Abstract
Purpose
Compare functional outcomes of radiotherapy (RT) concurrent with cetuximab (cet-RT) or with chemotherapy (chemo-RT) for comparable, good prognosis patients with Human Papillomavirus related (HPV+) oropharyngeal cancer (OPC).
Methods
Outcomes of patients with stage III/IV HPV+ OPC patients with minimal smoking history and non-T4/N3/N2C, treated on prospective protocol of RT concurrent with cetuximab (cet-RT), were compared to similar patients on prospective chemo-RT protocols. In both groups, videofluoroscopy (VF), observer rated dysphagia (ORD), and validated QOL questionnaires: Xerostomia Questionnaire (XQ), Head and Neck QOL, and University of Washington QOL, were performed periodically and compared to pretreatment. Mixed effects models with adjustment for baseline assessed differences between groups.
Results
26 cet-RT patients were compared to 27 chemo-RT patients with similar baseline characteristics. In the chemo-RT group, no recurrences occurred. In the cet-RT group, 1 patient had persistent microscopic disease on salvage neck dissection and 1 distant failure. Both groups had mild VF-based swallowing dysfunction pre-treatment, worsened at 3 months (P<0.02) and persisted at 12 months, not differing between groups (P>0.11). For both groups ORD was very low pretreatment, worsened at 3 months and improved at 12 months, without differences between treatment groups (P=0.26). QOL Summary and domain scores for eating were good pretreatment, worse at 3 mo, and then improved to near baseline at 12 months, without differences between the groups in any QOL domains (P>0.10).
Conclusion
Both groups had excellent clinical outcomes without significant differences in objective or subjective functions. These data question using cetuximab instead of chemotherapy for treatment de-intensification for HPV+ OPC.
Introduction
Human papillomavirus related (HPV+) oropharyngeal cancer (OPC) has emerged as a favorable subtype of head and neck cancer with improved locoregional control and survival compared to its smoking and drinking related counterparts (1,2). The high tumor control rates and survival amongst this population have led investigators to hypothesize that many patients are being over-treated and patients could have equivalent outcomes with reduced intensity treatment, thereby reducing adverse side effects and long-term sequelae of treatment (3,4). Efforts to reduce treatment intensity for this favorable population are ongoing with randomized clinical trials currently underway (5).
Replacing chemotherapy with cetuximab for patients undergoing radiation therapy for head and neck cancer has been proposed for treatment de-intensification for HPV-positive patients. Cetuximab has been shown in a randomized clinical trial to improve outcomes in head and neck patients compared to radiation alone, especially in OPC patients, with reported low rates of toxicity, comparable to the radiation alone arm, with regard to mucositis, dysphagia, gastrostomy dependence, or quality of life (6,7). No long term toxicity was reported for this study, nor were objective functional studies performed. Randomized trials comparing concurrent chemoradiation (chemo-RT) and cetuximab-radiation (cet-RT) are underway (6–8). Improvements in long-term toxicity, such as reduction in dysphagia or xerostomia, by using concurrent cetuximab instead of chemotherapy, have not yet been established.
Therefore, we sought to compare toxicity outcomes of matched patients treated at our institution who received either chemo-RT or cet-RT. The cet-RT patients participated in an institutional phase II trial investigating the toxicity and outcomes of stage III/IV HPV+ patients with minimal or no smoking history treated with cetuximab concurrent with IMRT. We compared the functional outcomes of these patients to a matched cohort of patients, the chemo-RT group, prospectively enrolled on intensity modulated radiotherapy IMRT concurrent with chemotherapy in phase II trials from 2003–2011. Radiotherapy goals in all trials were to spare the salivary glands as well as the swallowing structures. Longitudinal functional assessments were the same in both groups: patient-reported quality of life (QOL) emphasizing dysphagia, observer-rated dysphagia, and videofluoroscopy to assess the mechanics of swallowing after therapy compared with pre-therapy.
Methods
Patients and radiation treatments
The cet-RT protocol is an Institutional Review Board approved single arm phase II trial. Criteria for enrollment are HPV+ OPC, stage III or IV, non-T4, non-N3, with little or no smoking history (≤10 pack year). For all patients, HPV status was established by using either polymerase chain reaction for HPV DNA from banked tissue in prospectively assembled tissue microarrays or immunohistochemistry to detect p16 as previously described (4). P16 served as a surrogate for HPV positive disease as has been reported previously (9,10). Patients received a loading dose of cetuximab 400 mg/m2 followed with weekly 250 mg/m2 concurrent with RT. RT was designed to deliver 70 Gy to clinical tumor volume 1 (CTV1) and 59–56 Gy to CTV2-3 over 35 treatments, aiming to spare salivary glands, pharyngeal constrictors, larynx and upper esophagus to improve xerostomia and swallowing function. Two patients were excluded from analysis, one because of T4 disease and one because she received unilateral radiation.
95 OPC patients with stage III or IV disease from prospective phase II trials of chemo-RT (11,12) were reviewed to compare to the cetuximab patients (same PI for all studies). Of the 95 patients, 27 met the cet-RT protocol enrolment criteria and were included in the comparison analyses. Radiation planning principles were same as in the cet-RT group. Chemotherapy consisted of concurrent weekly carboplatin (AUC=1) and paclitaxel (30 mg/m2).
Direct laryngoscopy and contrast enhanced CT, PET/CT imaging, or both were used for pretreatment staging. All patients were treated with parotid sparing and swallowing organs sparing intensity modulated radiotherapy (IMRT) to decrease long term dysphagia and xerostomia. The mean parotid dose limit was 24 Gy and mean pharyngeal constrictor dose limit was 50 Gy whenever possible, however, doses to planning target volumes (PTV) took priority over limiting doses to organs at risk. In recent years, mean doses to the inferior constrictors, larynx and esophagus were limited to 20 Gy for patients without lower cervical lymphadenopathy. Mean doses to normal structures, including parotid glands, submandibular glands, oral cavity, constrictors (superior, middle and inferior), larynx, and esophagus, were compared between groups and used as independent factors in regression and correlation analyses as potential predictors of outcomes.
Patients were seen in follow-up (f/u) every 6–8 weeks for the first 3 years and every 3 months (mo) afterwards. Post-treatment imaging was obtained approximately 3 months post-radiation and was most often a PET/CT scan.
Videofluoroscopy
Modified Barium videofluoroscopies were performed by Speech/Swallow specialists (MH, TL) pretreatment and 3 and 12 months post-treatment. Patients were scored according to standard scales and evaluated under several swallowing conditions including solids, soft foods, thick liquids and thin liquids. Penetration/aspiration was scored based on direct visualization of any portion of a bolus present in either the laryngeal vestibule (penetration) or descending through the vocal folds into the tracheobronchial tree (aspiration). An overall measurement of material entering the airway was accomplished using the Penetration Aspiration Scale (PAS; Rosenbek, et.al. 1996) (13) which is a validated 8-point scale used to describe penetration/aspiration events (including the patient’s reflexive ability to expel material from the airway). These methods have been well established for evaluating swallowing function (14) and have been described previously in IMRT studies (15–17).
Observer Reported Mucositis and Dysphagia
Common Toxicity Criteria for Adverse Events (CTCAE_v.3) for functional mucositis was performed during each week of radiotherapy and 1 month post treatment. The standard scoring of 0–4 was used with 0 meaning no mucositis, 1- asymptomatic or mild symptoms, 2- moderate pain not interfering with oral intake, 3-severe pain interfering with oral intake, unable to adequately hydrate or aliment orally, 4- life threatening requiring hospitalization and parenteral or enteral nutrition or prophylactic intubation.
Observer- rated dysphagia (ORD) was performed pre-treatment and at every post treatment visit. A score of 0 meaning no difficulty, 1 - minimal change in swallowing without changing diet, 2- difficulty requiring modified diet (softs or liquids), 3- severe difficulty requiring feeding tube or parenteral nutrition and 4 - life threating swallow dysfunction such as perforation or obstruction.
Health related quality of life (QOL) Questionnaires
Three validated questionnaires assessed patient reported xerostomia, dysphagia, and quality of life, pretreatment and periodically post-treatment. We analyzed outcomes at pretreatment, 3 months and 12 months, corresponding to the timing of the objective VF studies. The questionnaires were the head and neck QOL (HNQOL)(18), xerostomia questionnaire (XQ)(19), and University of Washington QOL (UWQOL)(20). Summary and domain scores (SS) were calculated for each test. All QOL scores were normalized to a scale of 100 with lower scores indicating less dysfunction and higher scores indicating more severe dysfunction.
The HNQOL has communication, discomfort, eating, and emotion domains, with 3–4 questions within each domain. For the purposes of this study, we compared HNQOL summary scores (HNQOL-SS, taking all domains into account) and eating domains (HNQOL-ED) between patient groups. The UWQOL has a pain, disfigurement, activity, recreation, employment, eating, saliva, taste, speech, and phlegm domains. For the purposes of this study, only summary scores and eating/swallowing domains were compared between groups.
The XQ assesses dry mouth using 8 questions related to discomfort from dry mouth while talking, chewing, swallowing, and while not eating. A score averaging responses to all the questions was the overall XQ summary score (XQ-SS). Both xerostomia while-fasting domain (XQ-FD) and xerostomia while-eating domain (XQ-ED), four questions in each domain, were also analyzed.
Statistical Analysis
For mucositis outcomes, regression and correlation models were used for each time point (weeks 1–7 and 1 month) maximum mucositis and percent grade 3 mucositis. Logistic regression modeling of mucositis (none/mild vs moderate/severe) at each time point was performed with systemic therapy group, oral cavity dose and pharyngeal constrictor dose as potential variables in the models.
For the long term outcomes, Chi-square tests and t-tests at each time point assessed the differences between the two treatment groups for categorical and continuous baseline variables, respectively. Toxicity outcomes were defined as the change from baseline to either 3 or 12 months. Differences over time in each outcome between treatment groups were determined using mixed effects models (21) with adjustment for baseline values of the outcome, age, time, and within-subject correlation. Random subject level intercepts were included to account for correlation over time within each patient. We also fit similar mixed models assessing for treatment differences while adjusting for radiation doses to various organs known to be involved in swallowing and xerostomia. Additionally, at each time point, we applied multiple regression analysis with stepwise model selection using all available covariates to identify a set of significant predictors for each outcome. Analyses were performed using R (version 3.1.1; R Foundation for Statistical Computing, Vienna, Austria) and MedCalc Statistical Software version 14.12.0 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014).
Results
Clinical outcomes
Twenty seven patients treated with chemo-RT were compared to 26 cet-RT patients and had similar values of all baseline variables (Table 1) with the exception of the cet-RT patients being older (median age 60 vs 52). All patients received their full course of treatment with no treatment breaks. For the chemo-RT group (median f/u 52 mo), no failures were seen, neither loco-regionally or distantly. In the cet-RT group (median f/u 20 mo), one patient had persistent microscopic disease on salvage neck dissection and is currently without evidence of disease, and one had distant failure, with overall disease control 96%. These differences between groups were not statistically significant.
Table 1.
Characteristics of Patients receiving chemo-RT or cet-RT
| Characteristic | Chemo-RT: n (%) | Cet-RT: n (%) | P value |
|---|---|---|---|
| Patients | 27 (100%) | 26 (100%) | |
| Age (median/range) | 52 (45–70) | 60 (49–77) | 0.002 |
| Male Gender | 24 (89%) | 24 (92%) | 0.96 |
| Tonsil cancer | 17(71%) | 12 (46%) | 0.23 |
| T Stage | |||
| T1 | 6 (22%) | 7 (27%) | 0.53 |
| T2 | 15 (56%) | 15 (57%) | |
| T3 | 6 (22%) | 4 (15%) | |
| N Stage | P = 0.06 | ||
| N0/N1 | 3 (11%) | 7 (27%) | |
| N2 | 24(89%) | 19 (73%) | |
| N2A | 6 (22%) | 8 (31%) | |
| N2B | 18 (67%) | 11 (42%) | |
| Wt change during RT | 10% | 11% | 0.68 |
| NG/G-tube during therapy | 22% | 35% | 0.51 |
All patients in both groups had pre-therapy and 3 months complete VF studies, ORD, and patient reported outcomes, while 20 of 26 patients in the Cetuximab-RT group and 26 of 27 patients in the chemo-RT group had complete studies at 12 months post-treatment.
Doses to Normal Structures
In recent years we have made efforts to further reduce the mean doses to the normal structures in the cet-RT group (treated more recently), resulting in doses that were significantly lower than in the previously treated chemo-RT group (Supplemental Table 1). No differences were observed in the parotid gland doses. The doses to the normal structures were adjusted for in the analyses of outcomes using the statistical methods described above.
Mucositis
Physician reported mucositis for the entire group started low and began rising during weeks 2–4 of radiation therapy, peaked at weeks 5–7 and generally improved by 1 month post treatment. There was no significant difference in the observer rated maximum mucositis (p=0.90) nor in the percent grade 3 mucositis between groups (55% vs 46% P=0.69). There was also no difference in mucositis during weeks 4–7 of radiotherapy, however there were differences at week 3 and at 1 month post treatment, indicating an earlier onset and a longer lasting mucositis in the chemo-RT group (Supplemental Figure 1). However, upon partial correlation, taking into account the effect of oral cavity and pharyngeal constrictor dose, these time points are no longer significant (p=0.10, 0.092). Oral cavity and pharyngeal constrictor doses are the strongest predictors of grade 3 mucositis (P= 0.044 and 0.0024 respectively) and mucositis at each week starting at week 5 (R=0.3–0.4, P<0.05). Additionally, in a logistic regression comparing grades 0/1 (none to mild) to 2/3 (moderate to severe) mucositis, no differences between groups were observed at any time point except at 1 month but when doses to the pharyngeal constrictors and oral cavity are included in the analysis, the systemic therapy grouping was excluded from the model, and only the pharyngeal constrictor dose was included (odds ratio 1.18 confidence interval 1.05–1.33, p=0.004). Therefore, these slight apparent differences in mucositis between the chemo-RT and cet-RT group are related to differences in doses to organs at risk and are not related to the differences in systemic therapy. Also, there was no difference in weight loss (P=0.68) or the need for tube feedings (P=0.51) during radiation between groups (Table 1).
Videofluoroscopy (VF)
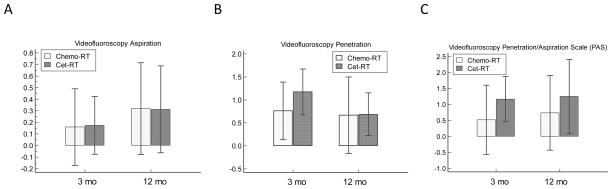
Overall, patients in both groups had only mild to moderate VF-based dysfunction before treatment, with scores of Aspiration: <1, Penetration: 1–2, and PAS: 2–3. For the entire cohort, there was a significant worsening of swallowing function from pretreatment to 3 months post-treatment (P<0.02) for all measures except aspirations (p=0.10) which remained <1. No significant differences in any measurements were evident between 3 mo and 12 months on paired T tests (p values 0.44–0.84). Likewise, Aspiration, Penetration and PAS were not different between the chemo-RT and cet-RT groups at the 3 or 12 months analyzed by T-tests (Figure 1, Table 2). Additionally we performed two repeated measure analysis mixed effect models adjusted for time, baseline measure and within-subject correlation, without (Model 1) or with (Model 2) dose effect (Table 3). The normal tissue doses adjusted for in Model 2 include the pharyngeal constrictors, glottis and esophagus. No significant differences in VF outcomes were evident between chemo-RT and Cet-RT groups when all relevant parameters are adjusted for. There was, however, a non-significant trend for worse toxicity in the cet-RT group in both the PAS and Penetration scores (Model 2, Table 3).
Figure 1.
Videofluoroscopy studies comparing chemotherapy and cetuximab groups: Bars are mean score changes from baseline, and whiskers are 95% confidence interval (CI). Higher scores denote worse function. A) Aspiration scores (ASP) at 3 and 12 months. B) Penetration scores (PEN) at 3 and 12 months. C) Penetration/Aspiration scores (PAS) at 3 months and 12 months. See supplementary table 2 for results of statistical analysis
Table 2.
Results from unadjusted analysis at 3 and 12 months. “Difference” column denotes difference in score between chemo-RT and cet-RT groups. Positive “Difference” means Cet-RT group is higher (more toxicity), negative difference means cet-RT group is lower (less toxicity). No statistical differences were seen between the cet-RT and chemo-RT groups at any time point for any outcome, except XQ-SS, and XQ-FD at 3 and 12 months, and XQ-ED at 3 months.
| A | ||||||
|---|---|---|---|---|---|---|
| T-Test | 3 months | 12 months | ||||
| Outcome | Difference | Std Err | P value | Difference | Std Err | P value |
| VF-Aspiration | 0.04 | 0.20 | P = 0.95 | 0.06 | 0.27 | P = 0.84 |
| VF-Penetration | 0.41 | 0.38 | P = 0.29 | 0.21 | 0.51 | P = 0.68 |
| VF-PAS | 0.65 | 0.63 | P = 0.31 | 0.51 | 0.81 | P = 0.53 |
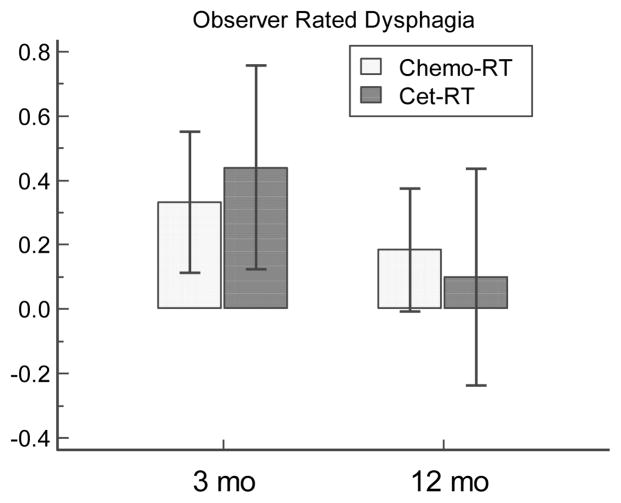
| ORD | 0.11 | 0.18 | P = 0.57 | −0.09 | 0.18 | P = 0.63 |
| XQ-SS | −17.77 | 6.66 | P = 0.01 | −13.24 | 5.84 | P = 0.03 |
| XQ-FD | −15.62 | 6.82 | P = 0.03 | −13.05 | 5.63 | P = 0.03 |
| XQ-ED | −17.72 | 7.33 | P = 0.02 | −12.93 | 6.86 | P = 0.07 |
| HNQOL-SS | −2.54 | 3.60 | P = 0.48 | −2.01 | 3.61 | P = 0.58 |
| HNQOL-ED | −3.22 | 4.62 | P = 0.49 | −2.08 | 5.44 | P = 0.71 |
| UWQOL-SS | −1.74 | 3.75 | P = 0.65 | −5.06 | 3.14 | P = 0.11 |
| UWQOL-ED | −9.13 | 5.96 | P = 0.13 | 0.21 | 4.84 | P = 0.97 |
ED=eating domain; FD=fasting domain; HNQOL=head & neck quality of life questionnaire; ORD=observer rated dysphagia; PAS=Penetration/Aspiration Score; SS= summary score; UWQOL=University of Washington quality of life questionnaire; VF=videofluoroscopy; XQ= xerostomia questionnaire
Table 3.
Multivariable repeated measures analysis of outcomes. “Estimate” denotes the predicted difference between cet-RT and Chemo-RT groups once other variables in the model are adjusted for. A negative value means chemo-RT worse, a positive value means cet-RT worse.
| Repeated Measures | Model 1 (without dose adjustment) | Model 2 (with dose adjustment) | ||||
|---|---|---|---|---|---|---|
| Outcome | Estimate | SE | p value | Estimate | SE | p value |
| VF−Aspiration | −0.06 | 0.20 | 0.78 | 0.08 | 0.23 | 0.72 |
| VF-Penetration | 0.50 | 0.35 | 0.15 | 0.57 | 0.40 | 0.16 |
| VF-PAS | 0.53 | 0.54 | 0.33 | 0.83 | 0.63 | 0.19 |
| ORD | 0.01 | 0.16 | 0.93 | 0.23 | 0.18 | 0.19 |
| XQ-SS | −11.77 | 5.78 | 0.04 | 0.24 | 6.75 | 0.97 |
| XQ- FD | −6.38 | 1.69 | <0.01 | −3.24 | 1.93 | 0.09 |
| XQ- ED | −7.23 | 2.19 | <0.01 | −3.59 | 2.70 | 0.18 |
| HNQOL-SS | −5.21 | 3.06 | 0.09 | 2.07 | 3.46 | 0.55 |
| HNQOL-ED | −5.85 | 4.50 | 0.19 | 2.43 | 5.23 | 0.64 |
| UWQOL-SS | −4.78 | 3.26 | 0.14 | 2.59 | 3.77 | 0.49 |
| UWQOL-ED | −6.40 | 4.37 | 0.14 | −3.87 | 5.43 | 0.48 |
Estimates of average difference between treatments groups across time (3 and 12 months) effect without (Model 1) or with (Model 2) adjustment for radiation doses. For VF outcomes, Model 2 adjusts for radiation doses to pharyngeal constrictors, glottis and esophagus. For ORD, Model 2 adjusts for radiation doses to pharyngeal constrictors, glottis and esophagus. For XQ outcomes, Model 2 adjusts for radiation doses to the oral cavity, parotid glands and contralateral submandibular gland. For HNQOL and UWQOL outcomes, Model 2 adjusts for doses to the pharyngeal constrictors, glottis, esophagus, oral cavity, parotid glands and contralateral submandibular gland. Once dose has been adjusted for, there were no significant differences between groups for any outcomes.
ED=eating domain; FD=fasting domain; HNQOL=head & neck quality of life questionnaire; ORD=observer rated dysphagia; PAS=Penetration/Aspiration Score; SS= summary score; UWQOL=University of Washington quality of life questionnaire; VF=videofluoroscopy; XQ= xerostomia questionnaire
Observer Rated Dysphagia
Pre-therapy CTCAE ORD scores were 0 in almost all patients except for score 1 in one and two patients in the cet-RT and chemo-RT groups, respectively. In both cohorts, there was significant worsening of ORD at 3 months (p=0.003), with 59%, 29%, and 12% grades 0,1 and 2, respectively, in the cet-RT patients, and 63% and 37% grades 0 and 1, respectively, in the chemo-RT patients. ORD then significantly improved at 12 month in both groups (P=0.005), with ORD score 3 in one patients and the rest 0 in the cet-RT group, and 23% grade 1, the rest 0, in the chemo-RT group. T-tests found no difference between chemo-RT and cet-RT groups at 3 mo (P=0.56) or 12mo (P=0.63) (Figure 2 and Table 2). Additionally, repeated-measures analysis with mixed effects modeling found no significant differences in ORD between groups with or without adjusting for doses to the constrictors, glottis and esophagus (Table 3).
Figure 2.
CTCAE observer-rated dysphagia comparison between chemo-RT and cet-RT at 3 and 12 months. Histogram representation of results. Bars are mean score changes from baseline, and whiskers are 95% CI. Higher scores denote worse observer rated dysphagia. See supplementary Table 2 for statistical analysis.
Head and Neck QOL Questionnaire
HNQOL-summary scores for all patients significantly worsened from pretreatment to 3 months: median 10 (range 0–45), vs 19 (0–63), P<0.0001. The scores then improved and were back to baseline at 12 months (median 7, range 0–47, P=0.3 compared to pretreatment value). For the eating domain, patients had low pretreatment values (median 0 (0–46)), that were worse at 3 months [21 (0–79), P<0.0001] and then improved by 12 months but were still not back to baseline [10 (0–71); P=0.0007 compared to 3 mo, and P=0.001 compared to pretreatment]. Using the change from pre-RT values, we compared summary scores and eating domains in the chemo-RT and cet-RT groups. Unadjusted analysis revealed no difference between groups at either 3 or 12 months (Figure 3, Table 2). Repeated measures analysis with mixed effect modeling also demonstrated no significant differences between the groups, with or without adjusting for doses to the constrictors, glottis, esophagus, parotid glands, contralateral submandibular gland and oral cavity (Table 3).
Figure 3.
Results of health related quality of life (QOL) questionnaires. Bars are mean score changes from baseline, and whiskers are 95% CI. Higher scores denote worse QOL. A–B) Head and Neck QOL (HNQOL) summary score (SS) and eating domain (ED). C–D) University of Washington QOL (UWQOL) SS and ED. See supplementary Table 2 for statistical analysis.
University of Washington QOL Questionnaire
For all patients, the UWQOL-summary score was initially low (mean 9), was significantly worse at 3 months (mean 24, paired T test P<0.0001), and was improved at 12 months (mean 14, P<0.0001). However, the summary score at 12 months was still significantly worse than the baseline score (P=0.004). A similar trend was seen for the eating/swallowing domain, with a pretreatment mean score of 5 that worsened to 18 at 3 months and then improved to 11 at 12 months (pre vs 3 mo, P=0.0008; 3 mo vs 12 mo, P=0.07; pre vs 12 mo, P=0.03). Unadjusted analysis revealed no differences in outcomes, either in summary score or eating domain, between treatment groups at 3 or 12 months (Figure 3, Table 2). Using repeated measures analysis with mixed effect modeling, there were no differences in summary scores or eating domains with or without adjusting for doses to the constrictors, glottis, esophagus, parotid glands, contralateral submandibular gland and oral cavity (Table 3).
Xerostomia Questionnaire (XQ)
For all patients, the XQ summary score (XQ-SS) was significantly worse from pretreatment (median= 1, range 0–33) to 3 months [47 (0–94), p<0.0001)]. XQ-SS then improved at 12 months (median 23, range 0–69). Unadjusted analysis demonstrated better summary scores as well as xerostomia while eating domain (XQ-ED) and xerostomia while fasting domain (XQ-FD) scores in cet-RT, compared with the chemo-RT group, that were significant at 3 and 12 months (P<0.05) (Supplemental Figure 2, Table 2). However, after adjusting for oral cavity and salivary gland doses no significant differences in XQ-SS, XQ-ED, or XQ- FD were seen (Table 3, Model 2). Multiple regression analysis using stepwise method revealed the only significant predictor of XQ outcomes at 3 months was dose to the contralateral submandibular gland (cSMG), while at 12 months both cSMG and oral cavity mean doses were significant for XQ-SS, cSMG for XQ-while-Fasting Domain, and oral cavity for XQ-while-Eating Domain (Supplemental Table 2). In summary, while univariate analysis demonstrated improved XQ scores in the cet-RT group, this difference appears to be attributable to lower doses to the major salivary glands and oral cavity (containing minor salivary glands) rather than to using cetuximab instead of chemotherapy.
Discussion
This is the first study that directly compares functional and QOL outcomes of patients treated with concurrent chemotherapy-RT or cetuximab-RT. Using a matched cohort of prospectively treated, favorable, HPV+ OPC patients, no differences between cet-RT and chemo-RT cohorts were apparent in objective or subjective measures of acute mucositis or longer term dysphagia or QOL, evaluated by VF, ORD, and QOL questionnaires up to one year.
Cetuximab concurrent with radiation is an effective treatment for head and neck cancer and for OPC specifically (22). In a recent metaanalysis comparing chemoradiation to cetuximab radiation, comprised mostly of retrospective studies, overall patients who received platinum based chemotherapy had improved outcomes (23). Selection of patients who are older or have co-morbidities to receive cetuximab instead of chemotherapy is prevalent in many retrospective series, and may explain part of this finding. Of note, patients with HPV+ OPC had equivalent 2 year survival and progression free survival with cetuximab as with chemotherapy (23), similar to the finding in our series where patients were similar in both groups. Cetuximab is currently being tested against platinum based chemotherapy in a randomized trial, the results of which are pending (6). In our study, tumor control rates for the chemo-RT and cet-RT patients were 100% and 96% respectively. Longer follow-up is needed for the cet-RT patients, but with such few events, it is unlikely that the long-term cure rates will be statistically different from the chemo-RT patients. Taking into account comparable cure rates, the relative toxicity must be assessed to know if the newer treatment, in this case cet-RT, provides any advantage.
In the initial report by Bonner et al (22), no differences in acute and early toxicities between cetuximab-radiation and radiation alone were reported, with the exception of cetuximab induced skin rash. Cetuximab appeared to have an excellent toxicity profile, similar to RT alone, as well as similar QOL (28). This contrasts with publications implicating concurrent chemotherapy in long-term dysphagia (24,25). While encouraging, this study had no chemoradiotherapy arm for comparison, did not distinguish between head and neck disease sites, and no objective swallowing function tests were administered. Other, smaller studies suggested a higher rate of toxicity following cet-RT (26). Therefore, whether or not cetuximab-RT is equivalent to or better than chemo-RT with regard to late toxicities, HRQOL, and objective dysphagia, remain unknown, and are addressed in the current study.
The standard chemotherapy for head and neck cancer patients including HPV+ disease, supported by level 1 evidence, is high dose cisplatin (100 mg/m2 every 3 weeks). The chemotherapy in our institutional phase II trials was weekly carboplatin and paclitaxel, with the hope that the addition of a taxane would improve outcomes. Several studies have demonstrated the equivalence of high dose cisplatin (Q3 weeks) and weekly regimens, including carboplatin regimens, with regard to patient outcome with less toxicity for patients treated weekly (27,28). While the older RTOG studies, such as 1016, used concurrent high-dose cisplatin in the chemotherapy arm, more recent NRG/RTOG studies have used weekly low-dose cisplatin (protocols RTOG 1221 and 1216) as they are widely used in the community and reduce toxicity. This may especially be true for patients with HPV+ disease. In our experience of HPV+ patients treated with carboplatin and paclitaxel, there was a 95% local control rate and more than 80% survival at 3 years (4), and the patients in the current trial had 100% disease control rate. High dose cisplatin is likely overtreatment in this patient population and it is possible that a weekly regimen will be considered standard of care in the near future. Thus, our weekly concurrent chemotherapy regimen is relevant to the current experience in the community.
In recently treated patients, doses to the contralateral submandibular gland were limited to 30–39 Gy whenever possible, based on recent data (30,31). All patients had their pharyngeal constrictor mean dose limited to 50 Gy as this was seen as a crucial cut-off in investigations of swallowing function and QOL by us and others (12,16,32,33). Additionally, in recent years we limited the glottic larynx, esophagus and inferior constrictor to 20 Gy as none of the patients had low neck lymphadenopathy). Consequently, doses to the normal structures, including contralateral SMG, inferior pharyngeal constrictors and oral cavity were lower in the more recently treated cet-RT group compared to the chemo-RT group. This reduced dose to the pharyngeal constrictors and oral cavity was significantly correlated with a reduction in duration of mucositis, though not its maximum severity or the rate of grade 3 toxicity. For the long term toxicities and QOL, however, the differences in dose between groups were on the shallow end of the normal tissue complication probability (NTCP) curves (18, 33) and are expected to be clinically indistinguishable. Indeed, there was no significant difference in most outcomes besides xerostomia. Differences in xerostomia outcomes were the result of efforts to decrease doses to the contralateral submandibular gland and non-involved oral cavity (containing minor salivary glands), benefiting the more recently treated cet-RT group. Doses to these organs appear to contribute to xerostomia in our study as well as others (22,29,30,34,35). Additionally, we adjusted for these mean doses in our mixed models analysis. We included these dose terms linearly and that it is possible that the true effect of dose on the outcome is non-linear. However, with our limited sample size we are not able to model more complex relationships. From our analysis, it appears the improved in toxicity outcomes in the cet-RT group was from mean dose reduction to OARs.
Our study focused on whether there was a statistical difference in outcomes between the groups attributable to the systemic therapy and we found none. Had we found one, we would have been more concerned as to whether the differences seen surpassed the threshold of minimal clinically important difference (MCID). In the metaanalysis of Binenbaum et al (34) evaluating the MCID of several QOL measurements including the UWQOL, the mean MCID for various patient reported QOL domains ranged from 8–18 points. It is therefore reasonable to extrapolate to our data that 10% change in outcomes can be used as the minimal clinically significant difference. The unadjusted data in Table 2 reveal several outcomes that may be clinically important. However, the adjusted analysis in Table 3, model 2 reveals no significant differences between groups and also does not reveal any difference in outcomes greater than 5%. Therefore, we do not think our study reveals any possible MCID between the two cohorts. Longer follow up and validation of our findings are needed.
Additional caveats to this study include the small number of patients in each group as well as the single arm, non-randomized nature of each of the two studies. Also, the follow-up for the cet-RT patients is relatively short. With failure free survival >95%, the long-term functional outcomes of these patients is crucial. Longer follow up in patients receiving chemo-RT aiming to reduce xerostomia and dysphagia showed that at median f/u of 6.5 years, no functional deterioration was observed compared to the initial 2 year f/u assessments (35). Longer follow-up will help determine whether similar functional stability is observed after cetuximab-RT.
In summary, these results suggest that cetuximab-RT patients had no significant improvements in objective or subjective measurements of outcome through 1 year post-therapy that can be attributed to the use of cetuximab instead of chemotherapy. While longer follow up is needed, these data question the use of concurrent cetuximab instead of concurrent chemotherapy as a strategy for treatment de-intensification. Other trials of de-intensifications using lower doses or protons are underway and have recently been reviewed (36).
Supplementary Material
Highlights.
We compared functional outcome of matched patients with good-prognosis oropharyngeal cancer [HPV (+), remote or no smoking] treated on prospective studies of 1) chemo-RT and 2) cetuximab-RT.
Longitudinal evaluations of swallowing by videofluoroscopy, observer and patient-reported dysphagia, and QOL, were made in both arms from pre-therapy through 12 months.
No significant differences between the arms were observed.
These data do not support replacing chemotherapy with cetuximab for treatment de-intensification in these patients.
Acknowledgments
Supported by NIH grant PO1CA59827 and by the Newman Family Foundation
Footnotes
Conflict of interest statement: None of the authors has conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stuart E. Samuels, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
Yebin Tao, Department of Biostatistics, University of Michigan, Ann Arbor, MI.
Teresa Lyden, Department of Speech Pathology, University of Michigan, Ann Arbor, MI.
Marc Haxer, Department of Speech Pathology, University of Michigan, Ann Arbor, MI.
Matthew Spector, Department of Otolaryngology, University of Michigan, Ann Arbor, MI.
Kelly M. Malloy, Department of Otolaryngology, University of Michigan.
Mark E. Prince, Department of Otolaryngology, University of Michigan, Ann Arbor MI.
Carol R. Bradford, Department of Otolaryngology, University of Michigan
Francis P. Worden, Department of Medicine – Medical Oncology Division, University of Michigan.
Matthew Schipper, Department of Radiation Oncology and Biostatistics, University of Michigan, Ann Arbor, MI.
Avraham Eisbruch, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
References
- 1.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ang KK, Sturgis EM. Semin radiat oncol. United States: A 2012 Elsevier Inc; 2012. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma; pp. 128–42. [DOI] [PubMed] [Google Scholar]
- 3.O’Sullivan B, Huang SH, Siu LL, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–50. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 4.Vainshtein JM, Spector ME, McHugh JB, et al. Refining risk stratification for locoregional failure after chemoradiotherapy in human papillomavirus-associated oropharyngeal cancer. Oral Oncol. 2014;50:513–9. doi: 10.1016/j.oraloncology.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masterson L, Moualed D, Liu ZW, et al. De-escalation treatment protocols for human papillomavirus-associated oropharyngeal squamous cell carcinoma: A systematic review and meta-analysis of current clinical trials. Eur J Cancer. 2014;50:2636–2648. doi: 10.1016/j.ejca.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Clinicaltrails.Gov- rtog 1016: Phase iii trial of radiotherapy plus cetuximab versus chemoradiotherapy in hpv-associated oropharynx cancer. 2013 Editor, editor^editors. Book Clinicaltrails.Gov- rtog 1016: Phase iii trial of radiotherapy plus cetuximab versus chemoradiotherapy in hpv-associated oropharynx cancer. http://clinicaltrials.gov/ct2/show/NCT01302834.
- 7.Eu clinical trials register. De-escalate hpv: Determination of epidermal growth factor receptor-inhibitor (cetuximab) versus standard chemotherapy (cisplatin) early and late toxicity events in human papillomavirus-positive oropharyngeal squamous cell carcinoma. 2013 Editor, editor^editors. Book Eu clinical trials register. De-escalate hpv: Determination of epidermal growth factor receptor-inhibitor (cetuximab) versus standard chemotherapy (cisplatin) early and late toxicity events in human papillomavirus-positive oropharyngeal squamous cell carcinoma. https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-005165-21/GB.
- 8.Clinicaltrials.Gov-trog 12.01: A randomised trial of weekly cetuximab and radiation versus weekly cisplatin and radiation in good prognosis locoregionally advanced hpv associated oropharyngeal squamous cell carcinoma. 2013 Http://clinicaltrials.Gov/ct2/show/nct01855451. Editor, editor^editors. Book Clinicaltrials.Gov-trog 12.01: A randomised trial of weekly cetuximab and radiation versus weekly cisplatin and radiation in good prognosis locoregionally advanced hpv associated oropharyngeal squamous cell carcinoma. Http://clinicaltrials.Gov/ct2/show/nct01855451 2013.
- 9.Gillison ML, Zhang Q, Jordan R, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J Clin Oncol. 2012;30:2102–11. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer CA, Zlobec I, Green E, et al. Is the improved prognosis of p16 positive oropharyngeal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126:1256–62. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 11.Hunter KU, Fernandes LL, Vineberg KA, et al. Parotid glands dose-effect relationships based on their actually delivered doses: Implications for adaptive replanning in radiation therapy of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2013;87:676–82. doi: 10.1016/j.ijrobp.2013.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: Clinical and functional results. J Clin Oncol. 2010;28:2732–8. doi: 10.1200/JCO.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration-aspiration scale. Dysphagia. 1996;11:93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 14.JA L. Evaluation and treatment of swallowing disorders. 2. Austin, TX: Pro-Ed; 1983. [Google Scholar]
- 15.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by imrt? Int J Radiat Oncol Biol Phys. 2004;60:1425–39. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 16.Eisbruch A, Kim HM, Feng FY, et al. Chemo-imrt of oropharyngeal cancer aiming to reduce dysphagia: Swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011;81:e93–9. doi: 10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gluck I, Feng FY, Lyden T, et al. Evaluating and reporting dysphagia in trials of chemoirradiation for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2010;77:727–33. doi: 10.1016/j.ijrobp.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terrell JE, Nanavati KA, Esclamado RM, et al. Head and neck cancer-specific quality of life: Instrument validation. Arch Otolaryngol Head Neck Surg. 1997;123:1125–32. doi: 10.1001/archotol.1997.01900100101014. [DOI] [PubMed] [Google Scholar]
- 19.Eisbruch A, Kim HM, Terrell JE, et al. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- 20.Hassan SJ, Weymuller EA. Assessment of quality of life in head and neck cancer patients. Head Neck. 1993;15:485–96. doi: 10.1002/hed.2880150603. [DOI] [PubMed] [Google Scholar]
- 21.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 22.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21–8. doi: 10.1016/S1470-2045(09)70311-0. [DOI] [PubMed] [Google Scholar]
- 23.Petrelli F, Coinu A, Riboldi V, et al. Concomitant platinum-based chemotherapy or cetuximab with radiotherapy for locally advanced head and neck cancer: A systematic review and meta-analysis of published studies. Oral Oncol. 2014;50:1041–8. doi: 10.1016/j.oraloncology.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal DI, Mendoza TR, Fuller CD, et al. Patterns of symptom burden during radiotherapy or concurrent chemoradiotherapy for head and neck cancer: A prospective analysis using the university of texas md anderson cancer center symptom inventory-head and neck module. Cancer. 2014;120:1975–84. doi: 10.1002/cncr.28672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen NP, Frank C, Moltz CC, et al. Analysis of factors influencing aspiration risk following chemoradiation for oropharyngeal cancer. Br j radiol England. 2009:675–80. doi: 10.1259/bjr/72852974. [DOI] [PubMed] [Google Scholar]
- 26.Pryor DI, Porceddu SV, Burmeister BH, et al. Enhanced toxicity with concurrent cetuximab and radiotherapy in head and neck cancer. Radiother oncol Ireland. 2009:172–6. doi: 10.1016/j.radonc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Dobrosotskaya IY, Bellile E, Spector ME, et al. Weekly chemotherapy with radiation versus high-dose cisplatin with radiation as organ preservation for patients with hpv-positive and hpv-negative locally advanced squamous cell carcinoma of the oropharynx. Head Neck. 2014;36:617–23. doi: 10.1002/hed.23339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilkins AC, Rosenfelder N, Schick U, et al. Equivalence of cisplatin and carboplatin-based chemoradiation for locally advanced squamous cell carcinoma of the head and neck: A matched-pair analysis. Oral Oncol. 2013;49:615–9. doi: 10.1016/j.oraloncology.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Samuels SE, Vainshtein J, Spector ME, et al. Impact of retropharyngeal adenopathy on distant control and survival in hpv-related oropharyngeal cancer treated with chemoradiotherapy. Radiother Oncol. 2015;116:75–81. doi: 10.1016/j.radonc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Little M, Schipper M, Feng FY, et al. Reducing xerostomia after chemo-imrt for head-and-neck cancer: Beyond sparing the parotid glands. Int J Radiat Oncol Biol Phys. 2012;83:1007–14. doi: 10.1016/j.ijrobp.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murdoch-Kinch CA, Kim HM, Vineberg KA, et al. Dose-effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:373–82. doi: 10.1016/j.ijrobp.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dirix P, Abbeel S, Vanstraelen B, et al. Dysphagia after chemoradiotherapy for head-and-neck squamous cell carcinoma: Dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2009;75:385–92. doi: 10.1016/j.ijrobp.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 33.Feng FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: Early dose-effect relationships for the swallowing structures. Int J Radiat Oncol Biol Phys. 2007;68:1289–98. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 34.Binenbaum Y, Amit M, Billan S, et al. Minimal clinically important differences in quality of life scores of oral cavity and oropharynx cancer patients. Ann Surg Oncol. 2014;21:2773–81. doi: 10.1245/s10434-014-3656-z. [DOI] [PubMed] [Google Scholar]
- 35.Vainshtein JM, Moon DH, Feng FY, et al. Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus-related oropharyngeal cancer. Int J Radiat Oncol Biol Phys. 2015;91:925–33. doi: 10.1016/j.ijrobp.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuels SE, Eisbruch A, Beitler JJ, et al. Management of locally advanced hpv-related oropharyngeal squamous cell carcinoma: Where are we? Eur Arch Otorhinolaryngol. 2015 doi: 10.1007/s00405-015-3771-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.