 3 or 4 disease, and starting all pregnant women on lifelong HAART independent of CD4 count or clinical indication (Option B+), or when not feasible offering maternal HAART for women not otherwise indicated for treatment throughout the pregnancy and breastfeeding periods (Option B) (9).
The Post-Exposure Prophylaxis of the Infant (PEPI) trial was conducted prior to the WHO updated guidelines and prior to the introduction of Option B+ in Malawi. PEPI demonstrated that 14 week extended infant antiretroviral prophylaxis significantly reduced postnatal MTCT (10). We previously reported that receipt of postnatal maternal HAART by women eligible for treatment significantly reduced postnatal MTCT risk between 14 weeks (after infant prophylaxis ceased) and 24 months compared to women eligible for treatment who did not receive HAART (11).
Preventing HIV infection alone is not the ultimate goal of successful prevention of MTCT (PMTCT) programs however. HIV-free survival –PMTCT coupled with a reduction in all-cause child mortality-- is endorsed by the WHO as the indicator critical to the success of PMTCT interventions and to the formulation of infant feeding guidelines (12). Assessment of the impact of maternal HAART on infant outcomes should thus consider the HIV-free survival endpoint. Furthermore, several reports indicate that HIV-exposed but uninfected children experience adverse outcomes at higher rates than HIV-unexposed children and that low maternal CD4 cell count during pregnancy is associated with higher mortality in HIV-exposed, uninfected children (13-16).
The primary objective of this analysis was to assess the impact of maternal HAART within the PEPI trial on child HIV-free survival; the secondary objective was to assess the impact of maternal HAART on 24-month child survival amongst HIV-exposed, uninfected infants. These data preceding the current recommendations for PMTCT may provide preliminary evidence of the potential impact of current PMTCT guidelines – particularly among women with lower CD4 cell counts.
MATERIALS AND METHODS
Study Design
This is a secondary analysis of data collected as part of the PEPI clinical trial which enrolled participants between April 2004-December 2007 and followed participants to September 2009. The trial has been described previously (2). Briefly, the PEPI trial screened pregnant women who attended antenatal clinics and labor wards in Blantyre, Malawi for HIV infection. HIV-infected mothers intending to breastfeed and not previously initiated on HAART were enrolled and infants were randomized at birth to receive one of three treatment strategies for PMTCT: i) single-dose nevirapine (sdNVP) at birth, plus one week of zidovudine (ZDV) (control arm); ii) control treatment plus daily NVP to age 14 weeks; or iii) control treatment plus daily NVP+ZDV to age 14 weeks.
Infant HIV infection status was assessed at 1, 6, 9 and 14 weeks after birth, and then subsequently at 6, 9, 12, 15, 18 and 24 months. HIV-testing was performed using DNA polymerase chain reaction (PCR) (Roche Amplicor, version 1.5, Roche Molecular Systems) through the first 12 months and then using HIV serology from 15 months forward. HIV infection was defined as a confirmed positive HIV test (PCR or serology). Maternal CD4 cell count was measured at delivery and 14 weeks, 12 months and 24 months post-partum.
When the PEPI-Malawi trial began in April 2004, antiretroviral treatment (ART) was not widely available. The Malawian government began scale-up of their free national treatment program in 2004 and involved HAART eligibility based on CD4 count <250 cells/mm3 or WHO Clinical Stage 3 or 4 disease (17). The national treatment program was introduced mid-way through the PEPI trial. Upon introduction of the national ART program, post-partum women eligible for treatment per national guidelines who participated in PEPI were referred to the government ART clinics. At the time when government ART program implementation started, coverage of ART-eligible individuals was largely determined by logistical factors. For example, the number of eligible participants in the government program was restricted to 150 new patients per month in Blantyre and access to treatment was on a first-come first-served basis (18). Therefore, the majority of eligible women from the PEPI study who received HAART from the government program started after 14 weeks post-partum. Assessment of maternal HAART use was based on maternal history collected at each visit on structured case report forms and verified using concomitant medication treatment logs and clinic records.
Women enrolled in the PEPI trial were regularly counseled to exclusively breastfeed for the first six months after delivery and initially advised to stop breastfeeding after 6 months. Following revisions in WHO breastfeeding guidance however, counseling was revised during the trial to advise women to breastfeed past 6 months if they could not safely substitute for breastfeeding. Breastfeeding status was collected at each study visit and was heterogeneous after six months post-partum due to guideline changes.
The PEPI study protocol was reviewed and approved by the relevant institutional review boards in Malawi and the United States (Research and Ethics Committee, University of Malawi College of Medicine; institutional review boards of the Johns Hopkins Bloomberg School of Public Health, Baltimore, and the Centers for Disease Control and Prevention, Atlanta). All women provided written informed consent.
Endpoint and Exposure Assessment
The primary outcome in this analysis is child HIV-free survival up to 24 months – where HIV-free survival is defined as survival to age 24 months without HIV infection. HIV-free survival is used to assess infant outcomes as breastfeeding exposes infants to HIV-infection, but also provides other child survival benefits. Infants contributed time-at-risk to the HIV-free survival analyses from time of birth until the point of HIV infection, mortality, loss-to-follow-up or administrative censoring at 24 months or the completion of the study, whichever occurred first. HIV infection was assigned to the visit of HIV diagnosis. Infants were included in the analysis if they were HIV uninfected at birth (PCR DNA negative).
To examine any additional benefit of maternal HAART beyond PMTCT, we separately assessed predictors of child mortality within the first 24 months of life among the HIV-exposed and uninfected children. The models in this analysis compared relative hazards of time to child-death across covariates. Infants HIV uninfected at birth contributed time-at-risk from birth until they had the outcome (mortality), were infected with HIV (and censored), lost to follow-up, administratively censored at 24 months, or the completion of the study. Results were stratified by 0-6 months postpartum and >6 months-24 months as nearly all women breastfed during the first 6 months post-partum and breastfeeding practices varied after 6 months.
The primary exposure of interest for the analyses was maternal HAART and CD4 status. Mothers were classified according to whether they were eligible for treatment and initiated on HAART at government clinics during study follow-up (HAART initiated), whether they had a CD4 count <250 cells/mm3 during study follow-up and remained HAART naïve for any reason (e.g. the woman did not attend the clinic or the clinic did not initiate treatment), or whether their CD4 count remained ≥250 cells/mm3 throughout study follow-up (HAART ineligible). As this was a longitudinal study, HAART exposure and CD4 classification were time-varying and although no women began the study on HAART, women could contribute time-at-risk to different exposure categories as treatment was later initiated among some mothers.
Statistical Analyses
Differences in means, medians and proportions were compared using analysis of variance (ANOVA), Wilcoxon rank-sum tests and chi-squared statistics respectively. Visual assessments were used to assess outliers and to confirm the normality of the distribution of continuous variables for each group for the ANOVA analyses; Levene's test was used to assess homogeneity of variances. Medians and non-parametric Wilcoxon rank-sum tests were reported when ANOVA assumptions were not met. Kaplan-Meier survival curves were plotted to assess child HIV-free survival across maternal HAART eligibility and treatment groups. Cox proportional hazards (PH) models were used to assess bivariate and multivariate predictors of HIV infection or child mortality in the cohort and separately to assess predictors of child mortality amongst HIV-exposed, uninfected children. In addition to the exposure of maternal HAART status, the covariates for both maternal and infant characteristics, including maternal age, body mass index (BMI), viral load and infant birth weight and household characteristics including electricity and running water. Covariates were retained for the multivariate models based on known associations or statistical associations of p≤0.10. Models also controlled for treatment assignment in the parent randomized clinical trial. As the two infant prophylaxis arms (NVP alone or NVP+ZDV) significantly reduced HIV infection and were equally effective in the PMTCT in the PEPI trial as compared to the control arm (2), the two infant prophylaxis arms were combined and adjusted for in this analysis. Proportionality of hazards were assessed using Schoenfeld residuals. All analyses were conducted using Stata 12.1 (StataCorp, College Park, Texas, USA).
RESULTS
Overall, 3,022 mother-infant pairs were included in the analysis. Amongst the 349 women starting HAART during study follow-up, the mean CD4 count at HAART initiation was 167 cells/mm3 (standard deviation [sd]: 100); 21% of women initiating HAART had CD4 counts ≤100 cells/mm3. The mean CD4 count of women eligible, but not yet initiated on HAART was 186 cells/mm3 (sd: 55); 10% had CD4 counts ≤100 cells/mm3 (p<0.01). As no women were initiated on HAART at enrollment into the study, Table 1 compares characteristics of women according to their CD4 cell count and HAART status at the end of follow-up. Women across treatment categories were different by baseline maternal age and body mass index (BMI), CD4 cell count (at baseline and endline), baseline log viral load, educational attainment, infant birth weight and breastfeeding duration.
Table 1.
Characteristics of mothers and infants according to final maternal treatment category, PEPI Study, Malawi, 2004-2009 (n=3,022)
| HAART naïve CD4 <250 cells/mm3 | HAART initiated | HAART ineligible | p-value | |
|---|---|---|---|---|
| Baseline Maternal & Household Characteristics | n=581 | n=349 | n=2092 | |
| Control arm, n (%) | 188 (32.4) | 98 (28.1) | 683 (32.6) | 0.24 |
| Age, Mean yrs (SD) | 27.2 (4.6) | 28.3 (4.7) | 25.7 (4.7) | <0.01 |
| Body Mass Index, Mean kg/m2 (SD) | 23.6 (3.5) | 23.6 (3.6) | 23.9 (4.0) | 0.05 |
| CD4 count at enrollment, Median cells/mm3, (IQR) | 211 (157-249) | 174 (112-242) | 485 (374-654) | <0.01 |
| Log10 viral load, Mean copies/ml (SD)a | 4.3 (0.8) | 4.4 (0.8) | 3.9 (0.8) | <0.01 |
| Married, n (%) | 538 (92.6) | 314 (90.0) | 1929 (92.2) | 0.31 |
| Parity, Median (IQR) | 3 (2-4) | 4 (3-5) | 3 (2-4) | <0.01 |
| Education, n (%) | ||||
| No schooling | 66 (11.4) | 43 (12.3) | 206 (9.8) | |
| Grade 1-8 | 289 (49.7) | 175 (50.1) | 1156 (55.3) | 0.02 |
| Secondary or beyond | 226 (38.9) | 131 (37.6) | 730 (34.9) | |
| Electricity in the house, n (%) | 192 (33.1) | 117 (33.5) | 682 (32.6) | 0.93 |
| Running water, n (%) | 144 (24.8) | 74 (21.2) | 449 (21.5) | 0.21 |
| Endline Maternal Characteristics | ||||
| Final CD4 count, Median cells/mm3, (IQR) | 209 (154-252-) | 270 (177-375) | 493 (372-662) | <0.01 |
| Infant Characteristics | ||||
| Combined infant treatment arm (NVP alone + NVP/ZDV), n (%) | 393 (67.6) | 251 (71.9) | 1409 (67.4) | 0.24 |
| Birth weight, Mean grams (SD) | 2993 (470) | 2970 (450) | 3052 (436) | <0.01 |
| Breastfeeding duration, Median days (IQR) | 181 (98-188) | 182 (180-185) | 182 (165-270) | <0.01 |
NVP = Nevirapine; ZDV = Zidovudine; SD = Standard deviation; IQR = Interquartile range. Differences in means, medians and proportions were compared using analysis of variance (ANOVA), Wilcoxon rank-sum tests and chi-squared statistics respectively.
Viral load n=2726
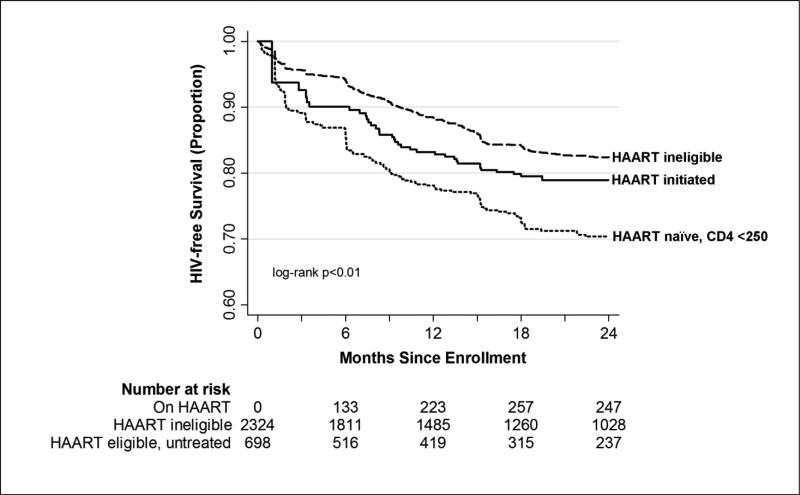
Infants contributed 4,214 person-years (PY) at-risk for HIV infection or death and experienced 532 events (incidence rate 12.6/100 PY, 95% confidence interval [CI] 11.6-13.7). Figure 1 shows the differences in Kaplan-Meier estimates for child HIV-free survival by time-varying HAART and CD4 status. Infants of mothers with CD4 counts ≥250 cells/mm3 throughout study follow-up (HAART ineligible) had the best survival outcomes, followed by infants with HAART initiated mothers. Infants of mothers with CD4 cell counts <250 cells/mm3 who remained HAART naïve during follow-up had the lowest HIV-free survival. Overall at 24 months, cumulative HIV-free child survival ranged from 70.4% among infants of HAART-naïve mothers with CD4 counts <250 cells/mm3 to 82.4% among infants of mothers ineligible for HAART per national guidelines.
Figure 1.
Time to Infant HIV Infection or Death by Time-varying Maternal HAART Status, PEPI Study, Malawi, 2004-2009 (n=3,022)
Baseline log viral load, maternal HAART status, infant birth weight and infant prophylaxis were associated with HIV-free survival in the bivariate Cox models (Table 2). Maternal HAART was associated with a 45% reduction in infant HIV infection or death in the adjusted model compared with infants of mothers who were HAART naïve and had CD4 counts <250 cells/mm3. Infants whose mothers were ineligible for HAART per national guidelines (CD4 ≥250 cells/mm3) also had improved HIV-free survival outcomes in the adjusted analysis (33% reduction in HIV infection or death) as compared to infants with HAART naïve mothers with CD4 counts <250 cells/mm3. Because viral load measurements were missing for 296 women, the multivariate analyses were repeated amongst the full cohort excluding viral load, however inferences were unchanged.
Table 2.
Bivariate and adjusted proportional hazards models for HIV infection or child mortality, PEPI Study, Malawi, 2004-2009, n=2726
| Characteristics | Proportional Hazards [95% CI] | p-value | Adjusted Proportional Hazards [95% CI]a | p-value |
|---|---|---|---|---|
| Maternal age, yrs | 1.00 [0.98-1.02] | 0.93 | ||
| Electricity in the house, yes vs. no | 0.88 [0.73-1.06] | 0.19 | ||
| Running water, yes vs. no | 0.88 [0.71-1.09] | 0.24 | ||
| Baseline maternal BMI, kg/m2 | 0.97 [0.94-0.99] | 0.01 | 0.99 [0.96-1.02] | 0.45 |
| Baseline viral load, log10 (copies/ml) | 1.66 [1.47-1.88] | <.01 | 1.63 [1.44-1.85] | <0.01 |
| Time-varying maternal HAART status | ||||
| HAART naïve, CD4 <250 cells/mm3 | REF | REF | ||
| HAART initiated | 0.59 [0.41-0.86] | <0.01 | 0.55 [0.37-0.82] | <0.01 |
| HAART ineligible, CD4 ≥250 cells/mm3 | 0.51 [0.42-0.61] | <0.01 | 0.67 [0.55-0.82] | <0.01 |
| Infant birth weight, per 1 kgb | ||||
| 0-6 months | 0.51 [0.39-0.67] | <0.01 | 0.58 [0.44-0.78] | <0.01 |
| >6 months – 24 months | 0.79 [0.60-1.04] | 0.10 | 0.92 [0.69-1.21] | 0.54 |
| Control armb | REF | REF | ||
| Combined infant prophylaxis arm | ||||
| 0-6 months | 0.59 [0.45-0.76] | <0.01 | 0.57 [0.44-0.75] | <0.01 |
| >6 months – 24 months | 1.07 [0.82-1.41] | 0.60 | 1.04 [0.79-1.36] | 0.78 |
Model restricted to women with available baseline viral loads
Variable is interacted with time due to violation of the proportional hazards assumption; results are provided for the effect of the variable at 0-6 months and the effect at >6-24 months.
To examine the impact of maternal HAART on child HIV-free survival according to breastfeeding status, we additionally conducted stratified Cox PH analyses of child HIV-free survival over two time periods, 0-6 months when nearly all women reported breastfeeding (88.6% at 6 months), and 6-24 months when women initially started cessation of breastfeeding. In both time periods, the risk of HIV infection or death was lower amongst infants with mothers initiated on HAART or ineligible for HAART, as compared to infants with mothers HAART naïve with CD4 cell counts <250 cells/mm3 (results not shown). In these models the magnitude of the protective effect of HAART for infants with treated mothers as compared to infants with eligible, but HAART naïve mothers was similar during the first 6 months after birth when nearly all mothers were breastfeeding (adjusted Hazard Ratio [aHR] 0.52 [95% CI: 0.25-1.08], p=0.08), to the effect observed in the model from 6-24 months when breast-feeding status was mixed (aHR 0.51 [95% CI: 0.29-0.89], p=0.02). Both of these models adjusted for maternal BMI, maternal viral load, infant birth weight and infant prophylaxis treatment arm; the 6-24 month model additionally adjusted for time-varying breastfeeding status as breastfeeding practices varied across women during this period. In these same two models, infants with mothers ineligible for HAART also trended toward having a reduced risk of infant HIV infection or death during 0-6 months (aHR 0.66 [95% CI: 0.51-0.85], p<0.01), and the 6-24 month time period (aHR 0.71 [95% CI: 0.50-1.01], p=0.06) as compared to infants of HAART naïve mothers with CD4 counts <250 cells/mm3.
HIV-exposed, but uninfected children in the PEPI cohort had more favorable child survival outcomes as compared to HIV-infected children. Even so, by two years post-partum, mortality amongst HIV-uninfected children whose mothers were eligible for HAART per national guidelines was greater than 10% (results not shown). Cox PH models were run to assess predictors of child mortality among HIV-exposed, but uninfected children (Table 3). Maternal viral load and maternal death were strongly associated with child mortality in the first six months in bivariate analyses, while an increase in infant birth weight was protective against child mortality. In the multivariate analysis, maternal viral load and infant birth weight remained significantly associated with child mortality during the 0-6 month strata. For infants >6 months, running water in the household and breastfeeding were associated with a statistically significant reduction in child mortality amongst HIV-exposed, uninfected infants. Maternal HAART use was not associated with child mortality in this sub-analysis.
Table 3.
Risks factors for child mortality amongst HIV-exposed, uninfected children, PEPI Study, Malawi, 2004-2009.
| 0-6 Months, n=2694 | ||||
|---|---|---|---|---|
| Hazard Ratio [95% CI] | p-value | Adjusted Hazard Ratio [95% CI] | p-value | |
| Maternal age, per one year | 0.97 [0.92-1.01] | 0.15 | ||
| Maternal BMI, per kg/m2 | 0.98 [0.92-1.04] | 0.45 | ||
| Maternal viral load at baseline, log (copies/ml) | 1.73 [1.27-2.36] | <0.01 | 1.49 [1.08-2.05] | 0.02 |
| Maternal HAART | ||||
| HAART naïve, CD4 <250 cells/mm3 | REF | REF | ||
| HAART initiated | 0.78 [0.18-3.34] | 0.74 | 0.50 [0.07-3.81] | 0.51 |
| HAART ineligible, CD4 ≥250 cells/mm3 | 0.52 [0.33-0.84] | 0.01 | 0.80 [0.46-1.37] | 0.41 |
| Maternal death | 8.18 [3.76-17.78] | <0.01 | 2.22 [0.86-5.73] | 0.10 |
| Infant birth weight, per kg | 0.31 [0.20-0.47] | <0.01 | 0.38 [0.24-0.62] | <0.01 |
| Infant prophylaxis treatment arm | 0.89 [0.56-1.43] | 0.64 | ||
| Electricity in the house | 1.00 [0.63-1.61] | 0.99 | ||
| Running water | 1.29 [0.78-2.12] | 0.33 | 1.52 [0.90-2.55] | 0.12 |
| >6 months – 24 months, n=2311 | ||||
|---|---|---|---|---|
| Hazard Ratio [95% CI] | p-value | Adjusted Hazard Ratio [95% CI] | p-value | |
| Maternal age, per one year | 1.00 [0.97-1.04] | 0.90 | ||
| Maternal BMI, per kg/m2 | 1.01 [0.97-1.05] | 0.64 | ||
| Maternal viral load, log(copies/ml) | 1.14 [0.93-1.39] | 0.20 | 1.11 [0.91-1.37] | 0.31 |
| Maternal HAART | ||||
| HAART naïve, CD4 <250 cells/mm3 | REF | REF | ||
| HAART initiated | 1.28 [0.74-2.21] | 0.38 | 1.03 [0.58-1.84] | 0.92 |
| HAART ineligible, CD4 ≥250 cells/mm3 | 0.90 [0.60-1.36] | 0.24 | 0.94 [0.61-1.47] | 0.80 |
| Maternal death | 1.00 [0.37-2.68] | 0.99 | 0.68 [0.21-2.15] | 0.51 |
| Infant birth weight, per kg | 0.72 [0.51-1.01] | 0.06 | 0.78 [0.54-1.13] | 0.19 |
| Infant prophylaxis treatment arm | 0.93 [0.66-1.30] | 0.66 | ||
| Electricity in the house | 0.79 [0.56-1.12] | 0.19 | ||
| Running water | 0.62 [0.40-0.96] | 0.03 | 0.57 [0.36-0.90] | 0.02 |
| Breastfeeding status | ||||
| No longer breastfeeding | REF | REF | ||
| Currently breastfeeding | 0.05 [0.01-0.19] | <0.01 | 0.05 [0.01-0.21] | <0.01 |
DISCUSSION
This study demonstrates the substantial effect of maternal HAART on improving HIV-free child survival outcomes in women with advanced HIV disease requiring therapy. Women with high CD4 cell counts who were ineligible for HAART had the best infant survival outcomes in the crude analysis. After controlling for maternal viral load and other important factors that differed amongst women by HAART status, infants with mothers on HAART and infants of mothers who were not yet eligible for HAART had respectively, an estimated 45% and 33% reduction in HIV infection or death as compared to infants with HAART naïve mothers with CD4 counts <250 cells/mm3 (Table 2). Greater than 10% of infants with mothers eligible for but not receiving HAART were infected with HIV or died by three months of age (Figure 1). The rapid initiation of HAART as part of the Option B+ program will hopefully eliminate this treatment delay and offer HIV-free child survival benefits for infants of women with low CD4 cell counts. Whether initiation of HAART would also provide benefit to infants of women with higher CD4 counts is unknown; in the HPTN 046 trial, HIV-free survival rates at 18 months were not significantly different for infants of women on HAART per national guidelines and infants of women with CD4 count ≥350 cells/mm3 not receiving HAART (96.3% and 95.2%, respectively, p=0.46) (19). However, while progression to CD4 <350 cells/mm3 is slow in women with high CD4 cell counts (CD4 ≥500-550 cells/mm3), nearly a third of women with CD4 350-499 cells/mm3 experience a drop below 350 cells/mm3 within 1-2 years postpartum, and the threshold for starting treatment is often missed in HIV-infected women after delivery (20, 21). Thus, given programmatic difficulties in ensuring appropriate postpartum follow-up of women, starting all women on HAART provides an alternative to ensure women who need therapy receive it.
Maternal HAART was not directly associated with reduced child mortality among HIV exposed, uninfected children, suggesting that child HIV-free survival benefits are largely conferred through prevention of mother-to-child transmission. However the PEPI data demonstrates indirect evidence for the potential of maternal HAART in reducing child mortality as earlier HAART initiation would result in decreased maternal mortality and increased breastfeeding duration, which were both independently associated with reductions in child mortality (Table 3). Indeed a major finding from the analysis of HIV-uninfected, exposed infants was that breastfeeding between the ages 6-24 months was associated with a 95% decrease in risk of child mortality (aHR 0.05 [95% CI 0.01-0.21]).This highlights the importance of breastfeeding for child survival amongst uninfected, exposed infants and underscores the benefits of breastfeeding in this environment when MTCT risks can be minimized through HAART initiation.
The study has some limitations. Because women were initiated on HAART at local clinics and not through study services, the study was only able to ascertain whether women were currently on HAART and not the exact date of HAART initiation. Thus we cannot account for any expected delay in the positive effect of HAART from time of HAART initiation to time of viral load suppression. Furthermore, among women with low CD4 cell counts (<250 cells/mm3), HAART was not universally initiated within the public sector and it is possible that women who were more ill may have been more likely to receive HAART. In fact, women initiated on HAART in our analysis were more likely to have CD4 counts <100 cells/mm3 at baseline as compared to women with low CD4 cell counts who remained HAART naïve. As a result, if women initiated on HAART were actually more ill than HAART naïve women with low CD4 counts, confounding-by-indication would be present in our analysis and we may have underestimated the protective effect of HAART in this population. There also may have been important unmeasured confounders such as geographic proximity to the clinic, which may have been associated with both initiation of HAART amongst eligible women and better infant outcomes. However a previous analysis from our cohort did not find any demographic factors to be associated with HAART initiation amongst post-partum women (18).
Despite the limitations, these results clearly demonstrate the importance of maternal HIV disease status on child HIV-free survival. Infants of mothers not meeting national treatment eligibility at the time of the study had the best HIV-free survival; for infants of mothers meeting treatment eligibility criteria, our results demonstrate the crucial impact of initiating maternal HAART on the HIV-free survival of children. Although we had expected greater survival benefits beyond PMTCT than what were observed, this finding may be due to the overall poorer health status of women eligible for HAART. Infants of mothers with higher CD4 cell counts had the best HIV-free survival outcomes. This suggests that even though a protective effect of HAART above PMTCT was not directly observed in this cohort of women initiating treatment <250 cells/mm3, earlier initiation of HAART at higher CD4 counts to prevent high viral burden, greater immune suppression and other concomitant maternal morbidities may prevent HIV transmission and independently maximize child survival outcomes.
The current Option B+ program in Malawi recommending lifelong HAART for pregnant and postpartum breastfeeding women was not introduced in Malawi based on effectiveness data from the field. So far only coverage and retention components of the Malawi program have been reported (22-24). In the PEPI study which preceded Option B+, high rates of HIV infection and mortality occurred during the first 3 months post-partum, reinforcing the need to treat earlier those women requiring HAART and providing evidence in support of Option B or B+ strategies which facilitate immediate HAART initiation without need to wait for CD4 test results. Importantly, the benefits of HAART in mothers with advanced HIV disease for infant HIV-free survival persist after 6 months post-partum when breastfeeding practices are more variable. For women with higher CD4 cell counts, initiation of HAART regardless of CD4 count would hopefully avoid the additional delays observed in starting ART after CD4 cell decline because of lack of immunologic monitoring in the postpartum period (21). A critical component of the Option B+ strategy related to both maternal and infant survival will be to ensure retention in care and provide support for adherence to therapy, particularly among women who would otherwise be not starting on HAART for their own health (23).
ACKNOWLEDGEMENTS
We are indebted to the mothers and children who participated in the PEPI -Malawi study. We are grateful to the nursing and technical staff in Malawi for their excellent collaboration throughout this study.
This work was supported by a Cooperative Agreement [grant number 5 U50 PS022061-05 and award # U50/CC0222061] from the Centers for Disease Control and Prevention and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health.
Footnotes
Conflicts of Interest and Source of Funding
There are no conflicts of interest to declare.
Disclaimer:
The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention or the National Institutes of Health. Use of trade names is for identification purposes only and does not constitute endorsement by the U.S. Centers for Disease Control and Prevention, National Institutes of Health, or the Department of Health and Human Services.
Clinical Trials Registration
This study is registered at http://clinicaltrials.gov/ under trial number NCT00115648.
REFERENCES
- 1.Mofenson LM. Antiretroviral drugs to prevent breastfeeding HIV transmission. Antiviral therapy. 2010;15:537–553. doi: 10.3851/IMP1574. [DOI] [PubMed] [Google Scholar]
- 2.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. The New England journal of medicine. 2008;359:119–129. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. The New England journal of medicine. 2010;362:2282–2294. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. The New England journal of medicine. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. The Lancet infectious diseases. 2011;11:171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 6.Kilewo C, Karlsson K, Massawe A, et al. Prevention of mother-to-child transmission of HIV-1 through breast-feeding by treating infants prophylactically with lamivudine in Dar es Salaam, Tanzania: the Mitra Study. J Acquir Immune Defic Syndr. 2008;48:315–323. doi: 10.1097/QAI.0b013e31816e395c. [DOI] [PubMed] [Google Scholar]
- 7.Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 8.Thomas TK, Masaba R, Borkowf CB, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding--the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS medicine. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a public health approach. 2013 [PubMed] [Google Scholar]
- 10.Taha TE, Li Q, Hoover DR, et al. Postexposure prophylaxis of breastfeeding HIV-exposed infants with antiretroviral drugs to age 14 weeks: updated efficacy results of the PEPI-Malawi trial. J Acquir Immune Defic Syndr. 2011;57:319–325. doi: 10.1097/QAI.0b013e318217877a. [DOI] [PubMed] [Google Scholar]
- 11.Taha TE, Kumwenda J, Cole SR, et al. Postnatal HIV-1 transmission after cessation of infant extended antiretroviral prophylaxis and effect of maternal highly active antiretroviral therapy. The Journal of infectious diseases. 2009;200:1490–1497. doi: 10.1086/644598. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. Guidelines on HIV and infant feeding. 2010 [Google Scholar]
- 13.Marinda E, Humphrey JH, Iliff PJ, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. The Pediatric infectious disease journal. 2007;26:519–526. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 14.Mermin J, Were W, Ekwaru JP, et al. Mortality in HIV-infected Ugandan adults receiving antiretroviral treatment and survival of their HIV-uninfected children: a prospective cohort study. Lancet. 2008;371:752–759. doi: 10.1016/S0140-6736(08)60345-1. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee A, Bosch RJ, Hunter DJ, Fataki MR, Msamanga GI, Fawzi WW. Maternal disease stage and child undernutrition in relation to mortality among children born to HIV-infected women in Tanzania. J Acquir Immune Defic Syndr. 2007;46:599–606. doi: 10.1097/QAI.0b013e31815a5703. [DOI] [PubMed] [Google Scholar]
- 16.Landes M, van Lettow M, Chan AK, Mayuni I, Schouten EJ, Bedell RA. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PloS one. 2012;7:e47337. doi: 10.1371/journal.pone.0047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malawi Ministry of Health Guidelines for the use of antiretroviral therapy in Malawi. (3rd edition.) 2008 [Google Scholar]
- 18.Kumwenda J, Matchere F, Mataya R, et al. Coverage of highly active antiretroviral therapy among postpartum women in Malawi. International journal of STD & AIDS. 2011;22:368–372. doi: 10.1258/ijsa.2011.010359. [DOI] [PubMed] [Google Scholar]
- 19.Fowler MG, Coovadia H, Herron CM, et al. Efficacy and safety of an extended nevirapine regimen in infants of breastfeeding mothers with HIV-1 infection for prevention of HIV-1 transmission (HPTN 046): 18-month results of a randomized, double-blind, placebo-controlled trial. J Acquir Immune Defic Syndr. 2014;65:366–374. doi: 10.1097/QAI.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watts DH, Brown ER, Maldonado Y, et al. HIV disease progression in the first year after delivery among African women followed in the HPTN 046 clinical trial. J Acquir Immune Defic Syndr. 2013;64:299–306. doi: 10.1097/QAI.0b013e3182a2123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coria A, Noel F, Bonhomme J, et al. Consideration of postpartum management in HIV-positive Haitian women: an analysis of CD4 decline, mortality, and follow-up after delivery. J Acquir Immune Defic Syndr. 2012;61:636–643. doi: 10.1097/QAI.0b013e31826abdd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention Impact of an innovative approach to prevent mother-to-child transmission of HIV--Malawi, July 2011-September 2012. MMWR Morbidity and mortality weekly report. 2013;62:148–151. [PMC free article] [PubMed] [Google Scholar]
- 23.Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women ('Option B+') in Malawi. AIDS. 2014;28:589–598. doi: 10.1097/QAD.0000000000000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matovu NJ NZ, Mubiru M, Musingye E, Kyarimpa M, Kakande A, Kamya S, Musoke P, Kamya M, Fowler MG. The implementation of Option B +. Sharing unique challenges for refusal to take Option B+ drugs as reported by HIV-infected mothers at Mulago National Referral Hospital, Kampala, Uganda.. 7th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Kuala Lumpur. 2013; Abstract TUAC0102. [Google Scholar]