Abstract
Thirty (1E,4E,6E)-1,7-diaryl-1,4,6-heptatrien-3-ones, featuring a central linear trienone linker and two identical nitrogen-containing heteroaromatic rings, were designed and synthesized as curcumin-based anticancer agents on the basis of their structural similarity to the enol-tautomer of curcumin, in addition to taking advantage of the possibly enhanced pharmacokinetic profiles contributed by the basic nitrogen-containing heteroaromatic rings. Their cytotoxicity and antiproliferative activity were evaluated towards both androgen-dependent and androgen-independent prostate cancer cell lines, as well as HeLa human cervical cancer cells. Among them, the ten most potent analogues are 5- to 36-fold more potent than curcumin in inhibiting cancer cell proliferation. The acquired structure-activity relationship data indicate (i) that (1E,4E,6E)-1,7-diaryl-1,4,6-heptatrien-3-ones represent a potential scaffold for development of curcumin-based agents with substantially improved cytotoxicity and anti-proliferative effect; and (ii) 1-alkyl-1H-imidazol-2-yl and 1-alkyl-1H-benzo[d]imidazole-2-yl serve as optimal heteroaromatic rings for increased in vitro potency of this scaffold. Two of most potent compounds displayed no apparent cytotoxicity toward MCF-10A normal mammary epithelial cells at 1 μM concentration. Treatment of PC-3 prostate cancer cells with the most potent compound led to appreciable cell cycle arrest at a G1/G0 phase and cell apoptosis induction.
Keywords: curcumin analogue; 1,7-diaryl-1,4,6-heptatrien-3-one; cytotoxicity; anti-proliferative activity
Graphical Abstract
1. Introduction
Curcumin, with (1E,6E)-1,7-bis(4-hydroxyl-3-methoxyphenyl)-1,6-heptadiene-3,5-dione as its IUPAC name, is a dietary natural product extracted from Curcuma longa of the Zingiberaceae family. Turmeric, the rhizomes of Curcuma longa, has long been used as a yellow spicy curry ingredient and as Ayuvedic, Chinese, and Hindu medicine. Curcumin was first reported to have anti-cancer potential in 1985 on the basis of in vitro cell-based and in vivo mice model experiments [1]. Its in vitro anti-prostate cancer activities were first explored in 2000 by Dorai et. al. [2]. The high safety profile of curcumin in humans has been validated by the Food and Drug Administration (FDA) in the USA [3,4]. The curcumin's mechanism of action as anticancer agents has been extensively investigated and known to be associated with its interacting with multiple signaling molecules within the cell [5]. Multiple clinical studies have been initiated for treatment of various human cancers [6-8]. However, its clinical advancement has been hampered by its moderate potency and extremely low bioavailability due to its poor water solubility and rapid in vivo metabolism. One phase I clinical study has verified its poor bioavailability, in which no curcumin plasma concentration was detected even though the oral dose was escalated to 450-3600 mg per day [9].
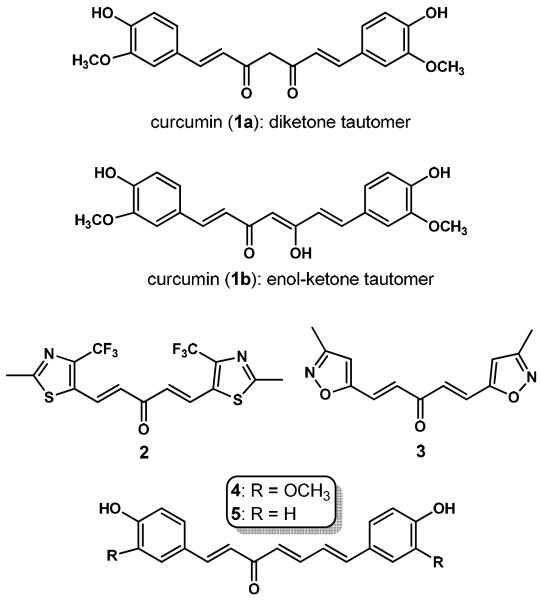
Structural modifications of curcumin may serve as a meaningful approach to discovering analogues with enhanced bioavailability and anticancer potential. Extensive research in this field has been conducted by several groups in search for effective curcumin-based anti-cancer agents with favorable safety profiles [10,11]. We have recently identified that (i) 1,5-diheteroarylpenta-1,4-diene-3-ones, exemplified by compounds 2 and 3 in Figure 1, serve as an optimal scaffold for developing potential curcumin-based anticancer agents due to their appreciably enhanced in vitro potency relative to curcumin; and (ii) (1E,4E)-1,5-bis(2-methyl-4-(trifluoromethyl)thiazol-5-yl)penta-1,4-dien-3-one (2) is a very promising lead compound because of its good in vitro potency and attractive in vivo pharmacokinetic profiles [12,13].
Figure 1.
Structures of curcumin and its analogues
In the present study, thirty 1,7-di-heteroaryl-1,4,6-heptatrien-3-ones (6-35, see Figure 2 and Table 1) that retained the 7-carbon spacer between the aromatic rings were designed as curcumin-based anticancer agents because of their similar shape and size as curcumin's enol-ketone tautomer in addition to having basic heteroaromatic scaffolds. As part of our ongoing project in search of effective curcumin-based chemotherapeutics, the aim of the present study was to investigate the in vitro cytotoxicity and antiproliferative activity of these 1,7-diheteroaryl-1,4,6-heptatrien-3-ones towards prostate and cervical cancer cells.
Figure 2.
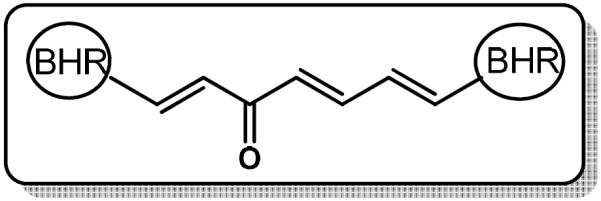
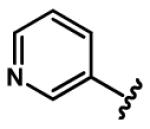
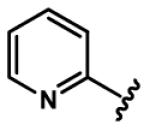
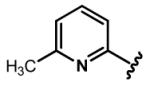
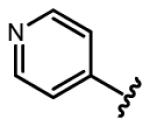
1,7-diheteroarylhepta-1,4,7-trien-3-ones (6-35). BHR: Basic nitrogen-containing heteroaromatic ring. For the specific structure of each BHR, refer to Table 1.
Table 1.
Structures of Basic Nitrogen-Containing Heteroaromatic Rings
| Compds | BHR | Compds | BHR |
|---|---|---|---|
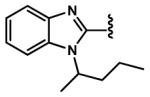
| 6, 36, 66, 96 |
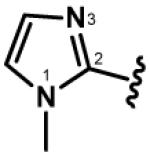
|
7, 37, 67,97 |
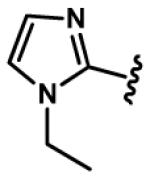
|
| 8, 38, 68, 98 |
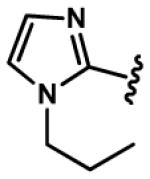
|
9, 39, 69, 99 |
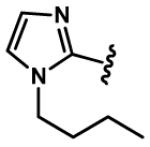
|
| 10, 40, 70, 100 |
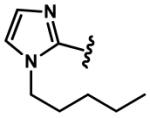
|
11, 41, 71, 101 |
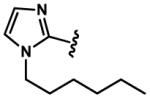
|
| 12, 42, 72, 102 |
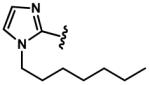
|
13, 43, 73, 103 |
|
| 14, 44, 74, 104 |
|
15, 45, 75, 105 |
|
| 16, 46, 76, 106 |
|
17, 47, 77, 107 |
|
| 18, 48, 78, 108 |
|
19, 49, 79, 109 |
|
| 20, 50, 80, 113 |
|
21, 51, 81, 114 |
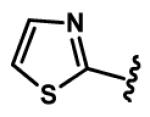
|
| 22, 52, 82, 115 |
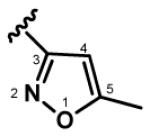
|
23, 53, 83, 116 |
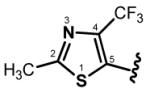
|
| 24, 54, 84, 117 |
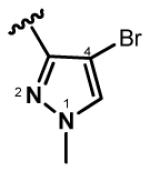
|
25, 55, 85, 118 |
|
| 26, 56, 86, 119 |
|
27, 57, 87, 120 |
|
| 28, 58, 88, 110 |
|
29, 59, 89, 121 |
|
| 30, 60, 90, 122 |
|
31, 61, 91, 123 |
|
| 32, 62, 92, 124 |
|
33, 63, 93, 125 |
|
| 34, 64, 94, 126 |
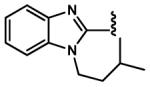
|
35, 65, 95, 127 |
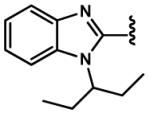
|
2. Results and Discussion
2.1 Design of Target Compounds
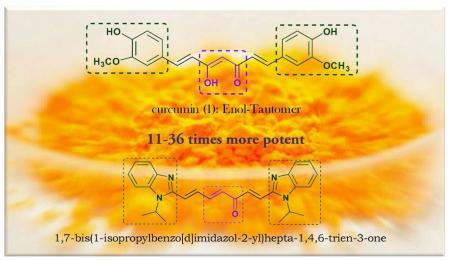
The structure of curcumin is characteristic of a central diketone moiety (1a) and two identical substituted phenyl groups. The central symmetric β-diketone moiety of curcumin in the solid state exists as the asymmetric keto-enol tautomer (1b), which has been confirmed by the X-ray crystallographic analysis in 1982 by Tonnesen et. al. [3]. The NMR data acquired by Payton and co-workers suggested the exclusive existence of the keto-enol tautomer (1b) in a diversity of solvents with various pH values ranging from 3 to 9 [14]. We envisioned that the (1E, 4E, 6E)-heptatrien-3-one motif might act as a good bioisostere of the keto-enol linker in curcumin because of the similar shape and size. So far, only two naturally-occurring curcumin analogues with trienone linker, 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,4,6-heptatrien-3-one (4) [15] and 1,7-bis(4-hydroxyphenyl)-1,4,6-heptatrien-3-one (5) [16], have been reported. It is worth noting that these two trienones have been isolated from Curcuma longa, the plant with curcumin as its major chemical component. These two natural trienones and a group of trienone analogues with two identical substituted phenyl groups have recently been evaluated by Chuprajob and co-workers for their cytotoxic activity against human oral cancer KB cell line [17]. This study indicated that the 1,7-diphenyl-1,4,6-trien-3-ones are more potent than curcumin towards oral cancer cells.
Consequently, thirty 1,7-di-heteroaryl-1,4,6-heptatrien-3-ones (6-35, see Figure 2 and Table 1) were designed as curcumin analogues because of their similar shape and size as curcumin's enol-ketone tautomer in addition to having basic heteroaromatic scaffolds. These curcumin analogues that possess two identical terminal nitrogen-containing heteroaromatic rings and a central trienone linear linker have been designed for synthesis and in vitro evaluation as potential anticancer agents towards prostate and cervical cancer cell lines. Our previous investigations on 1,5-diheteroarylpenta-1,4-diene-3-ones have demonstrated that replacement of substituted phenyl groups with nitrogen-containing heteroaromatic rings resulted in enhanced cytotoxic potency towards prostate and cervical cancer cells and better pharmacokinetic profiles. Among our designed 1,7-di-heteroaryl-1,4,6-heptatrien-3-ones, nineteen compounds contain 5-membered heteroaromatic scaffolds; five compounds contain 6-membered pyridine ring systems; and six compounds contain bulkier aromatic heterocycle scaffolds. All these target compounds are new except for one pyridine analogue --- 1,7-di-4-pyridinyl-1,4,6-heptatrien-3-one (28). This compound (CAS#: 121031-56-9) has been included in a patent regarding photopolymerization initiator compositions [18]. However, neither preparation nor any anti-cancer activity of this compound has been reported.
2.2 Chemistry
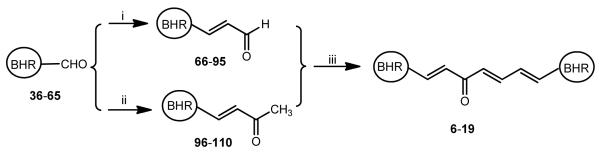
Fourteen curcumin analogues (6-19) containing a central trienone linker and two identical terminal 1-alkyl-1H-imidazol-2-yl groups have been synthesized through a sequence of two Wittig reactions and an aldol condensation reaction, as illustrated in Scheme 1. Specifically, they have been synthesized by an aldol condensation reaction of a (3E)-4-(BHR)-3-buten-2-one (96-110) with a (2E)-3-(BHR)-2-propenal (66-79) in the presence of sodium methoxide. (3E)-4-(BHR)-3-buten-2-ones (96-110) have been readily synthesized by Wittig reaction of the appropriate carboxaldehyde (36-49) with 1-(triphenylphosphoranylidene)-2-propanone in toluene under refluxing [19]. Similarly, (2E)-4-(BHR)-2-propenals (66-79) have been easily prepared via Wittig reaction of the appropriate carboaldehyde (36-49) with (triphenylphosphoranylidene)acetaldehyde at room temperature using DMF as solvent [20]. These reaction conditions can prevent the further Wittig reaction of the desired (2E)-4-(BHR)-2-propenals with (triphenylphosphoranylidene)acetaldehyde.
Scheme 1.
Synthesis of (2E)-3-aryl-2-propenals (66-95), (3E)-4-aryl-3-buten-2-ones (96-100), and 1,7-diheteroarylhepta-1,4,7-trien-3-ones (6-19). Reactants and conditions: (i) 2-(triphenylphnosphoranylidene)acetaldehyde, DMF, 25°C, 1-4 days; (ii) 1-(triphenylphosphoranylidene) propan-2-one, toluene, reflux 9 h; (iii) NaOCH3, CH3OH, 0 – 25 °C, 1 –12 h. BHR: Basic nitrogen-containing heteroaromatic ring. For the specific structure of each BHR, refer to Table 1.
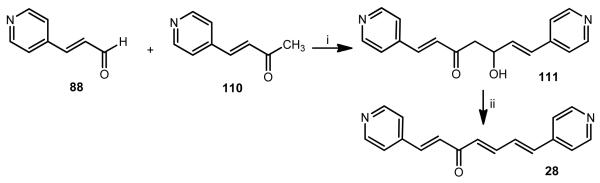
However, the synthesis of other 1,7-diaryl-1,4,6-heptatrien-3-ones (20-35) employing the same method was achieved in poor yields. Alternatively, compound 28 has been synthesized through an aldol addition reaction of (3E)-4-(pyridin-4-yl)-but-3-en-2-one (110) with (2E)-3-(pyridin-4-yl)-2-propenal (88) using LDA as base at −78°C, followed by dehydration of the generated aldol (Scheme 2) [21,22]. This dehydration reaction was achieved at room temperature for 20 days. Attempts to reach a more efficient elimination method were not successful so far.
Scheme 2.
Synthesis of 1,7-diheteroarylhepta-1,4,7-trien-3-one 28. Reactants and conditions: (i) LDA, THF, −78 °C; (ii) rt, 20 days.
After various explorations, the remaining 1,7-diaryl-1,4,6-heptatrien-3-ones (20-27, 29-35) were synthesized via the Horner-Wadsworth-Emmons reaction of (2E)-3-(BHR)-2-propenals (80-87, 89-95) with (E)-diethyl(2-oxo-4-heteroaryl-but-3-en-1-yl)phosphonates (113-127) using potassium carbonate as base (Scheme 3). The (E)-diethyl(2-oxo-4-heteroaryl-but-3-en-1-yl)phosphonates (113-127) were prepared by Horner-Wadsworth-Emmons reaction of 1 equivalent of 1,3-bis(diethylphosphonato) acetone with 1 equivalent of appropriate heteroarylformaldehydes (50-57, 59-65), using the reaction sequence illustrated in Scheme 3.
Scheme 3.
Synthesis of 1,7-diheteroarylhepta-1,4,7-trien-3-ones 20-27, 29-35. Reactants and conditions: (i) K2CO3, H2O-EtOH, 1h to overnight. BHR: Basic nitrogen-containing heteroaromatic ring. For the specific structure of each BHR, refer to Table 1.
2.3 Cytotoxicity towards prostate and cervical cancer cell lines
The in vitro cytotoxicity of 1,7-diaryl-1,4,6-heptatrien-3-ones (6-35) was determined using trypan blue dye exclusion assay (TB) against a panel of cancer cell lines (PC-3, DU145, LNCaP, and HeLa). Both PC-3 and DU145 cell lines are androgen-independent metastatic prostate cancer cell lines that cannot express prostate-specific antigen and functional androgen receptor [23,24]; while LNCaP cell line is androgen-dependent and is able to express prostate-specific antigen and functional androgen receptor [25]. They represent the most common cell-based models for in vitro assessment of potency and efficacy of anti-prostate cancer agents. Curcumin and DMSO were used as positive and negative control, respectively.
As shown in Table 2, with few exceptions, exposure of the cancer cells to the synthesized 1,7-diaryl-1,4,6-heptatrien-3-ones (6-35) at 1 and 10 μM concentrations decreases the viability of four cell lines. Ten (13, 14, 15, 17, 18, 30, 31, 32, 33, and 35) out of thirty test compounds demonstrated significantly improved ability to inhibit the growth of four cancer cell lines at both concentrations, as compared with curcumin. Nineteen compounds (6-12, 16, 19-25, 27-29, and 34) appeared to be slightly more effective than curcumin. Compound 26, with ortho pyridines as terminal aromatic rings, is apparently less effective than curcumin.
Table 2.
Cytotoxicity of 1,7-Diheteroarylhepta-1,4,6-trien-2-ones
| Compd | Inhibitory Rate (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| PC-3a | DU145b | LNCaPc | HeLad | |||||
|
| ||||||||
| 10 μM | 1 μM | 10 μM | 1 μM | 10 μM | 1 μM | 10 μM | 1 μM | |
| Curcumin | 55 | 2.5 | 59 | 7 | 66 | 29 | 50 | 14 |
| 6 | 83 | 35 | 66 | 21 | 55 | 41 | 93 | 18 |
| 7 | 85 | 32 | 84 | 34 | 62 | 23 | 98 | 43 |
| 8 | 91 | 23 | 84 | 25 | 95 | 38 | 98 | 34 |
| 9 | 87 | 33 | 91 | 35 | 92 | 47 | 92 | 44 |
| 10 | 86 | 36 | 88 | 43 | 94 | 44 | 95 | 39 |
| 11 | 85 | 11 | 86 | 34 | 76 | 31 | 98 | 32 |
| 12 | 92 | 28 | 93 | 25 | 70 | 12 | 99 | 13 |
| 13 | 92 | 48 | 86 | 46 | 79 | 69 | 97 | 62 |
| 14 | 86 | 47 | 88 | 52 | 81 | 67 | 95 | 62 |
| 15 | 86 | 60 | 93 | 77 | 79 | 29 | 94 | 83 |
| 16 | 89 | 32 | 88 | 32 | 75 | 33 | 95 | 31 |
| 17 | 92 | 56 | 87 | 65 | 89 | 74 | 95 | 85 |
| 18 | 95 | 70 | 82 | 55 | 88 | 58 | 97 | 87 |
| 19 | 87 | 35 | 75 | 0 | 70 | 38 | 94 | 66 |
| 20 | 86 | 25 | 46 | 8 | 57 | 21 | 96 | 20 |
| 21 | 86 | 28 | 65 | 2 | 80 | 28 | 94 | 11 |
| 22 | 88 | 25 | 79 | 25 | 70 | 13 | 97 | 17 |
| 23 | 88 | 24 | 80 | 13 | 92 | 37 | 96 | 12 |
| 24 | 91 | 25 | 86 | 42 | 63 | 38 | 95 | 52 |
| 25 | 86 | 24 | 87 | 24 | 61 | 2 | 97 | 20 |
| 26 | 20 | 17 | 37 | 11 | 7.2 | 0 | 24 | 21 |
| 27 | 91 | 35 | 75 | 12 | 57 | 50 | 94 | 40 |
| 28 | 84 | 33 | 81 | 60 | 50 | 20 | 95 | 22 |
| 29 | 84 | 19 | 81 | 36 | 66 | 31 | 94 | 26 |
| 30 | 88 | 80 | 77 | 57 | 77 | 52 | 89 | 95 |
| 31 | 89 | 67 | 79 | 42 | 48 | 5 | 97 | 76 |
| 32 | 82 | 86 | 75 | 73 | 72 | 54 | 95 | 96 |
| 33 | 87 | 75 | 89 | 64 | 76 | 10 | 95 | 68 |
| 34 | 93 | 58 | 97 | 21 | 60 | 15 | 97 | 53 |
| 35 | 89 | 83 | 75 | 56 | 64 | 45 | 94 | 89 |
Human androgen-insensitive prostate cancer cell line
Human androgen-insensitive prostate cancer cell line
Human androgen-sensitive prostate cancer cell line
Human aggressive cervical cancer cell line
2.4 Antiproliferative activity towards prostate and cervical cancer cell lines
To further determine the in vitro anticancer potency of the synthesized 1,7-diaryl-1,4,6-heptatrien-3-ones, ten compounds (13, 14, 15, 17, 18, 30, 31, 32, 33, and 35) with inhibitory rate greater than 45% at 1 μM toward three out of the four cancer cell lines were selected for WST-1 cell proliferation assay according to the procedure as described in the Experimental Section in three prostate and one cervical cancer cell lines. WST-1 is a water-soluble tetrazolium salt that can be converted to formazan catalyzed by the cellular mitochondrial dehydrogenases. Consequently, the amount of generated formazan dye directly correlates to the number of live cells in the culture. Curcumin was used as a positive control for comparison in the parallel experiments and the IC50 values were summarized in Table 3. These ten 1,7-diaryl-1,4,6-heptatrien-3-ones were suggested as promising anti-prostate and anti-cervical cancer agents by comparing their IC50 values with that of curcumin (Table 3). They are 10-36 times, 14-29 times, 5-13 times, and 9-34 times, respectively, more potent than curcumin towards PC-3, DU145, LNCaP, and HeLa cancer cell lines. This validates the promising scaffold containing two identical terminal basic nitrogen-containing heteroaromatic rings and a central linear trienone linker as novel curcumin mimics with promising cytotoxic and antiproliferative effects against cancer cells.
Table 3.
Anti-Proliferative Activity of Selected 1,7-diheteroarylhepta-1,4,6-trien-2-ones
| Compd | IC50 (μM)a | IC50 (curcumin) / IC50 (compd) | ||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| PC-3b | DU145c | LNCaPd | HeLae | PC-3b | DU145c | LNCaPd | HeLae | |
| Curcumin | 25.43 ± 2.15 | 26.23 ± 0.65 | 13.61 ± 2.69 | 12.11 ± 0.67 | 1 | 1 | 1 | 1 |
| 13 | 2.56 ± 0.14 | 1.62 ± 0.57 | 1.56 ± 0.12 | 0.98 ± 0.18 | 10 | 16 | 9 | 12 |
| 14 | 2.54 ± 0.10 | 1.82 ± 0.43 | 1.79 ± 0.63 | 1.04 ± 0.07 | 10 | 14 | 8 | 12 |
| 15 | 1.88 ± 0.32 | 1.07 ± 0.28 | 1.21 ± 0.21 | 0.82 ± 0.08 | 14 | 25 | 11 | 15 |
| 17 | 1.59 ± 0.37 | 0.94 ± 0.05 | 1.32 ± 0.14 | 0.75 ± 0.08 | 16 | 28 | 10 | 16 |
| 18 | 1.55 ± 0.04 | 0.91 ± 0.26 | 1.03 ± 0.11 | 0.71 ± 0.04 | 16 | 29 | 13 | 17 |
| 30 | 0.70 ± 0.08 | 0.91 ± 0.04 | 1.29 ± 0.51 | 0.36 ± 0.11 | 36 | 29 | 11 | 34 |
| 31 | 2.31 ± 0.35 | 1.61 ± 0.20 | 2.82 ± 0.09 | 1.35 ± 0.38 | 11 | 16 | 5 | 9 |
| 32 | 1.03 ± 0.07 | 1.01 ± 0.27 | 1.23 ± 0.52 | 0.82 ± 0.11 | 25 | 26 | 11 | 15 |
| 33 | 1.62 ± 0.11 | 1.85 ± 0.25 | 2.83 ± 0.80 | 1.11 ± 0.21 | 16 | 14 | 5 | 11 |
| 35 | 1.06 ± 0.08 | 1.09 ± 0.37 | 2.00 ± 0.45 | 1.02 ± 0.12 | 24 | 24 | 7 | 12 |
IC50 is the drug concentration effective in inhibiting 50% of the cell viability measured by WST-1 cell proliferation assay (WST-1) after 3 days exposure.
Human androgen-insensitive prostate cancer cell line
Human androgen-insensitive prostate cancer cell line
Human androgen-sensitive prostate cancer cell line
Human aggressive cervical cancer cell line
2.5 Structure-Activity Relationships
2.5.1. (1E, 4E, 6E)-1,7-diaryl-1,4,6-heptatrien-3-one scaffold
In prostate and cervical cancer cell models, among thirty (1E, 4E, 6E)-1,7-diaryl-1,4,6-heptatrien-3-ones (6-35), only compound 26 did not show increased or similar cytotoxicity as compared with curcumin (Table 2), implying that (1E, 4E, 6E)-1,7-diaryl-1,4,6-heptatrien-3-ones represent a promising scaffold for designing curcumin-based anticancer agents.
2.5.2. Terminal nitrogen containing heteroaromatic rings
-
(i)
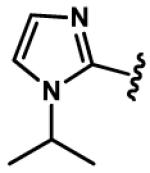
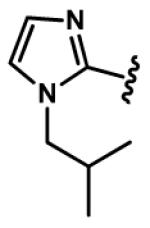
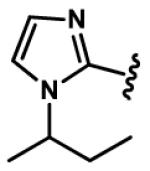
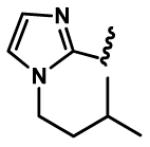
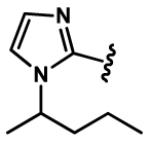
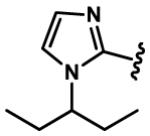
1-Alkyl-1H-imidazol-2-yl groups in compounds 6-19 represent the optimal terminal five-membered heteroaromatic rings for the potency in four cancer cell lines. α- and β-branched alkyl groups, such as isopropyl in compound 13, isobutyl in 14, sec-butyl in 15, pentan-2-yl in 17, and pentan-3-yl in 18, act as the most favorable alkyl groups on the nitrogen atom of 1H-imidazol-2-yl for the potency.
-
(ii)
Regarding six-membered heteroaromatic rings, no apparent effect can be bestowed by meta pyridyl (e.g. 25) and para pyridyl (e.g. 28 and 29). The ortho pyridyl (e.g. 26) abolishes the activity, but ortho pyridyl analogue 27 with an electron donating group (e.g. methyl) at para position is slightly more potent than curcumin.
-
(iii)
All six 1-alkyl-1H-benzo[d]imidazole-2-yl analogues (30-35) showed significantly enhanced potency, suggesting that 1-alkyl-1H-benzo[d]imidazole-2-yl serves as the beneficial terminal heteroaromatic ring for the scaffold of 1,7-diaryl-1,4,6-heptatrien-3-one as curcumin-based anticancer agents for the potential treatment of prostate and cervical cancer.
2.6 Cytotoxicity toward MCF-10A normal mammary epithelial cells
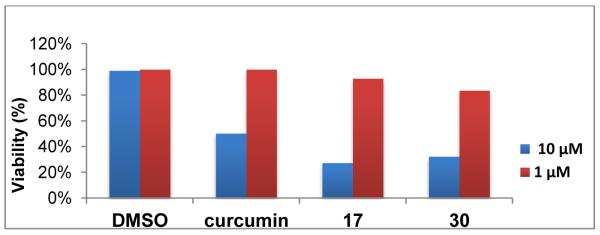
The high safety profile of curcumin in humans has been validated by a clinical study [4]. To test the safety profiles of the 1,7-diaryl-1,4,6-heptatrien-3-ones in vitro, compounds 17 and 30 were evaluated of their toxicity toward normal cells by trypan blue exclusion assay. The data, as illustrated in Figure 3, suggest no apparent cytotoxicity of these two compounds toward MCF-10A normal mammary epithelial cells at 1 μM concentration, with less than 16% inhibitory rate. As shown in Table 2, the inhibitory rate for these two compounds against four cancer cell lines are significant higher (in a range from 52% to 95%). These findings suggest that compounds 17 and 30 possess cytotoxic selectivity because they are more responsive to prostate cancer cells than normal cells in vitro.
Figure 3.
Cytotoxicity of two 1,7-diheteroarylheptatrienones (17 and 30) toward MCF-10A normal mammary epithelial cells.
2.7 Effects of compound 30 on PC-3 cell cycle progression and apoptosis
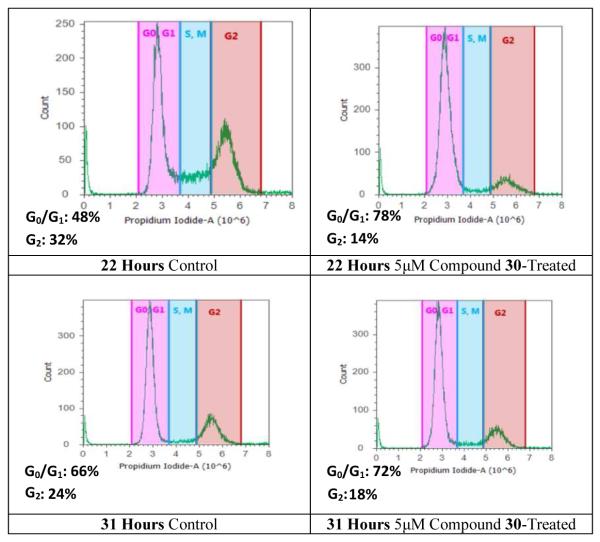
Curcumin has been reported to induce cell cycle regulation of PC-3 prostate cancer cells and other cells at a G1/S phase [26-30]. The effect of compound 30 on cell cycle was evaluated using flow cytometry analysis. Compound 30 was found to cause accumulation of PC-3 cells in a G0/G1 phase (Figure 4), while fewer cells were in the G2 phase. Compound 30 increased the population of PC-3 cells in the G0/G1 phase from 48% and 66% (control cells) at 22 hours and 31 hours, respectively, to 78% and 72%. The population of cells in the G2 phase decreased from 32% in control cells to 14% at 22 hours, and from 24% in control cells to 18% at 31 hours.
Figure 4.
Cell cycle analysis of PC-3 cells. PC-3 cancer cells were untreated or treated with compound 30. Cells were harvested after 22 and 31 hours then they were fixed, stained, and analyzed for DNA content. The distribution and percentage of cells in G1/G0, and G2 phase of the cell cycle are indicated.
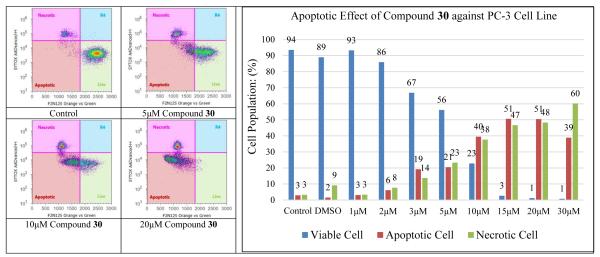
Curcumin has been demonstrated to be capable in inducing PC-3 prostate cancer cell apoptosis [2, 30]. Consequently, F2N12S and CYTOX AADvanced double staining flow cytometry-based assay was chosen to discriminate PC-3 cells dying from apoptosis from those dying from necrosis in response to increasing concentrations of compound 30. PC-3 cells were incubated with the test compound for 16 h. Staurosporine was used as specific apoptotic inducer and positive apoptotic control in all these experiments (not shown). As illustrated in Figure 5, compound 30 induced appreciable levels of apoptotic cell death in the androgen-insensitive PC-3 prostate cancer cell line in a dose-responsive manner after a 16-hour treatment. Specifically, 5 μM of compound 30 could induce detectable early phase of apoptosis in PC-3 cells as compared with control cells; treatment with 15 μM of compound 30 led to 51% early apoptotic cells and 47% late apoptotic/necrotic cells; 20 μM of compound 30 activated notable apoptosis as well, with 51% early apoptotic cells and 48% late apoptotic/necrotic cells. Both apoptotic and necrotic cell populations increased in response to increasing concentration of compound 30 (0-30 μM final concentration range). The apoptotic cell population reached maximum when exposure PC-3 cancer cells to compound 30 at 15 and 20 uM concentrations.
Figure 5.
Evolution of viable, apoptotic, and necrotic PC-3 cells populations in response to increasing dosages of compound 30.
3. Conclusion
In conclusion, thirty (1E,4E,6E)-1,7-diaryl-1,4,6-heptatrien-3-ones were designed as curcumin-based anticancer agents on the basis of their structural similarity to the enol-tautomer of curcumin, in addition to taking advantage of the enhanced water solubility conferred by the basic nitrogen-containing heteroaromatic rings. Aldol condensation and Horner-Wadsworth-Emmons reaction were chosen as the critical reactions to achieve these targets. The cytotoxicity of these 1,7-diaryl-1,4,6-heptatrien-3-ones were evaluated against both androgen-dependent and androgen-independent prostate cancer cell lines, as well as HeLa human cervical cancer cells. Ten compounds that significantly improved cytotoxicity towards four cancer cell lines were selected for the more in-depth evaluation of their anti-proliferative activities. Compared to curcumin, the ten analogues are 5- to 36-fold more potent in inhibiting cancer cell proliferation. The acquired structure-activity relationship data indicate (i) that (1E, 4E, 6E)-1,7-diaryl-1,4,6-heptatrien-3-ones represent a potential scaffold for developing curcumin-based agents with substantially improved cytotoxicity and anti-proliferative effect in prostate and cervical cancer cell models; and (ii) 1-alkyl-1H-imidazol-2-yl and 1-alkyl-1H-benzo[d]imidazole-2-yl serve as favorable heteroaromatic rings for increased in vitro potency. The robust structure-activity relationships (SAR) of (1E, 4E, 6E)-1,7-diaryl-1,4,6-heptatrien-3-ones obtained from this study will guide future designs of new curcumin-based chemotherapeutics. Compounds 17 and 30, two of most potent compounds, exhibit no apparent cytotoxicity toward MCF-10A normal mammary epithelial cells at 1μM concentration. Compound 30 was demonstrated to inhibit the proliferation of PC-3 cells through arresting cell cycle regulation at the G1/G0 phase and inducing cell apoptosis.
4. Experimental
4.1. General synthetic procedures
Anhydrous toluene was purified by PureSolv MD 7 Solvent Purification System from Innovative Technologies (MB-SPS-800). All other reagents and solvents were purchased from commercial sources and were used without further purification. Silica gel column chromatography was performed using silica gel (32-63 μm). Preparative thin-layer chromatography (PTLC) separations were carried out on 1000 μm AnalTech thin layer chromatography plates (Lot No.13401). HRMS were obtained on an Orbitrap mass spectrometer with electrospray ionization (ESI). NMR spectra were obtained on a Bruker Fourier 300 spectrometer or an Agilent-Varian 400 spectrometer in CDCl3 or CD3OD. The chemical shifts are given in ppm referenced to the respective solvent peak, and coupling constants are reported in Hz. Curcumin was synthesized by Claisen-Schmidt condensation of aromatic aldehyde with acetylacetone according to the procedure described in the literature [31]. 1-Alkyl-1H-imidazole-2-carbaldehydes (36-49) and 1-alkyl-1H-benzo[d]imidazole-2-carbaldehydes (60-65) were synthesized according to the procedure described in the literature [12,13].
4.2. General procedure for the synthesis of (E)-3-(aryl)-acrylaldehyde [20]
The reaction mixture of aldehyde (0.5 mmol) and 2-(triphenylphosphoranylidene)acetaldehyde (0.51 mmol) in DMF (0.5 mL) was stirred for 1-4 days. The reaction mixture was extracted with dichloromethane (10 mL × 3). The combined organic extracts were rinsed with brine (5 mL × 3) and dried over anhydrous magnesium sulfate, and the volatile components were evaporated under vacuum to give the crude product, which was subjected to the PTLC purification using hexanes/ethyl acetate (1/1, v/v) as eluent to give the respective product.
4.2.1. (E)-3-(1-Methyl-1H-imidazol-2-yl)acrylaldehyde (66)
Yellow oil, 29% yield; 1H NMR (300 MHz, CDCl3): δ 9.65 (d, J = 7.6 Hz, 1H), 7.29 (d, J = 15.5 Hz, 1H), 7.19 (s, 1H), 7.03 (s, 1H), 6.95 (dd, J = 15.5, 7.6 Hz, 1H), 3.78 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 193.0, 142.8, 135.5, 131.3, 129.6, 124.9, 33.4.
4.2.2. (E)-3-(1-Ethyl-1H-imidazol-2-yl)acrylaldehyde (67)
Yellow oil, 42% yield; 1H NMR (300 MHz, CDCl3): δ 9.67 (d, J = 7.6 Hz, 1H), 7.29 (d, J = 15.5 Hz, 1H), 7.22 (s, 1H), 7.10 (s, 1H), 7.00 (dd, J = 15.5, 7.6 Hz, 1H), 4.13 (q, J = 7.3 Hz, 2H), 1.46 (t, J = 7.3 Hz, 3H);13C NMR (75 MHz, CDCl3): δ 193.0, 141.9, 135.3, 131.5, 129.6, 122.9, 41.4, 16.7.
4.2.3. (E)-3-(1-Propyl-1H-imidazol-2-yl)acrylaldehyde (68)
Yellow oil, 28% yield; 1H NMR (300 MHz, CDCl3): δ 9.65 (d, J = 7.6 Hz, 1H), 7.27 (d, J = 15.4 Hz, 1H), 7.19 (s, 1H), 7.07 (s, 1H), 6.98 (dd, J = 15.4, 7.6 Hz, 1H), 4.02 (t, J = 7.2 Hz, 2H), 1.87–1.72 (m, 2H), 0.92 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 192.9, 142.2, 135.3, 131.2, 129.5, 123.7, 48.0, 24.7, 11.1.
4.2.4. (E)-3-(1-Butyl-1H-imidazol-2-yl)acrylaldehyde (69)
Yellow oil, 60% yield; 1H NMR (300 MHz, CDCl3): δ 9.62 (d, J = 7.6 Hz, 1H), 7.24 (d, J = 15.6 Hz, 1H), 7.15 (s, 1H), 7.04 (s, 1H), 6.95 (dd, J = 15.6, 7.6 Hz, 1H), 4.02 (t, J = 7.3 Hz, 2H), 1.75–1.65 (m, 2H), 1.33–1.22 (m, 2H), 0.88 (m, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 192.8, 142.0, 135.3, 131.1, 129.4, 123.6, 46.1, 33.3, 19.7, 13.4.
4.2.5. (E)-3-(1-Pentyl-1H-imidazol-2-yl)acrylaldehyde (70)
Yellow oil, 55% yield; 1H NMR (300 MHz, CDCl3): δ 9.60 (d, J = 7.2 Hz, 1H), 7.23 (d, J = 15.4 Hz, 1H), 7.13 (s, 1H), 7.03 (s, 1H), 6.95 (dd, J = 15.4, 7.2 Hz, 1H), 4.00 (t, J = 7.2 Hz, 2H), 1.80–1.58 (m, 2H), 1.35–1.10 (m, 4H), 0.80 (t, J = 7.0 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 192.8, 141.9, 135.3, 131.0, 129.3, 123.5, 46.3, 30.9, 28.5, 22.0, 13.7.
4.2.6. (E)-3-(1-Hexyl-1H-imidazol-2-yl)acrylaldehyde (71)
Yellow oil, 65% yield; 1H NMR (400 MHz, CDCl3): δ 9.68 (d, J = 7.4 Hz, 1H), 7.27 (d, J = 15.4 Hz, 1H), 7.23 (s, 1H), 7.07 (s, 1H), 7.04 (dd, J = 15.4, 7.4 Hz, 1H), 4.05 (t, J = 7.2 Hz, 2H), 1.81–1.74 (m, 2H), 1.33–1.26 (m, 6H), 0.87 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 192.9, 142.3, 135.2, 131.4, 129.7, 123.6, 46.7, 31.5, 31.3, 26.4, 22.6, 14.1.
4.2.7. (E)-3-(1-Heptyl-1H-imidazol-2-yl)acrylaldehyde (72)
Yellow oil, 77% yield; 1H NMR (300 MHz, CDCl3): δ 9.62 (d, J = 7.6 Hz, 1H), 7.24 (d, J = 15.3 Hz, 1H), 7.14 (s, 1H), 7.03 (s, 1H), 6.95 (dd, J = 15.3, 7.6 Hz, 1H), 4.00 (t, J = 7.2 Hz, 2H), 1.79–1.61 (m, 2H), 1.32–1.08 (m, 8H), 0.79 (t, J = 6.6 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 192.8, 142.0, 135.3, 131.0, 129.3, 123.5, 46.3, 31.4, 31.3, 28.6, 26.4, 22.4, 13.9.
4.2.8. (E)-3-(1-Isoprpoyl-1H-imidazol-2-yl)acrylaldehyde (73)
Yellow oil, 83% yield; 1H NMR (300 MHz, CDCl3): δ 9.66 (d, J = 7.6 Hz, 1H), 7.32 (d, J = 15.4 Hz, 1H), 7.22 (s, 1H), 7.15 (s, 1H), 7.03 (dd, J = 15.4, 7.6 Hz, 1H), 4.67–4.54 (m, 1H), 1.48 (d, J = 6.7 Hz, 6H); 13C NMR (75 MHz, CDCl3) δ 192.9, 141.7, 135.2, 131.5, 129.7, 119.4, 47.9, 23.8.
4.2.9. (E)-3-(1-Isobutyl-1H-imidazol-2-yl)acrylaldehyde (74)
Yellow oil, 52% yield; 1H NMR (400 MHz, CDCl3): δ 9.63 (d, J = 7.5 Hz, 1H), 7.24 (d, J = 15.2 Hz, 1H), 7.22 (s, 1H), 7.04 (s, 1H), 7.03 (dd, J = 14.9, 7.6 Hz), 3.80 (d, J = 7.4 Hz, 2H), 2.04–1.93 (m, 1H), 0.89 (s, 3H), 0.88 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 192.9, 142.5, 135.2, 131.2, 129.7, 124.1, 53.9, 30.7, 20.0.
4.2.10. (E)-3-(1-(sec-Butyl)-1H-imidazol-2-yl)acrylaldehyde (75)
Yellow oil, 74% yield; 1H NMR (300 MHz, CDCl3): δ 9.69 (d, J = 7.4 Hz, 1H), 7.32 (d, J = 15.3 Hz, 1H), 7.26 (s, 1H), 7.13 (s, 1H), 7.09 (dd, J = 15.3, 7.4 Hz, 1H), 4.36–4.27 (m, 1H), 1.88–1.72 (m, 2H), 1.48 (d, J = 6.7 Hz, 3H), 0.84 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 192.9, 142.2, 135.0, 132.2, 132.1, 119.5, 53.9, 31.0, 21.9, 10.7.
4.2.11. (E)-3-(1-Isopentyl-1H-imidazol-2-yl)acrylaldehyde (76)
Yellow oil, 52% yield; 1H NMR (400 MHz, CDCl3): δ 9.68 (d, J = 7.5 Hz, 1H), 7.26 (d, J = 15.5 Hz, 1H), 7.22 (s, 1H), 7.07 (s, 1H), 7.02 (dd, J = 15.5, 7.5 Hz, 1H), 4.05 (t, J = 7.3 Hz, 2H), 1.68–1.56 (m, 3H), 0.96 (d, J = 6.4 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 192.9, 142.2, 135.2, 131.4, 129.6, 123.5, 44.9, 40.3, 25.7, 22.3.
4.2.12. (E)-3-(1-(Pentan-2-yl)-1H-imidazol-2-yl)acrylaldehyde (77)
Yellow oil, 49% yield; 1H NMR (400 MHz, CDCl3): δ 9.68 (d, J = 7.5 Hz, 1H), 7.31 (d, J = 15.3 Hz, 1H), 7.25 (s, 1H), 7.12 (s, 1H), 7.07 (dd, J = 15.3, 7.5 Hz, 1H), 4.45–4.33 (m, 1H), 1.78–1.72 (m, 2H), 1.47 (d, J = 6.7 Hz, 3H), 1.26–1.16 (m, 2H), 0.88 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 192.9, 142.1, 135.2, 131.7, 129.8, 119.6, 52.2, 40.0, 22.3, 19.4, 13.7.
4.2.13. (E)-3-(1-(Pentan-3-yl)-1H-imidazol-2-yl)acrylaldehyde (78)
Yellow oil, 30% yield; 1H NMR (300 MHz, CDCl3): δ 9.71 (d, J = 7.2 Hz, 1H), 7.32 (d, J = 15.3, 1H), 7.31 (s, 1H), 7.15 (dd, J = 15.3, 7.2 Hz, 1H), 7.09 (s, 1H), 4.11–4.00 (m, 1H), 1.95–1.84 (m, 2H), 1.80– 1.64 (m, 2H), 0.81 (t, J = 7.4 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 192.9, 143.1, 135.3, 131.8, 129.9, 119.5, 60.1, 29.2, 10.6.
4.2.14. (E)-3-(1-(3-Methylbut-2-en-1-yl)-1H-imidazol-2-yl)acrylaldehyde (79)
Yellow oil, 44% yield; 1H NMR (300 MHz, CDCl3): δ 9.66 (d, J = 7.6 Hz, 1H), 7.28 (d, J = 15.5 Hz, 1H), 7.21 (s, 1H), 7.07 (s, 1H), 7.00 (dd, J = 15.5, 7.6 Hz, 1H), 5.28 (t, J = 6.2 Hz, 1H), 4.64 (d, J = 6.8 Hz, 2H), 1.79 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 193.0, 142.3, 138.6, 135.7, 131.3, 129.6, 123.3, 118.5, 44.6, 25.7, 18.3.
4.2.15. (E)-3-(4-Methylthiazol-2-yl)acrylaldehyde (80)
Yellow oil, 65% yield; 1H NMR (300 MHz, CDCl3): δ 9.65 (d, J = 7.6 Hz, 1H), 7.49 (d, J = 15.9 Hz, 1H), 7.05 (s, 1H), 6.78 (dd, J = 15.9, 7.6 Hz, 1H), 2.44 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 192.6, 162.1, 155.7, 142.7, 131.2, 117.8, 17.0.
4.2.16. (E)-3-(Thiazol-2-yl)acrylaldehyde (81)
Gray solid, 71% yield; 1H NMR (300 MHz, CDCl3): δ 9.74 (d, J = 7.6 Hz, 1H), 7.97 (d, J = 3.1 Hz, 1H), 7.62 (d, J = 15.9 Hz, 1H), 7.52 (d, J = 3.1 Hz, 1H), 6.87 (dd, J = 15.9, 7.6 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 192.7, 163.2, 145.4, 142.6, 131.9, 122.8.
4.2.17. (E)-3-(5-Methylisoxazol-3-yl)acrylaldehyde (82)
Brown crystal, 85% yield; 1H NMR (300 MHz, CDCl3): δ 9.66 (d, J = 7.7 Hz, 1H), 7.43 (d, J = 16.3 Hz, 1H), 6.58 (dd, J = 16.3, 7.7 Hz, 1H), 6.21 (s, 1H), 2.40 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 192.9, 170.8, 159.7, 138.8, 134.0, 99.4, 12.2.
4.2.18. (E)-3-(2-Methyl-4-(trifluoromethyl)thiazol-5-yl)acrylaldehyde (83)
Yellow solid, 63% yield; 1H NMR (300 MHz, CDCl3): δ 9.65 (d, J = 7.4 Hz, 1H), 7.73 (d, J = 15.7 Hz, 1H), 6.42 (dd, J = 15.7, 7.4 Hz, 1H), 2.74 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 192.1, 169.1, 142.6 (q, JCF = 36 Hz), 137.5, 135.3, 133.2, 120.5 (q, JCF = 270.0 Hz), 19.7.
4.2.19. (E)-3-(4-Bromo-1-methyl-1H-pyrazol-3-yl)acrylaldehyde (84)
Yellow solid, 68% yield; 1H NMR (300 MHz, CDCl3): δ 9.64 (d, J = 7.9 Hz, 1H), 7.44 (s, 1H), 7.35 (d, J = 16.1 Hz, 1H), 6.98 (dd, J = 16.1, 7.9 Hz, 1H), 3.91 (s, 3H); 13C NMR (75 MHz, CDCl3) δ 193.9, 144.5, 141.2, 132.3, 129.5, 96.1, 40.1.
4.2.20. (E)-3-(Pyridin-3-yl)acrylaldehyde (85)
Yellow oil, 40% yield; 1H NMR (300 MHz, CDCl3): δ 9.67 (d, J = 7.6 Hz, 1H), 8.72 (d, J = 1.8 Hz, 1H), 8.58 (dd, J = 4.7, 1.2 Hz, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.43 (d, J = 16.1 Hz, 1H), 7.32 (dd, J = 8.0, 4.8 Hz, 1H), 6.71 (dd, J = 16.1, 7.6 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 193.0, 151.8, 150.0, 148.5, 134.4, 130.1, 129.8, 123.9.
4.2.21. (E)-3-(Pyridin-2-yl)acrylaldehyde (86)
Yellow oil, 30% yield; 1H NMR (300 MHz, CDCl3): δ 9.75 (d, J = 7.8 Hz, 1H), 8.66 (d, J = 4.2 Hz, 1H), 7.73 (t, J = 7.6 Hz, 1H), 7.51 (d, J = 7.5 Hz, 1H), 7.49 (d, J = 15.8 Hz, 1H), 7.28 (dd, J = 7.2, 4.9 Hz, 1H), 7.04 (dd, J = 15.8, 7.7 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ 193.8, 152.8, 151.2, 150.5, 137.1, 131.8, 125.0, 124.3.
4.2.22. (E)-3-(6-Methylpyridin-2-yl)acrylaldehyde (87)
Yellow oil, 27% yield; 1H NMR (300 MHz, CDCl3): δ 9.71 (d, J = 7.9 Hz, 1H), 7.59 (t, J = 7.7 Hz, 1H), 7.44 (d, J = 15.8 Hz, 1H), 7.30 (d, J = 7.6 Hz, 1H), 7.12 (d, J = 7.7 Hz, 1H), 7.01 (dd, J = 15.8, 7.9 Hz, 1H), 2.53 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 193.7, 159.3, 151.9, 151.7, 137.0, 131.3, 124.7, 121.4, 24.5.
4.2.23. (E)-3-(Pyridin-4-yl)acrylaldehyde (88)
Brown wax, 85% yield; 1H NMR (300 MHz, CDCl3): δ 9.72 (d, J = 7.5 Hz, 1H), 8.65 (d, J = 6.0 Hz, 2H), 7.40 (d, J = 16.0 Hz, 1H), 7.38 (d, J = 6.0 Hz, 2H), 6.79 (dd, J = 16.1, 7.5 Hz, 1H).
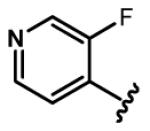
4.2.24. (E)-3-(3-Fluoropyridin-4-yl)acrylaldehyde (89)
Yellow off-solid, 45% yield; 1H NMR (300 MHz, CDCl3): δ 9.74 (d, J = 7.5 Hz, 1H), 8.54 (s, 1H), 8.46 (d, J = 4.9 Hz, 1H), 7.55 (d, J = 16.3 Hz, 1H), 7.43 (t, J = 5.6 Hz, 1H), 6.86 (dd, J = 16.3, 7.5 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 193.0, 156.9 (d, JCF = 261.8 Hz), 146.3 (d, JCF = 5.3 Hz), 141.2 (d, JCF = 2.3 Hz), 139.5 (d, JCF = 22.5 Hz), 134.0 (d, JCF = 5.3 Hz), 128.9 (d, JCF = 9.8 Hz), 121.6.
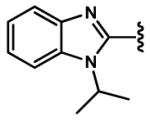
4.2.25 (E)-3-(1-Isopropyl-1H-benzo[d]imidazol-2-yl)acrylaldehyde (90)
Yellow oil, 44% yield; 1H NMR (300 MHz, CDCl3): δ 9.80 (d, J = 7.2 Hz, 1H), 7.81 (dd, J = 6.0, 3.2 Hz, 1H), 7.58 (d, J = 15.5 Hz, 1H), 7.58–7.56 (overlapped, 1H), 7.39 (dd, J = 15.4, 7.2 Hz, 1H), 7.33–7.28 (overlapped, 2H), 4.99–4.89 (m, 1H), 1.72 (d, J = 6.9 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 192.4, 146.7, 143.7, 135.5, 134.5, 134.3, 124.2, 123.5, 120.9, 112.2, 48.4, 22.1.
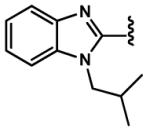
4.2.26. (E)-3-(1-Isobutyl-1H-benzo[d]imidazol-2-yl)acrylaldehyde (91)
Yellow oil, 66% yield; 1H NMR (400 MHz, CDCl3): δ 9.67 (d, J = 7.0 Hz, 1H), 7.72–7.69 (m, 1H), 7.34 (d, J = 15.4 Hz, 1H), 7.29–7.19 (overlapped, 4H), 3.97 (d, J = 7.6 Hz, 2H), 2.15–2.04 (m, 1H), 0.86 (d, J = 6.7 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 192.4, 147.3, 143.2, 136.2, 135.3, 133.5, 124.4, 123.5, 120.5, 110.5, 51.0, 30.0, 20.2.
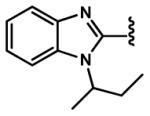
4.2.27. (E)-3-(1-(sec-Butyl)-1H-benzo[d]imidazol-2-yl)acrylaldehyde (92)
Yellow oil, 55% yield; 1H NMR (300 MHz, CDCl3): δ 9.79 (d, J = 7.3 Hz, 1H), 7.83–7.76 (m, 1H), 7.56 (d, J = 15.3 Hz , 1H), 7.55–7.50 (overlapped, 1H), 7.37 (dd, J = 15.3, 7.3 Hz, 1H), 7.31–7.25 (overlapped, 2H), 4.65–4.53 (m, 1H), 2.25–2.10 (m, 1H), 2.07–1.96 (m, 1H), 1.69 (d, J = 7.0 Hz, 3H), 0.78 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3) δ 192.4, 147.5, 144.0, 135.8, 134.7, 134.3, 124.2, 123.5, 121.1, 112.2, 54.6, 28.9, 20.5, 11.4.
4.2.28. (E)-3-(1-(Pentan-2-yl)-1H-benzo[d]imidazol-2-yl)acrylaldehyde (93)
Yellow oil, 60% yield; 1H NMR (300 MHz, CDCl3): δ 9.80 (d, J = 7.3 Hz, 1H), 7.83–7.77 (m, 1H), 7.56 (d, J = 15.3 Hz, 1H), 7.55–7.52 (overlapped, 1H), 7.38 (dd, J = 15.3, 7.3 Hz, 1H), 7.32–7.27 (overlapped, 2H), 4.75–4.63 (m, 1H), 2.22–2.10 (m, 1H), 1.98–1.86 (m, 1H), 1.69 (d, J = 7.0 Hz, 3H), 1.28–1.17 (m, 1H), 1.13–1.00 (m, 1H), 0.85 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 192.4, 147.3, 143.9, 135.8, 134.6, 134.2, 124.1, 123.4, 121.0, 112.1, 52.8, 37.8, 20.7, 20.0, 13.7.
4.2.29. (E)-3-(1-isopentyl-1H-benzo[d]imidazol-2-yl)acrylaldehyde (94)
Yellow oil, 43% yield; 1H NMR (300 MHz, CDCl3): δ 9.74 (d, J = 6.8 Hz, 1H), 7.78 (dd, J = 5.6, 3.1 Hz, 1H), 7.40 (d, J = 15.4 Hz, 1H), 7.36–7.25 (m, 4H), 4.24 (t, J = 7.5 Hz, 2H), 1.78–1.51 (m, 3H), 0.99 (d, J = 6.1 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 192.4, 146.9, 143.3, 135.8, 134.9, 133.7, 124.6, 123.7, 120.6, 110.1, 42.3, 39.5, 26.0, 22.5.
4.2.30. (E)-3-(1-(Pentan-3-yl)-1H-benzo[d]imidazol-2-yl)acrylaldehyde (95)
Yellow oil, 73% yield; 1H NMR (300 MHz, CDCl3): δ 9.81 (d, J = 7.0 Hz, 1H), 7.84–7.81 (m, 1H), 7.57 (d, J = 15.4 Hz, 1H), 7.55–7.51 (overlapped, 1H), 7.44 (dd, J = 15.4, 7.0 Hz, 1H), 7.34–7.27 (overlapped, 2H), 4.37–4.26 (m, 1H), 2.19–2.10 (m, 2H), 2.07–1.99 (m, 2H), 0.76 (t, J = 7.4 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 192.4, 148.1, 143.5, 135.4, 134.4, 124.2, 123.6, 120.8, 112.2, 61.3, 27.2, 11.2.
4.3. General procedure for the synthesis of (E)-3-(1-alkyl-1H-imidazol-2-yl)-but-3-en-2-ones (96-109) and (E)-4-(pyridin-4-yl)but-3-en-2-one (110)
The reaction of aldehyde (0.5 mmol) and 1-(triphenylphosphoranylidene)propan-2-one (0.52 mmol) in toluene (1.5 mL) was refluxed for 9 h. The solution was diluted 1 M HCl (10 mL) and extracted with dichloromethane (10 mL × 3). The collected aqueous layer was neutralized by saturated aqueous NaHCO3 and then extracted with dichloromethane (10 mL × 3). The combined organic extracts were dried over anhydrous magnesium sulfate, and the volatile components were evaporated under vacuum to give the respective product.
4.3.1. (E)-4-(1-Methyl-1H-imidazol-2-yl)but-3-en-2-one (96)
Brown wax, 96% yield; 1H NMR (300 MHz, CDCl3): δ 7.08 (d, J = 15.6 Hz, 1H), 6.84 (s, 1H), 6.78 (d, J = 15.6 Hz, 1H), 6.77 (s, 1H), 3.48 (s, 3H), 2.04 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 197.5, 142.7, 130.0, 127.4, 126.3, 124.1, 32.8, 28.5.
4.3.2. (E)-4-(1-Ethyl-1H-imidazol-2-yl)but-3-en-2-one (97)
Yellow oil, 91% yield; 1H NMR (300 MHz, CDCl3): δ 7.31 (d, J = 15.4 Hz, 1H), 7.10 (d, J = 15.4 Hz, 1H), 7.09 (s, 1H), 6.99 (s, 1H), 4.03 (q, J = 7.3 Hz, 2H), 2.27 (s, 3H), 1.36 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 197.6, 142.3, 130.6, 127.4, 125.9, 122.1, 41.1, 30.2, 29.5, 16.7, 16.4.
4.3.3. (E)-4-(1-Propyl-1H-imidazol-2-yl)but-3-en-2-one (98)
Yellow oil, 87% yield; 1H NMR (300 MHz, CDCl3): δ 7.25 (d, J = 15.4 Hz, 1H), 7.07 (d, J = 15.4 Hz, 1H), 7.04 (s, 1H), 6.93 (s, 1H), 3.90 (t, J = 7.2 Hz, 2H), 2.22 (s, 3H), 1.75–1.61 (m, 2H), 0.81 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 197.4, 142.5, 130.2, 127.3, 125.8, 122.8, 47.7, 29.3, 24.6, 10.9.
4.3.4. (E)-4-(1-Butyl-1H-imidazol-2-yl)but-3-en-2-one (99)
Yellow oil, 95% yield; 1H NMR (300 MHz, CDCl3): δ 7.23 (d, J = 15.3 Hz, 1H), 7.05 (d, J = 15.3 Hz, 1H), 7.02 (s, 1H), 6.92 (s, 1H), 3.91 (t, J = 7.2 Hz, 2H), 2.21 (s, 3H), 1.68–1.54 (m, 2H), 1.27–1.11 (m, 2H), 0.80 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 197.4, 142.4, 130.2, 127.3, 125.8, 122.7, 45.9, 33.2, 29.3, 19.6, 13.4.
4.3.5. (E)-4-(1-Pentyl-1H-imidazol-2-yl)but-3-en-2-one (100)
Yellow oil, 94% yield; 1H NMR (300 MHz, CDCl3): δ 7.21 (d, J = 15.4 Hz, 1H), 7.03 (d, J = 15.4 Hz, 1H), 6.98 (s, 1H), 6.89 (s, 1H), 3.84 (t, J = 7.2 Hz, 2H), 2.16 (s, 3H), 1.65–1.49 (m, 2H), 1.22–1.01 (m, 4H), 0.70 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 197.1, 142.1, 123.0, 127.2, 125.7, 122.6, 45.8, 30.7, 28.9, 28.2, 21.8, 13.5.
4.3.6. (E)-4-(1-Hexyl-1H-imidazol-2-yl)but-3-en-2-one (101)
Yellow oil, 50% yield; 1H NMR (400 MHz, CDCl3): δ 7.35 (d, J = 15.3 Hz, 1H), 7.17 (d, J = 15.3 Hz, 1H), 7.15 (d, J = 1.0 Hz, 1H), 7.00 (d, J = 1.0 Hz, 1H), 4.01 (t, J = 7.3 Hz, 2H), 2.32 (s, 3H), 1.76–1.70 (m, 2H), 1.30–1.24 (m, 6H), 0.86 (t, J = 6.8 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 197.7, 142.7, 130.5, 127.6, 125.9, 122.8, 46.4, 31.5, 31.3, 29.8, 26.3, 22.5, 14.0.
4.3.7. (E)-4-(1-Heptyl-1H-imidazol-2-yl)but-3-en-2-one (102)
Yellow oil, 53% yield; 1H NMR (300 MHz, CDCl3): δ 7.20 (d, J = 15.4 Hz, 1H), 7.09 (d, J = 15.4 Hz, 1H), 7.02 (s, 1H), 6.92 (s, 1H), 3.86 (t, J = 7.2 Hz, 2H), 2.19 (s, 3H), 1.64–1.57 (m, 2H), 1.22–1.01 (m, 8H), 0.72 (t, J = 6.7 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 197.1, 142.2, 129.9, 127.3, 125.6, 122.7, 45.9, 31.2, 31.1, 29.1, 28.4, 26.1, 22.2, 13.7.
4.3.8. (E)-4-(1-Isoprpoyl-1H-imidazol-2-yl)but-3-en-2-one (103)
Yellow oil, 99% yield; 1H NMR (400 MHz, CDCl3): δ 7.39 (d, J = 15.3 Hz, 1H), 7.18 (d, J = 15.3 Hz, 1H), 7.15 (d, J = 1.1 Hz, 1H), 7.08 (d, J = 1.1 Hz, 1H), 4.61–4.55 (m, 1H), 2.30 (s, 3H), 1.44 (d, J = 6.7 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 197.6, 142.1, 130.8, 127.4, 126.0, 118.6, 47.6, 29.7, 23.8.
4.3.9. (E)-4-(1-Isobutyl-1H-imidazol-2-yl)but-3-en-2-one (104)
Yellow oil, 62% yield; 1H NMR (400 MHz, CDCl3): δ 7.31 (d, J = 15.3 Hz, 1H), 7.16 (d, J = 15.3 Hz, 1H), 7.12 (d, J = 1.1 Hz, 1H), 6.96 (d, J = 1.1 Hz, 1H), 3.80 (d, J = 7.4 Hz, 2H), 2.30 (s, 3H), 2.04–1.93 (m, 1H), 0.88 (d, J = 6.7 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 197.6, 143.0, 130.4, 127.5, 126.0, 123.4, 53.7, 30.7, 29.8, 20.0.
4.3.10. (E)-4-(1-(sec-butyl)-1H-imidazol-2-yl)but-3-en-2-one (105)
Yellow oil, 80% yield; 1H NMR (300 MHz, CDCl3): δ7.40 (d, J = 15.3 Hz, 1H), 7.21 (d, J = 15.3 Hz, 1H), 7.18 (s, 1H), 7.05 (s, 1H), 4.38–4.26 (m, 1H), 2.32 (s, 3H), 1.85–1.67 (m, 2H), 1.43 (d, J = 6.7 Hz, 3H), 0.80 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 197.8, 142.7, 131.0, 127.5, 126.0, 118.8, 53.5, 30.9, 29.8, 21.9, 10.6.
4.3.11. (E)-4-(1-Isopentyl-1H-imidazol-2-yl)but-3-en-2-one (106)
Yellow oil, 79% yield; 1H NMR (400 MHz, CDCl3): δ 7.31 (d, J = 15.4 Hz, 1H), 7.12 (d, J = 15.4 Hz, 1H), 7.10 (d, J = 1.1 Hz, 1H), 6.97 (d, J = 1.1 Hz, 1H), 3.99 (t, J = 4.7 Hz, 2H), 2.28 (s, 3H), 1.61–1.58 (m, 4H), 0.90 (d, J = 6.4 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 197.6, 142.6, 130.5, 127.5, 125.9, 122.7, 44.7, 40.3, 29.6, 25.6, 22.3.
4.3.12. (E)-4-(1-(Pentan-2-yl)-1H-imidazol-2-yl)but-3-en-2-one (107)
Yellow oil, 83% yield; 1H NMR (400 MHz, CDCl3): δ 7.40 (d, J = 15.2 Hz, 1H), 7.22 (d, J = 15.2 Hz, 1H), 7.18 (d, J = 1.1 Hz, 1H), 7.06 (d, J = 1.1 Hz, 1H), 4.43–4.37 (m, 1H), 2.32 (s, 3H), 1.74–1.68 (m, 2H), 1.43 (d, J = 6.7 Hz, 3H), 1.26–1.08 (m, 2H), 0.86 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3); δ 197.7, 142.5, 130.3, 128.4, 125.3, 118.8, 52.2, 40.0, 30.0, 22.4, 19.6, 13.8.
4.3.13. (E)-4-(1-(Pentan-3-yl)-1H-imidazol-2-yl)but-3-en-2-one (108)
Yellow oil, 99% yield; 1H NMR (300 MHz, CDCl3): δ 7.40 (d, J = 15.2 Hz, 1H), 7.23 (d, J = 15.2 Hz, 1H), 7.26 (s, 1H), 7.01 (s, 1H), 4.14–3.93 (m, 1H), 2.32 (s, 3H), 1.94–1.58 (m, 4H), 0.76 (t, J = 7.3 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 197.6, 143.6, 131.1, 127.5, 126.0, 118.8, 59.8, 29.9, 29.2, 10.5.
4.3.14 (E)-4-(1-(3-Methylbut-2-en-1-yl)-1H-imidazol-2-yl)but-3-en-2-one (109)
Yellow oil, 83% yield; 1H NMR (300 MHz, CDCl3): δ 7.35 (d, J = 15.4 Hz, 1H), 7.12 (d, J = 15.4 Hz, 1H), 7.11 (s, 1H), 6.99 (s, 1H), 5.24 (t, J = 6.9 Hz, 1H), 4.59 (d, J = 6.9 Hz, 2H), 2.31 (s, 3H), 1.75 (d, J = 5.2 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 197.6, 142.7, 138.2, 130.5, 127.4, 126.4, 122.5, 118.7, 44.3, 29.4, 25.7, 18.1.
4.3.15 (E)-4-(Pyridin-4-yl)but-3-en-2-one (110)
Brown oil, yield 69%; 1H NMR (300 MHz, CDCl3): δ 8.47 (d, J = 5.3 Hz, 2H), 7.25 (d, J = 16.4 Hz, 3H), 7.22 (s, 2H), 6.67 (d, J = 16.4 Hz, 1H), 2.22 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 197.4, 150.2, 141.5, 139. 8, 130.4, 121.6, 27.5.
4.4. General procedure for the synthesis of (E)-diethyl (2-oxo-(pyridinyl)but-3-en-1-yl)phosphonates (113-127)
To the solution of tetraethyl (2-oxopropane-1,3-diyl)bis(phosphonate) (1 mmol) in 1 mL ethanol, potassium carbonate (1 mmol) in 1.5 mL water was added dropwise. After 30 min at room temperature, (E)-(pyridinyl)acrylaldehyde (1 mmol) in 0.5 mL ethanol was added to the solution dropwise. The inorganic solids were removed by filtration after 2 h, and the filtrate was diluted with with 1 M HCl (10 mL) and extracted with dichloromethane (10 mL × 3). The collected aqueous layer was neutralized by saturated aqueous NaHCO3 and then extracted with dichloromethane (10 mL × 3). The combined organic extracts were dried over anhydrous magnesium sulfate, and the volatile components were evaporated under vacuum to give the crude product, which was subjected to the PTLC purification using dichloromethane/methanol (100/5, v/v) as eluent to give the respective product.
4.4.1. (E)-Diethyl(4-(4-methylthiazol-2-yl)-2-oxobut-3-en-1-yl)phosphonate (113)
Yellow oil, 57% yield; 1H NMR (300 MHz, CDCl3): δ 7.63 (d, J = 15.9 Hz, 1H), 7.06 (d, J = 15.9 Hz, 1H), 7.02 (s, 1H), 4.21–4.06 (m, 4H), 3.29 (d, J = 22.6 Hz, 2H), 2.47 (s, 3H), 1.31 (t, J = 7.1 Hz, 6H). 13C NMR (75 MHz, CDCl3): δ 190.7 (d, JCP = 7.5 Hz), 162.5, 155.6, 135.3, 128.9, 117.3, 62.8 (d, JCP = 6.8 Hz), 41.4 (d, JCP = 127.5 Hz), 17.2, 16.5 (d, JCP = 7.5 Hz).
4.4.2. (E)-Diethyl(2-oxo-4-(thiazol-2-yl)but-3-en-1-yl)phosphonate (114)
Yellow oil, 38% yield; 1H NMR (300 MHz, CDCl3): δ 7.94 (d, J = 3.1 Hz, 1H), 7.71 (d, J = 15.9 Hz, 1H), 7.47 (d, J = 3.0 Hz, 1H), 7.11 (d, J = 15.9 Hz, 1H), 4.23–4.07 (m, 4H), 3.31 (d, J = 22.7 Hz, 2H), 1.31 (t, J = 7.0 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 190.7 (d, JCP = 7.5 Hz), 163.5, 145.2, 135.3, 129.4, 122.2, 62.9 (d, JCP = 7.5 Hz), 41.4 (d, JCP = 126.8 Hz), 16.4 (d, JCP = 7.5 Hz).
4.4.3. (E)-Diethyl(4-(5-methylisoxazol-3-yl)-2-oxobut-3-en-1-yl)phosphonate (115)
Light yellow oil, 69% yield; 1H NMR (300 MHz, CDCl3): δ 7.46 (d, J = 16.3 Hz, 1H), 6.77 (d, J = 16.3 Hz, 1H), 6.16 (s, 1H), 4.13–4.00 (m, 4H), 3.29 (s, 1H), 3.25 (d, J = 22.7 Hz, 2H), 2.37 (s, 3H), 1.24 (t, J = 7.1 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 190.8 (d, JCP = 7.5 Hz), 170.5, 159.8, 131.8, 131.6, 99.4, 62.8 (d, JCP = 7.5 Hz), 40.5 (d, JCP = 127.5 Hz), 39.9, 16.3 (d, JCP = 7.5 Hz), 12.2.
4.4.4. (E)-Diethyl (4-(2-methyl-4-(trifluoromethyl)thiazol-5-yl)-2-oxobut-3-en-1-yl) phosphonate (116)
Colorless oil, 61% yield; 1H NMR (300 MHz, CDCl3): δ 7.81 (d, J = 15.6 Hz, 1H), 6.63 (d, J = 15.6 Hz, 1H), 4.17–4.07 (m, 4H), 3.24 (d, J = 22.7 Hz, 2H), 2.71 (s, 3H), 1.30 (t, J = 7.1 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 189.9 (d, JCP = 7.5 Hz), 168.2, 143.8 (q, JCF = 35.3 Hz), 135.7, 130.7, 130.4, 120.7 (q, JCF = 270.8 Hz), 62.9 (d, JCP = 7.5 Hz), 41.4 (d, JCP = 126.8 Hz), 19.7, 16.4 (d, JCP = 7.5 Hz).
4.4.5. (E)-Diethyl(4-(4-bromo-1-methyl-1H-pyrazol-3-yl)-2-oxobut-3-en-1-yl)phosphonate (117)
Colorless oil, 81% yield; 1H NMR (300 MHz, CDCl3): δ 7.38 (d, J = 16.2 Hz, 1H), 7.37 (s, 1H), 7.05 (d, J = 16.2 Hz, 1H), 4.11–3.97 (m, 4H), 3.80 (s, 3H), ), 3.19 (d, J = 22.7 Hz, 2H), 1.21 (t, J = 7.1 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 191.0 (d, JCP = 7.5 Hz), 144.3, 133.0, 132.0, 126.5, 96.0, 62.5 (d, JCP = 7.5 Hz), 40.7 (d, JCP = 126.0 Hz), 39.9, 16.2 (d, JCP = 7.5 Hz).
4.4.6. (E)-Diethyl(2-oxo-4-(pyridin-3-yl)but-3-en-1-yl)phosphonate (118)
Yellow oil, 83% yield; 1H NMR (300 MHz, CDCl3): δ 8.75 (s, 1H), 8.59 (d, J = 3.6 Hz, 1H), 7.86 (d, J = 8.0 Hz, 1H), 7.60 (d, J = 16.2 Hz, 1H), 7.31 (dd, J = 7.9, 4.8 Hz, 1H), 6.94 (d, J = 16.2 Hz, 1H), 4.19–4.07 (m, 4H), 3.30 (d, J = 22.7 Hz, 2H), 1.30 (t, J = 7.1 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 190.7 (d, JCP = 7.5 Hz), 151.5, 150.3, 140.8, 134.6, 130.0, 127.4, 123.9, 62.8 (d, JCP = 7.5 Hz), 41.4 (d, JCP = 126.8 Hz), 16.4 (d, JCP = 7.5 Hz).
4.4.7. (E)-Diethyl(2-oxo-4-(pyridin-2-yl)but-3-en-1-yl)phosphonate (119)
Yellow oil, 86% yield; 1H NMR (300 MHz, CDCl3): δ 8.63 (d, J = 4.1 Hz, 1H), 7.71 (t, J = 7.6 Hz, 1H), 7.60 (d, J = 15.8 Hz, 1H), 7.47 (d, J = 7.7 Hz, 1H), 7.31 (d, J = 15.8 Hz, 1H), 7.29–7.25 (overlapped, 1H), 4.18–4.09 (m, 4H), 3.33 (d, J = 22.6 Hz, 2H), 1.30 (t, J = 7.0 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 191.5 (d, JCP = 7.5 Hz), 152.8, 150.3, 143.0, 137.0, 129.3, 125.0, 124.7, 62.8, 41.4 (d, JCP = 127.5 Hz), 16.5.
4.4.8. (E)-Diethyl(4-(6-methylpyridin-2-yl)-2-oxobut-3-en-1-yl)phosphonate (120)
Yellow oil, 61% yield; 1H NMR (300 MHz, CDCl3): δ 7.52 (t, J = 7.6 Hz, 1H), 7.51 (d, J = 15.7 Hz, 1H), 7.23 (d, J = 15.7 Hz, 1H), 7.21 (d, J = 7.4, 1H), 7.07 (d, J = 7.7 Hz, 1H), 4.18–3.99 (m, 4H), 3.27 (d, J = 22.6 Hz, 2H), 2.48 (s, 3H), 1.25 (t, J = 7.1 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 191.5 (d, JCP = 7.5 Hz), 159.0, 151.9, 143.4, 136.9, 128.8, 124.4, 122.0, 62.6 (d, JCP = 7.5 Hz), 41.0 (d, JCP = 127.5 Hz), 24.5, 16.3 (d, JCP = 7.5 Hz).
4.4.9. (E)-Diethyl(4-(3-fluoropyridin-4-yl)-2-oxobut-3-en-1-yl)phosphonate (121)
Yellow oil, 14% yield; 1H NMR (300 MHz, CDCl3): δ 8.55 (s, 1H), 8.47 (d, J = 4.8 Hz, 1H), 7.66 (d, J = 16.3 Hz, 1H), 7.48 (t, J = 5.6 Hz, 1H), 7.13 (d, J = 16.3 Hz, 1H), 4.22–4.08 (m, 4H), 3.33 (d, J = 22.8 Hz, 2H), 1.32 (t, J = 7.1 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 190.7 (d, JCP = 7.5 Hz), 145.8, 139.2, 138.8, 133.7, 132.0, 130.0, 122.5, 63.0 (d, JCP = 7.5 Hz), 41.8 (d, JCP = 126.8 Hz), 24.5, 16.5 (d, JCP = 7.5 Hz).
4.4.10. (E)-Diethyl(4-(1-isopropyl-1H-benzo[d]imidazol-2-yl)-2-oxobut-3-en-1-yl)phosphonate (122)
Yellow oil, 76% yield; 1H NMR (300 MHz, CDCl3): δ 7.75 (d, J = 15.2 Hz, 1H), 7.81–7.75 (overlapped, 1H), 7.59 (d, J = 15.2 Hz, 1H), 7.56–7.51 (m, 1H), 7.29–7.26 (overlapped, 2H), 4.98–4.89 (m, 1H), 4.22–4.12 (m, 4H), 3.34 (d, J = 22.6 Hz, 2H), 1.68 (d, J = 7.0 Hz, 6H), 1.33 (t, J = 7.1 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 190.7 (d, JCP = 7.5 Hz), 147.0, 143.7, 134.4, 131.6, 128.4, 123.8, 123.2, 120.7, 112.1, 62.8 (d, JCP = 7.5 Hz), 48.2 , 42.2 (d, JCP = 126.8 Hz), 22.0, 16.4 (d, JCP = 7.5 Hz).
4.4.11. (E)-Diethyl(4-(1-isobutyl-1H-benzo[d]imidazol-2-yl)-2-oxobut-3-en-1-yl)phosphonate (123)
Yellow oil, 61% yield; 1H NMR (400 MHz, CDCl3): δ 7.79–7.75 (m, 1H), 7.65 (d, J = 15.3 Hz, 1H), 7.60 (d, J = 15.3 Hz, 1H), 7.36–7.33 (m, 1H), 7.32–7.28 (overlapped, 2H), 4.20–4.12 (m, 4H), 4.07 (d, J = 7.6 Hz, 2H), 3.31 (d, J = 16.9 Hz, 2H), 2.22–2.15 (m, 1H), 1.32 (t, J = 7.1 Hz, 6H), 0.94 (d, J = 6.7 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 190.7 (d, JCP = 7.5 Hz), 147.8, 143.2, 136.2, 131.2, 128.0, 124.3, 123.6, 120.5, 110.5, 62.9 (d, JCP = 7.5 Hz), 51.1, 42.3 (d, JCP = 127.5 Hz), 30.1, 20.3, 16.5 (d, JCP = 7.5 Hz).
4.4.12. (E)-Diethyl(4-(1-(sec-butyl)-1H-benzo[d]imidazol-2-yl)-2-oxobut-3-en-1-yl)phosphonate (124)
Yellow oil, 65% yield; 1H NMR (300 MHz, CDCl3): δ 7.80–7.76 (m, 1H), 7.73 (d, J = 15.2 Hz, 1H), 7.59 (d, J = 15.2 Hz, 1H), 7.53–7.47 (m, 1H), 7.28–7.24 (overlapped, 2H), 4.66–4.54 (m, 1H), 4.21–4.10 (m, 4H), 3.33 (d, J = 22.6 Hz, 2H), 2.20–2.09 (m, 1H), 2.01–1.92 (m, 1H), 1.65 (d, J = 7.0 Hz, 3H), 1.32 (t, J = 7.1 Hz, 6H), 0.75 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 190.8 (d, JCP = 7.5 Hz), 147.7, 143.6, 134.5, 131.8, 128.4, 123.9, 123.4, 120.7, 112.2, 62.9 (d, JCP = 7.5 Hz), 54.5, 43.2, 42.3 (d, JCP = 127.5 Hz), 28.8, 20.4, 16.5 (d, JCP = 7.5 Hz), 11.3.
4.4.13 (E)-Diethyl(2-oxo-4-(1-(pentan-2-yl)-1H-benzo[d]imidazol-2-yl)but-3-en-1-yl)phosphonate (125)
Yellow oil, 36% yield; 1H NMR (400 MHz, CDCl3): δ 7.75–7.72 (m, 1H), 7.69 (d, J = 15.2 Hz, 1H), 7.56 (d, J = 15.2 Hz, 1H), 7.47 (d, J = 7.6 Hz, 1H), 7.26–7.18 (m, 2H), 4.70–4.62 (m, 1H), 4.23–4.03 (m, 4H), 3.29 (d, J = 17.0 Hz, 2H), 2.14–2.03 (m, 1H), 1.90–1.80 (m, 1H), 1.61 (d, J = 6.9 Hz, 3H), 1.28 (t, J = 7.0 Hz, 6H), 1.21–1.13 (m, 1H), 1.05–0.93 (m, 1H), 0.79 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 190.6 (d, JCP = 7.5 Hz), 147.6, 143.7, 134.5, 131.5, 128.5, 123.7, 123.1, 120.7, 112.0, 62.8 (d, JCP = 7.5 Hz), 52.5, 42.2 (d, JCP = 126.8 Hz), 37.6, 20.5, 19.9, 16.4 (d, JCP = 7.5 Hz), 13.6.
4.4.14. (E)-Diethyl(4-(1-isopentyl-1H-benzo[d]imidazol-2-yl)-2-oxobut-3-en-1-yl)phosphonate (126)
Yellow oil, 62% yield. 1H NMR (300 MHz, CDCl3): δ 7.77–7.71 (m, 1H), 7.63 (d, J = 15.3 Hz, 1H), 7.55 (d, J = 15.3 Hz, 1H), 7.35–7.29 (m, 1H), 7.29–7.24 (overlapped, 2H), 4.23 (t, J = 7.5 Hz, 2H), 4.19–4.09 (m, 4H), 3.31 (d, J = 22.5 Hz, 2H), 1.71–1.59 (m, 3H), 1.30 (t, J = 7.1 Hz, 6H), 0.96 (d, J = 6.2 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 190.6 (d, JCP = 7.5 Hz), 147.3, 143.1, 135.7, 131.3, 127.6, 124.3, 123.6, 120.4, 110.0, 62.9 (d, JCP = 7.5 Hz), 42.3, 42.2 (d, JCP = 126.8 Hz), 39.5, 26.0, 22.5, 16.4 (d, JCP = 7.5 Hz).
4.4.15. (E)-Diethyl(2-oxo-4-(1-(pentan-3-yl)-1H-benzo[d]imidazol-2-yl)but-3-en-1-yl) phosphonate (127)
Yellow oil, 76% yield; 1H NMR (300 MHz, CDCl3): δ 7.72–7.69 (m, 1H), 7.65 (d, J = 15.2 Hz, 1H), 7.52 (d, J = 15.2 Hz, 1H), 7.41 (d, J = 6.8 Hz, 1H), 7.22–7.14 (overlapped, 2H), 4.29–4.18 (m, 1H), 4.13–4.03 (m, 4H), 3.26 (d, J = 22.6 Hz, 2H), 2.14–1.99 (m, 2H), 1.94–1.85 (m, 2H), 1.23 (t, J = 7.1 Hz, 6H), 0.64 (t, J = 7.4 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 190.5 (d, JCP = 7.5 Hz), 148.5, 143.6, 131.5, 128.4, 123.6, 123.0, 120.5, 111.9, 62.6 (d, JCP = 7.5 Hz), 60.7, 42.0 (d, JCP = 127.5 Hz), 27.0, 16.2 (d, JCP = 7.5 Hz), 10.9.
4.5. Synthesis of (1E,6E)-5-hydroxy-1,7-di(pyridin-4-yl)hepta-1,6-dien-3-one (111)
To the freshly prepared 2M LDA in THF solution (6 mL) at −78 °C under argon were added 110 (241 mg, 1.64 mmol) in 2 mL anhydrous THF. The reaction solution was stirred at −78 °C for 50 min, to which was added 88 (255 mg) in THF (2 mL). The subsequent reaction mixture was stirred at the same temperature for 1.5 h prior to being quenched with saturated ammonium chloride. The mixture was extracted with ethyl acetate (15 mL × 3), the combined organic extracts were dried over anhydrous magnesium sulfate, and the organic solvents were removed under vacuum. The obtained crude product was subjected to column chromatography over silica gel, eluting with 5% methanol in DCM, to furnish the title compound as a yellow oil in 52% yield. 1H NMR (300 MHz, CDCl3): δ 8.43 (d, J = 5.3 Hz, 1H), 8.28 (d, J = 5.3 Hz, 2H), 7.32 (d, J = 16.3 Hz, 1H), 7.20 (d, J = 5.3 Hz, 1H), 7.04 (d, J = 5.3 Hz, 2H), 6.76 (d, J = 16.3 Hz, 1H), 6.59–6.30 (m, 2H), 5.14 (s, 1H), 4.83 (dd, J = 8.2, 4.4 Hz, 1H), 2.99 (dd, J = 16.3, 8.2 Hz, 1H), 2.81 (dd, J = 16.3, 4.3 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 198.0, 149.9, 149.2, 144.2, 141.5, 139.7, 136.8, 130.0, 126.7, 121.8, 120.8, 67.3, 53.4, 47.6.
4.6. Synthesis of (1E,4E,6E)-1,7-di(pyridin-4-yl)hepta-1,4,6-trien-3-one (28)
Compound 111 was kept at room temperature for 20 days. Most of it was converted to its eliminated product 28, which was purified by PTLC eluting with 5% methanol in DCM. Yellow oil, 60% yield. 1H NMR (300 MHz, MeOD): δ 8.57 (d, J = 5.7 Hz, 2H), 8.50 (d, J = 5.7 Hz, 2H), 7.67–7.31 (m, 8H), 7.08 (d, J = 15.5 Hz, 1H), 6.83 (d, J = 15.2 Hz, 1H); 13C NMR (75 MHz, MeOD): δ 190.5, 150.9, 150.6, 146.0, 144.7, 144.5, 141.1, 139. 6, 133.4, 132.5, 130.7, 123.9, 123.0; IR (neat) νmax: 3030, 2924, 1652, 1622, 1592, 1414, 1181, 1081 cm−1; HRMS (ESI): m/z calculated for C17H15N2O [M+H]+: 263.1184. Found: 263.1182.
4.7 General procedure for the synthesis of (1E,4E,6E)-1,7-diarylhepta-1,4,6-trien-3-ones
Method A [32]: To a solution of the starting aldehyde (0.5 mmol) and ketone (0.5 mmol) in methanol (4 mL) was added the solution of sodium methoxide in methanol (5.4 M, 0.5 mmol), and the mixture was stirred and monitored with TLC. When the reaction was completed, saturated solution of ammonium chloride was added, and the subsequent mixture was extracted with dichloromethane. The organic layer was dried over anhydrous MgSO4. The solvent was evaporated under vacuum to give a crude product, which was purified by preparative TLC (3-5% methanol in dichloromethane) or column chromatography (2-5% methanol in dichloromethane). Method B [33]: The procedure to synthesize curcumin analogues via Horner-Wadsworth-Emmons reaction: A 4 mL flask was charged with (E)-diethyl (2-oxo-4-pyridinylbut-3-en-1-yl)phosphonate (0.5 mmoL) and the appropriate heteroaromatic carboxaldehyde (0.55 mmoL). A solution of potassium carbonate (2.80 g, 20.3 mmoL) in water (2.5 mL) and ethanol (1.5 mL) were added and the biphasic mixture was stirred rapidly at room temperature for 1h to overnight. The solution was extracted with dichloromethane (10 mL × 3). The combined extracts were dried over anhydrous magnesium sulfate and concentrated. The residue was purified over preparative thin layer chromatography eluting with dichloromethane/methanol (5% methanol in dichloromethane, v/v) to give the respective product.
Compounds synthesized by method A
4.7.1 (1E,4E,6E)-1,7-Bis(1-methyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (6)
Brown syrup, 31% yield; 1H NMR (300 MHz, MeOD): δ 7.60 (d, J = 15.6 Hz, 1H), 7.58 (dd, J = 15.6, 8.7 Hz, 1H), 7.36 (d, J = 15.6 Hz, 1H), 7.27 (s, 1H), 7.26 (dd, J = 15.6, 8.7 Hz, 1H), 7.17 (s, 1H), 7.15 (s, 1H), 7.08 (s, 1H), 7.07 (d, J = 15.6 Hz, 1H), 6.75 (d, J = 15.6 Hz, 1H), 3.84 (s, 3H), 3.78 (s, 3H); 13C NMR (75 MHz, MeOD): δ 189.2, 144.4, 143.5, 143.2, 130.4, 129.7, 129.2, 128.4, 126.5, 126.1, 125.2, 124.8, 123.4, 32.2, 32.0; IR (neat) νmax: 3107, 2952, 1644, 1609, 1575, 1477, 1426, 1279, 1076, 997 cm−1; HRMS (ESI): m/z calculated for C15H17N4O [M+H]+: 269.1402. Found: 269.1404.
4.7.2. (1E,4E,6E)-1,7-Bis(1-ethyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (7)
Yellow oil, 40% yield; 1H NMR (300 MHz, CDCl3): δ 7.62–7.44 (m, 3H), 7.37 (dd, J = 14.6, 11.5 Hz, 1H), 7.18 (s, 1H), 7.13 (s, 1H), 7.04 (s, 1H), 6.96 (s, 1H), 6.77 (d, J = 14.8 Hz, 1H), 6.54 (d, J = 15.1 Hz, 1H), 4.17–3.97 (m, 4H), 1.43 (td, J = 7.3, 1.9 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 188.6, 143.9, 143.1, 131.9, 130.7, 130.4, 130.0, 126.1, 126.0, 124.9, 122.1, 121.0, 41.2, 41.0, 16.9, 16.7; IR (neat) νmax: 3105, 2978, 2935, 1644, 1607, 1508, 1443, 1273, 1078 cm−1; HRMS (ESI): m/z calculated for C17H21N4O [M+H]+: 297.1715. Found: 297.1716.
4.7.3. (1E,4E,6E)-1,7-Bis(1-propyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (8)
Yellow oil, 50% yield; 1H NMR (400 MHz, CDCl3): δ 7.62 (d, J = 15.1 Hz, 1H), 7.56 (dd, J = 15.2, 11.4 Hz, 1H), 7.49 (d, J = 15.1 Hz, 1H), 7.41 (dd, J = 14.9, 11.4 Hz, 1H), 7.19 (d, J = 1.0 Hz, 1H), 7.15 (d, J = 1.1 Hz, 1H), 7.03 (d, J = 1.0 Hz, 1H), 6.95 (d, J = 1.1 Hz, 1H), 6.78 (d, J = 14.8 Hz, 1H), 6.56 (d, J = 15.2 Hz, 1H), 4.06–4.00 (m, 2H), 3.95 (t, J = 7.2 Hz, 2H), 1.86–1.75 (m, 4H), 0.95 (t, J = 7.4 Hz, 3H), 0.94 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 188.6, 144.0, 143.2, 132.0, 130.5, 130.1, 130.0, 126.3, 125.8, 124.6, 122.8, 121.8, 48.0, 47.9, 24.8, 24.7, 11.2, 11.2; IR (neat) νmax: 3104, 2964, 2933, 2875, 1644, 1606, 1575, 1507, 1442, 1177, 1078 cm−1; HRMS (ESI): m/z calculated for C19H25N4O [M+H]+: 325.2028. Found: 325.2027.
4.7.4. (1E,4E,6E)-1,7-Bis(1-butyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (9)
Yellow oil, 45% yield; 1H NMR (400 MHz, CDCl3): δ 7.57 (d, J = 15.1 Hz, 1H), 7.54 (dd, J = 14.8, 10.0 Hz, 1H), 7.50 (d, J = 15.1 Hz, 1H), 7.18 (d, J = 0.9 Hz, 1H), 7.13 (d, J = 1.0 Hz, 1H), 7.02 (d, J = 1.1 Hz, 1H), 6.94 (d, J = 1.1 Hz, 1H), 6.77 (d, J = 14.8 Hz, 1H), 6.54 (d, J = 15.2 Hz, 1H), 4.05 (t, J = 7.3 Hz, 2H), 3.97 (t, J = 7.3 Hz, 2H), 1.79–1.70 (m, 4H), 1.41–1.29 (m, 4H), 0.95 (q, J = 7.4 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 188.6, 144.1, 143.3, 143.1, 131.9, 130.6, 130.2, 130.0, 126.2, 125.8, 125.0, 122.8, 121.8, 46.2, 46.0, 33.6, 33.5, 20.0, 13. 7; IR (neat) νmax: 3103, 2957, 2930, 2872, 1644, 1607, 1576, 1474, 1273, 1173, 1078 cm−1; HRMS (ESI): m/z calculated for C21H29N4O [M+H]+ : 353.2341. Found: 353.2342.
4.7.5. (1E,4E,6E)-1,7-Bis(1-pentyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (10)
Yellow oil, 42% yield; 1H NMR (400 MHz, CDCl3): δ 7.56 (d, J = 15.0 Hz, 1H), 7.53 (dd, J = 15.2, 11.4 Hz, 1H), 7.48 (d, J = 15.0 Hz, 1H), 7.37 (dd, J = 14.6, 11.6 Hz, 1H), 7.16 (d, J = 0.9 Hz, 1H), 7.12 (d, J = 1.0 Hz, 1H), 7.01 (d, J = 1.1 Hz, 1H), 6.93 (d, J = 1.1 Hz, 1H), 6.75 (d, J = 14.8 Hz, 1H), 6.53 (d, J = 15.2 Hz, 1H), 4.03 (t, J = 7.3 Hz, 2H), 3.95 (t, J = 7.3 Hz, 2H), 1.83–1.65 (m, 4H), 1.38–1.24 (m, 8H), 0.88 (q, J = 7.0 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 188.5, 143.9, 143.1, 143.0, 131.7, 130.4, 130.0, 129.77, 126.1, 125.7, 124.9, 122.7, 121.7, 46.2, 46.1, 31.13, 31.05, 28. 7, 28.6, 22.1, 13.9; IR (neat) νmax: 3014, 2955, 2929, 2859, 1645, 1607, 1577, 1444, 1273, 1078 cm−1; HRMS (ESI): m/z calculated for C23H33N4O [M+H]+: 381.2654. Found: 381.2654.
4.7.6. (1E,4E,6E)-1,7-Bis(1-hexyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (11)
Yellow oil, 22% yield; 1H NMR (400 MHz, CDCl3): δ 7.62 (d, J = 15.2 Hz, 1H), 7.55 (dd, J = 15.2, 11.5 Hz, 1H), 7.49 (d, J = 15.2 Hz, 1H), 7.42 (dd, J = 14.8, 11.4 Hz, 1H), 7.19 (d, J = 0.7 Hz, 1H), 7.15 (d, J = 0.9 Hz, 1H), 7.03 (d, J = 1.0 Hz, 1H), 6.95 (d, J = 1.1 Hz, 1H), 6.78 (d, J = 14.8 Hz, 1H), 6.56 (d, J = 15.2 Hz, 1H), 4.05 (t, J = 7.3 Hz, 2H), 3.97 (t, J = 7.3 Hz, 2H), 1.77–1.74 (m, 4H), 1.35–1.28 (m, 12H), 0.91–0.86 (m, 6H); 13C NMR (75 MHz, CDCl3): δ 188.6, 144.1, 143.3, 143.2, 132.1, 130.4, 130.0, 126.2, 126.0, 124.8, 122.8, 121.7, 46.5, 46.4, 31.6, 31.5, 31.38, 31.35, 26.44, 26.37, 22.60, 22.57, 14.1; IR (neat) νmax: 3108, 2954, 2927, 2857, 1647, 1608, 1510, 1447, 1377, 1264, 1169, 1100 cm−1; HRMS (ESI): m/z calculated for C25H37N4O [M+H]+: 409.2967. Found: 409.2968.
4.7.7. (1E,4E,6E)-1,7-Bis(1-heptyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (12)
Yellow oil, 42% yield; 1H NMR (400 MHz, CDCl3): δ 7.54 (d, J = 15.2 Hz, 1H), 7.51 (dd, J = 14.8, 11.1 Hz, 1H), 7.46 (d, J = 15.2 Hz, 1H), 7.34 (dd, J = 14.6, 11.7 Hz, 1H), 7.14 (d, J = 0.8 Hz, 1H), 7.09 (d, J = 1.0 Hz, 1H), 6.99 (d, J = 1.1 Hz, 1H), 6.91 (d, J = 1.1 Hz, 1H), 6.73 (d, J = 14.8 Hz, 1H), 6.51 (d, J = 15.2 Hz, 1H), 4.00 (t, J = 7.3 Hz, 2H), 3.93 (t, J = 7.3 Hz, 2H), 1.77–1.67 (m, 4H), 1.31–1.19 (m, 16H), 0.85–0.81 (m, 6H); 13C NMR (75 MHz, CDCl3): δ 188.5, 144.0, 143.2, 143.0, 131.8, 130.5, 130.2, 129.8, 126.1, 125.7, 125.0, 122.7, 121.7, 46.3, 46.2, 31.6, 31.54, 31.46, 28.8, 26.7, 26.6, 22.6, 14.1; IR (neat) νmax: 2927, 2856, 1647, 1610, 1579, 1474, 1445, 1274, 1099 cm−1; HRMS (ESI): m/z calculated for C27H41N4O [M+H]+: 437.3280. Found: 437.3278.
4.7.8. (1E,4E,6E)-1,7-Bis(1-isopropyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (13)
Yellow oil, 25% yield; 1H NMR (300 MHz, CDCl3): δ 7.59–7.50 (m, 3H), 7.40 (dd, J = 14.6, 11.5 Hz, 1H), 7.19 (s, 1H), 7.14 (s, 1H), 7.11 (s, 1H), 7.03 (s, 1H), 6.82 (d, J = 14.6 Hz, 1H), 6.54 (d, J = 15.0 Hz, 1H), 4.70–4.61 (m, 1H), 4.55–4.46 (m, 1H), 1.47 (d, J = 6.7 Hz, 12H); 13C NMR (75 MHz, CDCl3): δ 188.7, 143.6, 143.1, 142.7, 131.9, 130.8, 130.4, 130.2, 126.2, 126.1, 125.1, 118.5, 117.4, 47.7, 47.6, 23.9, 23.7; IR (neat) νmax: 3105, 2977, 2933, 2874, 1644, 1606, 1575, 1452, 1420, 1255, 1078 cm−1; HRMS (ESI): m/z calculated for C19H25N4O [M+H]+: 325.2028. Found: 325.2027.
4.7.9. (1E,4E,6E)-1,7-Bis(1-isobutyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (14)
Yellow oil, 45% yield; 1H NMR (400 MHz, CDCl3): δ 7.61 (d, J = 15.1 Hz, 1H), 7.55 (dd, J = 15.3, 11.7 Hz, 1H), 7.47 (d, J = 15.0 Hz, 1H), 7.40 (dd, J = 14.6, 11.6 Hz, 1H), 7.19 (d, J = 0.7 Hz, 1H), 7.14 (d, J = 0.9 Hz, 1H), 7.00 (d, J = 1.1 Hz, 1H), 6.92 (d, J = 1.1 Hz, 1H), 6.76 (d, J = 14.8 Hz, 1H), 6.54 (d, J = 15.2 Hz, 1H), 3.85 (d, J = 7.5 Hz, 2H), 3.78 (d, J = 7.4 Hz, 2H), 2.09–1.99 (m, 2H), 0.93 (t, J = 6.9 Hz, 12H); 13C NMR (75 MHz, CDCl3): δ 188.6, 144.4, 143.6, 143.2, 132.0, 130.3, 130.2, 130.0, 126.1, 126.0, 125.0, 123.3, 122.3, 53.7, 30.7, 20.1, 20.0; IR (neat) νmax: 3107, 2960, 2873, 1646, 1609, 1507, 1467, 1443, 1389, 1264, 1179, 1082 cm−1; HRMS (ESI): m/z calculated for C21H29N4O [M+H]+ : 353.2341. Found: 353.2342.
4.7.10. (1E,4E,6E)-1,7-Bis(1-(sec-butyl)-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (15)
Yellow oil, 36% yield; 1H NMR (400 MHz, CDCl3): δ 7.59 (d, J = 15.0 Hz, 1H), 7.54 (dd, J = 15.1, 11.4 Hz, 1H), 7.53 (d, J = 15.0 Hz, 1H), 7.40 (dd, J = 14.8, 11.5 Hz, 1H), 7.20 (d, J = 1.0 Hz, 1H), 7.15 (d, J = 1.1 Hz, 1H), 7.06 (d, J = 1.1 Hz, 1H), 6.99 (d, J = 1.2 Hz, 1H), 6.81 (d, J = 14.7 Hz, 1H), 6.54 (d, J = 15.2 Hz, 1H), 4.40–4.33 (m, 1H), 4.25–4.18 (m, 1H), 1.83–1.70 (m, 4H), 1.45 (d, J = 6.7 Hz, 3H), 1.44 (d, J = 6.7 Hz, 3H), 0.84 (d, J = 7.2 Hz, 3H), 0.81 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 188.6, 144.1, 143.3, 143.1, 131.8, 130.9, 130.5, 130.0, 126.3 125.9, 125.2, 118.7, 117.7, 53.40, 53.36, 30.9, 21.9, 21.7, 10.7, 10.6; IR (neat) νmax: 3105, 2969, 2933, 2876, 1645, 1606, 1576, 1504, 1456, 1419, 1337, 1258, 1111, 1078 cm−1; HRMS (ESI): m/z calculated for C21H29N4O [M+H]+ : 353.2341. Found: 353.2341.
4.7.11. (1E,4E,6E)-1,7-Bis(1-isopentyl-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (16)
Yellow oil, 20% yield; 1H NMR (400 MHz, CDCl3): δ 7.61 (d, J = 15.1 Hz, 1H), 7.55 (dd, J = 15.2, 11.6 Hz, 1H), 7.47 (d, J = 15.0 Hz, 1H), 7.40 (dd, J = 14.6, 11.6 Hz, 1H), 7.18 (d, J = 0.8 Hz, 1H), 7.13 (d, J = 0.9 Hz, 1H), 7.02 (d, J = 1.1 Hz, 1H), 6.94 (d, J = 1.1 Hz, 1H), 6.76 (d, J = 14.8 Hz, 1H), 6.55 (d, J = 15.2 Hz, 1H), 4.10–4.03 (m, 2H), 4.01–3.95 (m, 2H), 1.68–1.58 (m, 6H), 0.97 (t, J = 6.2 Hz, 12H); 13C NMR (75 MHz, CDCl3): δ 188.6, 144.0, 143.3, 143.1, 132.0, 130.6, 130.22, 130.15, 126.1, 126.0, 124.9, 122.6, 121.61, 44.8, 44.6, 40.4, 40.3, 25.8, 25.7, 22.5, 22.4; IR (neat) νmax: 3106, 2956, 2929, 2870, 1647, 1609, 1508, 1467, 1450, 1368, 1276, 1174, 1081 cm−1; HRMS (ESI): m/z calculated for C23H33N4O [M+H]+: 381.2654. Found: 381.2654.
4.7.12 (1E,4E,6E)-1,7-Bis(1-(pentan-2-yl)-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (17)
Yellow oil, 39% yield; 1H NMR (400 MHz, CDCl3): δ 7.61 (d, J = 15.0 Hz, 1H), 7.54 (dd, J = 15.1, 11.4 Hz, 1H), 7.53 (d, J = 15.0 Hz, 1H), 7.42 (dd, J = 14.6, 11.5 Hz, 1H), 7.21 (d, J = 1.0 Hz, 1H), 7.16 (d, J = 1.1 Hz, 1H), 7.07 (d, J = 1.1 Hz, 1H), 6.99 (d, J = 1.2 Hz, 1H), 6.81 (d, J = 14.6 Hz, 1H), 6.54 (d, J = 15.1 Hz, 1H), 4.50–4.42 (m, 1H), 4.35–4.27 (m, 1H), 1.76–1.69 (m, 4H), 1.45 (d, J = 6.7 Hz, 3H), 1.44 (d, J = 6.7 Hz, 3H), 1.27–1.14 (m, 4H), 0.89 (t, J = 7.2 Hz, 3H), 0.87 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 188.7, 144.0, 143.20, 143.15, 131.9, 130.9, 130.5, 130.3, 126.2, 125.1, 118.8, 117.7, 51.9, 51.8, 40.0, 22.4, 22.2, 19.5, 19.4, 13.80, 13.78; IR (neat) νmax: 2960, 2873, 1646, 1609, 1578, 1457, 1420, 1264, 1079 cm−1; HRMS (ESI): m/z calculated for C23H33N4O [M+H]+: 381.2654. Found: 381.2654.
4.7.13. (1E,4E,6E)-1,7-Bis(1-(pentan-3-yl)-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (18)
Yellow oil, 67% yield; 1H NMR (300 MHz, CDCl3): δ 7.59 (d, J = 15.0 Hz, 1H), 7.53–7.49 (overlapped, 1H), 7.51 (d, J = 15.0 Hz, 1H), 7.40 (dd, J = 14.4, 11.5 Hz, 1H), 7.21 (s, 1H), 7.17 (s, 1H), 7.01 (s, 1H), 6.93 (s, 1H), 6.79 (d, J = 14.5 Hz, 1H), 6.52 (d, J = 14.9 Hz, 1H), 4.15–4.03 (m, 1H), 3.98–3.86 (m, 1H), 1.92–1.77 (m, 4H), 1.75–1.61 (m, 4H), 0.76 (td, J = 7.3, 3.8 Hz, 12H); 13C NMR (75 MHz, CDCl3): δ 188.7, 145.0, 144.2, 143.1, 131.8, 131.1, 130.8, 130.0, 126.4, 126.0, 125.3, 118.7, 117.6, 59.8, 59.7, 29.2, 10.7, 10.6; IR (neat) νmax: 3103, 2965, 2933, 2867, 1644, 1605, 1574, 1504, 1454, 1270, 1175, 1078 cm−1; HRMS (ESI): m/z calculated for C23H33N4O [M+H]+: 381.2654. Found: 381.2656.
4.7.14. (1E,4E,6E)-1,7-Bis(1-(3-methylbut-2-en-1-yl)-1H-imidazol-2-yl)hepta-1,4,6-trien-3-one (19)
Yellow oil, 34% yield; 1H NMR (300 MHz, CDCl3): δ 7.56–7.45 (m, 3H), 7.36 (dd, J = 14.6, 11.6 Hz, 1H), 7.17 (s, 1H), 7.12 (s, 1H), 7.02 (s, 1H), 6.94 (s, 1H), 6.76 (d, J = 14.8 Hz, 1H), 6.55 (d, J = 15.0 Hz, 1H), 5.33–5.23 (m, 2H), 4.64 (d, J = 7.0 Hz, 2H), 4.57 (d, J = 6.6 Hz, 2H), 1.80 (s, 6H), 1.78 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 188.6, 144.2, 143.3, 143.0, 138.4, 137.8, 131.9, 130.6, 130.2, 130.0, 126.5, 125.9, 125.2, 122.4, 121.5, 119.0, 118.8, 44.3, 44.2, 25.7, 18.3, 18.2; IR (neat) νmax: 3107, 2968, 2916, 1646, 1609, 1579, 1469, 1441, 1376, 1258, 1163, 1081 cm−1; HRMS (ESI): m/z calculated for C23H29N4O [M+H]+: 377.2341. Found: 377.2343.
Compounds synthesized by method B
4.7.15. (1E,4E,6E)-1,7-Bis(4-methylthiazol-2-yl)hepta-1,4,6-trien-3-one (20)
Colorless oil, 30% yield; 1H NMR (300 MHz, CDCl3): δ 7.69 (d, J = 15.7 Hz, 1H), 7.49 (dd, J = 15.2, 10.7 Hz, 1H), 7.29 (d, J = 15.8 Hz, 1H), 7.20 (dd, J = 15.2, 10.7 Hz, 1H), 7.10 (d, J = 15.3 Hz, 1H), 7.04 (s, 1H), 6.93 (s, 1H), 6.67 (d, J = 15.2 Hz, 1H), 2.52 (s, 3H), 2.49 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 188.1, 164.7, 163.2, 155.4, 154.2, 142.2, 133.9, 132.1, 131.8, 128.3, 117.2, 115.4, 17.2, 17.0; IR (neat) νmax: 3103, 2959, 2921, 2854, 1647, 1609, 1580, 1506, 1436, 1329, 1294, 1074 cm−1; HRMS (ESI): m/z calculated for C15H15N2OS2 [M+H]+: 303.0626. Found: 303.0624.
4.7.16. (1E,4E,6E)-1,7-Di(thiazol-2-yl)hepta-1,4,6-trien-3-one (21)
Yellow oil, 41% yield; 1H NMR (300 MHz, CDCl3): δ 7.95 (d, J = 3.2 Hz, 1H), 7.87 (d, J = 3.2 Hz, 1H), 7.76 (d, J = 15.7 Hz, 1H), 7.49 (dd, J = 15.2, 9.7 Hz, 1H), 7.47 (d, J = 3.2 Hz, 1H), 7.37 (d, J = 3.2 Hz, 1H), 7.31 (d, J = 15.7 Hz, 1H), 7.19 (dd, J = 15.5, 9.8 Hz, 1H), 7.16 (d, J = 15.2 Hz, 1H), 6.68 (d, J = 15.2 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 188.0, 165.3, 164.0, 145.1, 144.4, 142.3, 134.0, 132.9, 131.8, 131.5, 128.7, 122.0, 120.4; IR (neat) νmax: 3112, 3077, 1647, 1610, 1578, 1476, 1394, 1247, 1153, 1075 cm−1; HRMS (ESI): m/z calculated for C13H11N2OS2 [M+H]+: 275.0313. Found: 275.0311.
4.7.17. (1E,4E,6E)-1,7-Bis(5-methylisoxazol-3-yl)hepta-1,4,6-trien-3-one (22)
Light yellow solid, mp. 162-163 °C, 54% yield; 1H NMR (300 MHz, CDCl3): δ 7.57 (d, J = 16.3 Hz, 1H), 7.46 (dd, J = 15.3, 9.7 Hz, 1H), 7.04–6.86 (m, 3H), 6.68 (d, J = 15.3 Hz, 1H), 6.23 (s, 1H), 6.19 (s, 1H), 2.47 (s, 3H), 2.45 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 188.4, 170.6, 170.1, 160.9, 160.2, 142.6, 133.0, 131.5, 130.5, 130.1, 128.9, 99.7, 99.2, 12.4; IR (neat) νmax: 3128, 2925, 1656, 1624, 1590, 1450, 1347, 1285, 1244, 1185, 1015 cm−1; HRMS (ESI): m/z calculated for C15H15N2O3 [M+H]+: 271.1083. Found: 271.1082.
4.7.18. (1E,4E,6E)-1,7-Bis(2-methyl-4-(trifluoromethyl)thiazol-5-yl)hepta-1,4,6-trien-3-one (23)
Yellow solid, mp. 147-148 °C, 64% yield; 1H NMR (300 MHz, CDCl3): δ 7.87 (d, J = 15.6 Hz, 1H), 7.40 (dd, J = 15.2, 11.0 Hz, 1H), 7.32–7.20 (m, 1H), 6.83 (d, J = 15.6, 1H), 6.69 (dd, J = 15.2, 11.0 Hz, 1H), 6.58 (d, J = 15.2 Hz, 1H), 2.74 (s, 3H), 2.72 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 187.1, 167.8, 166.5, 143.5 (q, JCF = 35.3 Hz), 142.4, 141.0 (q, JCF = 35.3 Hz), 137.6, 136.3, 132.7, 130.6, 130.3, 129.7, 129.6, 128.2, 120.8 (q, JCF = 270.8 Hz), 19.8, 19.7; IR (neat) νmax: 1650, 1601, 1590, 1483, 1359, 1325, 1205, 1161, 1099, 983 cm−1; HRMS (ESI): m/z calculated for C17H13F6N2OS2 [M+H]+: 439.0373. Found: 439.0376.
4.7.19. (1E,4E,6E)-1,7-Bis(4-bromo-1-methyl-1H-pyrazol-3-yl)hepta-1,4,6-trien-3-one (24)
Yellow oil, 31% yield; 1H NMR (300 MHz, CDCl3): δ 7.57 (d, J = 16.0 Hz, 1H), 7.48 (dd, J = 15.2, 11.3 Hz, 1H), 7.42 (s, 1H), 7.39 (s, 1H), 7.35 (d, J = 16.0 Hz, 1H), 7.25 (dd, J = 16.0, 11.3 Hz, 1H), 6.88 (d, J = 15.5 Hz, 1H), 6.59 (d, J = 15.2 Hz, 1H), 3.93 (s, 3H), 3.90 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 189.1, 146.4, 145.5, 143.4, 132.0, 131.7, 131.4, 130.2, 129.7, 128.8, 126.1, 96.3, 94.8, 40.1, 40.0; IR (neat) νmax: 3126, 2943, 1653, 1613, 1457, 1404, 1355, 1150, 1089 cm−1; HRMS (ESI): m/z calculated for C15H15Br2N4O [M+H]+: 424.9612. Found: 424.9613.
4.7.20. (1E,4E,6E)-1,7-Di(pyridin-3-yl)hepta-1,4,6-trien-3-one (25)
Yellow oil, 17% yield; 1H NMR (300 MHz, CDCl3): δ 8.82 (s, 1H), 8.72 (s, 1H), 8.62 (d, J = 4.6 Hz, 1H), 8.55 (d, J = 3.9 Hz, 1H), 7.91 (d, J = 7.9 Hz, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.68 (d, J = 16.0 Hz, 1H), 7.53 (ddd, J = 15.2, 8.0, 2.1 Hz, 1H), 7.36 (dd, J = 7.8, 3.0, Hz, 1H), 7.32 (dd, J = 4.8, 3.0 Hz, 1H), 7.07 (d, J = 16.0 Hz, 1H), 7.03–6.96 (m, 2H), 6.67 (d, J = 15.2 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 188.3, 151.3, 150.1, 149.2, 143.1, 139.6, 138.1, 134.7, 133.6, 131.9, 130.7, 129.8, 128.8, 127.1, 124.0, 123.9; IR (neat) νmax: 3031, 1650, 1617, 1576, 1476, 1415, 1196, 1085 cm−1; HRMS (ESI): m/z calculated for C17H15N2O [M+H]+: 263.1184. Found: 263.1183.
4.7.21. (1E,4E,6E)-1,7-Di(pyridin-2-yl)hepta-1,4,6-trien-3-one (26)
Yellow oil, 35% yield; 1H NMR (300 MHz, CDCl3): δ 8.66 (dd, J = 14.6, 4.2 Hz, 2H), 7.73-7.45 (overlapped, 7H), 7.39 (d, J = 7.6 Hz, 1H), 7.32-7.27 (m, 1H), 7.24-7.20 (m, 1H), 7.05 (d, J = 14.3 Hz, 1H), 6.72 (d, J = 14.6 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 189.3, 154.3, 153.3, 150.3, 150.1, 143.0, 141.6, 140.3, 137.0, 136.9, 131.7, 130.9, 128.3, 125.3, 124.5, 123.6, 123.4; IR (neat) νmax: 3050, 2924, 1652, 1621, 1528, 1470, 1432, 1151, 994 cm−1; HRMS (ESI): m/z calculated for C17H14N2O [M+H]+: 263.1184. Found: 263.1186.
4.7.22. (1E,4E,6E)-1,7-Bis(6-methylpyridin-2-yl)hepta-1,4,6-trien-3-one (27)
Yellow oil, 19% yield; 1H NMR (300 MHz, CDCl3): δ 7.65 (d, J = 15.5 Hz, 1H), 7.55 (d, J = 15.2 Hz, 1H), 7.64–7.39 (overlapped, 4H), 7.28 (d, J = 7.7 Hz, 1H), 7.08 (d, J = 15.5, 7.7 Hz, 2H), 7.08 (d, J = 7.3 Hz, 1H), 7.04 (d, J = 14.4 Hz, 1H), 6.74 (d, J = 14.8 Hz, 1H), 2.61 (s, 3H), 2.59 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 189.5, 159.2, 158.7, 153.6, 152.7, 143.0, 141.9, 140.1, 137.3, 137.2, 131.4, 131.1, 128.5, 124.4, 123.3, 122.3, 120.6, 24.7, 24.5; IR (neat) νmax: 3059, 2922, 2851, 1651, 1613, 1584, 1327, 1492, 1450, 1408, 1158, 1035 cm−1; HRMS (ESI): m/z calculated for C19H19N2O [M+H]+: 291.1497. Found: 291.1497.
4.7.23. 1E,4E,6E)-1,7-Bis(3-fluoropyridin-4-yl)hepta-1,4,6-trien-3-one (29)
Yellow semi-solid, 36% yield; 1H NMR (300 MHz, CDCl3): δ 8.56 (d, J = 2.1 Hz, 1H), 8.51 (d, J = 2.2 Hz, 1H), 8.48 (d, J = 5.1 Hz, 1H), 8.43 (d, J = 5.1 Hz, 1H), 7.70 (d, J = 16.2 Hz, 1H), 7.53 (dd, J = 15.2, 10.7 Hz, 1H), 7.49–7.42 (overlapped, 2H), 7.25 (d, J = 16.2 Hz, 1H), 7.28–7.19 (overlapped, 1H), 7.09 (d, J = 15.8 Hz, 1H), 6.75 (d, J = 15.3 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 188.1, 159.3, 155.8, 146.3, 145.9, 143.0, 139.6, 139.3, 139.2, 138.9, 133.8, 133.4, 131.5, 129.9, 129.7, 122.4, 121.3; IR (neat) νmax: 3038, 1655, 1600, 1416, 1087, 1000 cm−1; HRMS (ESI): m/z calculated for C17H13F2N2O [M+H]+: 299.0996. Found: 299.0997.
4.7.24. (1E,4E,6E)-1,7-Bis(1-isopropyl-1H-benzo[d]imidazol-2-yl)hepta-1,4,6-trien-3-one (30)
Yellow oil, 28% yield; 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 15.0 Hz, 1H), 7.81 (d, J = 15.3 Hz, 1H), 7.83–7.64 (overlapped, 4H), 7.58–7.56 (m, 1H), 7.54–7.51 (m, 1H), 7.32–7.26 (m, 4H), 7.12 (d, J = 14.4 Hz, 1H), 6.71 (d, J = 14.8 Hz, 1H), 5.04–4.97 (m, 1H), 4.91–4.84 (m, 1H), 1.71 (d, J = 7.0 Hz, 12H); 13C NMR (75 MHz, CDCl3): δ 188.2, 148.8, 147.8, 144.0, 143.9, 142.8, 134.7, 134.5, 134.3, 133.2, 130.5, 127.6, 126.2, 123.7, 123.3, 123.2, 122.9, 120.7, 120.3, 112.2, 111.7, 48.2, 48.0, 22.1; IR (neat) νmax: 3041, 2978, 2934, 2879, 1650, 1608, 1580, 1384, 1185, 1076, 996 cm−1; HRMS (ESI): m/z calculated for C27H29N4O [M+H]+: 425.2341. Found: 425.2341.
4.7.25. (1E,4E,6E)-1,7-Bis(1-isobutyl-1H-benzo[d]imidazol-2-yl)hepta-1,4,6-trien-3-one (31)
Yellow oil, 39% yield; 1H NMR (400 MHz, CDCl3): δ 7.95 (d, J = 15.1 Hz, 1H), 7.70 (d, J = 15.1 Hz, 1H), 7.83–7.64 (overlapped, 4H), 7.39–7.28 (m, 6H), 7.01 (d, J = 14.7 Hz, 1H), 6.69 (d, J = 15.1 Hz, 1H), 4.11 (d, J = 7.6 Hz, 2H), 4.03 (d, J = 7.6 Hz, 2H), 2.26–2.19 (m, 2H), 0.99 (d, J = 6.7 Hz, 6H), 0.97 (d, J = 6.7 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 188.2, 149.5, 148.6, 143.4, 143.2, 142.9, 136.3, 136.0, 134.3, 133.3, 129.9, 127.1, 125.6, 124.1, 123.62, 123.55, 123.2, 120.3, 120.0, 110.5, 110.2, 51.1, 30.1, 20.4, 20.3; IR (neat) νmax: 3050, 2960, 2872, 1649, 1608, 1578, 1444, 1404, 1319, 1081, 995 cm−1; HRMS (ESI): m/z calculated for C29H33N4O [M+H]+: 453.2654. Found: 453.2653.
4.7.26. (1E,4E,6E)-1,7-Bis(1-(sec-butyl)-1H-benzo[d]imidazol-2-yl)hepta-1,4,6-trien-3-one (32)
Yellow oil, 24% yield; 1H NMR (400 MHz, CDCl3): δ 7.92 (d, J = 15.0 Hz, 1H), 7.72 (d, J = 15.5 Hz, 1H), 7.84–7.64 (overlapped, 4H), 7.54 (dd, J = 6.6, 2.1 Hz, 1H), 7.49 (dd, J = 6.9, 1.8 Hz, 1H), 7.33–7.23 (m, 4H), 7.10 (d, J = 14.5 Hz, 1H), 6.70 (d, J = 15.0 Hz, 1H), 4.70–4.62 (m, 1H), 4.60–4.51 (m, 1H), 2.23–2.15 (m, 2H), 2.05–1.98 (m, 2H), 1.699 (d, J = 7.0 Hz, 3H), 1.696 (d, J = 6.9 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H), 0.80 (t, J = 7.4 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 188.3, 149.5, 148.5, 144.0, 143.8, 142.9, 134.7, 134.6, 134.4, 133.2, 130.5, 127.7, 126.3, 123.8, 123.3, 123.0, 120.7, 120.3, 112.2, 111.8, 54.4, 54.3, 28.9, 28.8, 20.4, 11.5, 11.4; IR (neat) νmax: 3052, 2967, 2875, 1648, 1607, 1578, 1489, 1443, 1382, 1185, 1080, 995 cm−1; HRMS (ESI): m/z calculated for C29H33N4O [M+H]+: 453.2654. Found: 453.2653.
4.7.27. (1E,4E,6E)-1,7-Bis(1-(pentan-2-yl)-1H-benzo[d]imidazol-2-yl)hepta-1,4,6-trien-3-one (33)
Yellow oil, 40% yield; 1H NMR (400 MHz, CDCl3): δ 7.85 (d, J = 15.0 Hz, 1H), 7.76–7.19 (m, 2H), 7.70–7.56 (overlapped, 2H), 7.65 (d, J = 15.1 Hz, 1H), 7.47–7.45 (m, 1H), 7.43–7.41 (m, 1H), 7.25–7.14 (m, 4H), 7.03 (d, J = 14.5 Hz, 1H), 6.63 (d, J = 14.9 Hz, 1H), 4.72–4.64 (m, 1H), 4.60–4.53 (m, 1H), 2.13–2.04 (m, 2H), 1.88–1.80 (m, 2H), 1.60 (d, J = 7.0 Hz, 6H), 1.22–1.14 (m, 2H), 1.07–0.96 (m, 2H), 0.80 (t, J = 7.3 Hz, 3H), 0.78 (t, J = 7.3 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 188.2, 149.3, 148.3, 143.9, 143.8, 142.8, 134.7, 134.6, 134.4, 133.1, 130.4, 127.6, 126.2, 123.7, 123.2, 122.9, 120.6, 120.3, 112.1, 111.7, 52.6, 52.4, 37.7, 20.6, 20.1, 20.0, 13.8; IR (neat) νmax: 3053, 2959, 2931, 2872, 1648, 1608, 1578, 1444, 1382, 1184, 1081 cm−1; HRMS (ESI): m/z calculated for C31H37N4O [M+H]+: 481.2967. Found: 481.2966.
4.7.28. (1E,4E,6E)-1,7-Bis(1-isopentyl-1H-benzo[d]imidazol-2-yl)hepta-1,4,6-trien-3-one (34)
Yellow oil, 13% yield; 1H NMR (400 MHz, CDCl3): δ 7.97 (d, J = 15.1 Hz, 1H), 7.78 (d, J = 14.5 Hz, 1H), 7.82–7.76 (overlapped, 2H), 7.69 (d, J = 15.1 Hz, 1H), 7.72–7.64 (overlapped, 1H), 7.38–7.31 (m, 4H), 7.30–7.26 (m, 2H), 6.99 (d, J = 14.8 Hz, 1H), 6.70 (d, J = 14.9 Hz, 1H), 4.28 (t, J = 5.3 Hz, 2H), 4.21 (t, J = 5.4 Hz, 2H), 1.71–1.68 (m, 6H), 1.02 (d, J = 6.2 Hz, 6H), 1.01 (d, J = 7.1 Hz, 6H); 13C NMR (75 MHz, CDCl3): δ 188.1, 149.0, 148.0, 143.2, 143.0, 142.9, 135.7, 135.4, 134.7, 133.4, 130.3, 126.6, 125.1, 124.2, 123.7, 123.4, 120.3, 120.0, 110.1, 109.8, 42.4, 42.2, 39.6, 39.4, 26.2, 26.1, 22.59, 22.55; IR (neat) νmax: 3063, 2956, 2929, 2870, 1689, 1654, 1614, 1467, 1410, 1331, 1154, 969 cm−1; HRMS (ESI): m/z calculated for C31H37N4O [M+H]+: 481.2967. Found: 481.2964.
4.7.29. (1E,4E,6E)-1,7-Bis(1-(pentan-3-yl)-1H-benzo[d]imidazol-2-yl)hepta-1,4,6-trien-3-one (35)
Yellow oil, 42% yield; 1H NMR (300 MHz, CDCl3): δ 7.92 (d, J = 15.0 Hz, 1H), 7.82–7.61 (m, 5H), 7.50–7.44 (m, 2H), 7.31–7.18 (m, 4H), 7.08 (d, J = 14.3 Hz, 1H), 6.68 (d, J = 14.7 Hz, 1H), 4.38–4.20 (m, 1H), 4.28–4.18 (m, 1H), 2.25–2.06 (m, 4H), 2.03–1.91 (m, 4H), 0.74 (q, J = 6.2 Hz, 12H); 13C NMR (75 MHz, CDCl3): δ 188.1, 150.2, 149.3, 143.7, 142.8, 134.6, 133.1, 130.4, 127.5, 126.1, 123.7, 123.2, 122.9, 120.5, 120.2, 112.0, 60.9, 27.1, 11.3, 11.1; IR (neat) νmax: 3064, 2966, 2933, 2875, 1650, 1609, 1580, 1383, 1081 cm−1; HRMS (ESI): m/z calculated for C31H37N4O [M+H]+: 481.2967. Found: 481.2968.
4.8 Cell culture
All cell lines were initially purchased from American Type Culture Collection (ATCC™). The PC-3 prostate cancer cell line, the LNCaP prostate cancer cell line, and the HeLa cervical cancer cell line were routinely cultured in RPMI-1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cultures were maintained in a high humidity environment supplemented with 5% carbon dioxide at a temperature of 37°C. The DU-145 prostate cancer cells were routinely cultured in Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% FBS and 1% penicillin/streptomycin.
4.9 Trypan blue dye exclusion assay
PC-3, DU145, LNCaP, or HeLa cells were plated in 24-well plates at a density of 20,000 each well in 10% FBS RPMI-1640 medium. The cells were then treated with curcumin, or synthesized analogues separately at different doses for 3 days, while equal treatment volumes of DMSO were used as vehicle control. Cell numbers were counted with a cell viability analyzer (VI-Cell XR, Beckman Coulter). The ratio of drug treated viable cell numbers to vehicle treated viable cell numbers was defined as percentage viability and variation between replicate experiments is not greater than 5%. The inhibitory rates in Table 2 were expressed as cell viability reduction rates (percentage viability of untreated cell culture control - percentage viability of drug-treated cell culture). The IC50 value is the concentration of each compound that inhibits cell growth by 50% under the experimental conditions and is the average from triplicate determinations that were reproducible and statistically significant. For calculation of the IC50 values, a linear relationship between % inhibition and treatment concentration was established using at least five dosages for each compound.
4.10. WST-1 cell proliferation assay
PC-3, DU-145, LNCaP, or HeLa cells were plated in 96-well plates at a density of 3,200 each well in 100 μL of culture medium. The cells were then treated with curcumin, or synthesized analogues separately at different doses from 0.1 μM to 1 μM for 3 days, while equal treatment volumes of DMSO were used as vehicle control. The cells were cultured in a CO2 incubator at 37 °C for three days. 10 μL of the premixed WST-1 cell proliferation reagent (Clontech) was added to each well. After mixing gently for one minute on an orbital shaker, the cells were incubated for additional 3 hours at 37 °C. To ensure homogeneous distribution of color, it is important to mix gently on an orbital shaker for one minute. The absorbance of each well was measured using a microplate-reader (Synergy HT, BioTek) at a wavelength of 430 nm. The IC50 value is the concentration of each compound that inhibits cell proliferation by 50% under the experimental conditions and is the average from triplicate determinations that were reproducible and statistically significant. For calculating the IC50 values, a linear proliferative inhibition was made based on at least five dosages for each compound.
4.11 Cell cycle analysis
PC-3 cells were plated in 24-well plates at a density of 200,000 each well in 400 μL of culture medium. After 3 hours of cell attachment, the cells were then treated with compound 30 at 5 μM for 15 hours, while equal treatment volumes of DMSO were used as vehicle control. The cells were cultured in CO2 incubator at 37°C for 22 hours and 31 hours, respectively. Both attached and floating cells were collected in a centrifuge tube by centrifugation at rcf value 450 g for 5 minutes. After discarding the supernatant, the collected cells were re-suspended with 500 μL 80% cold ethanol to fix for 30 minutes in 4°C. The fixed cell could store in −20°C for one week. After fixation, the ethanol was removed after centrifuging and the cells were washed with PBS. The cells were then re-suspend with 100μL of 100 mg/mL ribonuclease and were cultured at 37°C for 30 minutes to degrade all RNA. The cells were stained with 200 μL of 50μg/mL propidium iodide stock solution for 30 minutes at −20°C, and then the fluorescence intensity of PI was detected in individual PC-3 cells using an Attune flow cytometer (Life Technologies) within 0.5 to 1 hour after staining.
4.12 F2N12S and CYTOX AADvanced double staining assay
PC-3 cells were plated in 24-well plates at a density of 200,000 each well in 400 μl of culture medium. After 3 hours of cell attachment, the cells were then treated with each test compound at different concentration for 15 hours, while equal treatment volumes of DMSO were used as vehicle control. The cells were cultured in CO2 incubator at 37°C for 15 hours. Both attached and floating cells were collected in a centrifuge tube by centrifugation at rcf value 450 g for 5 to 6 minutes. The collected cells were re-suspended with 500 μl HBSS to remove proteins which may affect flow signal and centrifuged again. After discarding the supernatant, the collected cells were re-suspended with 300 μl HBSS and stained with 0.3 μl of F2N12S for 3-5 minutes followed by 0.3 μl Sytox AAdvanced for an additional 5 minutes. The fluorescence intensity of the two probes was further measured in individual PC-3 cells using an Attune flow cytometer (Life Technologies) 0.5 to 1 hour after staining.
4.13. Statistical analysis
All data are represented as the mean ± standard deviation (S.D.) for the number of experiments indicated. Other differences between treated and control groups were analyzed using the Student’s t-test. A p-value < 0.05 was considered statistically significant.
Supplementary Material
Highlights.
29 new and 1 known trienone analogues with heteroaromatic rings were prepared.
Analogues showed superior cytotoxicity against prostate and cervical cancer cells.
10 analogues are 5- to 36-fold more potent than curcumin.
A new scaffold was identified for the potential treatment of cancer.
The most potent compound induced G1/G0 cell cycle arrest and cell apoptosis.
Acknowledgments
This study was financially supported by California State University (CSU)-Fresno and CSU Program for Education and Research in Biotechnology (CSUPERB) New Investigator Award and Research Development Award. HRMS was supported by NIH RCMI program at Xavier University of Louisiana through Grant 2G12MD007595-064 (G.W.) and NIH-NIGMS through Grant 1U54GM104940 (G.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data related to this article including the representative 1H and 13C NMR spectra for final products can be found at http://dx.doi.org/
References
- [1].Kuttan R, Bhanumathy P, Nirmala K, George MC. Potential anticancer activity of turmeric (Curcuma longa) Cancer Lett. (Shannon, Ireland) 1985;29:197–202. doi: 10.1016/0304-3835(85)90159-4. [DOI] [PubMed] [Google Scholar]
- [2].Dorai T, Gehani N, Katz A. Therapeutic potential of curcumin in human prostate cancer. I. Curcumin induces apoptosis in both androgen-dependent and androgen-independent prostate cancer cells. Prostate Cancer Prostatic Dis. 2000;3:84–93. doi: 10.1038/sj.pcan.4500399. [DOI] [PubMed] [Google Scholar]
- [3].Toennesen HH, Karlsen J, Mostad A. Structural studies of curcuminoids. I. the crystal structure of curcumin. Acta. Chem. Scand., Ser. B. 1982;B36:475–479. [Google Scholar]
- [4].Teiten M-H, Gaascht F, Eifes S, Dicato M, Diederich M. Chemopreventive potential of curcumin in prostate cancer. Genes Nutr. 2010;5:61–74. doi: 10.1007/s12263-009-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gupta SC, Prasad S, Kim JH, Patchva S, Webb LJ, Priyadarsini IK, Aggarwal BB. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011;28:1937–1955. doi: 10.1039/c1np00051a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- [7].Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- [8].Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “curecumin”: from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- [9].Garcea G, Jones DJL, Singh R, Dennison AR, Farmer PB, Sharma RA, Steward WP, Gescher AJ, Berry DP. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br. J. Cancer. 2004;90:1011–1015. doi: 10.1038/sj.bjc.6601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Anand P, Thomas SG, Kunnumakkara AB, Sundaram C, Harikumar KB, Sung B, Tharakan ST, Misra K, Priyadarsini IK, Rajasekharan KN, Aggarwal BB. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008;76:1590–1611. doi: 10.1016/j.bcp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- [11].Chen Q-H. Curcumin-based anti-prostate cancer agents. Anti-Cancer Agents Med. Chem. 2015;15:138–156. doi: 10.2174/1871520615666150116102442. [DOI] [PubMed] [Google Scholar]
- [12].Samaan N, Zhong Q, Fernandez J, Chen G, Hussain AM, Zheng S, Wang G, Chen Q-H. Design, synthesis, and evaluation of novel heteroaromatic analogues of curcumin as anti-cancer agents. Eur. J. Med. Chem. 2014;75:123–131. doi: 10.1016/j.ejmech.2014.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang R, Chen C, Zhang X, Zhang C, Zhong Q, Chen G, Zhang Q, Zheng S, Wang G, Chen Q-H. Structure-activity relationship and pharmacokinetic studies of 1,5-diheteroarylpenta-1,4-dien-3-ones: a class of promising curcumin-based anticancer agents. J. Med. Chem. 2015 doi: 10.1021/acs.jmedchem.5b00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Payton F, Samdusky P, Alworth WL. NMR study of the solution structure of curcumin. J. Nat. Prod. 2007;70:143–146. doi: 10.1021/np060263s. [DOI] [PubMed] [Google Scholar]
- [15].Park S-Y, Kim DSHL. Discovery of natural products from Curcuma longa that protect cells from beta-amyloid insult: a drug discovery effort against Alzheimer's disease. J. Nat. Prod. 2002;65:1227–1231. doi: 10.1021/np010039x. [DOI] [PubMed] [Google Scholar]
- [16].Jang MK, Sohn DH, Ryu J-H. A curcuminoid and sesquiterpenes as inhibitors of macrophage TNF-α release from Curcuma zedoaria. Planta Med. 2001;67:550–552. doi: 10.1055/s-2001-16482. [DOI] [PubMed] [Google Scholar]
- [17].Chuprajob T, Changtam C, Chokchaisiri R, Chunglok W, Sornkaew N, Suksamrarn A. Synthesis, cytotoxicity against human oral cancer KB cells and structure-activity relationship studies of trienone analogues of curcuminoids. Bioorg. Med. Chem. Lett. 2014;24:2839–2844. doi: 10.1016/j.bmcl.2014.04.105. [DOI] [PubMed] [Google Scholar]
- [18].Goto Y, Nakayama M. Photopolymerization initiator compositions containing pyridyl ketones and aromatic per acid esters. Jpn. Kokai Koho. 1988 JP63265901A 19881102. [Google Scholar]
- [19].Le PQ, Nguyen TS, May JA. A general method for the enantioselective synthesis of α-chiral heterocycles. Org. Lett. 2012;14:6104–6107. doi: 10.1021/ol3030605. [DOI] [PubMed] [Google Scholar]
- [20].van Loevezijn A, Venhorst J, Iwema Bakker WI, de Korte CG, de Looff W, Verhoog S, van Wees J, van Hoeve M, van de Woestijne RP, van der Neut MAW, Borst AJM, van Dongen MJP, de Bruin NMWJ, Keizer HG, Kruse CG. N'-(Arylsulfonyl)pyrazoline-1-carboxamidines as novel, neutral 5-hydroxytryptamine 6 receptor (5-HT R) antagonists with unique structural features. J. Med. Chem. 2011;54:7030–7054. doi: 10.1021/jm200466r. [DOI] [PubMed] [Google Scholar]
- [21].MacDonald FK, Burnell DJ. 2,3-Dihydro-4H-pyran-4-ones and 2,3-dihydro-4-pyridinones by cyclizations of α,β-unsaturated 1,3-diketones. J. Org. Chem. 2009;74:6973–6979. doi: 10.1021/jo901355e. [DOI] [PubMed] [Google Scholar]
- [22].Kobayashi S, Semba T, Takahashi T, Yoshida S, Dai K, Otani T, Saito T. A novel and efficient stereo-controlled synthesis of hexahydroquinolinones via the diene-transmissive hetero-Diels-Alder reaction of cross-conjugated azatrienes with ketenes and electrophilic dienophiles. Tetrahedron. 2009;65:920–933. [Google Scholar]
- [23].Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Inves. Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- [24].Stone KR, Mickey DD, Wunderli H, Michey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU145) Int. J. Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305. [DOI] [PubMed] [Google Scholar]
- [25].Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GGP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- [26].Srivastava RK, Chen Q, Siddiqui I, Sarva K, Shankar S. Linkage of curcumin-induced cell cycle arrest and apoptosis by cyclin-dependent kinase inhibitor p21/WAF1/CIP1. Cell Cycle. 2007;6:2953–2961. doi: 10.4161/cc.6.23.4951. [DOI] [PubMed] [Google Scholar]
- [27].Chen HW, Huang HC. Effect of curcumin on cell cycle progression and apoptosis in vascular smooth muscle cells. Br. J. Pharmacol. 1998;124:1029–1040. doi: 10.1038/sj.bjp.0701914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Singh AK, Sidhu GS, Deepa T, Maheshwari RK. Curcumin inhibits the proliferation and cell cycle progression of human unbilical vein endothelial cell. Cancer Lett. 1996;107:109–115. doi: 10.1016/0304-3835(96)04357-1. [DOI] [PubMed] [Google Scholar]
- [29].Choudhuri T, Pal S, Das T, Sa G. Curcumin selectively induces apoptosis in deregulated cyclin D1-expressed cells at G2 phase of cell cycle in a p53-dependent manner. J. Biol. Chem. 2005;280:20059–20068. doi: 10.1074/jbc.M410670200. [DOI] [PubMed] [Google Scholar]
- [30].Hilchie AL, Furlong SJ, Sutton K, Richardson A, Robichaud MRJ, Giacomantonio CA, Ridgway ND, Hoskin DW. Curcumin-induced apoptosis in PC3 prostate carcinoma cells is caspase-independent and involves cellular ceramide accumulation and damage to mitochondria. Nutr. Cancer. 2010;62:379–389. doi: 10.1080/01635580903441238. [DOI] [PubMed] [Google Scholar]
- [31].Wichitnithad W, Nimmannit U, Wacharasindhu S, Rojsitthisak P. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules. 2011;16:1888–1900. doi: 10.3390/molecules16021888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yadav B, Taurin S, Rosengren RJ, Schumacher M, Diederich M, Somers-Edgar TJ, Larsen L. Synthesis and cytotoxic potential of heterocyclic cyclohexanone analogs of curcumin. Bioorg. Med. Chem. 2010;18:6701–6707. doi: 10.1016/j.bmc.2010.07.063. [DOI] [PubMed] [Google Scholar]
- [33].Sehnal P, Taghzouti H, Fairlamb IJS, Jutand A, Lee AF, Whitwood AC. Heteroaromatic analogs of dibenzylideneacetone (dba) and Pd02(het-dba)3 complexes: effect of a Thienyl moiety on the reactivity of Pd0(η2-thn-dba)(PPh3)2/Pd0(PPh3)2 (n = 1 or 2) and Pd0(η2-th2-dba)(dppe)/Pd0(dppe) in oxidative addition reactions with iodobenzene. Organometallics. 2009;28:824–829. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.