Abstract
Intramyocardial injection of various injectable hydrogel materials has shown benefit in positively impacting the course of left ventricular (LV) remodeling after myocardial infarction (MI). However, since LV remodeling is a complex, time dependent process, the most efficacious time of hydrogel injection is not clear. In this study, we injected a relatively stiff, thermoresponsive and bioabsorbable hydrogel in rat hearts at 3 different time points - immediately after MI (IM), 3 d post-MI (3D), and 2 w post-MI (2W), corresponding to the beginnings of the necrotic, fibrotic and chronic remodeling phases. The employed left anterior descending coronary artery ligation model showed expected infarction responses including functional loss, inflammation and fibrosis with distinct time dependent patterns. Changes in LV geometry and contractile function were followed by longitudinal echocardiography for 10 w post-MI. While all injection times positively affected LV function and wall thickness, the 3D group gave better functional outcomes than the other injection times and also exhibited more local vascularization and less inflammatory markers than the earlier injection time. The results indicate an important role for injection timing in the increasingly explored concept of post-MI biomaterial injection therapy and suggest that for hydrogels with mechanical support as primary function, injection at the beginning of the fibrotic phase may provide improved outcomes.
Keywords: cardiac tissue engineering, injectable materials, myocardial infarction, hydrogel, intervention timing
Introduction
The loss of functional myocardium after a myocardial infarction (MI) results in a rapid increase in loading conditions, causing a pattern of progressive remodeling that includes ventricular dilation and the formation of a discrete collagenous scar that generally coincides with a thinned ventricular wall [1]. While MI can lead to sudden death by arrhythmic and mechanical effects, individuals who survive the initial event often experience deteriorating cardiac function and progress toward end stage heart failure and its associated low survival rates [2].
A feedback loop between high ventricular wall stress driving pathological wall thinning, and wall thinning further raising local wall stress is believed to set up as ischemic cardiomyopathy progresses [3]. To interrupt the mechanical aspects of this pathway, several biomaterials-based strategies have been devised. These strategies aim to provide mechanical support to the damaged ventricle, by acting as a barrier to further dilation, or to reduce ventricular wall stress by effectively increasing the area over which the force is applied. The latter can be achieved, for instance, by biomaterial injection to thicken the infarcted wall [4, 5], or by placement of a patch over the infarcted tissue [6]. In the former case, injection of natural or synthetic hydrogel materials has been a methodology pursued by several researchers, with beneficial effects reported for various types of biomaterials including fibrin gel, hyaluronic acid-based hydrogels, and poly(N-isopropylacrylamide)-based thermally responsive hydrogels [5, 7–13]. Hydrogel injection therapy is attractive in that supporting materials can be delivered minimally invasively [14], avoiding surgical intervention. Furthermore, growth factors, drugs and cells might be delivered with the hydrogels to alleviate inflammation and promote tissue repair [5, 15–17]. Hydrogel injection therapy has recently progressed to clinical trials [18].
Ventricular wall remodeling after MI involves a complex series of interconnected processes including myocyte apoptosis and necrosis, acute and chronic inflammation, extracellular matrix degradation, and the elaboration of new fibrotic tissue [19]. With the onset and abatement of these different phenomena, the mechanical properties of the remodeling ventricular wall vary as well [20]. The remodeling process has been identified with three consecutive phases featuring alterations in both wall structure and mechanical behavior. In the necrotic phase, beginning a few hours after MI, the passive wall mechanical properties are influenced by the onset of edema. In the fibrotic phase, a rapid increase in fibroblasts and collagen deposition occurs. In the long-term remodeling phase, infarct stiffness gradually decouples from collagen content and correlates more with collagen crosslinking [21]. Since the primary function of hydrogel injection is to reduce the mechanical load on the LV wall, and the injected materials interfere with pathological events, it is hypothesized that the timing of injection significantly influences the therapeutic outcome and would thus be critical in designing a successful intervention.
The objective of this study was to examine the effect of injection timing for the injection of a thermoresponsive hydrogel in a setting where direct comparisons could be made between histological and functional parameters and where the material injection behavior would not vary substantially between groups. A relatively stiff, biodegradable hydrogel, poly(NIPAAm-co-HEMA-co-MAPLA; where HEMA = 2-hydroxyethyl methacrylate, and MAPLA = methacrylate-polylactide) [22], was injected into the infarcted ventricular wall immediately after and at 3 d and 2 w following MI to correspond with the beginning of the necrotic, fibrotic and chronic remodeling phases, respectively. Follow up through ten weeks post-MI was chosen to allow evaluation of the chronic therapeutic effects. Together with the analysis of control rats, the resulting data were expected to provide guidance for assessing when biomaterial injection approaches might be expected to provide a maximal contribution to the sustenance of cardiac function.
Materials and Methods
Materials
All chemicals were purchased from Sigma-Aldrich unless otherwise stated. N-isopropylacrylamide (NIPAAm) was purified by recrystallization from hexane and vacuum-dried. 2-Hydroxyethyl methacrylate (HEMA) was purified by vacuum distillation. Lactide, benzoyl peroxide (BPO), sodium methoxide (NaOCH3), methacryloyl chloride and other solvents were used as received.
Synthesis of poly(NIPAAm-co-HEMA-co-MAPLA)
Poly(NIPAAm-co-HEMA-co-MAPLA) was synthesized from NIPAAm, HEMA and MAPLA by free radical polymerization as previously described [22]. The feed ratios of NIPAAm, HEMA and MAPLA were 80/10/10. Monomers (0.066 mol) were dissolved in 180 mL of 1,4-dioxane containing 0.23 g BPO. The polymerization was carried out at 75°C for 20 h under argon protection. The copolymer was precipitated in hexane and further purified by precipitation from THF into diethyl ether and vacuum-dried, with yields of ~80%. The copolymer was then dissolved in PBS at 15 wt% at 4°C to form a viscous hydrogel solution. Fluorescently labeled copolymers were synthesized using the same reaction conditions with fluorescein O-methacrylate added at a feed ratio of an additional 2%, with all of the other monomer molar ratios remaining constant.
Animal model
10–12 week old adult female syngeneic Lewis rats (Harlan Sprague Dawley Inc., Indianapolis, IN, USA) weighing 200-F250 g were used for this study. The research protocol followed the National Institutes of Health guidelines for animal care and was approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Rats were divided into 3 injection treatment groups (immediately after MI (IM), 3 d after MI (3D) and 2 w after MI (2W)) and 2 control groups (healthy and MI without treatments) to study the short- and long-term effects of MI and hydrogel injection. MI with saline injection immediately after MI served as an additional control for cytokine quantification.
Left ventricular infarction and hydrogel injection
Left ventricular infarction was created by ligation of the proximal left anterior descending (LAD) coronary artery as previously described [23]. Briefly, rats were anesthetized with 3.0% isoflurane inhalation with 100% oxygen followed by intubation and respiratory support with a rodent volume-controlled mechanical ventilator (683 Ventilator, Harvard Apparatus, Holliston, MA, USA) at a tidal volume of 3 mL and 80 breaths/min. Rats were placed in the right decubitus position, and the chest was shaved and prepared with povidone-iodine solution. Procedures were performed in a sterile environment on a heating blanket. The heart was exposed through a 4th left thoracotomy with electrocardiogram monitoring. The proximal LAD coronary artery was ligated with 7-0 polypropylene. The incision was closed in layers with 5-0 polypropylene continuous sutures. The animals were allowed to recover from anesthesia and returned to their cages.
For the IM group, this injection occurred within 30 min of LAD ligation. Visual identification of infarction was used to include rats with large infarction sites as prolonged general anesthesia for echocardiography can cause death for infarcted rats. A total of 100 μL of poly(NIPAAm-co-HEMA-co-MAPLA) solution in PBS (15 wt%) was injected into the apical, proximal, lateral, and septal wall regions bordering the infarct as well as into the center of the infarct (5 injections, 20 μL per region). The volume of hydrogel injected was arrived at based on the authors’ experience performing biomaterial injections into this rat model, generally seeking to maximize injection volumes, but recognizing that hydrogel regurgitation will increasingly occur when too large of injection volumes are attempted. To increase the injection volume of hydrogel and achieve a more even distribution of hydrogel in the myocardium, the hydrogel was injected into 5 injection sites in and around the infarction area. The incision was closed in layers with 4-0 silk continuous sutures. For rats in the 3D and 2W groups, the rats were anesthetized and evaluated with echocardiography two days after MI to measure the infarct size in terms of the percentage of scar area (akinetic or dyskinetic regions) to LV free wall area. Only rats with infarction >25% of the LV free wall were included. At the time of injection the infarcted anterior surface of the rat heart was exposed through a left thoracotomy and the hydrogel was injected as described above.
Time points
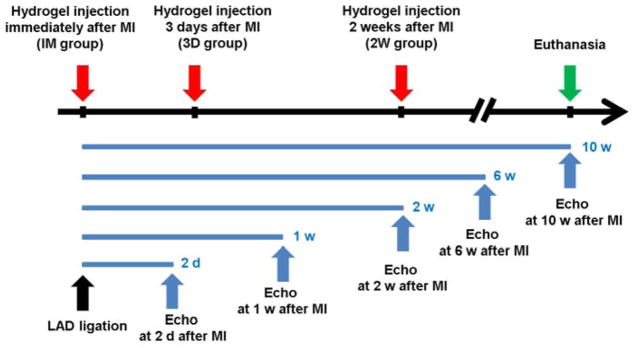
Rats in the treatment groups and the MI group were sacrificed within 2 w (short-term) and 10 w (long-term) after MI. Figure 1 shows the key time points for the experiments.
Figure 1.
Timeline of study.
Short-term: Rats with MI without hydrogel injections were assessed with echocardiography (n=10) and Masson trichrome & immunohistochemical staining (n=12) throughout the first 2 weeks after MI before sacrifice. Rats in the MI control group were sacrificed 30 min, 3 d, 1 w and 2 w after MI. 3 rats each from the IM group, 3D group and MI with saline injection were sacrificed 1 w after MI for acute immunohistochemical staining and cytokine quantification.
Long-term: 46 rats were used for the longer-term cardiac function and histology follow-up study: 12 rats for the IM group, 10 rats for 3D group, 14 rats for 2W group, and 10 rats for MI control group.
Echocardiography
Rats were anesthetized with 1.25–1.5% isoflurane inhalation with 100% oxygen. Standard transthoracic echocardiography was performed using the Acuson Sequoia C256 system with 13-MHz linear ultrasonic transducer (15L8; Acuson Corporation, Mountain View, CA, USA) in a phased array format. B-mode measurements on the LV short axis view (papillary muscle level) were performed. The end-diastolic (EDA) and end-systolic (ESA) LV internal cavity areas were measured by tracing the endocardial border. M-mode tracing images were also recorded from the same short axis view. The LV fractional area change (%FAC) was estimated as %FAC=[(EDA−ESA)/EDA]×100%. LV end-systolic volume (LVESV) and LV end-systolic volume (LVEDV) were estimated using the following formula of Teichholz: LV volume=7.0/(2.4+D)×D3 (D=diameter, measured by M-mode echocardiography at end-systolic or end-diastolic cardiac phase). LV ejection fraction (LVEF)=[(LVEDV−LVESV)/LVEDV]×100. Echocardiography and offline tracing processing were done by the operators, who were blinded to the treatment assignment.
Histology and immunohistochemistry
Rats were anesthetized, and the heart was exposed and arrested at the diastolic phase by injection into the apex of 2 mL of a hypothermic arresting solution including 10 U/mL of heparin, 68 mM NaCl, 1 M KCl, 36 mM NaHCO3, 2.0 mM MgCl2, 1.4 mM Na2SO4, 11 mM dextrose, and 30 mM 2,3-butanedione monoxime. The hearts were explanted and fixed in 2% paraformaldehyde for 2 h at 4°C and then embedded with optimal cutting temperature compound (Tissue-Tek, Torrance, CA, USA) followed by freezing at −80°C. Embedded, frozen LV tissues were serially sectioned at 8 μm in the LV transverse direction at the center of gel injected area and mounted on glass microscope slides. Sections of each heart were fixed in 4% paraformaldehyde, blocked with staining buffer for 1 h (2% goat serum in PBS) at room temperature, and incubated with mouse monoclonal antibody against CD68 (1:100, AbD Serotec, Oxford, UK) as a pan-macrophage marker, rabbit polyclonal antibody against alpha smooth muscle actin (α-SMA; 1:200, Abcam, Cambridge, MA, USA), rabbit polyclonal antibody against neutrophil elastase (1:200 Abcam), mouse monoclonal antibody against CD31(1:100 Abcam). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Vectashield, Burlingame, CA, USA). Sections that were stained with only the secondary antibody were used as negative controls. Slides were examined with an Olympus IX51 microscope and images captured using DP2-BSW software (Olympus America Inc., Center Valley, PA, USA). For each retrieved sample, 10 different microscopic fields at 200× magnification were photographed for α-SMA. To determine quantity of vessels or arterioles, the number of α-SMA-positive structures was measured using ImageJ at 200× magnification. Vessels were identified as tubular structures positively stained for α-SMA and CD31. Arterioles are defined α-SMA and CD31-positive structure, having visible lumen, and more than 10 μm in diameter. α-SMA-positive area was measured within infarcted area and this parameter includes not only clustered regions of α-SMA-positive tissue but also endocardial α-SMA-positive area. All measurements and assessments were performed using ImageJ.
Determination of infarction size, scar area, and LV anterior wall thickening
The cross-sectional surface after Masson’s trichrome staining was digitally photographed at the level of center of injection sites. Infarction size was defined as a percentage of the sum of the epicardial and endocardial infarct circumference divided by the sum of the total LV epicardial and endocardial circumferences. Scar area was measured as an infarction scar area using computer-based planimetry. LV anterior wall thickness was expressed as follows: scar area/[(epicardial circumference + endocardial circumference)/2]. Measurement of each parameter (n=6 each group) was performed using ImageJ.
Quantification of TNF-α, IL-1β and IL-6 in infarcted LV wall
The heart was explanted, quickly frozen in liquid nitrogen and stored at −80°C. 1 piece of sample was cut from the center of infarcted LV with the injected hydrogel. Sample was dissolved with cOmplete Lysis-M (Roche Diagnostics Corporation, Indianapolis, IN, USA) and the total protein content was measured with Protein BCA Protein Assay Kit (Thermo Fisher Scientific Inc., Pittsburgh, PA, USA). TNF-α, IL-6 and IL-1β amounts in the dissolved sample were measured with corresponding ELISA assay kits (TNF-α: ab100785; IL-6: ab100772; IL-1β: ab100768, Abcam). The measured TNF-α, IL-6 and IL-1β amounts were then divided by total protein content to give the expression level for the detected proteins.
Statistical analyses
Statistical analyses were performed using OriginPro 8. Results are presented as mean ± standard error of the mean. For the short-term effects of MI on cardiac function, a one-way repeated measures analysis of variance (ANOVA) followed by Bonferroni testing of specific differences was employed. Two-way ANOVA followed by Tukey’s test was applied for long-term cardiac function comparisons between treatment groups. One-way ANOVA followed by Tukey’s test was applied for all multi-group comparisons in the study. Differences were considered to be statistically significant at p<0.05.
Results
Short-term changes in LV after MI
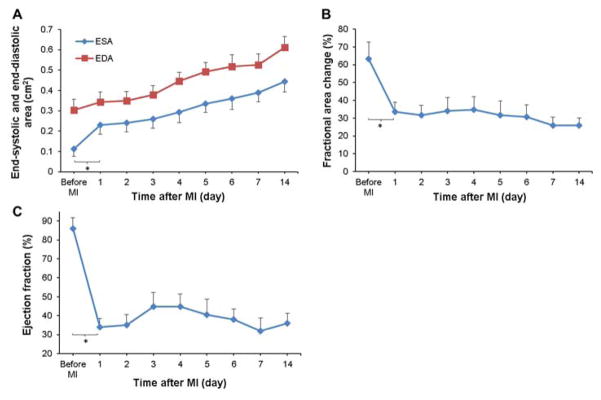
Cardiac function was followed during the first 2 weeks after MI. End-systolic and end-diastolic area increased significantly with time (Figure 2A). After an initial drop in fractional area change (%FAC) and ejection fraction (EF) within the first day after MI, both indicators generally remained stable except for a slight rebound in EF at day 3 followed by a deteriorating trend (Figure 2B,C).
Figure 2.
Short-term cardiac functional changes after MI measured by sequential echocardiography. Changes in (A) end-systolic area (ESA) and end-diastolic area (EDA), (B) fractional area change (%FAC), and (C) ejection fraction (EF) were determined temporally. For all of the parameters presented, a significant effect of time was observed.
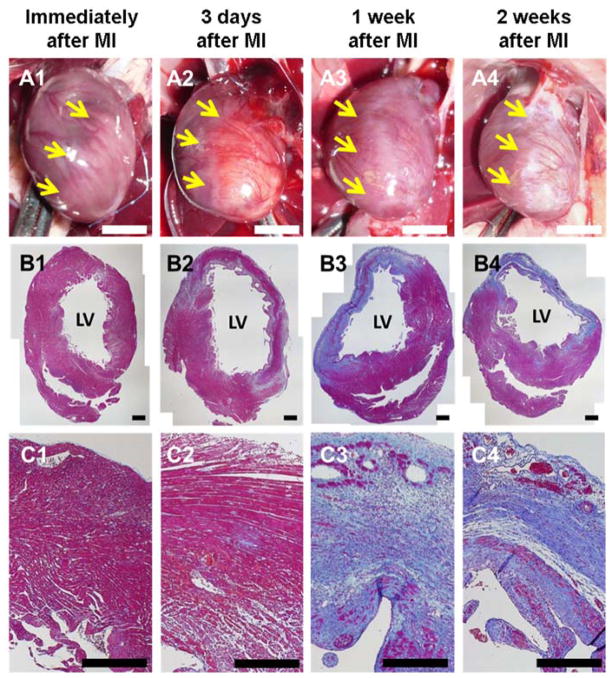
As shown in Figure 3A, the infarcted region of the LV was visually apparent 30 min after MI, and edema could be observed macroscopically at 3 d after MI. At 2 w after MI the infarcted region could be clearly differentiated visually. Histologically, no significant morphological changes were observed 30 min after MI (Figure 3B1,C1). At 3 d post-infarction, regions of the LV wall had lost cardiac muscle and tissue edemic effects were apparent (Figure 3B2). Only a small portion of cardiac muscle remained in the infarcted LV wall 1 w after MI, with a substantial increase in collagenous tissue (Figure 3B3,C3). Almost the entire infarction zone was occupied by fibrotic tissue 2 w after MI, and the LV wall was significantly thinner than the healthy counterpart (Figure 3B4,C4).
Figure 3.
Short-term histological changes after MI. Representative macroscopic images and Masson’s trichrome stained cross-sections of rat hearts: (A1, B1 and C1) immediately after MI, (A2, B2 and C2) 3 days after MI, (A3, B3 and C3) 1 week after MI and (A4, B4 and C4) 2 weeks after MI. LV: left ventricle. Yellow arrows: border between healthy tissue and the infarcted zone. Scale bar: 5 mm (white) and 500 μm (black). (Image quality improved)
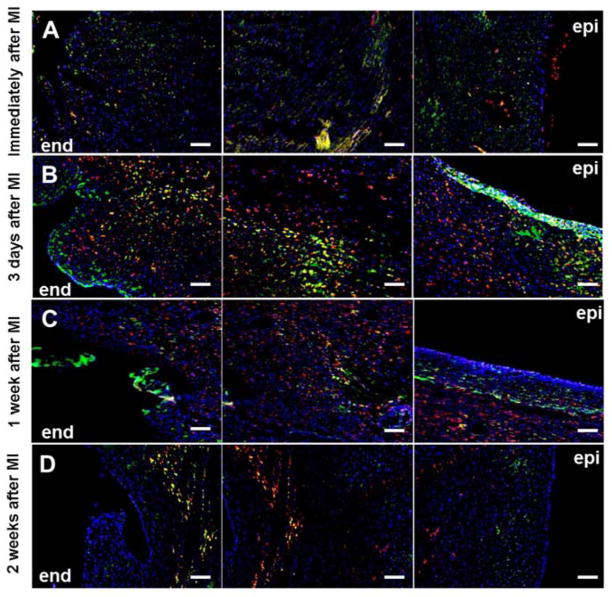
The infiltration of neutrophils and macrophages into the infarcted LV was characterized by staining marker proteins neutrophil elastase and CD68, respectively. As shown in Figure 4A, neutrophils responded rapidly and appeared in the LV myocardium shortly after the ischemic event with a transmural distribution at the 3 d time point. The neutrophil infiltrate was markedly reduced at the later 1 and 2 w time points (Figure 4B–D). Few macrophages were present in the infarction area shortly after MI (Figure 4A), while a greater macrophage infiltrate was observed at 3 d and a similar level of macrophage infiltration was maintained at least until the end of the first week after MI (Figure 4B,C). Fewer macrophages were seen to reside in the LV at 2 w after MI (Figure 4D).
Figure 4.
Short-term fluctuation in neutrophil and macrophage infiltration in infarcted LV. Green: neutrophil elastase, red: CD68, blue: nuclei. Each row from left to right: endocardial side (end) to epicardial side (epi). Scale bar=100 μm.
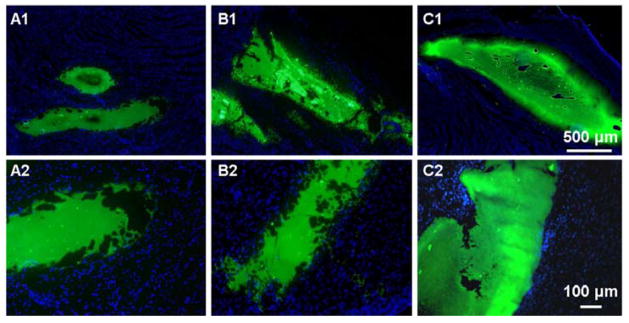
Distribution of injected hydrogel in the infarcted LV
At 3 d, 1 w and 2 w after MI, hydrogel that was fluorescently labeled by the incorporation of a green fluorescent monomer during polymer synthesis was injected into the infarction zone and rats were sacrificed within 30 min after the injections [24]. As shown in the tissue sections of Figure 5, the injected hydrogel formed distinct volumes in the LV wall. Clear boundaries between the injected hydrogel and the tissue could be observed in all 3 groups: no cell nuclear staining was apparent in the hydrogel region and hydrogel spreading into the peripheral tissue was not apparent, despite the diversity of composition and structure of the infarcted LV wall at different times following MI.
Figure 5.
Hydrogel morphology and distribution in infarcted LV right after injection. Hydrogels injected (A1,2) immediately after MI (IM), (B1,2) 3 days after MI (3D), (C1,2) 2 weeks after MI (2W). Green: hydrogel, blue: nuclei.
Zoomed out images added
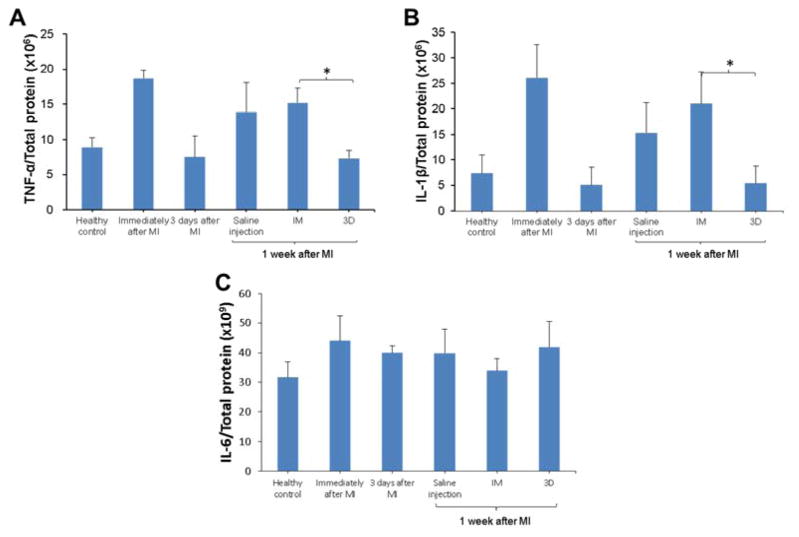
Effect of MI and hydrogel injection on the expression of inflammatory cytokines
The expression of inflammatory cytokines TNF-α, IL-1β and IL-6 during the first week after MI was measured by ELISA in LV samples with hydrogel injection. Compared to the saline-injected MI control immediately after MI or at 1 w after MI, hydrogel injection immediately after MI (IM) did not alter the expression level of TNF-α, IL-1β or IL-6. No significant effect was found for hydrogel injection 3 d after MI (3D), either. However, waiting 3 days for injection resulted in lower levels of TNF-α and IL-1β compared to injecting immediately after MI (Figure 6A,B).
Figure 6.
Effect of time and hydrogel injection on the expression of TNF-α, IL-1β and IL-6 in infarcted LV. * indicates significant differences between groups.
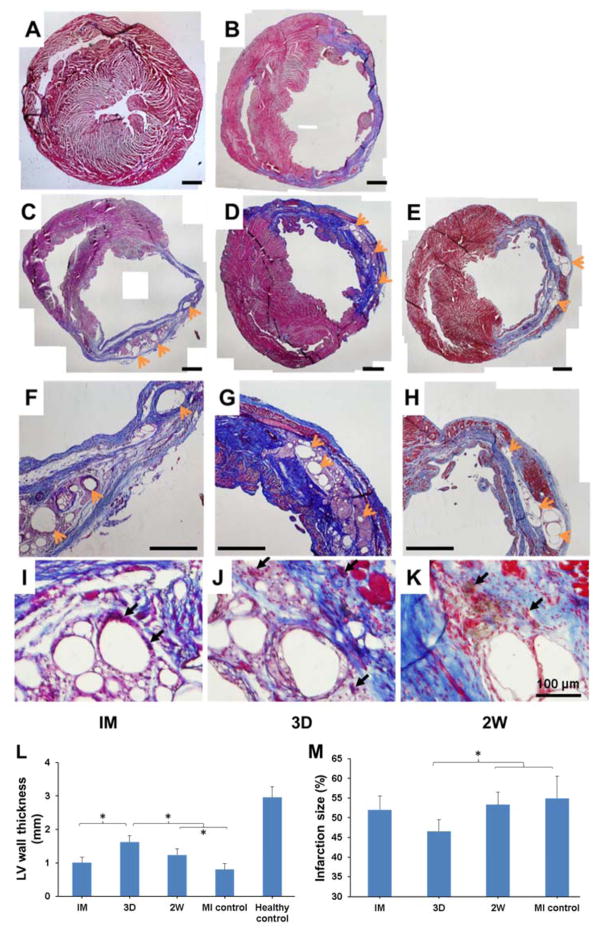
Long-term effect of injecting hydrogel at different time points
As shown in Figure 7, scar tissue rich in collagen could be found in all hydrogel injection groups and the MI control 10 w after MI. The hydrogels in all injection groups had separated into smaller regions. The remaining hydrogel was encompassed by tissue consistent with a foreign body response including multinucleate foreign body giant cells and collagen deposition. In terms of the wall thickness, the 3D and 2W groups showed a beneficial effect over non-treatment MI control with a thicker LV wall, with the outcome of injecting 3 d after MI (3D) being significantly more favorable between the two injection times (Figure 7L). 3D group also showed a smaller infarction size compared to MI control and 2W group (Figure 7M). In contrast, hydrogel injection immediately after MI (IM) failed to maintain the thickness of LV wall or limit the infarction size, making its therapeutic effect inferior to the other 2 injection times. In addition, more muscle-like tissue can be seen in the LV from 3D group and 2W group comparing to IM group.
Figure 7.
Ventricular wall histology for rat hearts 10 w after MI. Representative Masson’s trichrome stained cross-sections: (A) Healthy control, (B) MI control, (C, F, I) IM group, (D, G, J) 3D group, (E, H, K) 2W group. A–H scale bars=1 mm. Orange arrows point to the hydrogel residues, black arrows point to foreign body giant cells. Wall thickness (L) and infarction size (M) were measured from the complete set of these images. * indicates significant differences between groups.
I–K: added data; L: significant differences corrected; image quality improved
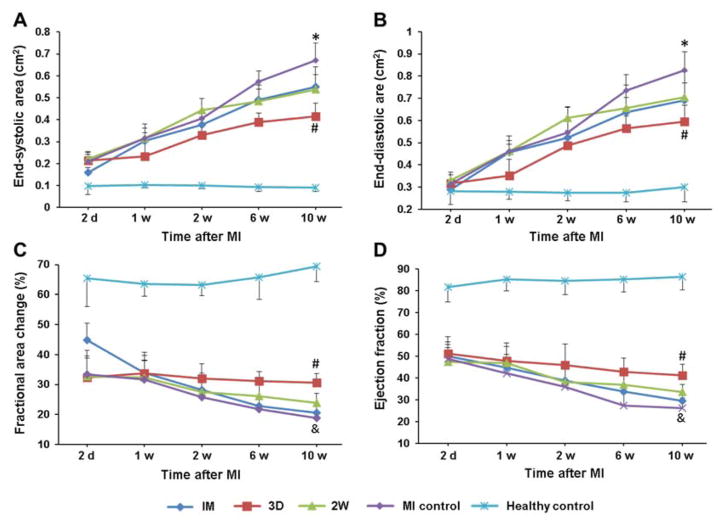
Echocardiographic functional assessment yielded similar trends at 10 w post-MI. ESA and EDA of the MI control group had significantly increased over time compared to the healthy control, a typical sign of pathological LV dilation (Figure 8A,B). All 3 hydrogel injection groups showed beneficial effects in preventing LV dilation to some extent, among which the 3D group was more effective than the other 2 hydrogel injection groups. As indicated by %FAC and EF, the pumping function of the heart showed the expected deterioration as a result of MI, with partial preservation of pumping by injecting hydrogels at 3 d or 2 w post-MI, but not injecting immediately after MI (Figure 8C,D). Again, the 3D group showed statistically superior cardiac function preservation among the three hydrogel injection groups, which was also confirmed by fractional shortening assessment (Supplemental Figure 2).
Figure 8.
Echocardiographic assessment of long-term cardiac functions: (A) End-systolic area (ESA), (B) End-diastolic area (EDA), (C) Fractional area change (%FAC), (D) Ejection fraction (EF). #indicates significant differences between 3D group versus other injection groups and MI control group. *indicates significant differences between MI control versus all injection groups. &indicates significant differences between MI control versus the 3D and 2W groups.
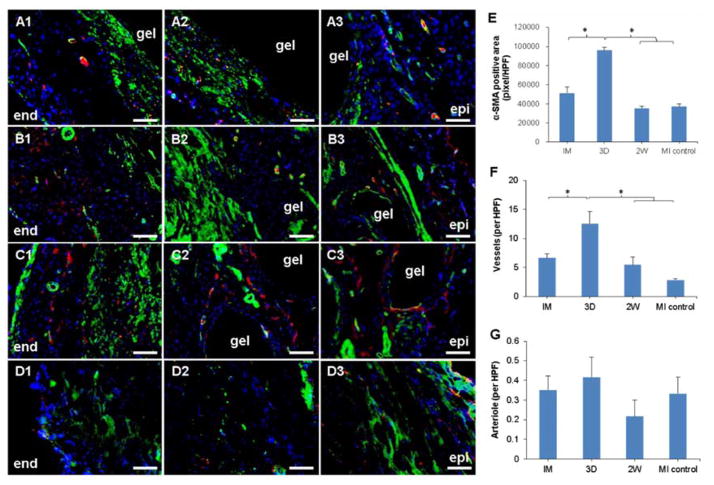
Consistent with the previous qualitative observation of more muscle-like tissue in the Masson’s trichrome stained sections from the 3D group comparing to IM and 2W groups, larger α-SMA positive area was found in immunohistochemical stained sections from the 3D group, as shown in Figure 9A–E. More α-SMA positive area was located near the endocardial side of the LV and underlying the injection sites. Vasculature structures could be found in sections from all hydrogel injection groups, among which the 3D group contained a significantly greater number of vessels in the infarction zone (Figure 9F).
Figure 9.
α-SMA (green), CD31 (red) and nuclear (blue) staining 10 weeks after MI. (A1–A3) IM group, (B1–B3) 3D group, (C1–C3) 2W group, (D1–D3) MI control. Each row from left to right: endocardial side (end) to epicardial side (epi). Scale bar=100 μm. (E) α-SMA-positive area in the infarction zone. (F, G) Density of α-SMA-positive vessels and arterioles in the infarction zone. * indicates significant differences.
D1–D3: added data; MI control in E–G: added data; image quality improved
Discussion
In clinical practice, reperfusion therapy is critical to eligible MI patients. According to AHA (American Heart Association) guideline, reperfusion therapy should be administered to all eligible patients with ST segment elevation myocardial infarction (STEMI) with symptom onset within the prior 12 h [25]. Primary percutaneous coronary intervention (PCI) is the recommended method of reperfusion for STEMI patients when it can be performed in a timely fashion, which is 90 min or less after initial medical contact [26]. Given the minimally invasive nature of PCI, qualified patients can be discharged in 30 h. Minimally invasive intramyocardial hydrogel injections could be delivered transendocardially (PCI) or transepicardially (catheter through a subxiphoid incision). A catheter equipped with a cooled injection line might be an effective means by which to keep the hydrogel in the liquid, injectable phase prior to delivery into the warm ventricular wall. Theoretically, either route does not have the risks associated with thoracotomy and could be in conjunction with reperfusion PCI or in a separate procedure at some point after PCI. In addition, diagnostic data given by MRI could provide the location and size of infarction, providing the basis for personalized injection strategies.
The theoretical mechanism by which hydrogel injection therapy is beneficial to the infarcted ventricular wall is by increasing the wall thickness and thus decreasing the wall stress in the myocardium, including the infarction zone and remote healthy areas [4]. Using MRI-derived diastolic strain data in a FE model, Dorsey et al. demonstrated that hydrogel injection increased infarct stiffness throughout a 12 week study when compared to saline-treated controls [27]. The mechanical load reduction may influence the biological pathways which dynamically regulate tissue necrosis and healing in a favorable way. Increased LV wall stress as a result of dilation is a powerful stimulus for intracellular signaling transduced by mechanoreceptors [1]. High wall stress activates of the local tissue renin-angiotensin system (RAS), which leads to the up-regulation of angiotensin II (Ang II). Ang II elevation augments cardiovascular inflammation through the production of inflammatory mediators like TNF-a, IL-6 and IL-1β; it also enhances subsequent fibrosis via matrix metalloproteinase (MMP)-mediated degradation of the extracellular matrix and production of collagen I and III [28–30]. In addition to mechanical effects, hydrogel injection also induces a local foreign body response which may interact with the time dependent signaling related to cardiac remodeling. In particular, this response may be associated with increased neovascularization and an extended period over which macrophages are recruited to, and are present in, the region of the infarct. In considering the results, it is assumed that the predominant effect of the injected hydrogel for this study is mechanical. For weaker hydrogels, or hydrogels with imparted bioactivity through loaded bioactive agents, this may not be the case and other pathways may dominate over those for this particular hydrogel.
Despite the considerable number of studies in the field of hydrogel injection therapy for MI, the influence of injection time is not well understood. A variety of times have been selected to pursue the intervention, making it difficult to draw broad conclusions. The most common times evaluated in the literature range from immediately after to 2 w post-MI [10, 12, 31–37], while a rationale for injection time selection is seldom provided. Consideration of morbidity and mortality from multiple thoracotomies and experimental expediency likely plays into the “immediately after” choice. For injections 1 to 2 w post-MI, an advantage is that the animal’s condition can be stabilized prior to the injection procedure. A number of cases showing beneficial impacts on LV remodeling have been reported for both time choices among various models involving different hydrogel materials and animal models [31, 33, 34, 36–38].
Two prior studies have each compared two time points in a rat model. Landa et al. showed that alginate gel injection was effective in positively altering LV remodeling in both recent and older infarcts (injected 1 w and 8 w after MI) [7]. However, since the time points at which the final assessments were performed were different, it is difficult to directly compare the effects of the 2 injection times. Kadner et al. directly compared the efficacies of different injection times. An MMP-sensitive polyethylene glycol (PEG) based hydrogel was injected into the rat LV immediately after and 1 w after MI. Beneficial functional effects in the 4 week study were observed only in the 1 w group, possibly due to the diffused form and fast degradation observed for this PEG hydrogel when injected immediately after MI [40]. In contrast, in the current study the injected hydrogels showed a consistent form after injection and appeared to exhibit similar degradation rates, but this is only based on qualitative observations of remaining hydrogel volume at the time of tissue recovery. Furthermore, the time points evaluated in the current study correspond with the distinct remodeling phases in rat ventricular remodeling after MI. In the mouse model, and using a non-synthetic, collagen-based material, Blackburn et al. recently reported injections at one time point in the necrotic phase (3 h) or one of two time points near the end of the fibrotic phase (1 or 2 w) [41]. They found that collagen injections at 3 h and 1 w after MI partially preserved cardiac function 4 w after treatment, and the 3 h injection favorably interacted with the early inflammatory response. However, this study also lacked of direct comparisons between consistent end points, since the final assessment time after MI was variable, based on the changing injection times. The distribution and absorption of injected collagen was also not followed.
The intramyocardial distribution and degradation of injected hydrogel did not appear to vary despite the obvious changes in the structure of the cardiac injection substrate at the different injection times. This might be related to the rapid sol-gel transition of hydrogel upon injection and the hydrolysis dominated degradation mechanism. These properties better allowed the investigation of the injection time effect alone, minimizing the interactive influence from inconsistent hydrogel behavior. For the wide variety of hydrogels employed in injection therapy studies, the permeability, modulus and degradation profile have in some cases been shown to have a significant, sometimes decisive influence on the efficacy of treatment [3, 39, 40]. Although deriving a universal conclusion covering all types of hydrogel injection candidates is not possible, the results should provide guidance for those hydrogels emphasizing a mechanical effect.
In this study we were guided by the temporal pattern of physiological reactions after MI in determining a more favorable, clinically relevant injection time. As characterized in the first part of the study, transmural infarction over a major portion of the LV was confirmed and a sequence of well-recognized events post-MI were identified, including the rapid decay of cardiac function immediately after MI, massive infiltration of inflammatory cells peaking 3 d after MI, and subsidence of acute inflammation around 2 w post-MI. Although it was anticipated that these events would occur according to the classical timeline described in the prior literature, it was confirmed that the chosen injection time points correlated to the cardiac remodeling stages post-MI in the chosen model (permanent ligation, no reperfusion). The responses to MI in human are similar to those in rats, and can also be divided into 3 phases, although the fibrotic phase begins at about 1 week post-MI in humans, as opposed to 3–5 days in rats [21].
In terms of preserving both long-term cardiac function and LV geometry, the 3D group was more effective than the 2W group, while the IM group showed no beneficial outcome compared to MI control. These results indicate that hydrogel injection at the end of the necrotic phase/beginning of the fibrotic phase rather than in the necrotic phase or in the remodeling phase is most likely to have the highest efficacy. The lack of efficacy of hydrogel injection immediately after MI has been previously reported with different hydrogels and animal models [32, 35], raising questions as to the interpretation of efficacy data from immediate intervention models in the literature. The IM injection time occurs when the LV myocardium is still temporarily intact in structure and viable cardiomyocytes are being challenged by low oxygen supply immediately after the ischemic event [42]. Locally injecting a hydrogel into this milieu may represent a secondary injury to the tissue and cells. In addition, the original purpose of hydrogel injection as a mechanical support could be attenuated by interstitial edema which stiffens the LV during the first several days after MI [43]. The hydrogel will also stimulate a foreign body response, regardless of when it is injected. The presence of this response in the early necrotic phase may stimulate inflammatory activity that is additive and detrimental, given the tissue substrate present at this time point.
Entering the fibrotic phase at 3 d, collagen deposition gradually dominates the mechanical behavior of infarcted LV [44]. The content of collagen I and III begins to increase and continues to increase for at least 3 weeks in rats [32]. Hydrogel injection at the beginning of the fibrotic phase may avoid the drawbacks of injecting immediately after MI and influence the infiltrated inflammatory cells and approaching wave of fibroblasts in a way which eventually alters the deposition of collagen fibers and LV remodeling. The relatively higher level of inflammatory factors after the first week for the IM group shows that injecting immediately after MI may interfere with subsequent fibrotic reactions in a negative way. In addition, after ischemic injury, fibroblasts can differentiate into myofibroblasts and the latter express α-SMA which peaks within 2 weeks after MI and decreases as scar tissues mature [45]. While it is possible that the α-SMA positive cells are myofibroblasts, it is worth noting that similar studies in the rat MI model where an epicardial patch was placed post-infarction similarly noted areas enriched in α-SMA positive cells [6, 46]. In one report evaluating these cells for other expression patterns it was found that, unlike myofibroblasts, several contractile smooth muscle markers (caldesmon, calponin, SM 22, and SMMHC-2) were present [6]. In a subsequent report these α-SMA cells were also shown to positively stain for sarcomeric α-actinin and cardiac-specific troponin-T as well as cardiac transcription factors Nkx-2.5 and GATA-4, in a manner that was consistent with that seen in developing embryonic myocardium [46]. These results, if translated to the biomaterial injection model, would suggest a non-myofibroblastic character for the α-SMA positive cells and caution against a direct conclusion that these cells are myofibroblasts. Without further evaluation in this model, the conclusions that can reached are limited, however it appears likely that the α-SMA cells, regardless of their characteristics, are contributory to the increased wall thickness observed with biomaterial injection.
Two weeks after MI, cardiac functions are substantially lower, few cardiomyocytes are left in the infarction zone, acute responses have diminished, and the LV has entered the remodeling phase and is dilated. Therefore, hydrogel injection 2 weeks after MI will miss the acute phases and can only affect the remodeling process. The 3D injection point led to the lowest infarction size among all groups, indicating an influence towards a less fibrotic healing response, and more local vascularization, suggesting a less responsive tissue substrate for later injection time points. The increased vascularization may have aided the remodeling process to better preserve local tissue resulting in a thicker LV wall and better preserved functionality.
In this study we focused on evaluating the differences between the different potential times of hydrogel injection, with the inclusion of one long-term control group without hydrogel or saline injection. However, since the injection procedure itself may cause an inflammatory response and affect the remodeling process, a group with saline injection alone could be considered for control purposes. Such a control was performed in a previous report from our group using the same rat MI model, and it was shown that saline injection did not provide improved functional outcomes versus hydrogel injection [5]. Since confirming the therapeutic benefit of hydrogel injection is not the primary objective of this study, and to reduce the number of animals utilized, further saline injection control experiments were not conducted.
Apart from the results presented in this study indicating that hydrogel injection at the beginning of the fibrotic phase is more beneficial than immediately after MI or at the beginning of the remodeling phase in a rat model, several limitations should be noted. Although one can speculate (as above), the mechanisms behind the improved outcomes associated with injection at the beginning of the fibrotic phase are unclear. Second, the size of the rat heart model means that the delivery techniques used and the overall amount of hydrogel injected would vary substantially from that needed for the human. A large animal model would better capture these effects, although the general course of cardiac remodeling would follow the same temporal trends. Finally, only three injection time points were studied, together with the non-injection control group. Although these time points were selected to correspond to distinct transitions in the remodeling pathway post-MI, including a higher frequency of injection times might indicate more effective times between the 3D and IM or 2W groups.
Conclusion
In this study, the effect that timing of thermoresponsive hydrogel injections had on cardiac remodeling and function post-MI was examined. Without injection, the first week after MI was associated with marked pathological and morphological changes and acute inflammation. Injection at the beginning of the fibrotic phase (3 d after MI) was found to be superior to injection immediately after MI or injection in the remodeling phase (2 w after MI) in terms of minimizing LV dilation and preserving cardiac function. Although associated with one particular type of hydrogel, this result provides guidance in defining the most efficacious time for hydrogel injection after MI and characterizes the associations between different intervention times and their impact on the ventricular remodeling process post-MI.
Supplementary Material
Acknowledgments
This study was supported by the National Institutes of Health (grant R01 HL105911). The authors thank Deanna Rhoads and Lori Walton for their excellent help with tissue histological assessment and Neil Turner for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sutton MGSJ, Sharpe N. Left Ventricular Remodeling After Myocardial Infarction: Pathophysiology and Therapy. Circulation. 2000;101:2981–8. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 2.Akhmedov AT, Marin-Garcia J. Myocardial regeneration of the failing heart. Heart Fail Rev. 2013;18:815–833. doi: 10.1007/s10741-012-9348-5. [DOI] [PubMed] [Google Scholar]
- 3.Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges. Acta Biomater. 2011;7:1–15. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006;114:2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 5.Nelson DM, Hashizume R, Yoshizumi T, Blakney AK, Ma Z, Wagner WR. Intramyocardial injection of a synthetic hydrogel with delivery of bFGF and IGF1 in a rat model of ischemic cardiomyopathy. Biomacromolecules. 2014;15:1–11. doi: 10.1021/bm4010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, et al. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49:2292–300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 8.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–9. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–48. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tous E, Ifkovits JL, Koomalsingh KJ, Shuto T, Soeda T, Kondo N, et al. Influence of injectable hyaluronic acid hydrogel degradation behavior on infarction-induced ventricular remodeling. Biomacromolecules. 2011;12:4127–35. doi: 10.1021/bm201198x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tous E, Purcell B, Ifkovits J, Burdick J. Injectable Acellular Hydrogels for Cardiac Repair. J Cardiovasc Transl Res. 2011;4:528–42. doi: 10.1007/s12265-011-9291-1. [DOI] [PubMed] [Google Scholar]
- 12.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, et al. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–68. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cittadini A, Monti MG, Petrillo V, Esposito G, Imparato G, Luciani A, et al. Complementary therapeutic effects of dual delivery of insulin-like growth factor-1 and vascular endothelial growth factor by gelatin microspheres in experimental heart failure. Eur J Heart Fail. 2011;13:1264–74. doi: 10.1093/eurjhf/hfr143. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Shi J, Wang Y, Yin Y, Wang L, Liu J, et al. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials. 2014 doi: 10.1016/j.biomaterials.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 17.Eckhouse SR, Purcell BP, McGarvey JR, Lobb D, Logdon CB, Doviak H, et al. Local Hydrogel Release of Recombinant TIMP-3 Attenuates Adverse Left Ventricular Remodeling After Experimental Myocardial Infarction. Sci Transl Med. 2014;6:223ra21. doi: 10.1126/scitranslmed.3007244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rane AA, Christman KL. Biomaterials for the treatment of myocardial infarction: a 5-year update. J Am Coll Cardiol. 2011;58:2615–29. doi: 10.1016/j.jacc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Jugdutt BI, Dhalla NS. Cardiac Remodeling: Molecular Mechanisms. Springer-Verlag; 2013. [Google Scholar]
- 20.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 21.Holmes JW, Borg TK, Covell JW. Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng. 2005;7:223–53. doi: 10.1146/annurev.bioeng.7.060804.100453. [DOI] [PubMed] [Google Scholar]
- 22.Ma Z, Nelson DM, Hong Y, Wagner WR. Thermally responsive injectable hydrogel incorporating methacrylate-polylactide for hydrolytic lability. Biomacromolecules. 2010;11:1873–81. doi: 10.1021/bm1004299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashizume R, Hong Y, Takanari K, Fujimoto KL, Tobita K, Wagner WR. The effect of polymer degradation time on functional outcomes of temporary elastic patch support in ischemic cardiomyopathy. Biomaterials. 2013;34:7353–63. doi: 10.1016/j.biomaterials.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Jiang H, Ye S-H, Yoshizumi T, Wagner WR. Tailoring the degradation rates of thermally responsive hydrogels designed for soft tissue injection by varying the autocatalytic potential. Biomaterials. 2015;53:484–93. doi: 10.1016/j.biomaterials.2015.02.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 26.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012 doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 27.Dorsey SM, McGarvey JR, Wang H, Nikou A, Arama L, Koomalsingh KJ, et al. MRI evaluation of injectable hyaluronic acid-based hydrogel therapy to limit ventricular remodeling after myocardial infarction. Biomaterials. 2015;69:65–75. doi: 10.1016/j.biomaterials.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lahera V, Cachofeiro V, de Las Heras N. Interplay of hypertension, inflammation, and angiotensin II. Am J Hypertens. 2011;24:1059. doi: 10.1038/ajh.2011.142. [DOI] [PubMed] [Google Scholar]
- 29.Haudek SB, Cheng J, Du J, Wang Y, Hermosillo-Rodriguez J, Trial J, et al. Monocytic fibroblast precursors mediate fibrosis in angiotensin-II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2010;49:499–507. doi: 10.1016/j.yjmcc.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bodiga S, Zhong JC, Wang W, Basu R, Lo J, Liu GC, et al. Enhanced susceptibility to biomechanical stress in ACE2 null mice is prevented by loss of the p47(phox) NADPH oxidase subunit. Cardiovasc Res. 2011;91:151–61. doi: 10.1093/cvr/cvr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada M, Payne TR, Oshima H, Momoi N, Tobita K, Huard J. Differential efficacy of gels derived from small intestinal submucosa as an injectable biomaterial for myocardial infarct repair. Biomaterials. 2010;31:7678–83. doi: 10.1016/j.biomaterials.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 32.Ravi S, Caves JM, Martinez AW, Xiao J, Wen J, Haller CA, et al. Effect of bone marrow-derived extracellular matrix on cardiac function after ischemic injury. Biomaterials. 2012;33:7736–45. doi: 10.1016/j.biomaterials.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon SJ, Fang YH, Lim CH, Kim BS, Son HS, Park Y, et al. Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel. J Biomed Mater Res B Appl Biomater. 2009;91:163–71. doi: 10.1002/jbm.b.31386. [DOI] [PubMed] [Google Scholar]
- 34.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, et al. Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction. J Am Coll Cardiol. 2012;59:751–63. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita M, Ishihara M, Morimoto Y, Simizu M, Saito Y, Yura H, et al. Efficacy of photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 in a rabbit model of chronic myocardial infarction. J Surg Res. 2005;126:27–33. doi: 10.1016/j.jss.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Wu J, Zeng F, Huang XP, Chung JC, Konecny F, Weisel RD, et al. Infarct stabilization and cardiac repair with a VEGF-conjugated, injectable hydrogel. Biomaterials. 2011;32:579–86. doi: 10.1016/j.biomaterials.2010.08.098. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Zhang X, Li Y, Ma Y, Zhang Y, Liu Z, et al. Improved myocardial performance in infarcted rat heart by co-injection of basic fibroblast growth factor with temperature-responsive Chitosan hydrogel. The J Heart Lung Transplant. 2010;29:881–7. doi: 10.1016/j.healun.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Lin YD, Yeh ML, Yang YJ, Tsai DC, Chu TY, Shih YY, et al. Intramyocardial peptide nanofiber injection improves postinfarction ventricular remodeling and efficacy of bone marrow cell therapy in pigs. Circulation. 2010;122:S132–41. doi: 10.1161/CIRCULATIONAHA.110.939512. [DOI] [PubMed] [Google Scholar]
- 39.Kadner K, Dobner S, Franz T, Bezuidenhout D, Sirry MS, Zilla P, et al. The beneficial effects of deferred delivery on the efficiency of hydrogel therapy post myocardial infarction. Biomaterials. 2012;33:2060–6. doi: 10.1016/j.biomaterials.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 40.Rane AA, Chuang JS, Shah A, Hu DP, Dalton ND, Gu Y, et al. Increased infarct wall thickness by a bio-inert material is insufficient to prevent negative left ventricular remodeling after myocardial infarction. PloS one. 2011;6:e21571. doi: 10.1371/journal.pone.0021571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blackburn NJ, Sofrenovic T, Kuraitis D, Ahmadi A, McNeill B, Deng C, et al. Timing underpins the benefits associated with injectable collagen biomaterial therapy for the treatment of myocardial infarction. Biomaterials. 2015;39:182–92. doi: 10.1016/j.biomaterials.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Kenneth Mallory G, White PD, Salcedo-Salgar J. The speed of healing of myocardial infarction: A study of the pathologic anatomy in seventy-two cases. Am Heart J. 1939;18:647–71. [Google Scholar]
- 43.Reimer KA, Jennings RB. The changing anatomic reference base of evolving myocardial infarction. Underestimation of myocardial collateral blood flow and overestimation of experimental anatomic infarct size due to tissue edema, hemorrhage and acute inflammation. Circulation. 1979;60:866–76. doi: 10.1161/01.cir.60.4.866. [DOI] [PubMed] [Google Scholar]
- 44.Gupta KB, Ratcliffe MB, Fallert MA, Edmunds LH, Jr, Bogen DK. Changes in passive mechanical stiffness of myocardial tissue with aneurysm formation. Circulation. 1994;89:2315–26. doi: 10.1161/01.cir.89.5.2315. [DOI] [PubMed] [Google Scholar]
- 45.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–9. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujimoto KL, Tobita K, Guan J, Hashizume R, Takanari K, Alfieri CM, et al. Placement of an elastic biodegradable cardiac patch on a subacute infarcted heart leads to cellularization with early developmental cardiomyocyte characteristics. J Card Fail. 2012;18:585–95. doi: 10.1016/j.cardfail.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.