Abstract
Background
MUC1 is a membrane-tethered mucin expressed on the surface of epithelial and hematopoietic cells. Previous studies have established that MUC1 attenuates airway inflammation in response to Pseudomonas aeruginosa (Pa) through suppression of Toll-like receptor (TLR) signaling. Here, we elucidate the mechanism through which the MUC1 cytoplasmic tail (CT) inhibits TLR5 signaling in response to Pa and its flagellin in primary normal human bronchial epithelial (NHBE) cells.
Methods
NHBE and human and mouse macrophages were stimulated with Pa or flagellin and transforming growth factor-α (TGF-α) and tumor necrosis factor-α (TNF-α) levels in cell culture supernatants were measured by ELISA. NHBE cells were stimulated with Pa, flagellin, or TNF-α and MUC1-CT, and epidermal growth factor receptor (EGFR) levels were measured by immunoblotting. NHBE cells were stimulated with Pa and MUC1-CT/TLR5 and MUC1-CT/EGFR association were detected by co-immunoprecipitation.
Results
Stimulation of NHBE cells with Pa and flagellin each increased release of the EGFR ligand, TGF-α, from NHBE cells. Both stimuli also activated EGFR tyrosine phosphorylation in these same cells. By contrast, stimulation of NHBE cells with Pa failed to induce TNF-α release, whereas stimulation of human or mouse macrophages with Pa promoted TNF-α release. Stimulation of NHBE cells with recombinant TNF-α increased both MUC1 and EGFR protein levels, and stimulation of these cells with Pa enhanced MUC1-CT tyrosine phosphorylation and increased MUC1-CT/TLR5 and MUC1-CT/EGFR protein association, in an EGFR-dependent manner.
Conclusions
These results indicate that in response to Pa or flagellin, EGFR associates with and tyrosine phosphorylates MUC1-CT in primary NHBE cells, leading to increased MUC1-CT association with TLR5. Based on prior studies in tumor cells, increased MUC1-CT/TLR5 association in NHBE cells is predicted to competitively inhibit Pa/flagellin-stimulated TLR5 activation, reduce TLR5-dependent cell signaling, and down-regulate airway inflammation. Given that MUC1 is a universal suppressor of TLR signaling, the results from this study suggest that abnormal interactions between MUC1 and EGFR or TLRs may lead to the development of chronic inflammatory diseases. Thus, this is an important finding from the clinical point of view.
Keywords: MUC1, EGFR, TLR5, Pseudomonas aeruginosa, flagellin, normal human bronchial epithelial cells, inflammation
Background
Pseudomonas aeruginosa (Pa) is a Gram-negative, opportunistic human pathogen responsible for a spectrum of acute and chronic respiratory tract infections. While Pa respiratory tract infection rarely occurs in healthy individuals, patients on mechanical ventilation or undergoing immunosuppression are particularly at risk of nosocomial infection (1). In these settings, Pa pneumonia is accompanied by excessive airway inflammation with high morbidity and mortality. Pa is also associated with exacerbation of airway disease symptoms, especially in patients with bronchiectasis (2) and chronic obstructive pulmonary disease (3). In spite of its documented clinical significance, the molecular mechanisms responsible for Pa pathogenesis, and the host response to infection, remain to be elucidated.
Inhaled pathogens are initially trapped by the airway surface liquid and subsequently removed from the airways by the mucociliary clearance process provided by respiratory epithelial cells. Pathogens breaching this first-line of defense are recognized by a family of innate immune receptors expressed by airway epithelial cells and resident leukocytes (4). Among these receptors, Toll-like receptors (TLRs) play a crucial role in host defence by sensing bacterial components and initiating an innate immune response (4). TLR5, in particular, recognizes bacterial flagellin, the major protein constituent of flagella, leading to nuclear factor-κB (NF-κB) activation and secretion of proinflammatory cytokines (e.g. tumor necrosis factor-α [TNF-α]) and chemokines (e.g. interleukin-8 [IL-8]) (5). Supporting the proinflammatory activity of airway epithelia, Pa phagocytosis by alveolar macrophages further contributes to bacterial clearance from the lungs (6). As with all human pathogens, these innate immune responses against Pa must be closely controlled to maintain homeostasis and prevent disease. For example, hyperactivation of alveolar macrophages by high-dose Pa infection leads to inflammasome activation with excessive production of IL-1β and immune cell apoptosis, thus impairing bacterial clearance (7). Similarly, uncontrolled inflammation in the absence of counter-regulatory mechanisms may cause detrimental, bystander damage to host tissues (7).
We previously reported that the transmembrane mucin, MUC1 (MUC1 in humans, Muc1 in animals), plays an anti-inflammatory role during Pa lung infection (8–11). MUC1 consists of two noncovalently associated subunits, a >250 kDa ectodomain containing a variable number of highly glycosylated tandem repeats, and a 25 kDa intracellular, tyrosine-phosphorylated cytoplasmic tail (CT) (12, 13). The MUC1 heterodimer is expressed on the apical surface of mucosal epithelial cells and hematopoietic cells (12). In mice, Muc1 expression inhibited ligand-specific TLR activation in both airway epithelial cells and alveolar macrophages (14). Although Muc1 expression levels are low in uninfected mouse lungs, Pa airway infection induced a dramatic increase in its expression in a TNF-α-dependent manner (15). Our recent studies with human A549 lung adenocarcinoma cells demonstrated that TGF-α induced EGFR activation stimulated MUC1-CT tyrosine phosphorylation and increased MUC1-CT/TLR5 interaction, resulting in decreased TLR5/MyD88 association, diminished TLR5-dependent signaling, and attenuated inflammation (11). However, since transformed and tumor-derived epithelial cells often display abnormal biochemical and physiologic responses, the current study was undertaken to assess Pa-stimulated EGFR activation, MUC1-CT tyrosine phosphorylation, and MUC1-CT/TLR5 association using primary NHBE cells.
Materials and methods
Reagents
All reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise stated. The sources of antibodies were: mouse monoclonal anti-phosphotyrosine antibody (P-Tyr-100), rabbit monoclonal anti-EGFR antibody (D38B1), and rabbit monoclonal anti-phospho-EGFR (Tyr1068) antibody (D7A5) (Cell Signaling Technology, Beverly, MA, USA); mouse monoclonal anti-TLR5 antibody (IMG-664A) (Imgenex, San Diego, CA, USA); rabbit polyclonal anti-β-actin antibody, normal rabbit IgG, normal mouse IgG, and normal Armenian hamster IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA); Armenian hamster anti-MUC1-CT antibody (CT2) (Dr. Sandra J. Gendler, Mayo Clinic College of Medicine, Scottsdale, AZ, USA); horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG and HRP-conjugated goat anti-rabbit IgG antibodies (Jackson ImmunoResearch, West Grove, PA, USA); and HRP-conjugated goat anti-Armenian hamster IgG antibody (KPL, Gaithersburg, MD, USA). AG1478 was from Cell Signaling Technology. Human recombinant TNF-α was from R&D Systems (Minneapolis, MN, USA).
Cell culture
NHBE cells were purchased from Lonza (Walkersville, MD, USA), and cultured in collagen-coated plates in LHC-9 medium (Life Technologies, Grand Island, NY, USA). Human THP-1 cells (American Type Culture Collection, Manassas, VA, USA) were cultured for 3 days with 200 nM phorbol 12-myristate 13-acetate to induce macrophage differentiation (16). Human alveolar macrophages were isolated by centrifugation of bronchoalveolar lavage fluid from healthy adult volunteers using a protocol approved by the University of Arizona College of Medicine Institutional Review Board. Bone marrow cells were harvested from the femurs and tibias of C57BL6/J mice, and peritoneal macrophages were isolated from thioglycolate-treated C57BL6/J mice as described (17) using a protocol approved by the University of Arizona Collage of Medicine Institutional Animal Care and Use Committee. Bone marrow cells were cultured for 7 days with 10 ng/ml of murine macrophage-colony stimulating factor (R&D Systems) to induce macrophage differentiation. THP-1 cells and murine macrophages were seeded at 2.5 × 106 cells/well in 6-well plates, and human alveolar macrophages were seeded at 5.0 × 105 cells/well in 24-well plates, and cultured in RPMI-1640 containing 2.0 mM glutamine, 1.0 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% FBS at 37°C in a humidified incubator containing 5% CO2 in air.
Stimulation of NHBE cells and macrophages with heat-inactivated Pa and purified flagellin
Heat-inactivated Pa strain K (PAK) was prepared by incubating 1.0 × 109 colony forming units [CFU]/ml of bacteria at 60°C for 30 min and stored at −80°C as described (11). NHBE cells and macrophages were washed twice with DPBS, pre-incubated in Opti-MEM (Life Technologies) for 2 h, and stimulated with 1.0 × 106, 1.0 × 107, or 1.0 × 108 CFU/ml of heat-inactivated PAK or 100 ng/ml of purified Pa flagellin (FLA-PA Ultrapure, InvivoGen, San Diego, CA, USA) as described (11).
ELISA
Human TGF-α, and human and mouse TNF-α, were quantified in cell culture supernatants by enzyme-linked immunosorbent assay (ELISA) using biotinylated detection antibodies (R&D Systems; eBioscience, San Diego, CA, USA) and HRP-labeled streptavidin (KPL, Gaithersburg, MD, USA). A standard curve for each protein was generated for each ELISA plate.
Immunoblot and co-immunoprecipitation analyses
Immunoblot and immunoprecipitation were performed as described previously (11). The EGFR immunoprecipitates were processed for immunoblotting with antibodies to phospho-EGFR (Tyr1068) and the MUC1-CT immunoprecipitates were processed for immunoblotting with antibodies to phosphotyrosine, TLR5, or EGFR. To control for protein loading and transfer, the blots were stripped and reprobed with the immunoprecipitating antibody.
Statistical analysis
Values were expressed as means ± SEM. Differences between means were compared using the Student’s t-test and considered significant if p<0.05.
Results
Pa and flagellin stimulate release of TGF-α from NHBE cells
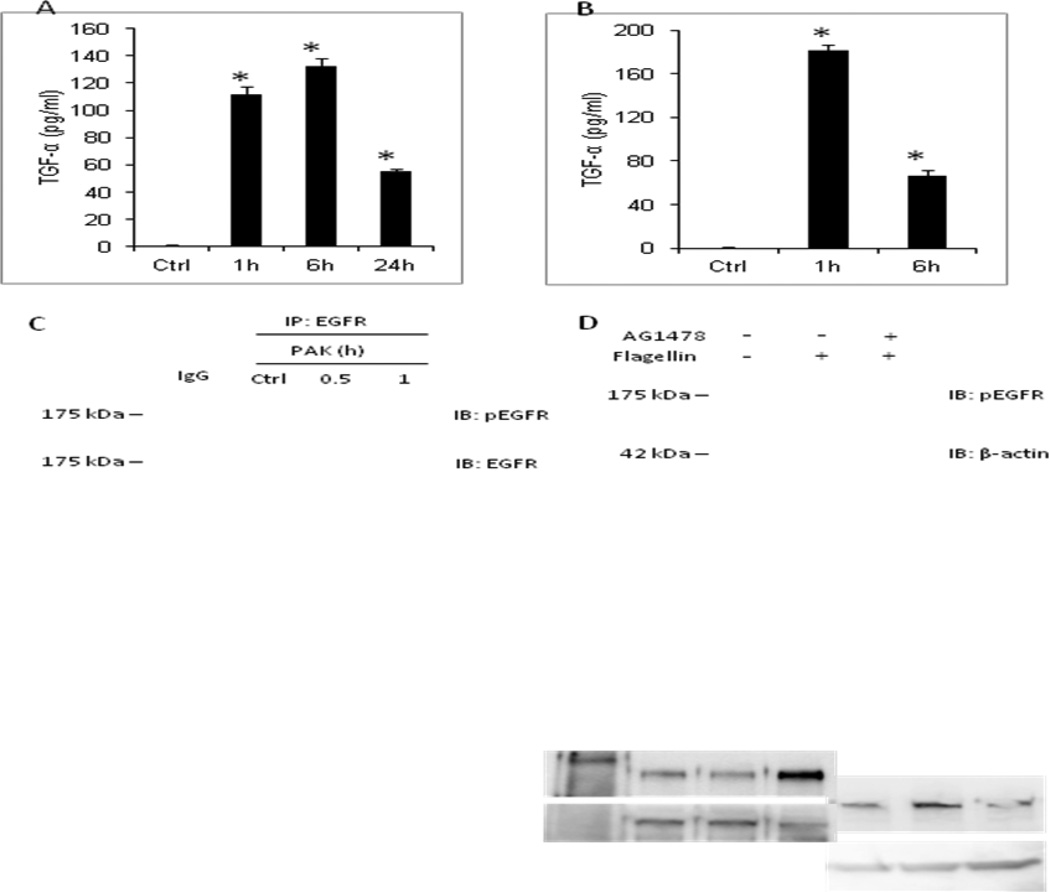
Yu et al. (18) previously reported that treatment of 16HBE cells, a SV40 transformed human bronchial epithelial cell line, with Pa flagellin stimulated TGF-α release, EGFR tyrosine phosphorylation, and MUC5AC expression in a TNF-α converting enzyme (TACE)-dependent manner. We asked whether stimulation of primary NHBE cells by Pa and/or its flagellin might also induce TGF-α release and EGFR tyrosine phosphorylation. Treatment of NHBE cells with 1.0 × 107 CFU/ml of heat-inactivated PAK or 100 ng/ml of flagellin for 1, 6, or 24 h increased TGF-α levels in cell culture supernatants (Figures 1A, 1B), and increased EGFR tyrosine phosphorylation (Figures 1C, 1D), compared with unstimulated controls. Flagellin stimulation in the presence of the EGFR selective inhibitor, AG1478, abrogated EGFR tyrosine phosphorylation (Figure 1D). Zhang Z et al. previously reported that heat-inactivated PAK mediates cytokine release from human airway epithelial cells through activation of TLR5 by its flagellin (5). Thus, in the present study, we used heat-inactivated PAK and flagellin interchangeably in stimulating TLR5 signaling.
Figure 1. Pa and flagellin stimulate TGF-α release from NHBE cells.
(A, B) NHBE cells were unstimulated (Ctrl) or stimulated for the indicated times with (A) 1.0 × 107 CFU/ml of heat-inactivated PAK or (B) 100 ng/ml of Pa flagellin and cell culture supernatants were processed for TGF-α levels by ELISA. Vertical bars represent mean ± SEM TGF-α levels (n=4). *, significantly increased TGF-α levels of Pa/flagellin-stimulated cells vs. unstimulated controls at p<0.05. (C) NHBE cells were unstimulated (Ctrl) or stimulated for the indicated times with 1.0 × 107 CFU/ml of heat-inactivated PAK. Cell lysates were processed for immunoprecipitation (IP) with anti-EGFR antibody or normal rabbit IgG as a negative control and immunoprecipitates were processed for phospho-EGFR (Tyr1068) and EGFR immunoblotting (IB). (D) NHBE cells were unstimulated (−) or stimulated for 1 h (+) with 100 ng/ml of Pa flagellin in the presence or absence of 100 nM of AG1478. Cell lysates were processed for phospho-EGFR (Tyr1068) immunoblotting. To control for protein loading and transfer, the blots were stripped and reprobed for β-actin. MW in kDa is indicated on the left. The results are representative of 3 independent experiments.
Pa and flagellin elicit TNF-α release from primary macrophages, but not NHBE cells
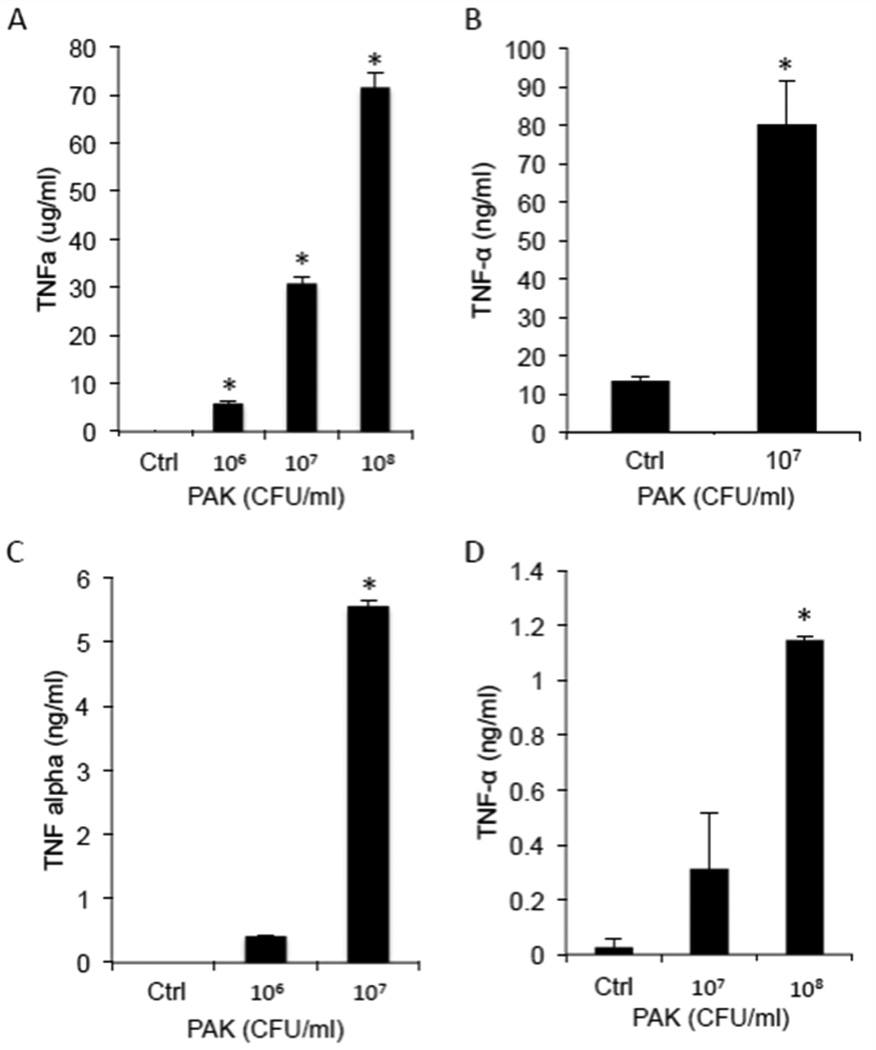
Our previous in vivo and in vitro studies demonstrated that TNF-α, another proinflammatory cytokine generated by TACE, up-regulates MUC1/Muc1 expression in human A549 cells [19] and Pa-infected mouse lungs (15, 19). Since A549 cells are a lung adenocarcinoma cell line, we tested whether stimulation of primary NHBE cells by Pa or flagellin also induce TNF-α release. The levels of TNF-α in culture supernatants of Pa- or flagellin-stimulated NHBE cells were undetected by ELISA over the course of 24 h (data not shown). On the other hand, stimulation of human alveolar macrophages increased TNF-α levels in cell culture supernatants in a dose-dependent manner (Figure 2A). Identical results were seen in other macrophages such as human THP-1-derived macrophages (Figure 2B), mouse peritoneal macrophages (Figure 2C), and mouse bone marrow-derived macrophages (Figure 2D). These results are consistent with a recent report that, whereas Pa or flagellin elicited TNF-α release by mouse alveolar macrophages, treatment of mouse primary airway epithelial cells by either stimulus failed to stimulate TNF-α release (20).
Figure 2. Pa stimulates TNF-α release from human and mouse macrophages.
(A) Human THP-1-derived macrophages, (B) human alveolar macrophages, (C) mouse peritoneal macrophages, and (D) mouse bone marrow-derived macrophages were stimulated for 4 h (mouse macrophages) or 24 h (human macrophages) with the indicated concentrations of heat-inactivated PAK or medium alone (Ctrl), and cell culture supernatants were processed for TNF-α levels by ELISA. Vertical bars represent mean ± SEM TNF-α levels (n=4). *, significantly increased TNF-α levels of cells stimulated with PAK vs. medium control at p<0.05. The results (A,C,D) are representative of 3 independent experiments.
Exogenous TNF-α, but not Pa, up-regulates MUC1 expression in NHBE cells
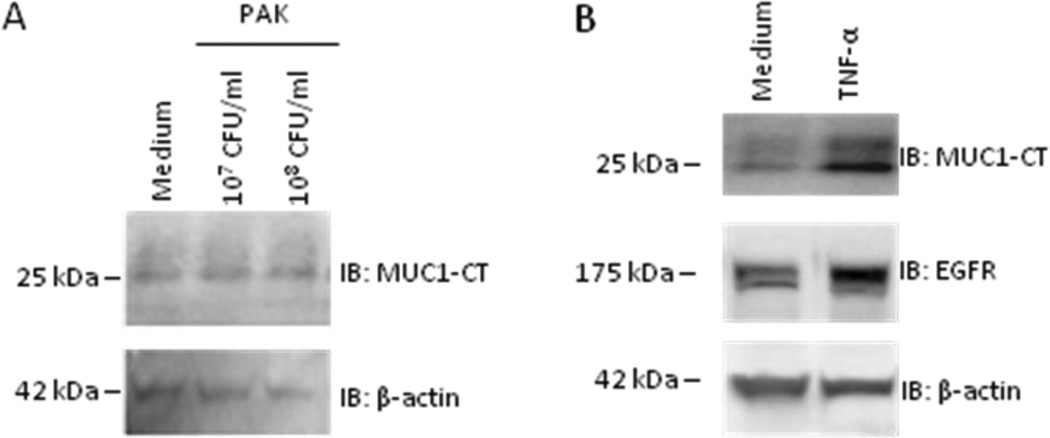
To directly assess the ability of Pa or TNF-α to up-regulate MUC1 expression, NHBE cells were stimulated for 24 h with 1.0 × 107 or 1.0 × 108 CFU/ml of heat-inactivated PAK, 50 ng/ml of human recombinant TNF-α, or medium controls and cell lysates were probed on immunoblots for MUC1-CT and EGFR. Whereas Pa failed to alter MUC1-CT expression, TNF-α stimulation increased both MUC1-CT and EGFR expression (Figure 3). Similarly, Pa failed to upregulate Muc1 expression in differentiated, primary mouse airway epithetical cells (data now shown).
Figure 3. Exogenous TNF-α, but not Pa, up-regulates MUC1 expression in NHBE cells.
NHBE cells were stimulated for 24 h with (A) the indicated doses of heat-inactivated PAK, (B) 50 ng/ml of TNF-α, or medium alone. Cell lysates were processed for MUC1-CT or EGFR immunoblotting (IB). To control for protein loading and transfer, the blots were stripped and reprobed for β-actin. MW in kDa is indicated on the left. The results are representative of 3 independent experiments.
Pa stimulation of NHBE cells increases tyrosine phosphorylation of MUC1-CT and augments MUC1-CT/TLR5 and MUC1-CT/EGFR association
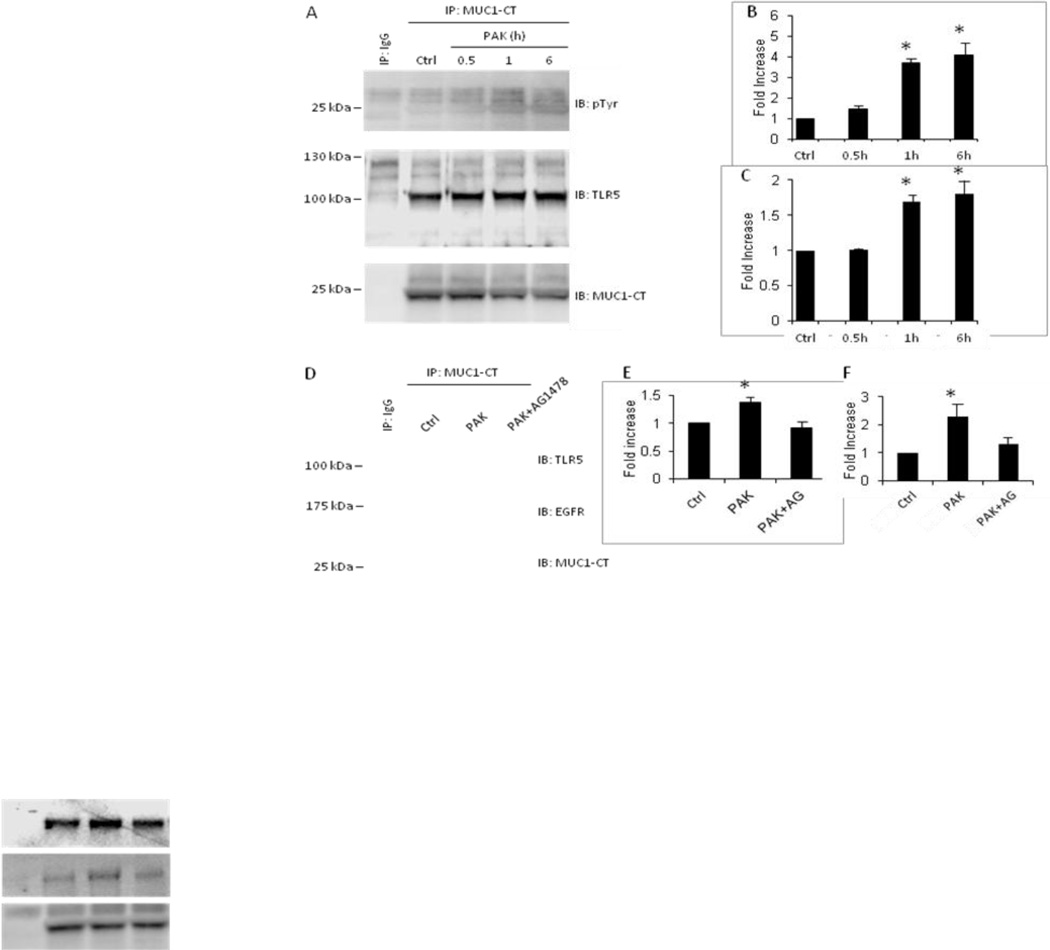
The MUC1-CT interacts with EGFR and EGFR tyrosine phosphorylates MUC1-CT at a Tyr-Glu-Lys-Val motif in breast adenocarcinoma cells (21–23). Further, tyrosine-phosphorylated MUC1-CT associates with TLR5 in lung cancer cells (11). We asked whether Pa stimulation of primary NHBE cells, which activates EGFR (Figure 1C), might increase MUC1-CT tyrosine phosphorylation and MUC1-CT association with TLR5 and/or EGFR. NHBE cells were pretreated for 24 h with 50 ng/ml of TNF-α, following which the cells were washed and either unstimulated or stimulated for increasing times with 1.0 × 108 CFU/ml of heat-inactivated PAK. Cell lysates were subjected to MUC1-CT immunoprecipitation and the immunoprecipitates processed for phosphotyrosine, TLR5, or EGFR immunoblotting. Pa stimulation of NHBE cells increased MUC1-CT tyrosine phosphorylation (Figure 4A, upper panel). When each MUC1-CT phosphotyrosine densitometry signal was normalized to the total MUC1-CT signal (Figure 4A, lower panel) in the same lane on the same blot, Pa stimulation for 0.5, 1, and 6 h increased MUC1-CT tyrosine phosphorylation by 1.5-, 3.7-, and 4.0-fold, respectively, compared with unstimulated controls (Figure 4B). Pa stimulation of NHBE cells also augmented MUC1-CT/TLR5 co-immunoprecipitation (Figure 4, middle panel). Densitometric analysis revealed 1.7- and 1.8 fold increased MUC1-CT/TLR5 association following 1 and 6 h Pa stimulation compared with unstimulated controls (Figures 4C). In other experiments, Pa stimulation of NHBE cells for 1 h increased MUC1-CT/TLR5 association by 1.4-fold and MUC1-CT/EGFR association by 2.3-fold compared with medium controls (Figures 4D–4F). Finally, Pa stimulation for 1 h in the presence of AG1478 protected against Pa-stimulated increases in both MUC1-CT/TLR5 and MUC1-CT/EGFR association (Figures 4D–4F). As negative controls, neither MUC1-CT tyrosine phosphorylation, nor MUC1-CT/TLR5 or MUC1-CT/EGFR co-immunoprecipitation, were seen using lysates of NHBE cells stimulated with Pa when normal Armenian hamster IgG was used in lieu of the MUC1-CT immunoprecipitating antibody (Figures 4A, 4D). Taken together, these data indicate that Pa stimulation of primary NHBE cells increases MUC1-CT tyrosine phosphorylation and enhances MUC1-CT association with both TLR5 and EGFR.
Figure 4. Pa stimulation of NHBE cells increases tyrosine phosphorylation of MUC1-CT and augments MUC1-CT/TLR5 and MUC1-CT/EGFR association.
(A) NHBE cells were unstimulated (Ctrl) or stimulated for the indicated times with 1.0 × 107 CFU/ml of heat-inactivated PAK. Cell lysates were processed for immunoprecipitation (IP) with anti-MUC1-CT antibody or normal Armenian hamster IgG and immunoprecipitates were processed for phosphotyrosine (pTyr) (upper panel) or TLR5 (middle panel) immunoblotting (IB). To control for protein loading and transfer, the blots were stripped and reprobed for MUC1-CT. (B) Densitometric analysis of the MUC1-CT phosphotyrosine blots in (A). (C) Densitometric analysis of the MUC1-CT/TLR5 co-immunoprecipitation in (A). Vertical bars represent mean ± SEM of (B) phosphotyroine or (C) TLR5 signals normalized to MUC1-CT signal in the same lane on the same blot (n=3) and expressed as fold increase over control. *, significantly increased normalized phosphotyrosine or TLR5 densitometry of Pa-stimulated cells vs. unstimulated cells at p<0.05. (D) NHBE cells were stimulated for 1 h with medium alone (Ctrl) or 1.0 × 107 CFU/ml of heat-inactivated PAK in the presence or absence of 100 nM of AG1478. Cell lysates were processed for immunoprecipitation with anti-MUC1-CT antibody or normal Armenian hamster IgG and immunoprecipitates were processed for TLR5 (upper panel) or EGFR (middle panel) immunoblotting. To control for protein loading and transfer, the blots were stripped and reprobed for MUC1-CT. (E) Densitometric analysis of the MUC1-CT/TLR5 co-immunoprecipitation in (D). (F) Densitometric analysis of the MUC1-CT/EGFR co-immunoprecipitation in (D). Vertical bars represent mean ± SEM of (E) TLR5 or (F) EFGR signals normalized to MUC1-CT signal in the same lane on the same blot (n=3) and expressed as fold increase over control. *, significantly increased normalized TLR5 or EGFR densitometry of PAK-stimulated cells vs. medium control at p<0.05. MW in kDa is indicated on the left. The results are representative of 3 independent experiments.
Discussion
MUC1/Muc1 expression attenuates airway inflammation during Pa lung infection (8, 19) and following intranasal challenge with Pa flagellin (8). This anti-inflammatory activity has been ascribed to the ability of MUC1/Muc1 to inhibit TLR signaling (8, 9, 14). However, the mechanistic details of this counter-regulatory effect remain to be elucidated. In this report, we now demonstrate that stimulation of primary NHBE cells with Pa or flagellin increased the release of TGF-α, but not TNF-α, from NHBE cells. This is in contrast to our previous reports in which treatment of A549 lung adenocarcinoma cells with pathogens stimulated TNF-α release which resulted in MUC1 upregulation in an autocrine fashion (24, 25). Pa/flagellin-stimulated TGF-α release led to activation of its cognate cell surface receptor, EGFR, in NHEB cells. By contrast, Pa stimulation of human or mouse macrophages enhanced TNF-α release, and stimulation of NHBE cells with exogenous, recombinant TNF-α increased both MUC1-CT and EGFR protein levels. Finally, immunoprecipitation experiments established that Pa stimulation enhanced MUC1-CT tyrosine phosphorylation, and increased MUC1-CT/TLR5 and MUC1-CT/EGFR protein association. Because both alveolar macrophages and NHBE cells express pattern recognition receptors (e.g. TLRs) (20), these results suggest a mechanism whereby inhaled Pa activate alveolar macrophages to release TNF-α, which in turn, increases MUC1 expression by NHBE cells in a paracrine fashion, while Pa stimulation of airway epithelial cells promotes TGF-α release, autocrine EGFR activation, MUC1-CT tyrosine phosphorylation, and MUC1-CT/TLR5 association (Figure 5). Based on our studies in HEK293 and A549 cells (11), we further propose that increased MUC1-CT/TLR5 association in NHBE cells diminishes Pa- and flagellin-driven, TLR5-dependent intracellular signaling and downstream inflammatory responses (Figure 5).
Figure 5. Proposed model for the roles of TGF-α, TNF-α, EGFR, MUC1, and TLR5 in the response of airway macrophages and epithelial cells to Pa.
Inhaled Pa activates alveolar macrophages (Mac) to release TNF-α, which increases MUC1 expression by airway epithelial cells (AEC). Pa stimulation of AEC drives TGF-α release, EGFR activation, MUC1-CT tyrosine phosphorylation, MUC1-CT/TLR5 association, and inhibition of airway inflammation.
EGFR regulates mucin secretion by airway goblet cells (26). Mechanistic studies revealed that Pa bacterial supernatants induce mucin production in human NCI-H292 airway epithelial cells through activation of TACE, release of TGF-α, and autocrine EGFR phosphorylation (27, 28). TGF-α is initially synthesized as a membrane-bound protein on the surface of airway epithelial cells. TACE proteolytically cleavages pro-TGF-α to the active ligand that engages EGFR, inducing its autotransactivation and proinflammatory signaling (29). The current study now demonstrates a second effect of Pa-stimulated EGFR activation in normal airway epithelia, i.e. MUC1-CT tyrosine phosphorylation and MUC1-CT/TLR5 association. These observations support prior reports showing EGFR tyrosine phosphorylates MUC1-CT in breast cancer cells (21), and that tyrosine-phosphorylated MUC1-CT interacts with TLR5 in lung cancer cells (11). Recently, suppression of TLR5-dependent proinflammatory responses by MUC1 also has been documented in telomerase-transformed, immortalized human corneal epithelial cells (30), MNK7 human gastric cancer cells (31), and murine dendritic cells (32). To our knowledge, however, this is the first report demonstrating both EGFR-driven MUC1-CT phosphorylation and MUC1-CT/TLR5 association in nontransformed, normal human airway epithelial cells.
While TLR activation is an essential defense mechanism, it must be tightly regulated by counter-balancing, anti-inflammatory pathways in order to prevent the development of autoimmune and hyper-inflammatory diseases. Three major anti-inflammatory mechanisms down-regulating TLR-driven inflammation have been described, dissociation of adaptor molecules, degradation of signaling proteins, and decreased gene transcription (33). We previously reported that Muc1 knockout mice exhibit greater airway inflammation following Pa lung infection compared with Muc1-expessing littermates (8). Subsequently, we demonstrated that overexpression of the full-length MUC1, but not a deletion mutant lacking the MUC1-CT, attenuated in vitro proinflammatory responses, thus mapping the anti-inflammatory effect to its intracellular domain (14). The results of the current study, and those reported elsewhere (11), now indicate that tyrosine phosphorylation of the MUC1-CT enhances its association with TLR5, thereby competitively inhibiting recruitment of the TLR adapter protein, MyD88, to TLR5, which is an additional anti-inflammatory mechanism down-regulating TLR-driven inflammation.
The observation that MUC1-CT also associates with EGFR in primary NHBE cells confirms previous reports of MUC1-CT/EGFR interaction in breast adenocarcinoma cells (21–23). Cross-talk between MUC1 and EGFR has been demonstrated in various cellular contexts. Anti-MUC1 antibody was shown to inhibit EGFR signaling (34), and targeting the MUC1-CT with a peptide that blocks its homodimerization diminished EGFR activation (35). Activated EGFR stimulated MUC1 expression (36), and suppression of MUC1 synthesis down-regulated EGFR expression (37). In the airways, MUC1 contributed to human bronchial epithelial cell transformation through facilitating EGFR activation (38). These examples of cross-talk between MUC1 and EGFR might be regulated, in part, through their molecular interaction. Whether MUC1-CT forms a protein complex with EGFR simultaneous with or independent of TLR5, and whether MUC1-CT association with TLR5 might influence MUC1-EGFR cross-talk (and vice versa), are currently unknown.
Conclusions
Stimulation of primary NHBE cells with the opportunistic human pathogen, Pa, or its flagellin, induces EGFR association with and tyrosine phosphorylation of the MUC1-CT. MUC1-CT tyrosine phosphorylation increases its association with TLR5. Based on other studies in lung adenocarcinoma cells (11), increased MUC1-CT/TLR5 association in NHBE cells is predicted to competitively inhibit Pa- or flagellin-stimulated TLR5 activation, reduce TLR5-dependent cell signaling, and diminish airway inflammatory responses.
Acknowledgments
This work was supported by National institutes of Health grant RO1 HL-047125. The authors thank Alec Hanss, Audriana Hurbon, and Nicole Morgan for technical assistance through their summer research program at the University of Arizona (Tucson, AZ).
List of abbreviations
- AEC
airway epithelial cell
- CFU
colony forming unit
- CT
cytoplasmic tail
- EGFR
epidermal growth factor receptor
- ELISA
enzyme-linked immunosorbent assay
- HRP
horseradish peroxidase
- IL
interleukin
- Mac
macrophage
- IB
immunoblot
- IP
immunoprecipitation
- NK-κB
nuclear factor-κB
- NHBE
normal human bronchial epithelial
- Pa
Pseudomonas aeruginosa
- PAK
Pseudomonas aeruginosa strain K
- PAMPs
pathogen-associated molecular patterns
- PI3K
phosphoinositide 3-kinase
- TACE
tumor necrosis factor-α converting enzyme
- TGF-α
transforming growth factor-α
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor-α
Footnotes
Competing interests
The authors declare that they have no competing interests.
Author’s contributions
KK and KCK designed the experiments; KK performed the experiments; KK, EPL, and KCK analyzed the data; KK, EPL, and KCK wrote the manuscript.
Contributor Information
Kosuke Kato, Email: kosukekato@oto.arizona.edu.
Erik P. Lillehoj, Email: elillehoj@peds.umaryland.edu.
Kwang Chul Kim, Email: kckim@oto.arizona.edu.
References
- 1.Zhuo H, Yang K, Lynch SV, Dotson RH, Glidden DV, Singh G, Webb WR, Elicker BM, Garcia O, Brown R, Sawa Y, Misset B, Wiener-Kronish JP. Increased mortality of ventilated patients with endotracheal Pseudomonas aeruginosa without clinical signs of infection. Crit Care Med. 2008;36(9):2495–2503. doi: 10.1097/CCM.0b013e318183f3f8. [DOI] [PubMed] [Google Scholar]
- 2.Chawla K, Vishwanath S, Manu MK, Lazer B. Influence of pseudomonas aeruginosa on exacerbation in patients with bronchiectasis. J Glob Infect Dis. 2015;7(1):18–22. doi: 10.4103/0974-777X.150885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347(7):465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 4.Basu S, Fenton MJ. Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol. 2004;286(5):L887–L892. doi: 10.1152/ajplung.00323.2003. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Z, Louboutin JP, Weiner DJ, Goldberg JB, Wilson JM. Human airway epithelial cells sense Pseudomonas aeruginosa infection via recognition of flagellin by Toll-like receptor 5. Infect Immun. 2005;73(11):7151–7160. doi: 10.1128/IAI.73.11.7151-7160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ojielo CI, Cooke K, Mancuso P, Standiford TJ, Olkiewicz KM, Clouthier S, Corrion L, Ballinger MN, Toews GB, Paine R, 3rd, Moore BB. Defective phagocytosis and clearance of Pseudomonas aeruginosa in the lung following bone marrow transplantation. J Immunol. 2003;171(8):4416–4424. doi: 10.4049/jimmunol.171.8.4416. [DOI] [PubMed] [Google Scholar]
- 7.Cohen TS, Prince AS. Activation of inflammasome signaling mediates pathology of acute P. aeruginosa pneumonia. J Clin Invest. 2013;123(4):1630–1637. doi: 10.1172/JCI66142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu W, Hisatsune A, Koga T, Kato K, Kuwahara I, Lillehoj EP, Chen W, Cross AS, Gendler SJ, Gewirtz AT, Kim KC. Cutting edge: enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J Immunol. 2006;176(7):3890–3894. doi: 10.4049/jimmunol.176.7.3890. [DOI] [PubMed] [Google Scholar]
- 9.Kato K, Lu W, Kai H, Kim KC. Phosphoinositide 3-kinase is activated by MUC1 but not responsible for MUC1-induced suppression of Toll-like receptor 5 signaling. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L686–L692. doi: 10.1152/ajplung.00423.2006. [DOI] [PubMed] [Google Scholar]
- 10.Umehara T, Kato K, Park YS, Lillehoj EP, Kawauchi H, Kim KC. Prevention of lung injury by Muc1 mucin in a mouse model of repetitive Pseudomonas aeruginosa infection. Inflamm Res. 2012;61(9):1013–1020. doi: 10.1007/s00011-012-0494-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato K, Lillehoj EP, Park YS, Umehara T, Hoffman NE, Madesh M, Kim KC. Membrane-Tethered MUC1 Mucin Is Phosphorylated by Epidermal Growth Factor Receptor in Airway Epithelial Cells and Associates with TLR5 To Inhibit Recruitment of MyD88. J Immunol. 2012;188:2014–2022. doi: 10.4049/jimmunol.1102405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia. 2001;6(3):339–353. doi: 10.1023/a:1011379725811. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, Lillehoj EP, Kim KC. Identification of four sites of stimulated tyrosine phosphorylation in the MUC1 cytoplasmic tail. Biochem Biophys Res Commun. 2003;310(2):341–346. doi: 10.1016/j.bbrc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 14.Ueno K, Koga T, Kato K, Golenbock DT, Gendler SJ, Kai H, Kim KC. MUC1 mucin is a negative regulator of toll-like receptor signaling. Am J Respir Cell Mol Biol. 2008;38(3):263–268. doi: 10.1165/rcmb.2007-0336RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi S, Park YS, Koga T, Treloar A, Kim KC. TNF-alpha is a key regulator of MUC1, an anti-inflammatory molecule, during airway Pseudomonas aeruginosa infection. Am J Respir Cell Mol Biol. 2011;44(2):255–260. doi: 10.1165/rcmb.2009-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5(1):e8668. doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;Chapter 14(Unit 14.1) doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu H, Zhou X, Wen S, Xiao Q. Flagellin/TLR5 responses induce mucus hypersecretion by activating EGFR via an epithelial cell signaling cascades. Exp Cell Res. 2012;318(6):723–731. doi: 10.1016/j.yexcr.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Koga T, Kuwahara I, Lillehoj EP, Lu W, Miyata T, Isohama Y, Kim KC. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: its signaling pathway and biological implication. Am J Physiol Lung Cell Mol Physiol. 2007;293(3):L693–L701. doi: 10.1152/ajplung.00491.2006. [DOI] [PubMed] [Google Scholar]
- 20.Raoust E, Balloy V, Garcia-Verdugo I, Touqui L, Ramphal R, Chignard M. Pseudomonas aeruginosa LPS or flagellin are sufficient to activate TLR-dependent signaling in murine alveolar macrophages and airway epithelial cells. PLoS One. 2009;4(10):e7259. doi: 10.1371/journal.pone.0007259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Ren J, Yu W, Li Q, Kuwahara H, Yin L, Carraway KL, 3rd, Kufe D. The epidermal growth factor receptor regulates interaction of the human DF3/MUC1 carcinoma antigen with c-Src and beta-catenin. J Biol Chem. 2001;276(38):35239–35242. doi: 10.1074/jbc.C100359200. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder JA, Thompson MC, Gardner MM, Gendler SJ. Transgenic MUC1 interacts with epidermal growth factor receptor and correlates with mitogen-activated protein kinase activation in the mouse mammary gland. J Biol Chem. 2001;276(16):13057–13064. doi: 10.1074/jbc.M011248200. [DOI] [PubMed] [Google Scholar]
- 23.Bitler BG, Goverdhan A, Schroeder JA. MUC1 regulates nuclear localization and function of the epidermal growth factor receptor. J Cell Sci. 2010;123(Pt 10):1716–1723. doi: 10.1242/jcs.062661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kyo Y, Kato K, Park YS, Gajhate S, Umehara T, Lillehoj EP, Suzaki H, Kim KC. Anti-inflammatory role of MUC1 mucin during nontypeable Haemophilus influenzae infection. Am J Respir Cell Mol Biol. 2012;46(2):149–156. doi: 10.1165/rcmb.2011-0142OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Dinwiddie DL, Harrod KS, Jiang Y, Kim KC. Anti-inflammatory effect of MUC1 during respiratory syncytial virus infection of lung epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol. 2010;298(4):L558–L563. doi: 10.1152/ajplung.00225.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeyama K, Dabbagh K, Lee HM, Agusti C, Lausier JA, Ueki IF, Grattan KM, Nadel JA. Epidermal growth factor system regulates mucin production in airways. Proc Natl Acad Sci U S A. 1999;96(6):3081–3086. doi: 10.1073/pnas.96.6.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohri K, Ueki IF, Shim JJ, Burgel PR, Oh YM, Tam DC, Dao-Pick T, Nadel JA. Pseudomonas aeruginosa induces MUC5AC production via epidermal growth factor receptor. Eur Respir J. 2002;20(5):1263–1270. doi: 10.1183/09031936.02.00001402. [DOI] [PubMed] [Google Scholar]
- 28.Shao MX, Ueki IF, Nadel JA. Tumor necrosis factor alpha-converting enzyme mediates MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A. 2003;100(20):11618–11623. doi: 10.1073/pnas.1534804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gooz M. ADAM-17: the enzyme that does it all. Crit Rev Biochem Mol Biol. 2010;45(2):146–169. doi: 10.3109/10409231003628015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menon BB, Kaiser-Marko C, Spurr-Michaud S, Tisdale AS, Gipson IK. Suppression of Toll-like receptor-mediated innate immune responses at the ocular surface by the membrane-associated mucins MUC1 and MUC16. Mucosal Immunol. 2015 doi: 10.1038/mi.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng YH, Triyana S, Wang R, Das I, Gerloff K, Florin TH, Sutton P, McGuckin MA. MUC1 and MUC13 differentially regulate epithelial inflammation in response to inflammatory and infectious stimuli. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.98. [DOI] [PubMed] [Google Scholar]
- 32.Williams MA, Bauer S, Lu W, Guo J, Walter S, Bushnell TP, Lillehoj EP, Georas SN. Deletion of the mucin-like molecule muc1 enhances dendritic cell activation in response to toll-like receptor ligands. J Innate Immun. 2010;2(2):123–143. doi: 10.1159/000254790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kondo T, Kawai T, Akira S. Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol. 2012;33(9):449–458. doi: 10.1016/j.it.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Hisatsune A, Nakayama H, Kawasaki M, Horie I, Miyata T, Isohama Y, Kim KC, Katsuki H. Anti-MUC1 antibody inhibits EGF receptor signaling in cancer cells. Biochem Biophys Res Commun. 2011;405(3):377–381. doi: 10.1016/j.bbrc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 35.Kharbanda A, Rajabi H, Jin C, Tchaicha J, Kikuchi E, Wong KK, Kufe D. Targeting the oncogenic MUC1-C protein inhibits mutant EGFR-mediated signaling and survival in non-small cell lung cancer cells. Clin Cancer Res. 2014;20(21):5423–5434. doi: 10.1158/1078-0432.CCR-13-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neeraja D, Engel BJ, Carson DD. Activated EGFR stimulates MUC1 expression in human uterine and pancreatic cancer cell lines. J Cell Biochem. 2013;114(10):2314–2322. doi: 10.1002/jcb.24580. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Wang L, Nunes DP, Troxler RF, Offner GD. Suppression of MUC1 synthesis downregulates expression of the epidermal growth factor receptor. Cancer Biol Ther. 2005;4(9):968–973. doi: 10.4161/cbt.4.9.1913. [DOI] [PubMed] [Google Scholar]
- 38.Xu X, Bai L, Chen W, Padilla MT, Liu Y, Kim KC, Belinsky SA, Lin Y. MUC1 contributes to BPDE-induced human bronchial epithelial cell transformation through facilitating EGFR activation. PLoS One. 2012;7(3):e33846. doi: 10.1371/journal.pone.0033846. [DOI] [PMC free article] [PubMed] [Google Scholar]