Abstract
Physical and functional interactions between mitochondria and the endoplasmic reticulum (ER) are crucial for cell life. These two organelles are intimately connected and collaborate to essential processes, such as calcium homeostasis and phospholipid biosynthesis. The connections between mitochondria and endoplasmic reticulum occur through structures named mitochondria associated membranes (MAMs), which contain lipid rafts and a large number of proteins, many of which serve multiple functions at different cellular sites. Growing evidence strongly suggest that alterations of ER-mitochondria interactions are involved in neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS), a devastating and rapidly fatal motor neuron disease. Mutations in proteins that participate in ER-mitochondria interactions and MAM functions are increasingly being associated with genetic forms of ALS and other neurodegenerative diseases. This evidence strongly suggests that, rather than considering the two organelles separately, a better understanding of the disease process can derive from studying the alterations in the their crosstalk. In this review we discuss normal and pathological ER-mitochondria interactions and the evidence that link them to ALS.
Keywords: Endoplasmic reticulum, calcium, MAMS, mitochondria, reactive oxygen species, protein misfolding, amyotrophic lateral sclerosis
Introduction
Cells depend on a compartmentalized system for biochemical processes and signaling responses to live and thrive. Mitochondria are double membrane organelles enclosing their own DNA (mitochondrial DNA) in the matrix. They are the main cellular energy producers through oxidative phosphorylation and the physical hubs for the majority of the enzymatic pathways of intermediate metabolism. The endoplasmic reticulum (ER) is a large membrane-bound network surrounding a single lumen that spreads throughout the cytoplasm. The ER is localized within reach of contact with all other membrane structures and organelles, including the nuclear envelope, plasma membrane, and mitochondria. The ER is particularly important for protein folding and intracellular calcium storage. Through physical and functional interactions, ER and mitochondria contribute to common crucial cellular processes, such as calcium homeostasis and lipid biosynthesis. Numerous lines of evidence suggest that dysfunction of these organelles participates in the pathogenesis of various neurodegenerative conditions, including amyotrophic lateral sclerosis (ALS). A large body of literature has addressed the involvement of ER and mitochondria individually. However, due to the emerging concept that these organelles are physically and functionally intertwined, it is logical to address ER-mitochondria interactions in discussing their roles in disease pathogenesis. This review article briefly describes established concepts supporting the involvement of mitochondria and ER in ALS, while delving deeper into emerging evidence for abnormal ER-mitochondria crosstalk in the context of ALS and discussing new perspectives in ER-mitochondria involvement in the pathogenesis of this disease.
Physiological mitochondria-ER interactions
Physical contacts between ER and mitochondria occur at specific sites called mitochondria associated membranes (MAMs). MAMs are specialized ER membranes tethered to mitochondria through a host of protein interactions. Many proteins have been identified in the MAMs (van Vliet et al., 2014). They can be broadly categorized into calcium signaling proteins, such as inositol 1,4,5-triphosphate (IP3) receptor (IP3R) and voltage dependent anion channel (VDAC), lipid metabolism, such as phosphatidylethanolamine N-methyltransferase 2 (PEMT2) and fatty acid-CoA ligase 4 (FACL4), autophagy related proteins, such as ATG15 and ATG4, and tethering proteins, such as mitofusin 2 (Mfn2).
Two fundamental mitochondria-ER functional interactions occurring at the MAMs are phospholipid biosynthesis and intracellular calcium handling. Enzymes involved in phospholipid biosynthesis are concentrated in MAMs, where they metabolize phospholipid intermediates both on the mitochondrial and the ER membranes (Vance, 2014). Phospholipid intermediates are shuttled back and forth between the two organelles during the biosynthetic process. For example, phosphatidylserine (PS) made in the ER is transferred to mitochondria to be converted to phospatidyletanolamine (PE), and PE goes back to the ER to be incorporated into biological membranes.
MAMs are also involved in ER-mitochondria interactions through calcium transfer. The ER is a reservoir for intracellular calcium, able to store up to hundreds of micromolar calcium. Particularly in non-excitable cells, calcium signaling pathways are activated by hormones that act through G protein-coupled receptors and production of inositol 3-phosphate (IP3), A puff of ER calcium released through IP3R activates calcium-induced calcium release (ICICR) by ryanodine receptors (RyRs) to increase cytosolic calcium and trigger signals regulating a multiplicity of calcium-dependent systems. ICICR through RyRs is particularly important in muscle cells, where it activates myofibers contraction, but it has been proposed that it is also involved in neuronal plasticity (Barbara, 2002), suggesting a role for this receptor in intracellular signaling in neurons. Mitochondria actively take up calcium through the mitochondrial calcium uniporter (MCU). MCU activity is membrane potential dependent, and requires low micromolar calcium to initiate uptake (Baughman et al., 2011; De Stefani et al., 2011). Close apposition of ER and mitochondrial membranes at MAMs, where ER calcium is released through IP3Rs and RyRs, allows for “hot spots” of calcium transfer from ER to mitochondria, because local calcium concentration is sufficient to trigger full MCU activity (Rizzuto et al., 1993; Rizzuto et al., 2004). Calcium entry into mitochondria boosts oxidative phosphorylation, as the dehydrogenases of the Krebs cycle are stimulated by calcium (Cardenas et al., 2010; McCormack and Denton, 1980). On the other hand, excessive mitochondrial calcium accumulation can cause the opening of the mitochondrial permeability transition pore (MPTP), which has been associated with activation of cell death pathways (Rasola and Bernardi, 2011). It was demonstrated experimentally that altering the physical distance between the opposing membranes affect calcium flow from ER to mitochondria and cell viability (Csordas et al., 2006).
In addition to the interactions mentioned above, it was recently proposed that ER plays a critical role in the regulation of mitochondrial dynamics, especially organellar fission. ER-associated mitochondrial division (ERMD), is a process whereby the ER tubules wrap around mitochondria at the sites where division occurs (Friedman and Nunnari, 2014). This process is highly conserved throughout evolution and in yeast it depends on proteins of the ER (ER)-mitochondria encounter structure (ERMES) complex (Kornmann and Walter, 2010). This complex also regulates mtDNA nucleoids maintenance. Orthologs of ERMES components have not yet been identified in mammalian cells, but it is likely that MAM proteins serve analogous purposes. Furthermore, MAMs are involved in the process of mitophagy, as ER-mitochondrial contacts are sites of phagophore membrane formation (Bockler and Westermann, 2014a; Bockler and Westermann, 2014b).
Mitochondria-ER interactions in ALS
ALS is a debilitating disease with aggressively progressive muscle paralysis leading to death within few years of diagnosis. Paralysis is caused by a prominent degeneration of upper and lower motor neurons that communicate with muscle cells. There is an urgent need for effective therapies, because currently there are essentially no treatments available for ALS patients besides Riluzole, which prolongs life for a few months, at best. Familial ALS (fALS) patients with known genetic mutations are relatively rare, and over 90 percent of cases occur sporadically. Mutations in the gene encoding for the antioxidant superoxide dismutase 1 (SOD1) were discovered over 20 years ago in fALS patients (Rosen et al., 1993). Studies of SOD1 mutants have provided insight into the pathogenic mechanisms of ALS, but limited to a subset of approximately 10% of the familial cases. However, in the past 5 years, with the advancement of powerful sequencing tools, there has been a tremendous increase in the identification of genetic mutations responsible for fALS. Many mutations across more than 20 genes have been conclusively or putatively associated with ALS (Marangi and Traynor, 2015). Abnormalities found in genes such as TAR-DNA binding protein 43 (TDP43), fused in sarcoma (FUS), ubiquilin 2 (Ubqln2), vesicle associated membrane protein associated protein B (VAPB), and valosin containing protein (VCP), point to RNA metabolism and protein degradation as potential pathogenic pathways in ALS. However, several other genes, involved in completely different cellular pathways, point to a complex heterogeneity of fALS genetics. Furthermore, the same mutations can often cause different clinical phenotypes, even within the same family. Such clinical phenotypes can range from frontotemporal dementia to ALS or a combination of both, as in the case of the hexanucleotide expansion in the first intron of C9Orf72, a gene with still unknown function, which causes the most common form of fALS (DeJesus-Hernandez et al., 2011; Renton et al., 2011). Symptoms in these syndromic forms of fALS can also include myopathy, such as in the case of mutations in VAPB (Nishimura et al., 2004), VCP (Johnson et al., 2010), and the recently identified mitochondrial protein CHCHD10 (Ajroud-Driss et al., 2015; Bannwarth et al., 2014; Johnson et al., 2014a). Nevertheless, despite the apparent clinical variability in terms of associations of symptoms, widely different genetic causes of fALS appear to ultimately converge into common pathogenic pathways that result in motor neuron degeneration.
Mitochondrial defects in ALS
Interestingly, there seem to be converging pathogenic pathways in ALS that involve mitochondria, as mitochondrial abnormalities are common to many forms of fALS and sALS. Mitochondrial involvement in ALS pathogenesis has been documented extensively (Cozzolino and Carri, 2012). Mitochondrial morphological abnormalities, reduced calcium uptake capacity, transport impairment, bioenergetic dysfunction, and aberrant reactive oxygen species (ROS) production, all appear to contribute to motor neuron demise. In mutant SOD1-fALS, the accumulation and aggregation of the mutant protein in mitochondria poses a detrimental effect on mitochondrial integrity and function (Tan et al., 2014). Mitochondrial abnormalities are also common in non-SOD1 familial cases as well as in sporadic ALS (Carri et al., 2015; Palomo and Manfredi, 2015). Mitochondrial dysfunction in mouse models of ALS appears prior to symptom onset, but it is not clear whether mitochondria are primary or secondary targets of toxicity. The recent discovery of fALS mutations in a gene encoding for a mitochondrial protein, CHCHD10 (Bannwarth et al., 2014; Johnson et al., 2014a), suggests that motor neuron toxicity can arise from mitochondria themselves. More work on decoding the function of CHCHD10, which is so far unknown, will likely reveal important clues on this issue.
ER stress in ALS
The most evident ER abnormality described in ALS is ER stress accompanied by upregulation of the unfolded protein response (UPR). This phenomenon has been amply described in ALS patients (Atkin et al., 2008; Kiskinis et al., 2014; Sasaki, 2010), as well as in cellular and animal models of ALS (C9Orf72 (Zhang et al., 2014), TDP43 (Walker et al., 2013), FUS (Farg et al., 2012), SOD1 (Prell et al., 2012), (Kanekura et al., 2009)). Furthermore, in mutant SOD1 transgenic mouse spinal cord, it was shown that ER stress is detected as one of the earliest pathological events, specifically in the most vulnerable fast fatigable motor neurons (Saxena et al., 2009). While UPR upregulation is undoubtedly a common response in many forms of ALS, it is unclear what exactly triggers its activation. It is likely that proteostasis dysregulation is involved. For example, it was suggested that SOD1 interferes with the ER associated degradation (ERAD) pathway (Nishitoh et al., 2008). Mutant proteins associated with ALS can cause UPR from within the ER lumen, since some of these proteins are found inside the ER or traffic through it. For example, SOD1 can be secreted through the ER-Golgi pathway to the extracellular milieu (Urushitani et al., 2006) and cause toxicity to neurons from the extracellular space (Sundaramoorthy et al., 2013; Walker and Atkin, 2011). Furthermore, mutant SOD1 can misfold in the ER, as demonstrated by the finding of high molecular weight aggregates of mutant SOD1 in ER fractions isolated from the spinal cord of transgenic mice (Urushitani et al., 2008). Recent work also suggested that BIP and its co-factor Sil1 may progressively decline in mutant SOD1 cells (Filezac de L’Etang et al., 2015). In addition to activating ER stress response directly, mutant SOD1 also induces alterations in the protein folding apparatus. Protein disulfide isomerase (PDI), a key oxidoreductase in the ER oxidative protein folding machinery, is S-nitrosylated in ALS, causing defective protein folding and presumably accumulation misfolded proteins (Jeon et al., 2014; Walker and Atkin, 2011). This phenomenon can further exacerbate ER stress in ALS. Mislocalization and aggregation in the cytosol of proteins normally localized in the nucleus, such as TDP43, may cause ER dysfunction or disruption of the ER-mitochondrial contacts (Stoica et al., 2014). Moreover, C9Orf72 mutant motor neurons derived from human induced pluripotent stem cells (iPSC) revealed markers of ER stress (Kiskinis et al., 2014). In iPSC-derived motor neurons, aberrant peptides generated through repeat-associated non-ATG (RAN) translation from the expanded hexanucleotide repeat of C9Orf72 could be responsible for cell toxicity through ER stress (Zhang et al., 2014).
MAM proteins in ALS
Here we explored the evidence for mitochondrial-ER connections in relationship to proteins that physically or functionally relate to ER and MAMs, and are genetically associated with ALS or other neurodegenerative diseases (table 1).
Table 1.
MAM-localized proteins that are associated with ALS and other neurodegenerative conditions.
| Protein | Function | Association with Disease | Reference |
|---|---|---|---|
| Sigma1R | Calcium signaling | Mutations cause fALS | (Al-Saif et al., 2011) |
| VAPB | Membrane interactions, vesicular trafficking | Mutations cause fALS | (Nishimura et al., 2004) |
| IP3R | Calcium signaling | Polymorphisms associated with ALS | (van Es et al., 2008) |
| VDAC | Calcium signaling | Mutant SOD1 affects protein function | (Israelson et al., 2010) |
| Bcl-2 | Anti-apoptotic protein | Mutant SOD1 affects protein function | (Pedrini et al., 2010) |
| SIL1/BIP | ER molecular chaperone | SIL1 reduced in ALS motor neurons Mutations cause Marinesco–Sjögren syndrome |
(Filezac de L’Etang et al., 2015) |
| PERK | Unfolded protein response | Activated in ALS | (Wang et al., 2011) |
| Mfn2 | Mitochondrial fusion | Mutations cause neuropathy | (Ranieri et al., 2013) |
| DRP1 | Mitochondrial fission | Mutations cause fatal encephalopathy | (Waterham et al., 2007) |
| PACS2 | ER localization of transmembrane proteins | Involved in Aβ toxicity? | (Hedskog et al., 2013) |
| α-synuclein | Synaptic function | Mutations (linked to PD) decrease MAMs | (Guardia-Laguarta et al., 2014) |
| Presenilin 1/2 | Gamma secretase complex | Mutations (linked to AD) increase MAMs | (Area-Gomez et al., 2012) |
| Grp75 | Links VDAC and IP3R | Interacts with MATR3 and with FUS | (Osman and van Loveren, 2014) |
Sigma 1R
Sigma1R is a chaperone protein localized in MAMs that involved in lipid export and calcium signaling through IP3R regulation (Hayashi and Su, 2007). A homozygous E102Q mutation in a conserved transmembrane region of Sigma1R was found to be responsible for a juvenile autosomal recessive form of ALS (ALS16 (Al-Saif et al., 2011)). Furthermore, in various forms of fALS, Sigma1R was found to accumulate in large structures on the plasma membrane of spinal cord motor neurons and to co-localize with ubiquitin inclusions (Prause et al., 2013). In sALS spinal cords, Sigma1R levels were lower than in the healthy population (Prause et al., 2013). Experimentally, downregulation of Sigma1R was shown to alter intracellular calcium handling (Hayashi and Su, 2007). Moreover, in primary neurons from Sigma1R knockout mice, the ER ultrastructure was altered and loss of ER-mitochondrial contact sites was observed (Bernard-Marissal et al., 2015). As a consequence of decreased ER-mitochondrial contact and lipid rafts modifications, ER calcium efflux was increased in Sigma1R deficient cells, together with an increase in autophagic lysosome accumulation (Vollrath et al., 2014). Sigma1R deficiency affects mitochondrial transport and dynamics (Bernard-Marissal et al., 2015). Furthermore, E102Q mutant Sigma1R expression causes mitochondrial dysfunction with a decline of ATP production, which results in decreased proteasome activity and ER stress-induced neuronal death in cultured neuronal cells (Fukunaga et al., 2015). Therefore, it is plausible that a loss of function of Sigma1R, either by mutation or by mislocalization, plays a role in ALS pathogenesis. A decline in Sigma1R function could contribute to ALS pathology by causing abnormal ER morphology, lipid raft destabilization, calcium signaling dysregulation, and impaired ER-Golgi trafficking.
VAPB
VAPB is an integral ER protein, whose mutation at a conserved proline residue to serine is causative of ALS8 (Nishimura et al., 2004). VAPB localizes to MAMs and interacts with a mitochondrial protein localized to the outer membrane, protein tyrosine phosphatase-interacting protein-51 (PTPIP51). VAPB-PTPIP51 interaction is important for the maintenance of mitochondrial-ER contacts (Stoica et al., 2014), as deletion of either of the two proteins results in loss of contacts. The Pro56Ser mutation in VAPB associated with ALS increases its affinity for PTPIP51, thereby enhancing calcium transfer into mitochondria (De Vos et al., 2012). Furthermore, ER calcium dysregulation in VAPB mutant neurons results in decreased mitochondrial transport, possibly because of the effects of calcium on Miro1, the cargo adaptor that connects mitochondria to kinesins and microtubules (Morotz et al., 2012). Interestingly, overexpression of wild type or ALS-linked mutants of TDP43 result in mislocalization of the protein in the cytosol and interferes with VAPB-PIPT51 interactions, thereby disrupting ER-mitochondrial contacts (Stoica et al., 2014).
While protein tethers are important for physical contacts between ER and mitochondria, lipids are also critical for the regulation of MAMs and their functions. For example, pharmacological depletion of cholesterol results in increased ER-mitochondria contacts (Fujimoto et al., 2012). It was shown that in ALS spinal cord levels of sphingomyelin, ceramides, and cholesterol esters are increased, presumably due to oxidative stress (Cutler et al., 2002). Therefore, it could be hypothesized that in ALS spinal cord, increased production of cholesteryl ester from cholesterol by lecithin cholesterol esterase results in lower levels of cholesterol at MAMs and increased ER-mitochondrial contacts. Moreover, VAPB has been associated with phosphoinositide alterations. In drosophila, the VAPB ortholog VAP33 was shown to interact with oxysterol binding protein (OSBP), and proper localization of OSBP in the ER depends on VAPB. It was shown that ALS mutants of VAPB do not interact with OSBP and prevent its ER localization (Moustaqim-Barrette et al., 2014). Interaction of OSBP and VAP proteins through the FFAT binding domain is important for ceramide transport into the Golgi, and a depletion of VAP proteins can affect various lipids including phosphatidylinositol 4-phosphate, sphingomyelin, and diacylglycerol (Peretti et al., 2008). Since mutant VAPB can sequester wild type VAPB protein (Teuling et al., 2007), it is possible that this interaction can depletion of VAPB can lead to alteration of lipid composition in various membranes and vesicle formation in ALS. Phosphoinositide phosphatase Sac1 is another fly VAP binding partner. In a fly model of ALS8, mutant VAP caused neurodegeneration associated with increased phosphoinositide levels, which could be prevented by reducing phosphoinositide levels (Forrest et al., 2013).
SOD1
Although not a bona fide MAM protein, mutant SOD1 has been shown to cause abnormal calcium release from the ER in primary astrocytes, as a consequence of disrupted store operated calcium entry (SOCE) regulation (Kawamata et al., 2014). In motor neurons, clearance of cytosolic calcium largely depends on mitochondria (Lautenschlager et al., 2013; Tadic et al., 2014), and mitochondrial calcium capacity is diminished in the spinal cord of transgenic mutant SOD1 mice (Damiano et al., 2006). Increasing mitochondrial calcium capacity by genetic ablation of the permeability transition facilitator, cyclophilin D, prevented mitochondrial dysfunction in SOD1 mutant mice, but did not ameliorate the disease outcome (Kim et al., 2012; Parone et al., 2013), suggesting that, rather than focusing on mitochondrial calcium handling alone, we need to consider calcium dynamics involving ER, mitochondria and other cell compartments, in the whole cell context. The aberrant interactions of mutant SOD1 with Bcl-2 (Pasinelli et al., 2004; Pedrini et al., 2010), which is found both at the mitochondrial and ER membranes (Janiak et al., 1994), could in part define the mechanisms whereby mutant SOD1 affects calcium regulation, as Bcl-2 has been proposed to modulate IP3R activity (Eckenrode et al., 2010).
Other MAM proteins
In addition to the MAM proteins discussed above, other MAM resident proteins have been shown to be associated with ALS in various models, although no mutations have yet been linked to the disease. VDAC, a major component of the MAM, was shown to be partially inactivated by the physical association with mutant SOD1 aggregates, although the impact on MAM structure and function has not been investigated directly (Israelson et al., 2010). Another protein found both in mitochondria and ER is Bcl-2. Interestingly, it was shown that upon interactions with mutant SOD1 Bcl-2 undergoes a conformational change that exposes the pro apoptotic BH3 domain of the protein and results in toxicity (Pedrini et al., 2010). Lastly, polymorphisms in the IP3R have been associated with increased risk for ALS in a large genome wide study, suggesting that calcium abnormalities at MAM could predispose to the disease (van Es et al., 2008). However, this finding was not confirmed in a different patient cohort (Fernandez-Santiago et al., 2011).
MAM proteins in different neurodegenerative diseases
It is worth noting that mutations in proteins that are clearly associated with neurodegenerative diseases, such as presenilin 1 and 2 (PS1/2) in Alzheimer disease (AD) and α-synuclein in Parkinson disease (PD), lead to alterations of the mitochondria-ER contacts and calcium dynamics. While mutant PS1/2 cause increase contacts between the two organelles (Area-Gomez et al., 2012), mutant α-synuclein results in decreased MAMs (Guardia-Laguarta et al., 2014). Another class of MAM proteins involved in neurodegeneration comprises components of the mitochondrial fusion and fission machinery. Mutations in dynamin-related protein 1 (Drp1), the GTPase involved in mitochondrial fission, result in severe forms of encephalopathy (Waterham et al., 2007). Mutations in Mfn2, one of the two GTPases involved in outer mitochondrial membrane fusion and a regulator of ER-mitochondria tethering, are among the most common genetic causes of familial peripheral neuropathies (Reviewed in (Ranieri et al., 2013)). Although mutations of these proteins are not commonly associated with ALS phenotypes, these observations strongly support the concept that MAMs may be “hot spots” for neurodegeneration.
Potential consequences of MAM alterations in ALS
Taken together, the observations described above suggest that MAMs and ER-mitochondrial communications, especially lipid metabolism and calcium signaling between the two organelles, are logical points of intersection in the pathogenesis of different forms of ALS. Based on the extensive functional and physical interactions between the two organelles, discussed above, one could hypothesize multiple detrimental consequences of impaired ER-mitochondria communication. Decreased ER-mitochondrial interaction could result in insufficient calcium transfer from the ER stores to mitochondria and defective bioenergetic coupling. It could also alter the autophagic process, because of impaired vesicle biosynthesis. In the early phases of ER stress response, there is increased coupling of ER-mitochondrial contacts with mitochondrial bioenergetics to increase intracellular ATP content (Bravo et al., 2011). However, abnormally increased or persistent ER-mitochondria contact might result in enhanced calcium flux into mitochondria, triggering mitochondrial permeability transition and apoptosis. The latter scenario has not been explored in the context of ALS, but it has been demonstrated in other pathologies, such as obesity (Arruda et al., 2014).
Oxidative stress and mitochondria-ER involvement in ALS
One putative mechanism underlying alteration in mitochondria-ER contacts could involve redox signaling and imbalance, resulting in post-translational modification of proteins that are critical for inter-organellar interactions. Cysteine residues are crucial to regulation of redox responses, because they are readily oxidized under oxidative conditions. Formation of intra- or intermolecular disulfide bonds, sulfenic acid intermediates, or mixed disulfides with glutathione or nitric oxide, are some of the reversible thiol oxidation reactions that can alter protein functions. Thus, cysteine residue modifications by S-nitrosylation or S-glutathionylation are likely outcomes of redox signaling alterations in neurodegeneration. While many of these thiol-modifications are reversible and thought to protect proteins from permanent oxidative damages, the function of the thiol-modified proteins can be altered, causing further downstream effects. For example, we found that in mutant SOD1 astrocytes persistent S-glutathionylation of STIM1, an ER resident protein involved in SOCE, caused dysregulation of ER calcium signaling and calcium-dependent secretion that affected motor neuron viability (Kawamata et al., 2014).
Many proteins can be modified by S-nitrosylation or S-glutathionylation in the context of neurodegenerative processes (Halloran et al., 2013). Here we focus on those that are potentially involved in ALS and mitochondria-ER connections (table 2). Mutations in VCP have been associated with ALS (Abramzon et al., 2012; Johnson et al., 2010). VCP belongs to the AAA protein family and is involved in various cellular processes including ERAD and Pink1/Parkin-mediated mitochondrial quality control. Mutations in VCP result in reduced VCP recruitment to mitochondria when Parkin ubiquitinates mitochondrial proteins (Kim et al., 2013). Oxidant induced S-glutathionylation of VCP leading to inactivation of its ATPase activity has been proposed to be a regulatory mechanism to convert cellular oxidative stress signals into an ER stress response (Noguchi et al., 2005). While there are no reports on S-glutationylation of VCP in ALS, mutations in VCP that introduce cysteine residues have been reported (Abramzon et al., 2012), which may cause further oxidation opportunities and may be detrimental to VCP function.
Table 2.
ER and mitochondrial proteins for which thiol oxidation modifications are potentially involved in neurodegeneration.
| Protein | Modification | Normal Function | Functional consequences of oxidation | Reference |
|---|---|---|---|---|
| Drp1 | S-nitrosylation | Mitochondrial fission | Hyperactive GTPase; increased mitochondrial fission | (Cho et al., 2009) (Haun et al., 2013) |
| Parkin | S-nitrosylation | E3 ubiquitin ligase | Loss of activity; Increased protein aggregates | (Yao et al., 2004) |
| PDI | S-nitrosylation | Protein folding Chaperone |
Loss of activity; accumulation of misfolded proteins | (Walker and Atkin, 2011) (Jeon et al., 2014) |
| STIM1 | S-glutathionylation | SOCE | Loss of ER calcium sensitivity; unregulated SOCE | (Hawkins et al., 2010), (Kawamata et al., 2014) |
| VCP | S-glutathionylation | Protein quality control | Reduced mitochondrial recruitment for mitophagy | (Noguchi et al., 2005) |
Mutations in matrin3 (MATR3) have been recently linked to ALS (Johnson et al., 2014b). Through proteomic and protein interaction studies it was shown that MATR3 associates with glutathione transferase π 2, GRP78/BIP, and GRP75. This study was performed in thymoma cells, and did not directly demonstrate glutahtionylation of MATR3 (Osman and van Loveren, 2014). However, it is possible that MATR3 could be oxidatively modified (Chiang et al., 2012) in ALS. Interestingly, the study also pointed out that MATR3, a nuclear matrix protein whose function includes transport of RNA interacts with proteins in the ER (GRP78) and MAMs (GRP75).
Other proteins involved in mitochondrial quality control and dynamics are also known to be S-nitrosylated and could play a role in pathogenic processes. Drp1 becomes hyperactive when S-nitrosylated. Nitrosative stress due to mutant proteins such as huntingtin and the Aβ peptide is known to nitrosylate Drp1, causing accumulation of persistent small and de-energized mitochondria (Cho et al., 2009; Haun et al., 2013). Parkin is also S-nitrosylated (Yao et al., 2004), and excessive Parkin oxidation can disrupt its ligase activity and cause protein aggregation (Meng et al., 2011). While it is not yet known whether SNO-Drp1 or SNO-Parkin are involved in ALS, it would not be surprising to find that such alterations underlie some of the abnormalities in mitochondrial dynamics and quality control observed in ALS.
The involvement of PDI in ALS pathogenesis is debated (Jaronen et al., 2014), but S-nitrosylation of PDI in SOD1-associated models and ALS patient spinal cords have been reported (Jeon et al., 2014; Walker et al., 2010). S-nitrosylation of PDI at the active site cysteine residue is thought to reduce both chaperone and isomerase PDI activity, and result in accumulation of misfolded proteins and cell death (Jeon et al., 2014). While PDI levels increase as a part of the UPR in ALS (Atkin et al., 2006; Atkin et al., 2008), this PDI could be inactive allowing for accumulation of misfolded mutant SOD1. In G93A rats, PDI levels were upregulated early on in disease course, whereas at end stage, there was a marked reduction of PDI (Ahtoniemi et al., 2008). More recently, genetic variants in PDIA1 (PDI) and PDIA3 (ERp57) have been reported (Gonzalez-Perez et al., 2015), as well as intronic variants of PDIA1 as a genetic risk factor for ALS (Kwok et al., 2013), which warrants further attention to the involvement of this key protein in oxidative folding in the pathogenesis of ALS.
RyRs are also susceptible to oxidative modifications. RyRs contain cysteine residues that are modified by oxidants, affecting muscle contractile properties (Sun et al., 2013). RyR oxidation in neurons was also shown to regulate brain function (Kakizawa et al., 2012). In motor neurons, RyRs can modulate glutamatergic stimulation (Jahn et al., 2006), and it is possible that RyRs are dysregulated in ALS causing abnormal cytosolic and mitochondrial calcium influx (Grosskreutz et al., 2010). However, while the RyR inhibitor dantrolene showed protective effects on SOD1 mutant motor neurons in culture, it did not extend survival of G93A mice (Staats et al., 2012). Therefore, modulating the oxidative state of the RyR might provide a better approach than inhibiting it.
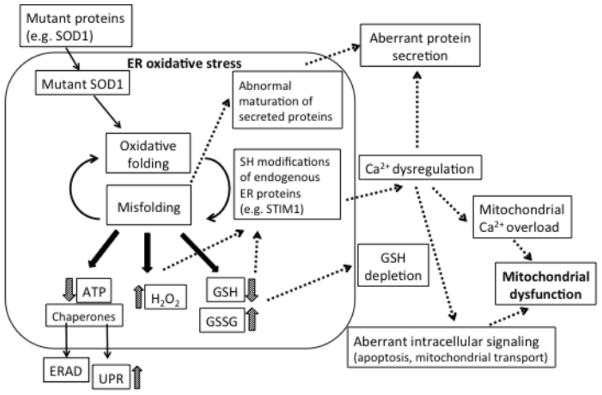
What could cause redox alterations in ALS cells? Many evidence exist for mutant SOD1 effects on redox. Oxidative stress has been long involved in ALS pathogenesis (Albers and Beal, 2000), where mitochondrial ROS production was considered the main culprit, possibly contributing to ER stress (Ilieva et al., 2007). Several mutant forms of SOD1 retain dismutase activity to varying degrees (Borchelt et al., 1994), but aberrant ROS production from mutant SOD1 has also been proposed (Bogdanov et al., 1998; Ferrante et al., 1997), and peroxynitrite formation due to zinc deficiency in SOD1 increases motor neuron vulnerability (Estevez et al., 1998). In mitochondria, SOD1 localizes in the intermembrane space (IMS) and can be protective from superoxide produced locally (Kawamata and Manfredi, 2008; Vijayvergiya et al., 2005). However, accumulation of mutant SOD1 in the IMS can cause havoc (Igoudjil et al., 2011; Magrane et al., 2009; Son et al., 2008; Son et al., 2007) and mitochondrial proteins can be abnormally oxidized (Mattiazzi et al., 2002). Furthermore, increased superoxide generation through NADPH oxidase (NOX) activity in mutant SOD1 microglia have been demonstrated (Harraz et al., 2008; Marden et al., 2007). PDI upregulation upon ER stress has also been associated with NOX activation in non-neuronal cells (Janiszewski et al., 2005; Laurindo et al., 2008; Santos et al., 2009). Another major source of oxidative stress is through ER protein folding involving PDI and Ero1. Since the proper folding of intramolecular disulfide bond produces a molecule of hydrogen peroxide (H2O2) by the activity of Ero1, futile cycles of protein folding have been suggested to cause oxidative stress in the ER (Haynes et al., 2004). As mentioned above, hyper-oxidation of the ER can result in thiol modifications of ER resident proteins that can alter their functions. In addition, futile oxidative protein folding cycles could result in depletion of glutathione (GSH) and ATP consumed by chaperones, further worsening the oxidative stress and energy imbalance in ALS (figure 1). Changes in GSH pool could be sensed by the organelles, because their redox state depends on the balance between reduced and oxidized GSH. The ER environment is highly oxidative and contains GSH:GSSG ratios that are significantly lower than the cytosol and mitochondria. In the early phases of ER stress, mitochondria increase their bioenergetic functions and ATP production (Bravo et al., 2011). The increased need for ATP could be due to the fact that the function of chaperones like BIP, which bind and release misfolded substrate proteins, depend on ATP hydrolysis. Furthermore degradation of misfolded proteins by the ERAD and the proteasome is also energy dependent. Thus, under increased burden of misfolded proteins, the ER may signal to mitochondria via calcium to boost ATP synthesis. Based on these putative mechanisms, it is logical to hypothesize that likely candidates for inter-organellar communication that go in disarray in ALS are small molecules, such as calcium, ROS, particularly the diffusible ones, H2O2 and nitric oxide, GSH, and ATP.
Figure 1. ER oxidative protein folding could be the hub of multiple pathogenic pathways in ALS.
SOD1 misfolding or other ALS-related proteins could cause ER oxidative stress, which could represent a common origin of a multitude of pathogenic pathways that have been identified in ALS, including ER stress, GSH depletion, aberrant calcium handling, protein secretion, and mitochondrial dysfunction.
Conclusions
The involvement of impaired mitochondria-ER contacts in ALS pathogenesis remains to be fully elucidated. Perturbation of proteins at contact sites, such as VAPB, PTPIP51, and Sigma1R has been implicated in ALS. While it is unlikely that all MAM protein alterations result in motor neuron degeneration and ALS, MAM abnormalities have also been linked to other neurodegenerative conditions, such as Parkinson disease with α-synuclein mutations (Guardia-Laguarta et al., 2014) and Alzheimer disease with presenilin mutations (Area-Gomez et al., 2012), suggesting that ER-mitochondrial contacts are vulnerable targets in neuronal degeneration.
ER-mitochondrial communication in ALS could be globally affected in all cell types, but cell-type-specific outcomes may be diverse. Motor neurons appear particularly sensitive to perturbations. Sensitivity to calcium dysregulation may be affected by calcium binding proteins, since motor neurons have lower amounts of these proteins compared to ALS-spared neurons (Bernard-Marissal et al., 2012). It is also possible that regulatory mechanisms and proteins involved may be different among different cell types. For example, while non-excitable cells, such as astrocytes, utilize SOCE dependent on STIM1 and Orai1 channels, in neurons the situation may be different. Studies have suggested that STIM2 is prevalent over STIM1 in neurons (Gruszczynska-Biegala and Kuznicki, 2013). On the other hand, neurons could be less sensitive to changes in intracellular calcium stores, compared to non-excitable cells. The enhanced ER calcium signaling and excess calcium-dependent secretion in mutant SOD1 astrocytes suggest that ER oxidative stress and altered STIM1 function underlies non-cell autonomous toxicity to motor neurons (Kawamata et al., 2014).
In fALS, and likely in sporadic cases as well, the underlying mutant proteins exists from conception, but it takes many years for motor neurons to die and ALS to manifest. During this long pre-symptomatic period, protein misfolding, ER stress, mitochondrial dysfunction and faulty mitochondria-ER communication could be taking place, but cells are able to cope. The timing of cellular dysfunction and its manifestations may be different in different cell types, or coping mechanism may fail at the same time but the effects may differ among cell types, with motor neurons degenerating and dying while glia becomes toxic. Together, these effects can create a perfect storm scenario, leading to the rapid development of the disease. Understanding the sequence of events that lead to this scenario over the lifetime of affected individuals and specifically the transition from ER-mitochondria functional to dysfunctional interactions would certainly provide ample windows of therapeutic opportunities. Targeting ER oxidative stress early on and preventing the accumulation of misfolded proteins in mitochondria and ER could synergistically help to maintain healthy ER-mitochondria interactions and prolong the pre-symptomatic phase of the disease and possibly prevent it entirely.
Acknowledgments
This work is supported by grants 2R01NS051419-05, 1R01NS062055-01, 1R01NS084486-01 from NIH/NINDS, and grant 255345 from MDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramzon Y, et al. Valosin-containing protein (VCP) mutations in sporadic amyotrophic lateral sclerosis. Neurobiol Aging. 2012;33:2231 e1–2231 e6. doi: 10.1016/j.neurobiolaging.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahtoniemi T, et al. Mutant SOD1 from spinal cord of G93A rats is destabilized and binds to inner mitochondrial membrane. Neurobiol Dis. 2008;32:479–85. doi: 10.1016/j.nbd.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Ajroud-Driss S, et al. Mutation in the novel nuclear-encoded mitochondrial protein CHCHD10 in a family with autosomal dominant mitochondrial myopathy. Neurogenetics. 2015;16:1–9. doi: 10.1007/s10048-014-0421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Saif A, et al. A mutation in sigma-1 receptor causes juvenile amyotrophic lateral sclerosis. Ann Neurol. 2011;70:913–9. doi: 10.1002/ana.22534. [DOI] [PubMed] [Google Scholar]
- Albers DS, Beal MF. Mitochondrial dysfunction and oxidative stress in aging and neurodegenerative disease. J Neural Transm Suppl. 2000;59:133–54. doi: 10.1007/978-3-7091-6781-6_16. [DOI] [PubMed] [Google Scholar]
- Area-Gomez E, et al. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J. 2012;31:4106–23. doi: 10.1038/emboj.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda AP, et al. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427–35. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkin JD, et al. Induction of the unfolded protein response in familial amyotrophic lateral sclerosis and association of protein-disulfide isomerase with superoxide dismutase 1. J Biol Chem. 2006;281:30152–65. doi: 10.1074/jbc.M603393200. [DOI] [PubMed] [Google Scholar]
- Atkin JD, et al. Endoplasmic reticulum stress and induction of the unfolded protein response in human sporadic amyotrophic lateral sclerosis. Neurobiol Dis. 2008;30:400–7. doi: 10.1016/j.nbd.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Bannwarth S, et al. A mitochondrial origin for frontotemporal dementia and amyotrophic lateral sclerosis through CHCHD10 involvement. Brain. 2014;137:2329–45. doi: 10.1093/brain/awu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbara JG. IP3-dependent calcium-induced calcium release mediates bidirectional calcium waves in neurones: functional implications for synaptic plasticity. Biochim Biophys Acta. 2002;1600:12–8. doi: 10.1016/s1570-9639(02)00439-9. [DOI] [PubMed] [Google Scholar]
- Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–5. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Marissal N, et al. Dysfunction in endoplasmic reticulum-mitochondria crosstalk underlies SIGMAR1 loss of function mediated motor neuron degeneration. Brain. 2015;138:875–90. doi: 10.1093/brain/awv008. [DOI] [PubMed] [Google Scholar]
- Bernard-Marissal N, et al. Reduced calreticulin levels link endoplasmic reticulum stress and Fas-triggered cell death in motoneurons vulnerable to ALS. J Neurosci. 2012;32:4901–12. doi: 10.1523/JNEUROSCI.5431-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockler S, Westermann B. ER-mitochondria contacts as sites of mitophagosome formation. Autophagy. 2014a;10:1346–7. doi: 10.4161/auto.28981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockler S, Westermann B. Mitochondrial ER contacts are crucial for mitophagy in yeast. Dev Cell. 2014b;28:450–8. doi: 10.1016/j.devcel.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Bogdanov MB, et al. Elevated “hydroxyl radical” generation in vivo in an animal model of amyotrophic lateral sclerosis. J Neurochem. 1998;71:1321–4. doi: 10.1046/j.1471-4159.1998.71031321.x. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, et al. Superoxide dismutase 1 with mutations linked to familial amyotrophic lateral sclerosis possesses significant activity. Proc Natl Acad Sci U S A. 1994;91:8292–6. doi: 10.1073/pnas.91.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci. 2011;124:2143–52. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas C, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–83. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carri MT, et al. Oxidative stress and mitochondrial damage: importance in non-SOD1 ALS. Front Cell Neurosci. 2015;9:41. doi: 10.3389/fncel.2015.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang BY, et al. In vivo tagging and characterization of S-glutathionylated proteins by a chemoenzymatic method. Angew Chem Int Ed Engl. 2012;51:5871–5. doi: 10.1002/anie.201200321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho DH, et al. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–5. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino M, Carri MT. Mitochondrial dysfunction in ALS. Prog Neurobiol. 2012;97:54–66. doi: 10.1016/j.pneurobio.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Csordas G, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–21. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, et al. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Ann Neurol. 2002;52:448–57. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- Damiano M, et al. Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem. 2006;96:1349–61. doi: 10.1111/j.1471-4159.2006.03619.x. [DOI] [PubMed] [Google Scholar]
- De Stefani D, et al. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–40. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJ, et al. VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum Mol Genet. 2012;21:1299–311. doi: 10.1093/hmg/ddr559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckenrode EF, et al. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem. 2010;285:13678–84. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez AG, et al. Role of endogenous nitric oxide and peroxynitrite formation in the survival and death of motor neurons in culture. Prog Brain Res. 1998;118:269–80. doi: 10.1016/s0079-6123(08)63214-8. [DOI] [PubMed] [Google Scholar]
- Farg MA, et al. Mutant FUS induces endoplasmic reticulum stress in amyotrophic lateral sclerosis and interacts with protein disulfide-isomerase. Neurobiol Aging. 2012;33:2855–68. doi: 10.1016/j.neurobiolaging.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Fernandez-Santiago R, et al. No evidence of association of FLJ10986 and ITPR2 with ALS in a large German cohort. Neurobiol Aging. 2011;32:551 e1–4. doi: 10.1016/j.neurobiolaging.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Ferrante RJ, et al. Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem. 1997;69:2064–74. doi: 10.1046/j.1471-4159.1997.69052064.x. [DOI] [PubMed] [Google Scholar]
- Filezac de L’Etang A, et al. Marinesco-Sjogren syndrome protein SIL1 regulates motor neuron subtype-selective ER stress in ALS. Nat Neurosci. 2015;18:227–38. doi: 10.1038/nn.3903. [DOI] [PubMed] [Google Scholar]
- Forrest S, et al. Increased levels of phosphoinositides cause neurodegeneration in a Drosophila model of amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22:2689–704. doi: 10.1093/hmg/ddt118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–43. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, et al. The role of cholesterol in the association of endoplasmic reticulum membranes with mitochondria. Biochem Biophys Res Commun. 2012;417:635–9. doi: 10.1016/j.bbrc.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, et al. The role of SIGMAR1 gene mutation and mitochondrial dysfunction in amyotrophic lateral sclerosis. J Pharmacol Sci. 2015;127:36–41. doi: 10.1016/j.jphs.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perez P, et al. Identification of rare protein disulfide isomerase gene variants in amyotrophic lateral sclerosis patients. Gene. 2015;566:158–65. doi: 10.1016/j.gene.2015.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosskreutz J, et al. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165–74. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Gruszczynska-Biegala J, Kuznicki J. Native STIM2 and ORAI1 proteins form a calcium-sensitive and thapsigargin-insensitive complex in cortical neurons. J Neurochem. 2013;126:727–38. doi: 10.1111/jnc.12320. [DOI] [PubMed] [Google Scholar]
- Guardia-Laguarta C, et al. alpha-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci. 2014;34:249–59. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halloran M, et al. The role of s-nitrosylation and s-glutathionylation of protein disulphide isomerase in protein misfolding and neurodegeneration. Int J Cell Biol. 2013;2013:797914. doi: 10.1155/2013/797914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz MM, et al. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–70. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haun F, et al. S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington’s disease. Antioxid Redox Signal. 2013;19:1173–84. doi: 10.1089/ars.2012.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins BJ, et al. S-glutathionylation activates STIM1 and alters mitochondrial homeostasis. J Cell Biol. 2010;190:391–405. doi: 10.1083/jcb.201004152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Haynes CM, et al. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell. 2004;15:767–76. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Hedskog L, et al. Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models. Proc Natl Acad Sci U S A. 2013;110:7916–21. doi: 10.1073/pnas.1300677110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igoudjil A, et al. In vivo pathogenic role of mutant SOD1 localized in the mitochondrial intermembrane space. J Neurosci. 2011;31:15826–37. doi: 10.1523/JNEUROSCI.1965-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilieva EV, et al. Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis. Brain. 2007;130:3111–23. doi: 10.1093/brain/awm190. [DOI] [PubMed] [Google Scholar]
- Israelson A, et al. Misfolded mutant SOD1 directly inhibits VDAC1 conductance in a mouse model of inherited ALS. Neuron. 2010;67:575–87. doi: 10.1016/j.neuron.2010.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn K, et al. Temporospatial coupling of networked synaptic activation of AMPA-type glutamate receptor channels and calcium transients in cultured motoneurons. Neuroscience. 2006;142:1019–29. doi: 10.1016/j.neuroscience.2006.07.034. [DOI] [PubMed] [Google Scholar]
- Janiak F, et al. Assembly of Bcl-2 into microsomal and outer mitochondrial membranes. J Biol Chem. 1994;269:9842–9. [PubMed] [Google Scholar]
- Janiszewski M, et al. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J Biol Chem. 2005;280:40813–9. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- Jaronen M, et al. ER stress and unfolded protein response in amyotrophic lateral sclerosis-a controversial role of protein disulphide isomerase. Front Cell Neurosci. 2014;8:402. doi: 10.3389/fncel.2014.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon GS, et al. Potential effect of S-nitrosylated protein disulfide isomerase on mutant SOD1 aggregation and neuronal cell death in amyotrophic lateral sclerosis. Mol Neurobiol. 2014;49:796–807. doi: 10.1007/s12035-013-8562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JO, et al. Mutations in the CHCHD10 gene are a common cause of familial amyotrophic lateral sclerosis. Brain. 2014a;137:e311. doi: 10.1093/brain/awu265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JO, et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron. 2010;68:857–64. doi: 10.1016/j.neuron.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JO, et al. Mutations in the Matrin 3 gene cause familial amyotrophic lateral sclerosis. Nat Neurosci. 2014b;17:664–6. doi: 10.1038/nn.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, et al. Nitric oxide-induced calcium release via ryanodine receptors regulates neuronal function. EMBO J. 2012;31:417–28. doi: 10.1038/emboj.2011.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekura K, et al. ER stress and unfolded protein response in amyotrophic lateral sclerosis. Mol Neurobiol. 2009;39:81–9. doi: 10.1007/s12035-009-8054-3. [DOI] [PubMed] [Google Scholar]
- Kawamata H, Manfredi G. Different regulation of wild-type and mutant Cu,Zn superoxide dismutase localization in mammalian mitochondria. Hum Mol Genet. 2008;17:3303–17. doi: 10.1093/hmg/ddn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata H, et al. Abnormal Intracellular Calcium Signaling and SNARE-Dependent Exocytosis Contributes to SOD1G93A Astrocyte-Mediated Toxicity in Amyotrophic Lateral Sclerosis. J Neurosci. 2014;34:2331–48. doi: 10.1523/JNEUROSCI.2689-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, et al. The mitochondrial calcium regulator cyclophilin D is an essential component of oestrogen-mediated neuroprotection in amyotrophic lateral sclerosis. Brain. 2012;135:2865–74. doi: 10.1093/brain/aws208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NC, et al. VCP is essential for mitochondrial quality control by PINK1/Parkin and this function is impaired by VCP mutations. Neuron. 2013;78:65–80. doi: 10.1016/j.neuron.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis E, et al. Pathways disrupted in human ALS motor neurons identified through genetic correction of mutant SOD1. Cell Stem Cell. 2014;14:781–95. doi: 10.1016/j.stem.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Walter P. ERMES-mediated ER-mitochondria contacts: molecular hubs for the regulation of mitochondrial biology. J Cell Sci. 2010;123:1389–93. doi: 10.1242/jcs.058636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok CT, et al. Association studies indicate that protein disulfide isomerase is a risk factor in amyotrophic lateral sclerosis. Free Radic Biol Med. 2013;58:81–6. doi: 10.1016/j.freeradbiomed.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Laurindo FR, et al. Novel role of protein disulfide isomerase in the regulation of NADPH oxidase activity: pathophysiological implications in vascular diseases. Antioxid Redox Signal. 2008;10:1101–13. doi: 10.1089/ars.2007.2011. [DOI] [PubMed] [Google Scholar]
- Lautenschlager J, et al. Overexpression of human mutated G93A SOD1 changes dynamics of the ER mitochondria calcium cycle specifically in mouse embryonic motor neurons. Exp Neurol. 2013;247:91–100. doi: 10.1016/j.expneurol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Magrane J, et al. Mutant SOD1 in neuronal mitochondria causes toxicity and mitochondrial dynamics abnormalities. Hum Mol Genet. 2009;18:4552–64. doi: 10.1093/hmg/ddp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marangi G, Traynor BJ. Genetic causes of amyotrophic lateral sclerosis: New genetic analysis methodologies entailing new opportunities and challenges. Brain Res. 2015;1607:75–93. doi: 10.1016/j.brainres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden JJ, et al. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–9. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiazzi M, et al. Mutated human SOD1 causes dysfunction of oxidative phosphorylation in mitochondria of transgenic mice. J Biol Chem. 2002;277:29626–33. doi: 10.1074/jbc.M203065200. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Denton RM. Role of calcium ions in the regulation of intramitochondrial metabolism. Properties of the Ca2+-sensitive dehydrogenases within intact uncoupled mitochondria from the white and brown adipose tissue of the rat. Biochem J. 1980;190:95–105. doi: 10.1042/bj1900095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng F, et al. Oxidation of the cysteine-rich regions of parkin perturbs its E3 ligase activity and contributes to protein aggregation. Mol Neurodegener. 2011;6:34. doi: 10.1186/1750-1326-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morotz GM, et al. Amyotrophic lateral sclerosis-associated mutant VAPBP56S perturbs calcium homeostasis to disrupt axonal transport of mitochondria. Hum Mol Genet. 2012;21:1979–88. doi: 10.1093/hmg/dds011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustaqim-Barrette A, et al. The amyotrophic lateral sclerosis 8 protein, VAP, is required for ER protein quality control. Hum Mol Genet. 2014;23:1975–89. doi: 10.1093/hmg/ddt594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura AL, et al. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75:822–31. doi: 10.1086/425287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitoh H, et al. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008;22:1451–64. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi M, et al. ATPase activity of p97/valosin-containing protein is regulated by oxidative modification of the evolutionally conserved cysteine 522 residue in Walker A motif. J Biol Chem. 2005;280:41332–41. doi: 10.1074/jbc.M509700200. [DOI] [PubMed] [Google Scholar]
- Osman AM, van Loveren H. Matrin 3 co-immunoprecipitates with the heat shock proteins glucose-regulated protein 78 (GRP78), GRP75 and glutathione S-transferase pi isoform 2 (GSTpi2) in thymoma cells. Biochimie. 2014;101:208–14. doi: 10.1016/j.biochi.2014.01.015. [DOI] [PubMed] [Google Scholar]
- Palomo GM, Manfredi G. Exploring new pathways of neurodegeneration in ALS: the role of mitochondria quality control. Brain Res. 2015;1607:36–46. doi: 10.1016/j.brainres.2014.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parone PA, et al. Enhancing mitochondrial calcium buffering capacity reduces aggregation of misfolded SOD1 and motor neuron cell death without extending survival in mouse models of inherited amyotrophic lateral sclerosis. J Neurosci. 2013;33:4657–71. doi: 10.1523/JNEUROSCI.1119-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinelli P, et al. Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron. 2004;43:19–30. doi: 10.1016/j.neuron.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Pedrini S, et al. ALS-linked mutant SOD1 damages mitochondria by promoting conformational changes in Bcl-2. Hum Mol Genet. 2010;19:2974–86. doi: 10.1093/hmg/ddq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretti D, et al. Coordinated lipid transfer between the endoplasmic reticulum and the Golgi complex requires the VAP proteins and is essential for Golgi-mediated transport. Mol Biol Cell. 2008;19:3871–84. doi: 10.1091/mbc.E08-05-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prause J, et al. Altered localization, abnormal modification and loss of function of Sigma receptor-1 in amyotrophic lateral sclerosis. Hum Mol Genet. 2013;22:1581–600. doi: 10.1093/hmg/ddt008. [DOI] [PubMed] [Google Scholar]
- Prell T, et al. The unfolded protein response in models of human mutant G93A amyotrophic lateral sclerosis. Eur J Neurosci. 2012;35:652–60. doi: 10.1111/j.1460-9568.2012.08008.x. [DOI] [PubMed] [Google Scholar]
- Ranieri M, et al. Mitochondrial fusion proteins and human diseases. Neurol Res Int. 2013;2013:293893. doi: 10.1155/2013/293893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasola A, Bernardi P. Mitochondrial permeability transition in Ca(2+)-dependent apoptosis and necrosis. Cell Calcium. 2011;50:222–33. doi: 10.1016/j.ceca.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Renton AE, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R, et al. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993;262:744–7. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, et al. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci STKE. 2004;2004:re1. doi: 10.1126/stke.2152004re1. [DOI] [PubMed] [Google Scholar]
- Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Santos CX, et al. Protein disulfide isomerase (PDI) associates with NADPH oxidase and is required for phagocytosis of Leishmania chagasi promastigotes by macrophages. J Leukoc Biol. 2009;86:989–98. doi: 10.1189/jlb.0608354. [DOI] [PubMed] [Google Scholar]
- Sasaki S. Endoplasmic reticulum stress in motor neurons of the spinal cord in sporadic amyotrophic lateral sclerosis. J Neuropathol Exp Neurol. 2010;69:346–55. doi: 10.1097/NEN.0b013e3181d44992. [DOI] [PubMed] [Google Scholar]
- Saxena S, et al. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627–36. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- Son M, et al. Isolated cytochrome c oxidase deficiency in G93A SOD1 mice over-expressing CCS protein. J Biol Chem. 2008 doi: 10.1074/jbc.M708523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son M, et al. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc Natl Acad Sci U S A. 2007;104:6072–7. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staats KA, et al. Dantrolene is neuroprotective in vitro, but does not affect survival in SOD1(G(9)(3)A) mice. Neuroscience. 2012;220:26–31. doi: 10.1016/j.neuroscience.2012.06.050. [DOI] [PubMed] [Google Scholar]
- Stoica R, et al. ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43. Nat Commun. 2014;5:3996. doi: 10.1038/ncomms4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QA, et al. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor/Ca2+ release channel (RyR1): sites and nature of oxidative modification. J Biol Chem. 2013;288:22961–71. doi: 10.1074/jbc.M113.480228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaramoorthy V, et al. Extracellular wildtype and mutant SOD1 induces ER-Golgi pathology characteristic of amyotrophic lateral sclerosis in neuronal cells. Cell Mol Life Sci. 2013;70:4181–95. doi: 10.1007/s00018-013-1385-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadic V, et al. The ER mitochondria calcium cycle and ER stress response as therapeutic targets in amyotrophic lateral sclerosis. Front Cell Neurosci. 2014;8:147. doi: 10.3389/fncel.2014.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, et al. Role of mitochondria in mutant SOD1 linked amyotrophic lateral sclerosis. Biochim Biophys Acta. 2014;1842:1295–301. doi: 10.1016/j.bbadis.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuling E, et al. Motor neuron disease-associated mutant vesicle-associated membrane protein-associated protein (VAP) B recruits wild-type VAPs into endoplasmic reticulum-derived tubular aggregates. J Neurosci. 2007;27:9801–15. doi: 10.1523/JNEUROSCI.2661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushitani M, et al. The endoplasmic reticulum-Golgi pathway is a target for translocation and aggregation of mutant superoxide dismutase linked to ALS. FASEB J. 2008;22:2476–87. doi: 10.1096/fj.07-092783. [DOI] [PubMed] [Google Scholar]
- Urushitani M, et al. Chromogranin-mediated secretion of mutant superoxide dismutase proteins linked to amyotrophic lateral sclerosis. Nat Neurosci. 2006;9:108–18. doi: 10.1038/nn1603. [DOI] [PubMed] [Google Scholar]
- van Es MA, et al. Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nat Genet. 2008;40:29–31. doi: 10.1038/ng.2007.52. [DOI] [PubMed] [Google Scholar]
- van Vliet AR, et al. New functions of mitochondria associated membranes in cellular signaling. Biochim Biophys Acta. 2014;1843:2253–62. doi: 10.1016/j.bbamcr.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta. 2014;1841:595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Vijayvergiya C, et al. Mutant superoxide dismutase 1 forms aggregates in the brain mitochondrial matrix of amyotrophic lateral sclerosis mice. J Neurosci. 2005;25:2463–70. doi: 10.1523/JNEUROSCI.4385-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath JT, et al. Loss of function of the ALS protein SigR1 leads to ER pathology associated with defective autophagy and lipid raft disturbances. Cell Death Dis. 2014;5:e1290. doi: 10.1038/cddis.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, Atkin JD. Mechanisms of neuroprotection by protein disulphide isomerase in amyotrophic lateral sclerosis. Neurol Res Int. 2011;2011:317340. doi: 10.1155/2011/317340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AK, et al. Protein disulphide isomerase protects against protein aggregation and is S-nitrosylated in amyotrophic lateral sclerosis. Brain. 2010;133:105–16. doi: 10.1093/brain/awp267. [DOI] [PubMed] [Google Scholar]
- Walker AK, et al. ALS-associated TDP-43 induces endoplasmic reticulum stress, which drives cytoplasmic TDP-43 accumulation and stress granule formation. PLoS One. 2013;8:e81170. doi: 10.1371/journal.pone.0081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. The unfolded protein response in familial amyotrophic lateral sclerosis. Hum Mol Genet. 2011;20:1008–15. doi: 10.1093/hmg/ddq546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterham HR, et al. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–41. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- Yao D, et al. Nitrosative stress linked to sporadic Parkinson’s disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–4. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YJ, et al. Aggregation-prone c9FTD/ALS poly(GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol. 2014;128:505–24. doi: 10.1007/s00401-014-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]