Abstract
Phonemic paraphasias are a common presenting symptom in aphasia and are thought to reflect a deficit in which selecting an incorrect phonemic segment results in the clear-cut substitution of one phonemic segment for another. The current study re-examines the basis of these paraphasias. Seven left hemisphere-damaged aphasics with a range of left hemisphere lesions and clinical diagnoses including Broca’s, Conduction, and Wernicke’s aphasia, were asked to produce syllable-initial voiced and voiceless fricative consonants, [z] and [s], in CV syllables followed by one of five vowels [i e a o u] in isolation and in a carrier phrase. Acoustic analyses were conducted focusing on two acoustic parameters signaling voicing in fricative consonants: duration and amplitude properties of the fricative noise. Results show that for all participants, regardless of clinical diagnosis or lesion site, phonemic paraphasias leave an acoustic trace of the original target in the error production. These findings challenge the view that phonemic paraphasias arise from a mis-selection of phonemic units followed by its correct implementation, as traditionally proposed. Rather, they appear to derive from a common mechanism with speech errors reflecting the co-activation of a target and competitor resulting in speech output that has some phonetic properties of both segments.
Introduction
Historically, the study of speech errors in both normal and aphasic populations has informed and challenged theories of the processes involved in spoken word production. In the study of normal populations, speech errors have been viewed as providing evidence for the psychological reality of phonemes and phonological features, which in turn has been used to support various stages of speech production. Here, production of a word is considered to be a serial process occurring in separate stages, with the sound shape of a lexical entry accessed, followed by phonological planning, and ultimately articulatory implementation. Speech production models based on speech errors in normals (cf. Meyer, 1992) located most speech errors at the phonological planning level, thereby taking into account the fact that (a.) most sound errors are perceived by the listener as clear-cut substitutions of one phoneme segment for another, (b.) they typically occur in single segments, and (c.) if an erroneous segment is temporally shifted, it is well-formed (Fromkin, 1971; Shattuck-Hufnagel & Klatt, 1979; Shattuck-Hufnagel, 1987). The separation of the phonological encoding level from the phonetic or articulatory implementation level (Dell, 1986; Dell, Juliano, & Govindjee, 1993; Levelt, Roelofs, and Meyer, 1999) resulted in articulatory implementation being viewed as essentially independent of phonological encoding processes.
Research focusing on speech production deficits of aphasic patients has similarly used the construct of the psychological reality of phonemes and features in the attempt to elucidate error types in aphasic speech. In particular, the phoneme errors produced by both anterior and posterior aphasics have been viewed as including the addition, deletion, substitution, or incorrect sequencing of one or more phonemes within a word. Both patients with anterior lesions, clinically diagnosed as Broca’s or non-fluent aphasics, and patients with posterior lesions, clinically diagnosed as Conduction and Wernicke’s aphasics, show similar patterns: phoneme substitution errors are the most common error type; there are more consonant than vowel errors; and more single feature substitutions occur than multiple feature substitutions (Blumstein 1973; Burns & Canter 1977; Haley, Jacks, & Cunningham, 2013; Halpern, Keith & Darly, 1976; Lecours & Lhermitte, 1969). These phoneme errors in aphasic populations are subsumed under the category of phonemic or literal paraphasias.
Despite similarities in phonological errors, anterior and posterior aphasics have been differentiated in the aphasia literature on the basis of the presence (or absence) of phonetic errors (for review see Blumstein, 2000). While posterior aphasics have been described as having fluent speech with multiple phonemic paraphasias, the speech of anterior aphasics has been characterized as slow, labored, with phonetic distortions and initiation difficulties. The occurrence of phonetic distortions is not systematic in that they do not appear in every utterance nor do they routinely occur in the same phonemes.
Importantly, acoustic analyses of speech production deficits in anterior aphasics have shown that despite articulatory output deficits, the patients maintain the underlying phonological distinctions. In particular, although anterior aphasics have shown deficits in the production of voice-onset time (VOT), a temporal cue distinguishing voiced and voiceless stop consonants (Blumstein, Cooper, Goodglass, Statlender & Gottlieb, 1980; Blumstein, Cooper, Zurif, & Caramazza, 1977; Freeman, Sands, & Harris, 1978; Gandor & Dardarananda, 1984; Itoh, Sasanuma, Hirose, Yoshioka, & Ushijima, 1980; Shewan, Leeper, & Booth, 1984), they can appropriately implement the voiced-voiceless distinction in the production of word final voiced and voiceless stop consonants utilizing the duration of the preceding vowel (Baum, Blumstein, Naeser, & Palumbo, 1990). Similarly, anterior aphasics have shown impaired production of voiced fricatives (Baum et al, 1990; Harmes, Daniloff, Hoffman, Lewis, Kramer, & Absher, 1984). However, Kurowski, Hazen and Blumstein (2003) found that these patients were able to coordinate the articulatory gestures for voicing to distinguish voiced and voiceless fricatives, but exhibited abnormal glottal patterning in voiced fricatives due to weak glottal excitation. In summary, it may be said that, in the aphasia literature, there has been an established dichotomy between phonological selection/planning deficits characteristic of posterior aphasics and articulatory implementation deficits characteristic of anterior aphasics (see Blumstein, 2000 for a review).
Nonetheless, it is worth noting that, even in the context of this dichotomy, anterior aphasics do make phonemic paraphasias, suggesting that they too display phonological selection and planning deficits (Blumstein, 1973; Haley et al., 2013), and posterior aphasics show phonetic changes in their speech output, suggesting that they have subtle articulatory implementation deficits (Baum et al., 1990). Taken together, these findings suggest that some phonemic paraphasias reflect phonetic (articulatory) rather than phonological (selection) impairments, consistent with the view that the processes involved in phonological selection/planning and articulatory implementation stages may not be as independent as suggested in the literature, but rather they are inextricably linked. Indeed, in a series of review papers, Buckingham (Buckingham, 1986; 1992a; Buckingham & Yule, 1987) challenged the view that phonemic paraphasias were clear-cut substitutions. Rather, he proposed that many phonemic paraphasias were what he called ‘phonemic false evaluations,’ whereby the speaker produced a phonetically distorted target phoneme that was subsequently misperceived by the listener. In his argument, Buckingham (Buckingham & Yule, 1987) referred to the results of several studies suggesting that both anterior and posterior aphasics showed articulatory impairments in the production of target phonemes (Blumstein, Cooper, Goodglass, Statlender, & Gottlieb, 1980; MacNeilage, Hutchinson & Lasater, 1981; Tuller, 1984).
Indeed, in the last two decades, a number of instrumental studies of normal speech has provided evidence against the simple division of speech errors into phonological versus phonetic categories that had been based primarily on transcription evidence. Boucher (1994) showed in x-ray films of target and speech error tokens intermediate articulations as evidenced by tongue overshoot and undershoot in word-initial consonants. Mowrey and MacKay (1990), using electromyographic recordings to observe speech motor activity in a tongue twister task, found that one third of the tokens showed intermediate articulations between the error and the target (i.e. two motor patterns produced at the same time) as well as graded (sub-phonemic) motor activity which in some cases resulted in an intermediate percept. They claimed that their results supported Laver’s (1980) hypothesis that induced vocalic errors including both English and non-English diphthongs might originate from the speaker’s indecision, sending neuromuscular commands simultaneously for two canonical forms resulting in an intermediate vowel. Mowrey and MacKay (1990) suggest further that models of speech production need to provide for “parallel” processing of two structures to the level of motor specification, allowing for “simultaneous and graded execution” (p. 1310).
The issue of gradient errors and the output stage they originate from has become a focus in the more recent speech error literature. Using the SLIP technique (Spoonerisms of Laboratory Induced Predisposition) to elicit speech errors, Pouplier (2007) applied articulography to examine intrusion and reduction speech errors which were described as “errors in phasing between the interacting consonant gestures” (p. 311) and concluded that her data showed “a gestural intrusion bias, leading to the simultaneous production of the intended and an intruding errorful consonant gesture” (p. 336) (see also Möller, Jansma, Rodriguez-Fornells, & Münte, 2007).
Using the gestural model, Goldstein, Pouplier, Chen, Saltzman, & Byrd (2007) elicited speech errors in a repetition task and analyzed them kinematically (cf. also Pouplier, Chen, Goldstein, & Byrd, 1999). Goldstein et al speculated that “gradient gestural errors (subsegmental)… are occurring at the speech planning level rather than purely at the level of low-level articulatory execution” (p. 408). Using acoustic analyses of fricative voicing, duration, and amplitude, Frisch and Wright (2002) focused on word-initial [s] and [z] in tongue twisters. Their results were consistent with the view that speech errors reflected a gradient phonetic level with low-level components of individual phonetic features being distorted. These low-level errors would be potentially classified as articulatory and not phonological in nature. They also pointed out that, in order to account for these gradient speech errors, speech production models must include processes of activation and competition among phonetic articulatory plans.
One of the models that specifically includes both activation and competition among articulatory plans is the cascading activation model (Goldrick, 2006; Goldrick & Chu, 2013; Rapp & Goldrick, 2000). This model allows an earlier lexical processing stage to generate multiple lexical representations including the target word and potential lexical competitors. These competing representations are sent downstream with their corresponding phonological representations partially activated. In cases where there remains partial activation of both the target word and competitor, articulatory implementation reflects phonetic properties of both representations.
Basing their study within the framework of cascading activation, Goldrick and Blumstein (2006) explored the relationship between phonological planning and articulatory processes by inducing speech errors using tongue twisters that focused on word-initial [k] and [g] (see also McMillan & Corey, 2010). Acoustic analysis of voice-onset time (VOT) in these stop consonants revealed traces of the target token in the VOT durations of error tokens: that is, when compared to their non-tongue twister baseline productions, the VOT of target voiced tokens were longer and the VOT of target voiceless tokens were shorter. These traces were hypothesized to be the result of cascading activation of the phonological representations of competing lexical candidates to articulatory processes.
The hypothesis that there is co-activation and hence competition between lexical candidates is an inherent property of Dells’ model of spoken word production (1986). Here, interactive spreading activation occurs between lexical and phonological levels, with activation spreading from word units to lower level constituent phonemes, and activation of these lower level constituents in turn feeding back to the lexical level. As a result, the activation of a target word is increased by the activation of its phonological neighbors (cf. also Dell & Gordon, 2003). The greater the number of neighbors, the more likely the target word will be selected and encoded correctly at the phonological level.
Applying this framework to lexical access in aphasia, Gordon (2002) showed that the extent of competition among words in the lexicon influences access to target lexical candidates. In particular, the occurrence of paraphasic errors in aphasia was influenced by neighborhood density; the greater the number of phonologically similar words, the fewer phonological errors made by the aphasic participants. While these findings support the view that the co-activation of lexical candidates influences lexical access in aphasia (cf. also Dell, Schwartz, Martin, Saffran, & Gagnon, 1997), it remains unclear whether co-activation of lexical candidates cascades to and influences articulatory processes in aphasic speech, and hence whether acoustic traces would be found in the production of a phonological paraphasia.
It is the goal of the current study to examine this question. In particular, we investigated whether phonemic paraphasias arise from: a). a mis-selection and planning of phonemic units, as traditionally proposed, b). a combination of mis-selection and planning of phonemic units and articulatory (phonetic) impairments, as proposed by Buckingham (1986; 1992a; Buckingham & Yule, 1987), or c). whether they reflect the co-activation of a target and competitor resulting in speech output that has some phonetic properties of both. To this end, syllable-initial voiced and voiceless fricative consonants, [z] and [s], were produced in CV syllables by both anterior and posterior aphasics. Acoustic analyses were conducted focusing on two acoustic parameters signaling voicing in fricative consonants: duration and amplitude properties of the fricative noise (Crystal & House, 1988: Stevens., Blumstein, Glicksman, Burton & Kurowski, 1992).
Our hypothesis is that paraphasias will show acoustic ‘traces’ of the target stimulus, i.e. voiceless paraphasias, [z] → [s*], will be more [z]-like, and voiced paraphasias, i.e. [s] → [z*], will be more [s]-like. Additionally, we hypothesize that both anterior and posterior aphasics will show similar patterns of performance, challenging the strict dichotomy in the aphasia literature claiming that posterior aphasics have phonological selection/planning deficits and anterior aphasics have articulatory implementation deficits. Such findings have important implications for the nature of speech output deficits in aphasia, for they suggest that phonemic paraphasias arise not from phonological selection stages, but result from co-activation of representations which ultimately influence articulatory implementation.
Method
Subjects
Seven aphasic subjects participated: three Broca’s aphasics (2 male, one female), three Conduction aphasics (2 males, one female), and one Wernicke’s aphasic (male). These patients were recruited from the Harold Goodglass Aphasia Research Center at the Boston VA Medical Center (n = 2) as well as from various Rhode Island hospitals: the Providence VA Medical Center (n = 2), Roger Williams Medical Center (n = 2), and Memorial Hospital (n = 1). All of the subjects were right-handed, native speakers of English. Aphasia diagnosis was made on the basis of clinical examination using the Boston Diagnostic Aphasic Examination (BDAE) (Goodglass & Kaplan 1983). Table 1 provides the BDAE scores for overall fluency, articulation, and auditory comprehension, in addition to a description of the lesion localization for each patient.
Table 1.
Clinical and lesion profile of aphasic patients (B= Broca’s, C= Conduction, and W = Wernicke’s aphasia)
| Subj. | Gender | Age at testing | Time post onset | BDAE Subtests | Lesion |
|---|---|---|---|---|---|
| B1 | Female | 58 | 13 yrs. | Fluency = +0.58 Aud Comp = +0.95 Articulation = 50% |
Large left insular lesion extending to anterior temporal lobe, sparing both Wernicke’s area and the anterior region of Broca’s area. |
| B2 | Male | 57 | 15 yrs | Fluency = +0.55 Aud Comp = +0.96 Articulation = 45% |
Involvement of the entire lenticostriate artery distribution of the MCA territory including the left caudate nucleus, globus pallidus, and the intervening anterior internal capsule. The infarct extends laterally to involve the medial temporal cortex and insula and superiorly to involve the PVWM on the left anteriorly. Lucency encroaches upon the posterior limbic internal capsule. The lesion involves the anterior temporal lobe but not mesial temporal structures. There is a separate small lesion in the area of the sub-thalamic nucleus and spinol-thalamic tract; the lesion spares Broca’s and Wernicke’s areas. Low density in the entire region between Broca’s and Wernicke’s areas from subcortical through insula and into temporal cortex. |
| B3 | Male | 74 | 25 yrs. | Fluency = +0.40 Aud Comp = +0.77 Articulation = 40% |
Left frontal lesion involving the posterior half of Broca’s area and most of the middle frontal gyrus with deep extension to the border of the left frontal horn. The lesion involves the head of the caudate and anterior limb of the internal capsule. It extends superiorly into the pre-motor, motor and sensory cortex areas and the white matter deep to these areas including the periventricular white matter adjacent to the body of the left lateral ventricle and undercutting fibers of the supplementary motor area. There is a clip in the anterior communicating artery. |
| C1 | Male | 57 | 2 yrs. | Fluency = +0.64 Aud Comp = +0.52 Articulation = 60% |
Left posterior temporoparietal lesion involves the insula and 1/4 of Wernicke’s area with superior extension into the supramarginal and angular gyrus areas and the white matter deep to them. The lesion continues up into the superior parietal lobule. There is slight bilateral frontal atrophy. |
| C2 | Female | 88 | 1 yr. | Fluency = +0.61 Aud Comp = +0.84 Articulation = 60% |
New left posterior temporoparietal infarct post left craniotomy for chronic bifrontal subdural hematoma (R>L). Small left hemisphere lesion in the white matter deep to the posterior portion of the middle temporal gyrus and deep to the posterior half of Wernicke’s area. There are also multiple scattered high signal intensities throughout the white matter bilaterally. There are also bilateral subdural hematomas present. In the right hemisphere, the subdural is very large and is present along the entire convexity from anterior (frontal) to posterior (occipital). On the left, the subdural is present in the frontal and parietal regions. |
| C3 | Male | 66 | 1 yr. | Fluency = +0.64 Aud Comp = +0.53 Articulation = 65% |
Left temporo-parietal lesion involving a little less than half of Wernicke’s area with superior extension into the supramarginal and angular gyri and the white matter deep to these areas. The lesion is in part of Wernicke’s area but in all of SMG. No Broca’s area involvement. No evidence of hemorrhage. There is moderate atrophy. |
| W1 | Male | 73 | 2 yrs. | Fluency = +0.66 Aud Comp = −1.65 Articulation = 60% |
Large focal area of low attenuation in the left fronto-parietal area consistent with subacute cortical infarct in L MCA; |
Stimuli
The target stimuli consisted of CV syllables: an initial alveolar fricative consonant [s, z] followed by one of five vowels [i e a o u]. These CV syllables were spoken in two experimental conditions: isolation and context. In the isolation condition, the target CV syllables were produced in citation form. This condition provided a means for examining productions independent of context and then to explore potential differences in initial consonant production as a function of the preceding context. Every fricative-vowel combination occurred once in each of eight separately randomized blocks of tokens, yielding a total of 80 token trials.
In the context condition, the CV syllables were spoken in two carrier phrases (“Please speak _ again”; “Say a big _ again”), which allowed for examination of potential changes in voicing of fricative consonants as a function of the voicing of the preceding consonant The context condition was also employed to reduce difficulties in initiating speech often encountered by Broca’s aphasics. Every combination of carrier phrase and CV target appeared once in each of eight randomly ordered blocks of sentences, yielding a total of 160 tokens. (The context condition results are reported in the supplementary materials).
The stimuli were printed in orthographic form on 3×5 cards which the subjects were instructed to read, speaking naturally. If a subject was unable to produce the target syllable by reading the card, the experimenter read the token aloud and asked the subject to repeat it. If the patient was unable to repeat the full sentence context, it was reduced to “Speak __” and “Big __”. Tokens that the subject could not produce were presented once more at the end of the stimulus block. Short breaks were provided at the end of each block and as needed.
Given that the goal of this study is to examine whether the acoustic properties of paraphasic errors show a ‘voicing’ trace of the intended consonant target, acoustic analyses were performed only on those utterances that were perceived by the examiners as either exemplars of the target CV syllable or as exemplars of a voicing paraphasia [s] → [z*] or [z] → [s*]. Thus, utterances in which the patient made other types of paraphasias on the CV target syllable (including the vowel) were excluded from analysis. All of the analyzed tokens were labeled as to whether a paraphasia occurred on the patient’s first attempt or second attempt to produce the target syllable. The first and second attempt utterances were analyzed separately for the following reason. It was unclear whether a second attempt reflected a failure to produce the originally presented target, or the influence of the production or perception of the initial first attempt on the second attempt by the participant. Hence, the ‘source’ of the paraphasia in the second utterance may not have been the same as that of the first utterance (cf. also Buckingham (1992a) for a similar view in considering multiple attempts or conduite d’approche produced by Conduction aphasics). For this reason, we report only the results of the first utterance.
In total, there were 95 first attempt voicing phonemic paraphasias produced by the Broca’s aphasics (B1=14; B2=35; B3=46), 106 produced by the Conduction aphasics ((C1=35; C2=28; C3=43), and 13 by the one Wernicke’s aphasic. With respect to second attempt voicing paraphasias, there were 80 produced by the Broca’s aphasics (B1= 19; B2 = 30; B3 = 31), 67 produced by the Conduction aphasics (C1= 20; C2 = 9; C3 = 38), and 11 by the Wernicke’s aphasic.
Procedure
Stimuli were recorded using a Sony Walkman Professional tape recorder and a Sony stereo microphone. The recorded stimuli were then digitized at a sampling rate of 20 kHZ with a 9.0-kHZ lowpass filter (Butterworth 24 dB/octave), and a 12 bit quantization.
To analyze the acoustic parameters in the fricative noise, cursors were set at the onset and offset of the fricative noise using a software waveform editor (Mertus, 2000). Frication was acoustically defined as the presence of high frequency noise in the range between 5 and 10 kHz. Using a 15 msec full-Hamming window, the onset cursor was placed at the earliest position on the waveform at which DFT and LPC analyses showed an increase of at least 10 dB within the high frequency range (5 to 10 kHz) when compared to the background noise level. The offset of frication noise was determined by the absence of high frequency noise in the waveform, the emergence of the harmonic spectrum of the following vowel, and by an increase of at least 10 dB in spectral energy at or above 2 kHz. Again, both LPC and DFT analyses were conducted using a 15 msec full-Hamming window to make this determination. In addition, a drop of at least 10 dB in the 5 to 10 kHz frequency range was used to confirm the cessation of frication noise.
Given the production difficulties inherent in aphasic speech, epenthetic vowels and other forms of vocal cord activity sometimes preceded the onset of frication. In these cases, the vocalic activity was not included as part of the fricative duration unless there was co-occurring frication noise evident in the waveform of the vocalic activity, as determined by the criteria described above. In addition, the vocalic activity with co-occurring frication had to be contiguous with the syllable waveform.
Utilizing the two fricative endpoint cursors, the acoustic parameters of fricative duration and amplitude difference were measured in the tokens. Fricative duration was measured as the time interval (in msec) between the fricative cursors in each alveolar consonant in the isolation and context conditions. The amplitude parameter measured the amplitude of the first harmonic (H1) as determined by DFT analysis in the fricative noise interval relative to that of the vowel onset. Prior research (Pirello, Blumstein & Kurowski, 1997; Stevens et al., 1992) showed that voiced fricatives displayed a 10 dB difference or less between the fricative and vowel that was sustained over at least 30 msec over the frication noise (i.e. three consecutive 30-msec windows advanced in 10 msec intervals during the frication noise duration), and voiceless fricatives displayed greater than a 10 dB difference between the amplitude of the fricative noise interval and the following vowel. In the present study, the amount and extent of glottal excitation as determined by H1 was charted throughout the duration of the fricative noise using a 30-msec full-Hamming window which was advanced in 10-msec steps across the entire frication interval. The overall mean and standard deviation of the amplitude differences obtained across the fricative duration was determined for each token.
Results
As indicated earlier, paraphasic utterances were classified and analyzed in terms of whether they were the first or second attempt to produce the target. Discussion of the results will be limited to only the first paraphasic error that the patient produced.
Duration Measure
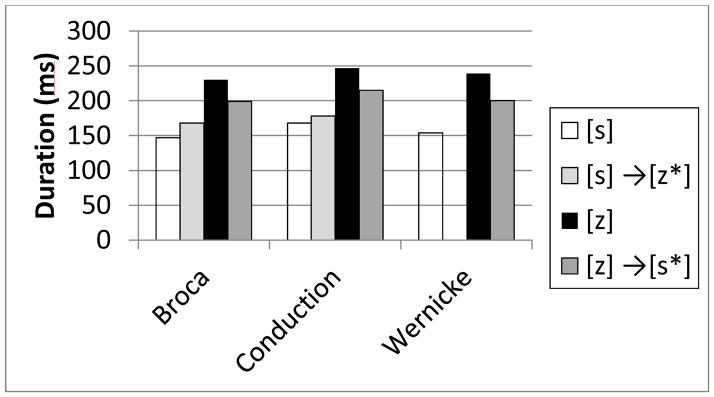
Figure 1 shows the overall mean duration of the fricative noise collapsed across vowel ([i e a o u] and conditions (isolation and the 2 context conditions) for the correctly produced (baseline) and paraphasic [z] and [s] productions for the Broca’s (n=3), Conduction (n=3), and Wernicke’s aphasics (n=1). (The Wernicke’s aphasic did not make any [s] → [z*] paraphasic errors). The baseline tokens indicate that for all groups, similar to normals, the duration of the voiceless fricative [s] is longer than that of the voiced fricative [z]. Importantly, the paraphasic errors for all groups show an acoustic trace of the original target; that is, the [s] → [z*] paraphasias are longer in duration than the baseline [z], and hence are more [s]-like, and similarly, the [z] → [s*] paraphasias are shorter than the [s] baseline, and hence are more [z]-like.
Figure 1.
Duration (in ms) of the fricative noise for baseline (correctly produce) [s] and [z] tokens and [s] →[z*] and [z] → [s*] phonemic paraphasias produced by participants clinically diagnosed with Broca’s, Conduction, and Wernicke’s aphasia.
Although the pattern of the overall data suggests that the paraphasic errors reflect a trace of the original target, there were too few subjects per group to conduct statistical analyses. Moreover, it is not clear from the means whether all subjects within a group showed this pattern. For this reason, we conducted two sets of additional analyses. In the first, we examined whether the overall pattern across all of the participants was statistically reliable. We then analyzed each subject’s production data individually to determine whether the pattern of responses for that subject reflect acoustic traces.
A one-way within-subjects parametric analysis of variance (ANOVA) was conducted to examine whether there were reliable differences across conditions (baseline [s], baseline [z], paraphasic [z] → [s*], and paraphasic [s] → [z*]). Assumptions of normality and homogeneity of variance were satisfied as shown by Mauchly’s test for sphericity (p >.05). Results of the ANOVA showed a significant effect (F(3, 18) = 71.233, p<.0001). Post-hoc Duncan pair-wise t-tests using the Bonferroni correction for multiple comparisons (p <.0083) revealed statistically significant differences between all conditions, except for the [z] vs. [s] → [z*] comparison (p<.02). Thus, on balance, considering the aphasic patients as a group, phonemic paraphasias contained an acoustic trace of their original target: the trace of a target [s] lengthens the fricative duration of the [z] error, while the trace of a target [z] shortens the fricative duration of the [s] paraphasia.
The individual data for the aphasics is shown in Table 2. As can be seen, similar to normals, all patients showed longer baseline voiceless fricative tokens than baseline voiced fricative tokens. With respect to the [s → z*] voicing paraphasias, the mean durations indicate that a [z] produced instead of the intended target [s] was longer than the baseline [z] for all subjects except C2. For [z → s*], the [s] produced when the intended target was a [z] had a shorter mean duration than that of the baseline [s] for all subjects.
Table 2.
Mean duration measures (in ms) across the five vowels and three conditions (isolation, [k]-context, [g] context) for the baseline and paraphasic productions for each subject.
| Aphasia Type | Subjects | [z] | [s→z*] | [s] | [z→s*] |
|---|---|---|---|---|---|
| Broca | B1 | 115 | 136 | 215 | 184 |
| B2 | 137 | 152 | 212 | 204 | |
| B3 | 188 | 216 | 261 | 209 | |
| Mean | 147 | 168 | 228 | 199 | |
| Conduction | C1 | 191 | 208 | 269 | 237 |
| C2 | 157 | 143 | 225 | 208 | |
| C3 | 155 | 181 | 247 | 229 | |
| Mean | 168 | 178 | 247 | 230 | |
| Wernicke | W1 | 154 | - | 239 | 200 |
To evaluate whether the error tokens produced by each aphasic subject showed statistically reliable evidence of traces of the target, separate Wilcoxon (signed rank sum test) analyses were performed on duration values for each subject, pairing individual target and error productions. In these paired analyses, the target and error tokens were matched for vowel and condition (isolation, /k/ context, /g/ context). A minimum of five matched pairs of tokens were used in performing the Wilcoxon analysis on each error type ([s] → [z*]; [z] → [s*]). Because there were often fewer than 5 matched tokens (i.e. tokens that shared vowel context in the same condition in the isolation and [k] and [g] context conditions), the matched tokens for each error type were combined across isolation and context conditions.
Each of the error tokens was paired with both a correctly produced baseline [s] and [z] token in order to determine if the error token was significantly different from both the target and the baseline tokens that matched the error production. For example, if the paraphasic error was [s] → [z*], then this [z*] should be different in duration from the target [s], since it was perceived as a [z]. The more critical question is whether the paraphasic [z*] is significantly different from a baseline [z] in duration, indicating the presence of a trace of the target [s]. The left half of Table 3 shows the Wilcoxon results for duration. With the exception of patients C1 and C2, each aphasic showed significant changes in the duration of the fricative noise when producing a paraphasic error, thus showing the presence of an acoustic trace in their voicing paraphasias.
Table 3.
Wilcoxon results for the duration and amplitude comparing the paraphasic error to the baseline that corresponded to it. For [s] → [z*], the corresponding baseline is [z]; for [z]→ [s*], the corresponding baseline is [s]).
| SUBJECTS: | DURATION MEASURES | AMPLITUDE MEASURES | ||
|---|---|---|---|---|
| [s→z*] | [z→s*] | [s→z*] | [z→s*] | |
| B1 | * | - | ||
| B2 | * | - | ||
| B3 | * | * | * | * |
| C1 | - | - | * | * |
| C2 | - | * | ||
| C3 | * | * | * | * |
| W1 | * | * | ||
An asterik (*) in the cell indicates a statistically significant result; a minus sign (−) indicates a non-significant result. Blank indicates that there were too few paraphasic tokens (or none) to perform a statistical analysis.
Amplitude Measure
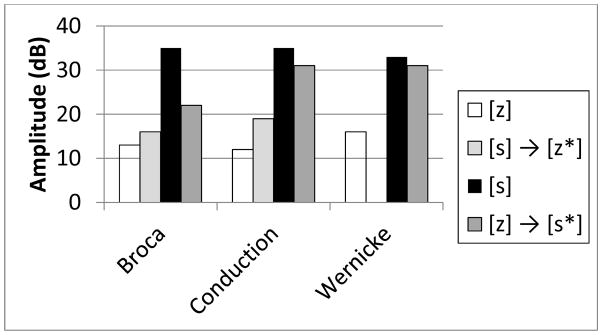
Figure 2 displays the group mean amplitude differences for baseline (correctly produced target) fricatives and for the voiced ([s → z*]) and voiceless ([z → s*]) paraphasias collapsed across vowels and context conditions. (The Wernicke’ aphasic did not make any [s] → [z*] paraphasias). For each group of patients, the baseline [s] and [z] amplitude measure shows, as do normals, smaller amplitude differences between the amplitude of the fricative noise and the vowel for the [z] target tokens compared to the [s] target tokens. Critically, there is an acoustic trace of the target in the paraphasias; [s] → [z*] paraphasias show an increase in the amplitude parameter rendering the [z] more [s]-like, and conversely, [z] → [s*] paraphasias show a decrease in amplitude rendering the [s] paraphasias more [z]-like.
Figure 2.
Amplitude measure (in dB) of voicing for baseline (correctly produce) [s] and [z] tokens and [s] →[z*] and [z] → [s*] phonemic paraphasias produced by participants clinically diagnosed with Broca’s, Conduction, and Wernicke’s aphasia.
Further analyses were conducted analogous to those carried out on the duration measures. A one-way ANOVA, collapsing across the 3 patient groups (Broca, Conduction, and Wernicke), vowel, and context conditions, examined whether the overall amplitude effects were statistically reliable. Similar to the duration analysis, assumptions of homogeneity of variance were satisfied. Results of the ANOVA showed a significant effect F(3, 18) = 18.455 (p<.0001). Post-hoc pairwise t-tests using the Bonferroni correction for multiple comparisons (p< .0083) revealed statistically reliable differences only for the [z] vs.[s] and the [s] vs. [s] → [z*]. The remaining comparisons showed the following significance levels: [z] vs. [z] → [s*], p < .01, [s] → [z*] vs. [z] → [s*], p< .024, and [z] vs. [s] → [z*] and [s] vs. [z] → [s*] p< .066.
The individual amplitude data for each patient is shown in Table 4. Similar to normals, all patients showed a smaller amplitude difference between the first harmonic in the fricative noise and the vowel for the voiced fricative [z] compared to the voiceless fricative [s]. With respect to the paraphasic errors, all patients showed an acoustic trace in the [z] → [s*] paraphasia, with the paraphasic [s*] showing a decrease (and hence more voicing) in the amplitude measure compared to the correctly produced [s]. Evidence for a trace in the [s] → [z*] paraphasias was weaker, failing to emerge for 2 of the 3 Broca’s aphasics (B1 and B2) and showing only a 1 dB difference for C1.
Table 4.
Mean amplitude measures (in dB) across the five vowels and three conditions (isolation, [k]-context, [g] context) for the baseline and paraphasic productions for each subject.
| Aphasia Type | Subjects | [z] | [s→z*] | [s] | [z→s*] |
|---|---|---|---|---|---|
| Broca | B1 | 17 | 14 | 29 | 16 |
| B2 | 9 | 9 | 39 | 12 | |
| B3 | 15 | 24 | 39 | 37 | |
| Mean | 13 | 16 | 35 | 22 | |
| Conduction | C1 | 11 | 12 | 41 | 36 |
| C2 | 12 | 24 | 37 | 35 | |
| C3 | 13 | 22 | 26 | 24 | |
| Mean | 12 | 19 | 35 | 31 | |
| Wernicke | W1 | 16 | - | 33 | 31 |
Wilcoxon analyses of the individual subject data were conducted following the same procedures as those done for the duration measure by matching individual target and error productions across vowel and condition. The right portion of Table 3 shows the results. With the exception of B1 and B2, each aphasic showed evidence of an acoustic trace in his/her paraphasias in the amplitude measure. Neither C2 nor W1 had the minimum number of matched token pairs for [s] → [z*] paraphasias to perform the Wilcoxon analysis.
Discussion
The results of this study show that the production of phonemic paraphasias in aphasic patients leaves an acoustic trace of the target phoneme. In particular, although voicing paraphasias in fricative consonants were both perceptually and acoustically distinct in voicing from their target (and hence were paraphasic errors), the acoustic manifestation of these paraphasias contained a voicing ‘trace’ of the original target; [s] paraphasias were more [z]-like, and [z] paraphasias were more [s]-like. Thus, although an [s] → [z*] paraphasia was produced as a [z], and acoustically it was voiced, it was more ‘voiceless’ (as shown by both duration and amplitude measures of voicing) than correctly produced [z] tokens. Similar effects emerged in voiceless fricative paraphasias. These findings indicate that phonemic paraphasias are not clear-cut substitutions of one phoneme for another reflecting the selection of an incorrect phoneme which is then implemented correctly nor are they a combination of some phonemic substitutions and other articulatory (phonetic) implementation errors. Instead, acoustic properties of the original target are manifested in paraphasic productions.
Importantly, all aphasic patients, regardless of clinical diagnosis or lesion localization showed this effect. Considering the clinical diagnosis, the classical aphasic literature made a distinction between the speech output of anterior and posterior aphasics. Anterior aphasics were characterized as having articulatory implementation impairments, and posterior aphasics were characterized as having selection impairments. Within the posterior aphasias, phonemic paraphasias are a clinical feature of Conduction aphasics (Pradat-Diehl, Tessier, Vallat, Mailhan, et al. (2001). Although Wernicke’s aphasics produce them as well, their occurrence is much less frequent (Goodglass & Kaplan, 1983). Nonetheless, despite these clinical distinctions, traces appeared in the paraphasic productions across the aphasia syndromes.
As to lesion localization, examination of the lesion localization across patients (as shown in Table 1) reveals an extensive pattern of lesions including frontal, parietal, and temporal lobes as well as subcortical areas. And as has been shown in earlier research, a clinical diagnosis of Broca’s aphasia does not always require a lesion in Broca’s area (the inferior frontal gyrus) (Willmes & Poeck, 1993). Indeed, only B3 showed a lesion in this area. There is insufficient data to determine whether damage to particular neural areas would fail to show evidence for traces in phonemic paraphasias. Suffice to say, the large number of neural areas damaged across the patients studied suggests that the presence of acoustic traces in phonemic paraphasias may be pervasive in aphasia irrespective of lesion.
It is the case that depending upon the acoustic parameter there were differences across the patients in the extent to which traces emerged. For example, the amplitude measure failed to show evidence of traces for 2 of the 3 Broca’s aphasics (B1 and B2). These findings are not surprising given that prior research has shown that Broca’s aphasics exhibit weak glottal excitation in the production of fricative consonants compared to normal controls (Kurowski et al., 2003). As a consequence, a measure relying on amplitude differences within an utterance (amplitude of the fricative noise relative to the amplitude of the vowel) was likely not sensitive enough to pick up traces in two of the Broca’s aphasics.
With regard to the duration measure, two of the three Conduction aphasics (C1 and C2) failed to show this acoustic trace, although they did use duration to distinguish correctly produced voiced and voiceless target productions. Previous research has shown deficits in posterior aphasics including Conduction aphasics in the production of a number of duration parameters of speech including fricatives (Baum et al., 1990). These same patients, however, do not show deficits in laryngeal control. As a result, it is not surprising that a duration parameter failed to show evidence of traces for the two Conduction aphasics while acoustic traces emerged in their data using the amplitude measure.
The presence of acoustic traces in phonemic paraphasias challenges the traditional explanation for the basis of these errors. That is, they do not reflect the selection of the wrong phoneme followed by its correct articulatory implementation. Rather, it appears as though the presence of acoustic traces reflects the selection and co-activation of both the target and voicing competitor which are ultimately sent downstream to articulatory processes. As described earlier, current models of spoken word production propose that the selection of a lexical (or syllable) candidate activates not only the target but also competitors which share either sound structure or semantic properties (Dell, 1986; Dell et al., 1997; Rapp & Goldrick, 2000). Under normal circumstances, the production of the target phoneme requires that the correct phonological segment and its features be activated and competitors be inhibited. Phonemic paraphasias appear to arise from the activation of the phonological representation of both the target and competitor.
There are two possible processes that may give rise to the paraphasia and trace. One possibility is that the incorrect segment is selected along with reduced activation of the target phoneme resulting in the incorrect production and a trace of the target. Alternatively, the correct segment is selected and there is overactivation of the competitor resulting in the production of the incorrect segment and a trace of the intended target. While we cannot distinguish between these two possibilities on the basis of the current data, we favor the first alternative since it is consistent with the findings of recent research suggesting that aphasic participants show a lexical access impairment characterized by a failure to resolve lexical competition (Blumstein, 2011; Yee, Blumstein, & Sedivy, 2008). Here, it has been proposed that aphasics fail to inhibit competitors owing to a deficit in which representations are weakly activated, or a deficit in which they cannot sufficiently inhibit partially activated representations.
That traces occur across all patients is consistent with the view that the neural substrates underlying spoken word production are broadly distributed and the functional architecture of this system reflects cascading processes. Consistent with this is evidence from both the aphasia and functional neuroimaging literature.
Past research examining the acoustic substrates of speech production in aphasia has shown that not only do anterior aphasics display articulatory deficits but posterior aphasics also display a subtle phonetic impairment affecting a range of phonetic parameters (cf. Blumstein 2000, for review). In the latter case, the impairments are not evident in the patient’s productions but are only revealed with instrumental analyses of speech output (cf. Baum et al., 1990; Vijayan and Gandour, 1995). Given the current study, what is less clear is the basis of the deficits that had been proposed. For example, in a series of studies conducted in the 70’s and 80’s investigating the production of voicing in stop consonants in Broca’s, Conduction, and Wernicke’s aphasics (Blumstein et al., 1977, 1980; Gandour & Dardarananda, 1984), VOT measures were taken of target productions independent of their perception. Thus, the VOT value for a particular target was measured whether it was perceived as a voiced or voiceless consonant. Results showed three types of productions: those that fell within the expected range for a voiced and voiceless stop consonant and were assumed to be normal productions, those that fell within the range of the contrasting phonetic category (the VOT of a [t] target fell within the VOT range of the [d] category) and assumed to be phonemic paraphasias, and those that fell between the two voiced and voiceless phonetic categories and assumed to be articulatory implementation errors. All 3 groups made both types of errors, although the Wernicke’s aphasics made considerably fewer errors. It is possible that many of these productions actually reflected traces of the original target rather than, as originally proposed, two distinct types of errors.
Looking at fMRI findings, evidence suggests that spoken word production recruits a broadly distributed neural system (Indefrey & Levelt, 2004), and activation patterns within this system support models of cascading activation. Using the presence or absence of minimal pair voicing contrasts as a proxy for lexical density, behavioral results show longer VOT values for words beginning with voiceless stop consonants that have voiced minimal pairs compared to words that do not (e.g. tense-dense vs. tenth, *denth is not a word) (Baese-Berk & Goldrick, 2009; Fox, Reilly, & Blumstein, 2015). Peramunage et al. (2011) examined the neural substrates of this lexically conditioned phonetic variation and showed cascading activation throughout the spoken word production system with modulatory effects under these conditions in the posterior superior temporal and supramarginal gyri (implicated in phonological and lexical selection), the inferior frontal gyrus (implicated in phonological planning) and the precentral gyrus (implicated in articulatory processes) (cf. also Okada & Hickok, 2006).
Taken together, the findings of the current study suggest that phonemic paraphasias reflect the integrity of cascading processes in spoken word production. They also suggest that these paraphasias reflect the selection of a phonemic segment with the concurrent co-activation of potential sound-shape competitors, and the continued partial activation of the competitor, resulting in the presence of a trace of the original target segment. For such patterns to emerge requires a system in which phonological properties of sound segments are represented in a gradient fashion rather than as binary units in which a phonological feature is either present or absent. Of importance, these results show unique insights from aphasia. Whereas the presence of acoustic traces in normals emerges using tasks that ‘stress’ the system, e.g. tongue twisters, where the test stimuli contain the competing sound segments, such is not the case in phonemic paraphasias. Here, the productions emerge ‘naturally’ and often, occurring under all types of speaking conditions ranging from natural speech to the task used in the current experiment. Thus, competition effects that induce traces in phonemic paraphasias are clearly intrinsic to the phonological and lexical systems being accessed, and are neither induced nor necessarily exacerbated by extrinsic factors.
Summary
The results of this study show that phonemic paraphasias contain an acoustic trace of the original target in the error production and appear to derive from a common mechanism with speech errors (c.f. also Buckingham, 1992b). The presence of a trace of the original target suggests that there is co-activation of lexical representations in the selection of a target word which influence downstream processes of articulation. Slips of the tongue, however, are not common, but are typically induced by utilizing tasks such as requiring subjects to produce tongue twisters which contain phonologically similar, and hence, competing phonological representations. In contrast, phonemic paraphasias are a common presenting symptom in aphasia, and occur in spontaneous speech as well as in reading and/or repeating individual words or syllables. Importantly, unlike slips of the tongue, aphasic paraphasic errors typically occur in the absence of competing representations present in the stimulus. Although left hemisphere damage may exacerbate the occurrence of paraphasias, they reflect ‘natural’ processes of speech production and cut across clinical types of aphasia as well as lesion site.
Supplementary Material
Table S1. Percentage of C3’s tokens in each condition (Isolation; [k] context; [g] context) that exhibited a voicing ‘trace’ prior to frication (e.g. epenthetic vowel, full vowel or prevoicing).
Acknowledgments
This research was supported in part by NIH grants R01 DC000314 to Brown University and P50 DC000081 to the Boston University School of Medicine. This material is the result of work supported with resources and the use of facilities at the Department of Veterans Affairs Medical Centers in Boston, MA and Providence, RI. The content is solely the responsibility of the authors and does not necessarily represent the official views or policy of the Department of Veterans Affairs, the National Institute on Deafness and Other Communication Disorders, or the National Institutes of Health. Thanks to Sahil Luthra for technical assistant and comments on an earlier draft of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baese-Berk M, Goldrick M. Mechanisms of interaction in speech production. Language and Cognitive Processes. 2009;24(4):527–554. doi: 10.1080/01690960802299378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum SR, Blumstein SE, Naeser MA, Palumbo CL. Temporal dimensions of consonant and vowel production: An acoustic and CT scan analysis of aphasic speech. Brain and Language. 1990;39(1):33–56. doi: 10.1016/0093-934x(90)90003-y. [DOI] [PubMed] [Google Scholar]
- Blumstein SE. A Phonological Investigation of Aphasic Speech. The Hague: Mouton; 1973. [Google Scholar]
- Blumstein SE. Deficits of speech production and speech perception in aphasia. In: Berndt R, editor. Handbook of Neuropsychology. 2. Vol. 2. The Netherlands: Elsevier Science; 2000. [Google Scholar]
- Blumstein SE. Neural systems underlying lexical competition in auditory word recognition and spoken word production: Evidence from aphasia and functional neuroimaging. In: Gaskell G, Zwitserlood P, editors. Lexical Representation: A Multidisciplinary Approach. The Hague: Mouton; 2011. [Google Scholar]
- Blumstein SE, Cooper WE, Goodglass H, Statlender S, Gottlieb J. Production deficits in aphasia: A voice-onset time analysis. Brain and Language. 1980;9(2):153–170. doi: 10.1016/0093-934x(80)90137-6. [DOI] [PubMed] [Google Scholar]
- Blumstein SE, Cooper WE, Zurif EB, Caramazza A. The perception and production of voice-onset time in aphasia. Neuropsychologia. 1977;15(3):371–383. doi: 10.1016/0028-3932(77)90089-6. [DOI] [PubMed] [Google Scholar]
- Boucher VJ. Alphabet-related biases in psycholinguistic enquiries: Considerations for direct theories of speech production and perception. Journal of Phonetics. 1994;(22):1–18. [Google Scholar]
- Buckingham HW. The scan-copier mechanism and the positional level of language production: Evidence from phonemic paraphasia. Cognitive Science. 1986;10:195–217. [Google Scholar]
- Buckingham HW. Phonological production deficits in Conduction aphasia. In: Kohn SE, editor. Conduction Aphasia. Hillsdale, New Jersey: Lawrence Erlbaum; 1992a. [Google Scholar]
- Buckingham HW. The mechanisms of phonemic paraphasia. Clinical Linguistics & Phonetics. 1992b;6(1–2):41–63. doi: 10.3109/02699209208985518. [DOI] [PubMed] [Google Scholar]
- Buckingham HW, Yule G. Phonemic false evaluation: Theoretical and clinical aspects. Clinical Linguistics and Phonetics. 1987;1(2):113–125. [Google Scholar]
- Burns MS, Canter GJ. Phonemic behavior of aphasic patients with posterior cerebral lesions. Brain and Language. 1977;4(4):492–507. doi: 10.1016/0093-934x(77)90041-4. [DOI] [PubMed] [Google Scholar]
- Crystal TH, House AS. Segmental durations in connected-speech signals: current results. Journal of the Acoustical Society of America. 1988;83:1553–1573. doi: 10.1121/1.388251. [DOI] [PubMed] [Google Scholar]
- Dell GS. A spreading-activation theory of retrieval in sentence production. Psychological Review. 1986;93(3):283. [PubMed] [Google Scholar]
- Dell GS, Gordon JK. Neighbors in the Lexicon: Friends or foes? In: Schiller NO, Meyer AS, editors. Phonetics and Phonology in Language Comprehension and Production: Differences and similarities. New York: Mouton de Gruyter; 2003. [Google Scholar]
- Dell GS, Juliano C, Govindjee A. Structure and content in language production: A theory of frame constraints in phonological speech errors. Cognitive Science. 1993;17(2):149–195. [Google Scholar]
- Dell GS, Schwartz MF, Martin N, Saffran EM, Gagnon DA. Lexical access in aphasic and nonaphasic speakers. Psychological Review. 1997;104(4):801. doi: 10.1037/0033-295x.104.4.801. [DOI] [PubMed] [Google Scholar]
- Fox NP, Reilly M, Blumstein SE. Phonological neighborhood competition affects spoken word production irrespective of sentential context. Journal of Memory and Language. 2015;83:97–117. doi: 10.1016/j.jml.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman FJ, Sands ES, Harris KS. Temporal coordination of phonation and articulation in a case of verbal apraxia: A voice onset time study. Brain and Language. 1978;6(1):106–111. doi: 10.1016/0093-934x(78)90048-2. [DOI] [PubMed] [Google Scholar]
- Frisch SA, Wright R. The phonetics of phonological speech errors: An acoustic analysis of slips of the tongue. Journal of Phonetics. 2002;30(2):139–162. [Google Scholar]
- Fromkin Victoria A. The non-anomalous nature of anomalous utterances. Language. 1971:27–52. [Google Scholar]
- Gandour J, Dardarananda R. Voice onset time in aphasia: Thai II. Production. Brain and Language. 1984;23(2):177–205. doi: 10.1016/0093-934x(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Goldrick M. Limited interaction in speech production: Chronometric, speech error, and neuropsychological evidence. Language and Cognitive Processes. 2006;21(7–8):817–855. [Google Scholar]
- Goldrick M, Blumstein SE. Cascading activation from phonological planning to articulatory processes: Evidence from tongue twisters. Language and Cognitive Processes. 2006;21(6):649–683. [Google Scholar]
- Goldrick M, Chu K. Gradient co-activation and speech error articulation: Comment on Pouplier and Goldstein (2010) Language, Cognition and Neuroscience. 2014;29(4):452–458. [Google Scholar]
- Goldstein L, Pouplier M, Chen L, Saltzman E, Byrd D. Dynamic action units slip in speech production errors. Cognition. 2007;103(3):386–412. doi: 10.1016/j.cognition.2006.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Gordon JK. Phonological neighborhood effects in aphasic speech errors: Spontaneous and structured contexts. Brain and Language. 2002;82:113–145. doi: 10.1016/s0093-934x(02)00001-9. [DOI] [PubMed] [Google Scholar]
- Haley KL, Jacks A, Cunningham KT. Error variability and the differentiation between apraxia of speech and aphasia with phonemic paraphasia. Journal of Speech, Language, and Hearing Research. 2013;56(3):891–905. doi: 10.1044/1092-4388(2012/12-0161). [DOI] [PubMed] [Google Scholar]
- Halpern H, Keith RL, Darley FL. Phonemic behavior of aphasic subjects without dysarthria or apraxia of speech. Cortex. 1976;12(4):365–372. doi: 10.1016/s0010-9452(76)80040-8. [DOI] [PubMed] [Google Scholar]
- Harmes S, Daniloff R, Hoffman P, Lewis J, Kramer M, Absher R. Temporal and articulatory control of fricative articulation by speakers with Broca’s aphasia. Journal of Phonetics. 1984;12:367–385. [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Itoh M, Sasanuma S, Hirose H, Yoshioka H, Ushijima T. Abnormal articulatory dynamics in a patient with apraxia of speech: X-ray microbeam observation. Brain and Language. 1980;11(1):66–75. doi: 10.1016/0093-934x(80)90110-8. [DOI] [PubMed] [Google Scholar]
- Kurowski K, Hazen E, Blumstein SE. The nature of speech production impairments in anterior aphasics: An acoustic analysis of voicing in fricative consonants. Brain and Language. 2003;84(3):353–371. doi: 10.1016/s0093-934x(02)00555-2. [DOI] [PubMed] [Google Scholar]
- Laver J. Slips of the tongue as neuromuscular evidence for a model of speech production. In: Dechert HW, Raupach M, editors. Temporal variables in speech: Studies in honour of Frieda Goldman-Eisler. The Hague: Mouton; 1980. [Google Scholar]
- Lecours AR, Lhermitte F. Phonemic paraphasias: Linguistic structures and tentative hypotheses. Cortex. 1969;5(3):193–228. doi: 10.1016/s0010-9452(69)80031-6. [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behavioral and brain sciences. 1999;22(01):1–38. doi: 10.1017/s0140525x99001776. [DOI] [PubMed] [Google Scholar]
- McMillan CT, Corley M. Cascading influences on the production of speech: Evidence from articulation. Cognition. 2010;117(3):243–260. doi: 10.1016/j.cognition.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertus J. BLISS (Brown Lab Interactive Speech System) software editor. 2000 @ http://www.Mertus.org/Bliss/
- Meyer AS. Investigation of phonological encoding through speech error analyses: Achievements, limitations, and alternatives. Cognition. 1992;42(1):181–211. doi: 10.1016/0010-0277(92)90043-h. [DOI] [PubMed] [Google Scholar]
- Möller J, Jansma BM, Rodriguez-Fornells A, Münte TF. What the brain does before the tongue slips. Cerebral Cortex. 2007;17(5):1173–1178. doi: 10.1093/cercor/bhl028. [DOI] [PubMed] [Google Scholar]
- Mowrey RA, MacKay IR. Phonological primitives: Electromyographic speech error evidence. The Journal of the Acoustical Society of America. 1990;88(3) doi: 10.1121/1.399706. [DOI] [PubMed] [Google Scholar]
- Okada K, Hickok G. Identification of lexical–phonological networks in the superior temporal sulcus using functional magnetic resonance imaging. Neuroreport. 2006;17(12):1293–1296. doi: 10.1097/01.wnr.0000233091.82536.b2. [DOI] [PubMed] [Google Scholar]
- Peramunage D, Blumstein SE, Myers EB, Goldrick M, Baese-Berk M. Phonological neighborhood effects in spoken word production: An fMRI study. Journal of Cognitive Neuroscience. 2011;23(3):593–603. doi: 10.1162/jocn.2010.21489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirello K, Blumstein SE, Kurowski K. The characteristics of voicing in syllable-initial fricatives in American English. The Journal of the Acoustical Society of America. 1997;101(6):3754–3765. doi: 10.1121/1.418334. [DOI] [PubMed] [Google Scholar]
- Pouplier M. Articulatory Perspectives on Errors. In: Schütze Carson T, Ferreira Victor S., editors. The state of the art in speech error research. Proceedings of the LSA Institute Workshop. MIT Working Papers in Linguistics. Vol. 53 2007. [Google Scholar]
- Pouplier M, Chen L, Goldstein L, Byrd D. Kinematic evidence for the existence of gradient speech errors. The Journal of the Acoustical Society of America. 1999;106(4):2242–2242. [Google Scholar]
- Pradat-Diehl P, Tessier C, Vallat C, Mailhan L, Mazevet D, Lauriot-Prevost MC, Bergego C. Conduction aphasia and phonemic disorder. Revue neurologique. 2001;157(10):1245–1252. [PubMed] [Google Scholar]
- Rapp B, Goldrick M. Discreteness and interactivity in spoken word production. Psychological Review. 2000;107(3):460. doi: 10.1037/0033-295x.107.3.460. [DOI] [PubMed] [Google Scholar]
- Shattuck-Hufnagel S. The role of word-onset consonants in speech production planning: New evidence from speech error patterns. In: Keller E, Gopnik M, editors. Motor and Sensory Processes of Language. Hillsdale, New Jersey: Lawrence Erlbaum; 1987. [Google Scholar]
- Shattuck-Hufnagel Stefanie, Klatt Dennis H. The limited use of distinctive features and markedness in speech production: Evidence from speech error data. Journal of Verbal Learning and Verbal Behavior. 1979;18(1):41–55. [Google Scholar]
- Shewan CM, Leeper H, Booth J. An analysis of voice onset time (VOT) in aphasic and normal subjects. In: Rosenbek J, McNeill M, Aronson A, editors. Apraxia of speech. San Diego, CA: College-Hill Press; 1984. pp. 197–220. [Google Scholar]
- Stevens KN, Blumstein SE, Glicksman L, Burton M, Kurowski K. Acoustic and perceptual characteristics of voicing in fricatives and fricative clusters. The Journal of the Acoustical Society of America. 1992;91(5):2979–3000. doi: 10.1121/1.402933. [DOI] [PubMed] [Google Scholar]
- Vijayan A, Gandour J. On the notion of a” subtle phonetic deficit” in fluent/posterior aphasia. Brain and Language. 1995;48(1):106–119. doi: 10.1006/brln.1995.1004. [DOI] [PubMed] [Google Scholar]
- Willmes K, Poeck K. To what extent can aphasic syndromes be localized? Brain. 1993;116(6):1527–1540. doi: 10.1093/brain/116.6.1527. [DOI] [PubMed] [Google Scholar]
- Yee E, Blumstein SE, Sedivy JC. Lexical-semantic activation in Broca’s and Wernicke’s aphasia: Evidence from eye movements. Journal of Cognitive Neuroscience. 2008;20:592–612. doi: 10.1162/jocn.2008.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Percentage of C3’s tokens in each condition (Isolation; [k] context; [g] context) that exhibited a voicing ‘trace’ prior to frication (e.g. epenthetic vowel, full vowel or prevoicing).