Abstract
Little is known about the development and course of depressive symptoms through emerging adulthood among individuals with a childhood history of attention-deficit/hyperactivity disorder (ADHD). The aim of this study was to examine if a history of ADHD in childhood significantly predicted depressive symptoms during emerging adulthood (i.e., ages 18–25 years), including the initial level of depressive symptoms, continued levels of depressive symptoms at each age year, and the rate of change in depressive symptoms over time. 394 participants (205 with ADHD and 189 without ADHD; 348 males and 46 females) drawn from the Pittsburgh ADHD Longitudinal Study (PALS) completed annual self-ratings of depressive symptoms between the ages of 18 and 25 years. Childhood history of ADHD significantly predicted a higher initial level of depressive symptoms at age 18, and higher levels of depressive symptoms at every age year during emerging adulthood. ADHD did not significantly predict the rate of change in depressive symptoms from age 18 to age 25. Childhood history of ADHD remained a significant predictor of initial level of depressive symptoms at age 18 after controlling for comorbid psychiatric diagnoses, but not after controlling for concurrent ADHD symptoms and psychosocial impairment. Participants with childhood histories of ADHD experienced significantly higher levels of depressive symptoms than non-ADHD comparison participants by age 18 and continued to experience higher, although not increasing, levels of depressive symptoms through emerging adulthood. Clinical implications and directions for future research are discussed.
Keywords: ADHD, depression, emerging adulthood, longitudinal outcome
Children, adolescents, and emerging adults with ADHD may be at risk of unipolar depressive disorders at rates higher than expected by chance (Chronis-Tuscano et al., 2010; Meinzer et al., 2013). A recent meta-analysis reported that ADHD and depression were positively associated but the size of their association varied considerably across subgroup analyses (Meinzer, Pettit, & Viswesvaran, 2014). For example, a medium positive association was reported for cross-sectional studies but a small and unreliable association was reported for longitudinal studies. These mixed findings, as well as the paucity of longitudinal studies that have tracked children with ADHD into emerging adulthood, highlight the need for research into the association between ADHD and depression, especially research using prospective longitudinal designs to track youth with ADHD through adolescence and emerging adulthood.
Emerging adulthood is a time of transition marked by increasing independence and autonomy from one’s family of origin without the normative responsibilities of adulthood (Arnett, 2000). It is often operationalized as the time period from age 18 years through 25 years (Arnett, 2000; Galambos, Barker, & Krahn, 2006), although there are likely individual differences in the timing of the assumption of adulthood responsibilities (e.g., establishing financial independence, becoming a parent, etc.). Emerging adulthood is generally a period of increasingly positive well-being. For example, during emerging adulthood, depressive symptoms tend to decrease (e.g., Galambos, et al., 2006; Pettit, Roberts, Lewinsohn, Seeley, & Yaroslavsky, 2011; Radloff, 1977) and levels of social support from family and friends tend to increase (Pettit, et al., 2011). Despite this normative increase in well-being, some individuals experience elevated depressive symptoms that persist through emerging adulthood and predict a host of negative outcomes in adulthood (Stoolmiller, Kim, & Capaldi, 2005). The variability in the levels of depressive symptoms highlights the need to identify risk factors that predict elevated levels of depression during this developmental period.
As noted above, ADHD may be one such risk factor. Recent findings demonstrate that individuals with a history of ADHD have a difficult time navigating the developmental tasks of emerging adulthood, as evidenced by low rates of college attendance (Barkley, Fischer, Smallish, & Fletcher, 2006; Kuriyan et al., 2013) and high rates of alcohol use disorders (Molina et al., 2007), risky sexual behaviors (Barkley, et al., 2006; Flory, Molina, Pelham, Gnagy, & Smith, 2006), intimate partner violence (Wymbs et al., 2012), and negative driving outcomes (Barkley, Guevremont, Anastopoulos, DuPaul, & Shelton, 1993). These difficulties experienced by individuals with ADHD could promote the development and maintenance of depressive symptoms during emerging adulthood.
To our knowledge, there are no published studies on the influence of ADHD on the development and course of depression during emerging adulthood. This is an important gap in the knowledge base given (1) the difficulties individuals with ADHD have mastering the developmental tasks of emerging adulthood, (2) research suggesting an elevated risk of depression among individuals with ADHD, and (3) the impairment and disability associated with ongoing depressive experiences during emerging adulthood. Impairment secondary to depressive experiences can exist even when symptoms are at subclinical levels (Lewinsohn, Solomon, Seeley, & Zeiss, 2000). Further, recent research demonstrated that elevated levels of depressive symptoms through emerging adulthood predicted a host of negative outcomes by age 30, including lower levels of educational attainment and income and higher levels of impairment in family relationships, non-familial social relationships, and work (Yaroslavsky, Pettit, Lewinsohn, Seeley, & Roberts, 2013). Understanding the influence of ADHD on levels of depressive symptoms during emerging adulthood may inform screening, prevention, or intervention efforts in this at-risk population.
The present study sought to advance understanding in this area by addressing two research questions. First, does a history of ADHD in childhood significantly predict depressive symptoms during emerging adulthood (i.e., ages 18–25 years), including the level of depressive symptoms at age 18 (intercept), levels of depressive symptoms at each age year during emerging adulthood, and the rate of change in symptoms over time (slope)? Based on prior research suggesting an elevated risk of depression among adolescents and young adults with ADHD compared to those without ADHD, we hypothesized that the presence of ADHD would significantly predict a higher mean level of depressive symptoms at age 18 (intercept) as well as higher levels of depressive symptoms at each year throughout emerging adulthood. As reviewed above, evidence has been found for a normative decrease in depressive symptom severity during emerging adulthood. To the extent that children diagnosed with ADHD continue to display clinically significant impairment in adolescence and early adulthood (Sibley et al., 2012), it seems plausible to hypothesize the slope of depressive symptoms would differ between individuals with ADHD and those without ADHD. Thus, we expected probands with ADHD would experience a significantly slower rate of decrease in depressive symptom severity from ages 18–25, as compared to those without ADHD.
Second, if a significant predictive effect of ADHD on depressive symptoms is found, does the effect remain significant when controlling for other predictors of depressive symptoms? Prior research and theory highlight several variables, both proximal and distal, that may partially explain the association between ADHD and depression. Past research has explored the possibility that distal variables of co-occurring psychiatric diagnoses may explain the association between ADHD and depression. Epiphenomenal comorbidity refers to the idea that multiple disorders are all associated with one another and that one of the pairwise associations represents the mathematical product of the others (Angold, Costello, & Erkanli, 1999). One study found support for epiphenomenal comorbidity (Angold, et al., 1999), but other studies found a robust association between ADHD and depression even after controlling for comorbid diagnoses (e.g., Meinzer et al., 2013). This latter finding suggests epiphenomenal comorbidity is not entirely responsible for the co-occurrence of ADHD and depression.
A theory of demoralization, a proximal explanation for the covariation between ADHD and depression, proposes the mood disturbances experienced by children with histories of ADHD arise secondary to the academic and social impairment associated with ADHD (Biederman, Mick, & Faraone, 1998). In two studies, this theory did not receive empirical support; childhood ADHD significantly predicted persistence of depression (Biederman, et al., 1998) and prospective risk of MDD onset (Meinzer, et al., 2013) even after controlling for academic and social impairment. However, the demoralization effect has not been rigorously tested in that in past studies narrow definitions of impairment were used. It may be that a broader, more comprehensive definition of impairment and a more inclusive measure of impairment secondary to ADHD accounts for the covariation between ADHD and depression.
Persistence of ADHD symptoms represents another proximal explanation for the covariation between ADHD and depression. Meinzer and colleagues (2013) found that continued ADHD symptoms into young adulthood partially accounted for the association between childhood ADHD and subsequent MDD onset. Continued ADHD symptoms may influence depressive symptoms via demoralization. Alternatively, shared etiological factors such as shared genetic risk may influence both ADHD symptoms and depressive symptoms. If persistence of ADHD symptoms remains a significant predictor of depression after accounting for concurrent psychosocial impairment, it would suggest shared etiologic factors (e.g., emotion regulation deficits, disturbed reward responsivity) may be contributing to both ADHD and depression.
To address the possibilities that epiphenomenal comorbidity, a demoralization effect, and/or ADHD symptom persistence may account for the association between childhood ADHD and depressive symptoms in emerging adulthood, we statistically controlled for comorbid lifetime psychiatric diagnoses and concurrent impairment and ADHD symptoms at age 18. If the association between childhood ADHD and depressive symptoms does not remain significant after controlling for comorbid diagnoses, it would suggest intervening on comorbid diagnosis may hold promise in reducing depressive symptoms. Alternatively, if the association between childhood ADHD and depressive symptoms does not remain significant after controlling for ADHD symptoms and impairment at age 18, it would suggest intervening on ongoing ADHD may hold promise in reducing depressive symptoms. Finally, if the association between childhood ADHD and depressive symptoms remains significant after controlling for comorbid diagnoses and ADHD symptoms and impairment, it would suggest that treatment of ADHD and comorbid diagnoses may not be sufficient to prevent depressive experiences among emerging adults with histories of ADHD.
Method
Participants
ADHD Probands
As described in Molina et al. (2012), DSM–III–R or DSM–IV ADHD criteria were used to diagnose childhood ADHD at the ADD Clinic, Western Psychiatric Institute and Clinic (WPIC), in Pittsburgh between 1987 and 1996. The Disruptive Behavior Disorder Rating Scale (DBD), a standardized parent and teacher DSM-III-R and DSM-IV symptom rating scale (Pelham, Gnagy, Greenslade, & Milich, 1992), and a standardized semi-structured diagnostic interview administered to parents by a Ph.D. level clinician were used to obtain diagnostic information on ADHD (see Molina et al., 2012, for details). Mean age at initial assessment was 9.40 years (SD=2.27 years, range=5.0–16.92). ADHD probands participated in an 8-week summer treatment program for children with ADHD that included behavioral modification, parent training, and medication trials where indicated (Pelham & Hoza, 1996).
Five hundred and sixteen youth with ADHD were eligible for follow-up in the Pittsburgh ADHD Longitudinal Study (PALS) and 70.5% (n=364) participated (M=8.35 years after childhood diagnosis, SD=2.79). A minority could not be located (n=23); 129 refused or failed to participate. Participating and nonparticipating ADHD probands were compared on 14 demographic, diagnostic, and related symptomatology variables collected in childhood, with only one of 14 comparisons statistically significant (p< .05). Participating probands had slightly lower average CD symptom ratings (participating probands M=.43, non-participating probands M=.53). The first PALS follow-up interview occurred on a rolling basis between 1999 and 2003; mean age was 17.75 years, SD=3.39 years, range=11 to 28 (three subjects were 26–28 years old), 89.6% were male, and 18.4% were racial/ethnic minorities.
Non-ADHD Comparison Group
Individuals without ADHD were recruited into the PALS at the same time as the ADHD probands’ recruitment into the follow-up study (n=240). Non-ADHD comparison participants were recruited on a rolling basis to ensure demographic similarity to the probands as a group (age within one year, sex, race, highest parental education; see Molina et al., 2012 for details). A telephone screening with parents was used to gather demographic characteristics, history of diagnosis and treatment for ADHD and other behavior problems and a checklist of ADHD symptoms. Young adults (18+) also provided self-report. Those who met DSM–III–R criteria for ADHD were excluded. There were no statistically significant differences between the 364 ADHD probands and 240 non-ADHD comparison participants on age, sex, ethnicity/racial minority status, or highest parental education. As with the ADHD probands, the non-ADHD comparison participants were interviewed on an annual basis once recruited into the PALS.
Subsample for the Current Study
Due to the present study’s emphasis on depressive symptoms during emerging adulthood, only participants who completed a self-report depressive symptom measure (Centers for Epidemiological Studies Depression Scale; CES-D) at least four times between ages 18 and 25 were retained (i.e., data had to be available at ≥50% of the assessment points). So, for example, participants in the PALS study who were 28 at the initial assessment were not retained for the present study. The purpose of this requirement was to focus on the developmental period of emerging adulthood. This resulted in a final sample of 394 (65.23% of the total PALS sample), including 205 probands with childhood ADHD and 189 participants without childhood ADHD. Of the 394 participants in the subsample for the current study 348 were males and 46 were females (see Table 1 for additional demographics). Of those with ADHD, the 205 probands with CES-D data available at four or more waves did not significantly differ on rates of comorbid diagnoses, gender, or intelligence as compared to the 159 excluded probands. Of those without ADHD, the 189 included did not differ on rates of comorbid diagnoses, gender, or intelligence as compared to the 51 excluded participants. Those excluded and those retained did not differ significantly on CES-D scores at any wave.
Table 1.
Demographic and Diagnostic Information by ADHD Status in Childhood
| ADHD (n=205) | Non-ADHD (n=189) | |
|---|---|---|
| Gender (Male) | 180 (87.80%) | 168 (88.89%) |
| Race/Ethnicity | ||
| Caucasian | 165 (80.49%) | 163 (86.24%) |
| African American | 24 (11.71%) | 17 (9.00%) |
| Hispanic | 2 (0.98%) | 1 (0.53%) |
| Other | 14 (6.83%) | 8 (4.23%) |
| Parent Education Status | ||
| High School | 33 (11.39%) | 14 (7.45%) |
| Some College/Vocational Training | 72 (35.64%) | 57 (30.32%) |
| College and/or Graduate Training | 107 (52.97%) | 117 (62.23%) |
| ADHDsx | M= 6.36 (SD=5.22) | M= 1.47 (SD=2.31) |
| MDD | 34 (16.59%) | 16 (8.47%) |
| ANX | 78 (38.05%) | 44 (23.28%) |
| ODD | 96 (46.83%) | 0 (0%) |
| CD | 67 (32.68%) | 0 (0%) |
| ALC | 35 (17.16%) | 20 (10.70%) |
Note. M= mean; SD= standard deviation; ADHDsx= symptoms of attention-deficit hyperactivity disorder reported at age 18; MDD= lifetime history of major depressive disorder; ANX= lifetime history of an anxiety disorder; ODD= oppositional defiant disorder; CD= conduct disorder; ALC= lifetime history alcohol use disorder.
In addition to retention analyses, demographic information collected at the beginning of the study was compared between the ADHD group and non-ADHD comparison group in the subsample. T-tests revealed that the ADHD and non-ADHD comparison groups did not significantly differ on gender, ethnicity, parent education status, or parent marital status. Educational and occupational attainment was significantly lower for adults with ADHD compared to adults without ADHD (for details, see Kuriyan et al., 2013). Similarly, ADHD and non-ADHD comparison groups did not significantly differ on the rate of antidepressant medication usage. Forty-one participants (10.4%) reported taking antidepressant medication at some point between ages 18–25; 14 in the non-ADHD comparison group (7.4% of the comparison group) and 27 in the ADHD group (13.2% of probands). Fifty-three participants (13.5%) reported taking stimulant medication at some point between ages 18–25; five in the non-ADHD comparison group (2.6% of the comparison group) and 48 in the ADHD group (23.4% of probands). The ADHD group reported a significantly higher rate of stimulant medication usage than the non-ADHD comparison group.
Procedure
All study procedures were approved by the Institutional Review Board and all parents and adolescents provided consent and assent, respectively. Follow-up interviews in adolescence and emerging adulthood were conducted by post-baccalaureate research staff. In cases where distance prevented participant travel to the center, information was collected through mail, telephone correspondence, and home visits.
Measures
Depressive Symptoms
The CES-D is a 20-item self-report scale on which participants rate the frequency of depressive symptoms experienced in the past week. Items are rated on a 0–3 Likert scale ranging from rarely or none of the time (less than 1 day) to most or all of the time (5–7 days). Scores range from 0–60, with a recommended clinical cut-off of 16 (Radloff, 1977). The CES-D has demonstrated excellent reliability and validity in many samples, including young adults (Joiner, Walker, Pettit, Perez, & Cukrowicz, 2005). In the present sample, alpha ranged from .88 to .91 across assessment waves.
ADHD symptoms
Young adult ADHD symptoms were assessed with an unpublished measure administered to participants and their parents (provided by R. Barkley; Barkley, Murphy, & Fischer, 2008). This measure included the eighteen DSM-IV symptoms of ADHD. Comparable to the DBD, responses on the adult ADHD measure were rated on a scale from 0 (never or rarely) to 3 (very often). A symptom was counted as present if the participant or parent endorsed either (2) often or (3) very often. In the present sample, alpha was .96.
Impairment
A self-report version of the Impairment Rating Scale (IRS; Fabiano et al., 2006) was used in which participants placed an X along a continuum from no problem (definitely does not need treatment or special services) to extreme problem (definitely needs treatment or special services) for each of 12 items (11 items assessing impairment specific to interpersonal, academic, occupational, and self-evaluative domains and 1 item asking participants to rate their overall general impairment). Each item is scored between 0 (no problem) and 6 (extreme problem) depending where the X is placed; scores > 3 indicate clinically significant impairment (Fabiano, et al., 2006). Because we did not make predictions regarding subdomains of impairment, a global impairment variable was used. The global impairment variable was created by taking the average score on the 11 items assessing specific domains of impairment. This choice to use the average score was driven by a desire to maximize power of the test with this variable by using the full dimensional range of the impairment scores. The IRS has demonstrated good concurrent validity and ability to discriminate between children with and without ADHD (Fabiano et al., 2006). In the present sample, alpha ranged from .83 to .96.
Diagnostic Status
Participant lifetime psychiatric diagnosis at age 18 was measured using the Structured Clinical Interview for DSM Disorders, Non-Patient Edition (SCID-NP). The SCID-NP was administered to participants at the first wave they completed following their 18th birthday (unless they were older at study entry, at which time they completed the SCID-NP at the time of study enrollment). Dichotomous variables were created to represent the presence or absence of participants’ lifetime MDD, alcohol use disorder, and any anxiety disorder.
Diagnoses of oppositional defiant disorder (ODD) or conduct disorder (CD) were gathered using the DBD in childhood for the ADHD probands by parent and teacher report. For control participants, diagnoses of ODD and CD were gathered retrospectively at Wave 1 using the DBD by parent and self-report on each symptom of ODD and CD for at least six months during childhood. The DBD lists the DSM-III-R and DSM-IV symptoms of ADHD, ODD, and CD and asks to provide ratings of (0) not at all, (1) just a little, (2) pretty much, or (3) very much for each symptom. Item level scores of (2) or (3) signified a positive indication of a symptom. Positive indications of symptoms were summed to create a total symptom count for CD and ODD, respectively, and then DSM criteria were applied to total symptom counts to establish diagnoses of CD and ODD. The maximum endorsement was taken across reporter, and the maximum rating was used to determine symptom presence across the reporters. Following the DSM-III-R or DSM-IV guidelines, a diagnosis was made if a sufficient number of symptoms were endorsed to result in diagnosis by considering information gathered via both parent- and teacher-report for probands and parent- and self-report for non-ADHD comparison participants.
Analytic Approach
Latent growth curve modeling (LGM) was used to examine depressive symptoms from ages 18 to 25. Due to the presence of age heterogeneity at each assessment wave, an age based approach was used to examine depressive symptoms from ages 18 to 25 (Mehta & West, 2000). Depressive symptoms at each age year from 18 to 25 were entered as separate variables. Participants were coded as having missing data for ages for which data were not provided. Table 2 presents the number of participants who had data available at each age year. Little’s test of missing completely at random (MCAR; Little, 1988) was not significant, and CES-D scores were assumed to be missing completely at random at each age year. Less than 5% of participants had missing data for each covariate utilized (i.e., participant diagnostic variables and participant psychosocial impairment). Missing data for scores were estimated using full information maximum likelihood estimation (FIML) which can effectively recover parameter estimates with missing data (Enders, 2011). Analyses were conducted in the Mplus 6.12 (Muthen & Muthen, 2010) software program.
Table 2.
Observed Means and Standard Deviations on Depressive Symptoms
| Total Sample | ADHD | Non-ADHD | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Age | N | CES-D | n | CES-D | n | CES-D |
| 18 | 195 | 11.42 (9.00) | 94 | 13.04 (9.90) | 101 | 9.91 (7.82) |
| 19 | 255 | 11.80 (8.47) | 127 | 13.33 (9.16) | 128 | 10.27 (7.46) |
| 20 | 300 | 11.41 (8.43) | 152 | 12.65 (9.38) | 148 | 10.13 (7.13) |
| 21 | 323 | 11.55 (9.45) | 168 | 13.42 (10.49) | 155 | 9.52 (7.71) |
| 22 | 306 | 10.85 (8.87) | 168 | 12.01 (9.36) | 138 | 9.43 (8.03) |
| 23 | 275 | 11.19 (9.13) | 145 | 13.22 (10.22) | 130 | 8.92 (7.11) |
| 24 | 220 | 11.01 (9.00) | 111 | 13.03 (9.85) | 109 | 8.95 (7.51) |
| 25 | 173 | 11.14 (9.25) | 94 | 13.30 (9.70) | 79 | 8.58 (8.02) |
Note. N=394. Standard deviations are reported in parentheses. ADHD= attention-deficit hyperactivity disorder; CES-D= Center for Epidemiological Studies Depression Scale.
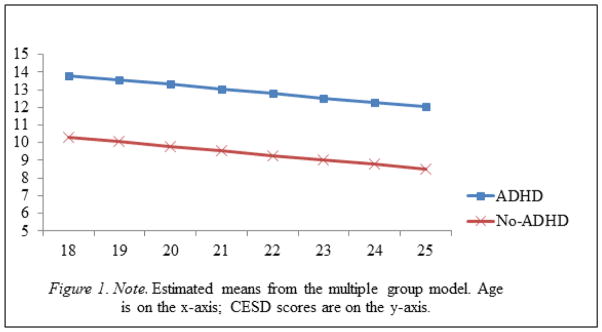
A variety of model fit indices was evaluated. Comparative Fit Index (CFI) and the Tucker Lewis Index (TLI) values ≥ .95 indicate acceptable model fit, as do root-mean-square error of approximation (RMSEA) values ≤ .05 (Browne & Cudeck, 1993). An unconditional growth model was first tested to determine whether a linear or nonlinear growth pattern best fit the depression data from ages 18 to 25. For the remainder of the manuscript, the model with no predictors will be referred to as the unconditional model. A linear growth model that specified time scores to age was fit and used in subsequent analyses, χ2(31)=57.25, p<.05, CFI=.97, TLI=.97, RMSEA=.05. For descriptive purposes, a multiple group model was analyzed in which the intercepts and slopes of depressive symptoms were estimated separately for ADHD probands and non-ADHD comparison participants (Figure 1). We then examined prediction from ADHD history and the other variables to depressive symptoms by regressing the intercept and slope of depressive symptoms on each predictor variable individually. Following the identification of significant univariate predictor variables, a growth model including distal predictor variables (comorbid diagnoses) was analyzed prior to a growth model that also included the proximal predictor variables of impairment and ADHD symptoms at age 18. This three-step process allowed us to evaluate what variables (distal and proximal) may account for the differences in depressive symptoms between ADHD probands and comparison participants.
Figure 1.
Emerging Adulthood Depressive Symptom Levels at Each Age Year
Results
Mean scores of depressive symptoms consistently fell below the recommended clinical cut-off in both the ADHD group (M=13.0, SD=0.5) and the non-ADHD group (M=9.5, SD=0.6) (Table 2). The mean percentage of participants across all age waves that fell above the clinical cut-off was 32.5% (SD=3.5) in the ADHD group and 19.2% (SD=2.1) in the non-ADHD comparison group.
Model fit indices are presented in Table 3 for the unconditional linear growth model. The significant and negative slope factor indicates that depressive symptoms decreased in severity in a linear manner from age 18 to 25. The intercept and slope factors were significantly different from zero and the variances of the intercept and slope factors were statistically significant. Given the statistically significant variance factors of both intercept and slope, predictors of intercept and slope were tested. To examine the predictive effect of childhood ADHD on depressive symptoms, we simultaneously regressed the intercept and slope of depressive symptoms on childhood ADHD. This model provided an acceptable fit to the data (CFI= .98, TLI= .98, RMSEA= .04). Childhood ADHD significantly predicted depressive symptoms intercept (estimate=2.99, standard error=0.88, critical ratio= 3.38, p<.01; Table 3), such that ADHD probands had significantly higher levels of depressive symptoms at age 18 than non-ADHD comparison participants. Childhood ADHD did not significantly predict depressive symptoms slope (estimate= 0.17, standard error= 0.17, critical ratio= 1.00, p=ns), indicating the rate of change in depressive symptoms from ages 18 to 25 was unrelated to childhood ADHD.
Table 3.
Model Fit Indices, Parameter Estimates, and Standard Errors for the Unconditional Linear Growth Model
| χ2, df | 57.25, 31 | ||
| CFI | .97 | ||
| TLI | .97 | ||
| RMSEA | .05 | ||
| Est. | SE | Critical Ratio | |
| CESD Intercept | |||
| Mean | 12.10 (1.78) | 0.45 (0.13) | 26.99 (14.02)* |
| Variance | 47.39 | 5.88 | 8.05 * |
| CESD Factor Slope | |||
| Mean | −0.24(−0.32) | 0.08(0.12) | −2.89 (−2.59)* |
| Variance | 0.58 | 0.20 | 2.90* |
| CES-D Factor Covariance INT with Slope | −1.25 (−0.24) | 0.91 (0.13) | −1.38 |
Note. N=394. Est= unstandardized model estimate; standardized estimates in parentheses; SE= standard error of estimated parameter; Critical Ratio= estimate divided by standard error; CFI=Comparative Fit Index; TLI=Tucker-Lewis Index; RMSEA= Root-Mean Square Error of Approximation; CES-D= Center for Epidemiological Studies-Depression Scale; INT= intercept;
p<.05.
To describe the intercepts and slopes for the ADHD group and non-ADHD comparison group, a multiple group model was run in which the depressive symptoms intercept was freed to vary across the ADHD and non-ADHD comparison groups, but the depressive symptoms slope was constrained to equality across the two groups. Figure 1 displays the estimated trajectories of depressive symptoms separately for ADHD probands and non-ADHD comparison participants. ADHD probands displayed higher mean levels of depressive symptoms at age 18 through age 25 than control participants, and both groups experienced comparable rates of decrease in depressive symptoms over time. To empirically test whether the mean level of depressive symptoms at each age year was higher among ADHD probands, we recentered the data in separate models at each age year from 18 through 25. In doing so, the intercept parameter shifted from age 18 to age 19, age 20, and so on up to age 25. We then simultaneously regressed the intercept and slope of depressive symptoms on childhood ADHD. In every model, childhood ADHD significantly predicted depressive symptoms intercept and did not significantly predict depressive symptoms slope. The predictive effects on intercept indicate that the mean difference in depressive symptoms between the ADHD group and non-ADHD comparison group was statistically significant at each age year.
In returning to the single group model with childhood ADHD diagnosis as a predictor, we took a three-step approach to test whether the significant effect of childhood ADHD on the depressive symptoms intercept remained significant when controlling for the distal predictor variables of comorbid diagnoses and the proximal predictor variables of impairment and ADHD symptoms at age 18. First, we regressed depressive symptoms intercept and slope on each lifetime diagnosis (ODD, CD, MDD, anxiety, alcohol use disorder), ADHD symptoms at age 18, and impairment at age 18 individually to identify significant univariate predictors. In addition to ADHD, 6 variables emerged as significant univariate predictors of the intercept but not slope of depressive symptoms: lifetime diagnoses of alcohol use disorder, anxiety, ODD, and MDD, as well as ADHD symptoms and impairment at age 18 (Table 4). Intercept and slope of depressive symptoms were regressed on stimulant and antidepressant medication usage at age 18 as well as usage at some point between the ages of 18 and 25. None of the four medication variables predicted depressive symptoms intercept or slope (not shown in Table 4 for ease of presentation).
Table 4.
Depressive Symptom Intercept (Age 18) Regressed on Individual Predictor Variables
| Est. | SE | Critical Ratio | |
|---|---|---|---|
| ADHD | 2.99 (0.43) | 0.88 (0.13) | 3.38 (3.48)** |
| MDD | 7.38 (1.06) | 1.36 (0.18) | 5.44 (5.92)*** |
| ANX | 2.24 (0.33) | 1.03 (0.15) | 2.17 (2.18)* |
| ODD | 2.35 (0.34) | 1.04 (0.15) | 2.27 (0.02)* |
| CD | 1.13 (0.17) | 1.20 (0.17) | 0.95 (0.95) |
| ALC | 4.99 (0.72) | 1.35 (0.19) | 3.69 (3.84) ** |
| ADHDsx | 0.41 (0.06) | 0.09 (0.01) | 4.44 (4.67) *** |
| IMP | 3.35 (0.34) | 0.59 (0.06) | 5.69 (5.89)*** |
Note. Est= unstandardized model estimate; standardized estimates in parentheses; SE= standard error of estimated parameter; Critical ratio= estimate divided by standard error; ADHD= attention-deficit hyperactivity disorder; MDD= lifetime history of major depressive disorder; ANX= lifetime history of an anxiety disorder; ODD= oppositional defiant disorder; CD= conduct disorder; ALC= lifetime history alcohol use disorder; ADHDsx= ADHD symptoms at age 18; IMP= impairment at age 18;
p≤.05,
p≤.01,
p≤.001
Second, the depressive symptoms intercept was regressed simultaneously on childhood ADHD and the lifetime diagnoses that were significant univariate predictor variables to identify predictors that remained significant. We did not regress slope on these variables because none of the variables significantly predicted slope in univariate models. Childhood ADHD and lifetime diagnoses of MDD and alcohol use disorder remained significant predictors of depressive symptoms intercept in the multivariate model (Table 5).
Table 5.
Model Fit Indices, Parameter Estimates, and Standard Errors for a Conditional Growth Model with ADHD and Comorbid Psychopathology Predicting Intercept of Depressive Symptoms
| χ2, df | 90.25, 61 | ||
| CFI | .97 | ||
| TLI | .97 | ||
| RMSEA | .04 | ||
| Est. | SE | Critical Ratio | |
| CES-D INT on ADHD | 2.17 (0.31) | 1.04 (0.15) | 2.09 (2.11)* |
| CES-D INT on MDD | 6.16 (0.89) | 1.38 (0.19) | 4.56 (4.69)* |
| CESD INT on ANX | 0.68 (0.10) | 0.96 (0.14) | 0.71 (.71) |
| CES-D INT on ODD | 0.05 (0.01) | 1.20 (0.14) | 0.05 (0.71) |
| CES-D INT on ALC | 3.56 (0.51) | 1.33 (0.19) | 2.68 (2.73)* |
Note. N=391. Est= unstandardized model estimate; standardized parameter estimates in parentheses; SE= standard error of estimated parameter; Critical ratio= estimate divided by standard error; CFI= Comparative Fit Index; TLI=Tucker-Lewis Index; RMSEA= Root-Mean Square Error of Approximation; CES-D= Center for Epidemiological Studies-Depression Scale; INT= intercept; ADHD= attention-deficit hyperactivity disorder; MDD= lifetime history of Major Depressive Disorder; ANX= lifetime history of an anxiety disorder; ODD= oppositional defiant disorder symptoms; ALC= lifetime history alcohol use disorder; CES-D intercept and slope were simultaneously predicted by the above variables but there were no significant associations regarding any predictor and CES-D slope;
p<.05.
Third, we examined whether the influence of childhood ADHD on the depressive symptoms intercept remained statistically significant after controlling for proximal predictor variables (i.e., ADHD symptoms and impairment at age 18). In a multivariate model including significant predictors from the previous step (ADHD, MDD, alcohol use disorder) and ADHD symptoms and impairment at age 18, only MDD, alcohol use disorder, ADHD symptoms, and impairment were significantly associated with depressive symptoms intercept (Table 6). Childhood ADHD was no longer a statistically significant predictor.
Table 6.
Model Fit Indices, Parameter Estimates, and Standard Errors for a Conditional Growth Model with ADHD, Comorbid Psychopathology, Impairment, and ADHD Symptoms Predicting Intercept of Depressive Symptoms
| CES-D | |||
|---|---|---|---|
| χ2, df | 101.01, 61 | ||
| CFI | .96 | ||
| TLI | .96 | ||
| RMSEA | .04 | ||
| Est. | SE | Critical Ratio | |
| CESD INT on ADHD | 0.11 (0.01) | 0.99 (0.07) | 0.91 (0.11) |
| CESD INT on MDD | 5.23 (0.26) | 1.28 (0.63) | 2.65 (4.09)*** |
| CESD INT on ALC | 3.39 (0.17) | 1.28 (0.06) | 2.65 (2.68)** |
| CESD INT on ADHDsx | 0.28 (0.19) | 0.10 (0.07) | 2.69 (2.72)** |
| CESD INT on IMP | 2.3 (0.23) | 0.60 (0.06) | 3.82 (3.81)*** |
Note. N=390. Est= unstandardized model estimate; standardized estimates are in parentheses; SE= standard error of estimated parameter; Critical ratio= estimate divided by standard error; CFI= Comparative Fit Index; TLI=Tucker-Lewis Index; RMSEA= Root-Mean Square Error of Approximation; ADHD= attention-deficit/hyperactivity disorder; MDD= lifetime history of major depressive disorder; ALC= lifetime history alcohol use disorder; ADHDsx= ADHD symptoms at age 18; IMP= impairment at age 18; INT= intercept. CES-D intercept and slope were simultaneously predicted by the above variables but there were no significant associations regarding any predictor and CES-D slope;
p<.05,
p≤.01,
p≤.001.
Discussion
Consistent with prior research (Galambos, et al., 2006; Pettit, et al., 2011; Radloff, 1977), levels of depressive symptoms decreased through emerging adulthood, indicating more positive emotional well-being as individuals progressed from age 18 to age 25. Individuals with a childhood history of ADHD displayed significantly higher levels of depressive symptoms at every age year throughout emerging adulthood. Although the rate of change in depressive symptoms was not different across the ADHD and non-ADHD groups, childhood ADHD history significantly predicted levels of depressive symptoms at age 18 through age 25. Consistent with past research (e.g., Chronis-Tuscano et al., 2010; Meinzer, et al., 2013), the association between childhood history of ADHD and depressive symptoms at age 18 remained statistically significant when controlling for the distal variables of comorbid diagnoses. However, when proximal variables measured at age 18 (i.e., ADHD symptoms and impairment) were added to the model, childhood ADHD no longer remained a significant predictor of depressive symptoms at age 18. In the final multivariate model, a lifetime history of alcohol use disorder and MDD also significantly and robustly predicted depressive symptoms at age 18.
The finding that childhood ADHD did not significantly predict the level of depressive symptoms at age 18 after controlling for concurrent ADHD symptoms and impairment is consistent with the possibility that ongoing impairment secondary to ADHD may account for its association with depressive symptoms (i.e., demoralization; Biederman et al., 1998). Given evidence for ADHD symptoms and impairment predicting depressive symptoms, improvements in psychosocial functioning might reduce levels of depressive symptoms in emerging adults with histories of ADHD. Psychosocial and pharmacological treatments represent two potential avenues for reduction of ADHD symptoms and impairment. Several studies have demonstrated positive effects of Cognitive-Behavioral Therapy (CBT) or Meta-Cognitive Therapy in reducing the severity of ADHD symptoms among adults with ADHD (Safren et al., 2010; Solanto et al., 2010). Further, there is preliminary evidence that CBT may lead to reductions in depressive symptoms in adults medicated for ADHD (Safren et al., 2005). In addition to administering CBT to emerging adults with histories of ADHD with the goal of preventing depressive symptoms, a possible avenue for future research would be to adapt a treatment program such as Supporting Teens’ Academic Needs Daily (STAND; Sibley et al., 2013) to fit the developmental level and tasks of emerging adulthood (e.g., readiness for college and/or employment) given the difficulties experienced by emerging adults with ADHD in those domains (Kuriyan et al., 2013). To the extent that such an intervention could promote reductions in ADHD symptoms and impairment, it also may prevent depressive symptoms.
Multiple FDA approved pharmacological treatments exist for the treatment of ADHD in adults and treatment has been found to reduce disease related disability (Waxmonsky et al., 2014; Wigal et al., 2010). However, adherence to stimulant medication tends to wane with age to the degree that most patients have discontinued medication by the time they enter young adulthood (Molina et al., 2009; Sibley, Kuriyan, Evans, Waxmonsky, & Smith, 2014). Future research should investigate whether maintenance of stimulant treatment into emerging adulthood is effective for preventing depression.
It will be important for future research to use a multi-informant assessment approach when evaluating impairment. Youth with ADHD often lack insight into their own impairment, showing a positive illusory bias (Hoza et al., 2004; Hoza, Murray-Close, Arnold, Hinshaw, & Hechtman, 2010). Some studies have found that adolescents and young adults with histories of ADHD also underreport symptoms and impairment (Sibley et al., 2012), while other studies have found that this bias tends to diminish in adolescence (Hoza, et al., 2010). At least some of this variability in bias may be accounted for by the presence of co-occurring depressive symptoms; children with ADHD who endorse depressive symptoms do not show a positive illusory bias (Hoza et al., 2004). To the extent that self-reports on impairment are biased by the presence or absence of depressive symptoms, caution is needed when drawing conclusions from self-report data on the role of impairment in the association between ADHD and depressive symptoms.
Future research is encouraged to examine when depressive symptoms first appear to further inform the developmental timing of depression screening and prevention programs for youth with a history of ADHD. Prior research has found mixed results on whether rates of MDD or levels of depressive symptoms differ in adolescents with ADHD compared to their peers without ADHD (Meinzer et al., 2013; Molina et al., 2009). However, as evidenced by the current study, differences in depressive symptoms are present by the end of adolescence and persist through emerging adulthood. Therefore, the transition from late adolescence to emerging adulthood appears to be a crucial time for depression screening and prevention among individuals with a history of ADHD.
A lifetime history of alcohol use disorder significantly predicted the level of depressive symptoms at age 18. This finding suggests that alcohol use problems in adolescence may portend problems with depression in emerging adulthood. A large literature on alcohol use and depression shows high rates of concurrent co-occurrence of these disorders (Grant & Harford, 1995) and longitudinal studies demonstrate a prediction of depression to alcohol use disorders in both the general population (e.g., Kuo, Gardner, Kendler, & Prescott, 2006) and within ADHD samples (e.g., Lansford et al., 2008). However, results of the current study indicate that a lifetime history of alcohol use disorders predicted depressive symptoms at age 18, even after controlling for a prior history of MDD. These results suggest that adolescents with alcohol use problems represent good candidates for depression prevention efforts. There is a need for longitudinal research on alcohol use and depressive symptoms and their reciprocal effects on each other over time, particularly for those with and without ADHD and/or self-reported impairment.
In contrast to the robust association between alcohol use disorder and depressive symptoms at age 18, the association between ODD and depressive symptoms did not remain statistically significant when controlling for other diagnoses including histories of MDD and ADHD. Future research should examine the extent to which the association between ODD in childhood and depressive symptoms in emerging adulthood is accounted for by co-occurring externalizing problems like ADHD and alcohol use or by early onset MDD.
We did not find differences in the slope of depression symptoms between the groups. On the one hand, this finding is encouraging because participants with histories of ADHD did not spiral into more severe depressive symptoms in emerging adulthood. On the other hand, this finding is concerning because participants with histories of ADHD did not narrow the gap in depressive symptoms; the higher mean level of depressive symptoms remained present through emerging adulthood. However, the analytic approach in the current study did not take into account heterogeneous trajectories. Future research should examine the possibilities that (a) distinct subgroups or classes of depressive symptom trajectories exist within emerging adults with ADHD, and (b) risk factors and outcomes may differ across their classes of trajectories.
Several limitations should be considered when evaluating the findings of this study. First, the sample was predominately male and white. Future research is encouraged to replicate these findings in racially and ethnically diverse samples with higher representation of women. As previously mentioned, depressive experiences are likely to be heterogeneous among individuals with ADHD. Future research is encouraged to identify patterns of heterogeneity in the course of depressive symptoms and examine variables that may explain such heterogeneity. One such variable may be gender. Women relative to men are at higher odds of experiencing persistently elevated levels of depressive symptoms in emerging adulthood (Yaroslavsky et al., 2013). Whether or not women with ADHD relative to men with ADHD are at elevated risk of a persistent course of depressive symptoms in emerging adulthood is unknown. The present study was unable to examine this issue given the preponderance of male participants. Lastly, the presence of a positive illusory bias may have led to a minimization of the severity of self-reported depressive symptoms and impairment among ADHD probands.
In summary, the current study demonstrated that individuals with histories of ADHD experienced significantly higher levels of depressive symptoms than their non-ADHD comparison peers at age 18 and then displayed comparable decreases in depressive symptoms through emerging adulthood. The predictive effect of childhood ADHD on depressive symptoms at age 18 remained statistically significant when controlling for comorbid diagnoses, but not when controlling for concurrent ADHD symptoms and psychosocial impairment at age 18. Adolescents with ADHD may therefore be good candidates for continued ADHD treatment as well as preventive interventions aimed at reducing depressive symptoms. It is also important to note that probands with ADHD displayed higher levels of depressive symptoms as compared to non-ADHD comparison participants in spite of receiving intensive behavioral treatment as children and in some instances medication for ADHD in childhood or adolescence (Pelham & Hoza, 1996). Thus, short-term treatment of ADHD is not sufficient to reduce the risk of depressive experiences in emerging adulthood (see also Molina, et al., 2009). Rather, for some children with ADHD, ongoing treatment that incorporates evidenced based interventions for the prevention of depression may be needed to impact adult outcomes.
Acknowledgments
This research was principally supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA011873 and AA00202) and the National Institute on Drug Abuse (DA12414), awarded to Drs. Brooke Molina and William Pelham, funded the Pittsburgh ADHD Longitudinal Study, which was the data source for this project. Additional funding was provided by AA12342, AA000202, MH50467, MH12010, ES05015-08, DA016631, MH065899, KAI-118-S1, DA85553, MH077676, MH069614, MH62946, MH53554, MH069434, MH099030, IES LO3000665A, IESR324B060045, & NS39087. This work was supported in part by an Emerging Scholars Fellowship from Active Minds awarded to Michael C. Meinzer.
In the past three years, Dr. James Waxmonsky served on the advisory panel for Noven Pharmaceuticals and the speaker’s board for Quintiles and received research funding from Janssen Pharmaceuticals. Additionally, Drs. Waxmonsky and Pelham received research funding from Noven Pharmaceuticals.
References
- Angold A, Costello EJ, Erkanli A. Comorbidity. Journal of Child Psychology and Psychiatry and Allied Disciplines. 1999;40:57–87. [PubMed] [Google Scholar]
- Arnett JJ. Emerging adulthood: A theory of development from the late teens through the twenties. American Psychologist. 2000;55:469–480. [PubMed] [Google Scholar]
- Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult outcome of hyperactive children: adaptive functioning in major life activities. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(2):192–202. doi: 10.1097/01.chi.0000189134.97436.e2. S0890-8567(09)62004-9. [DOI] [PubMed] [Google Scholar]
- Barkley RA, Guevremont DC, Anastopoulos AD, DuPaul GJ, Shelton TL. Driving-related risks and outcomes of attention deficit hyperactivity disorder in adolescents and young adults: A 3- to 5-year follow-up survey. Pediatrics. 1993;92:212–218. [PubMed] [Google Scholar]
- Barkley RA, Murphy KR, Fischer M. ADHD in adults: What the science says. New York, NY: Guilford; 2008. [Google Scholar]
- Biederman J, Mick E, Faraone SV. Depression in attention deficit hyperactivity disorder (ADHD) children: “True” depression or demoralization? Journal of Affective Disorders. 1998;47:113–122. doi: 10.1016/s0165-0327(97)00127-4. S0165-0327(97)00127-4. [DOI] [PubMed] [Google Scholar]
- Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bolen K, Long J, editors. Testing Structural Equation Models. Beverly Hills, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Chronis-Tuscano A, Molina BS, Pelham WE, Applegate B, Dahlke A, Overmyer M, Lahey BB. Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2010;67:1044–1051. doi: 10.1001/archgenpsychiatry.2010.127. 67/10/1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK. Analyzing longitudinal data with missing values. Rehabilitation Psychology. 2011;56:267–288. doi: 10.1037/a0025579. 2011-22474-001. [DOI] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE, Jr, Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, Burrows-Maclean L. A practical measure of impairment: psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child and Adolescent Psychology. 2006;35:369–385. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Flory K, Molina BS, Pelham WE, Jr, Gnagy E, Smith B. Childhood ADHD predicts risky sexual behavior in young adulthood. Journal of Clinical Child and Adolescent Psychology. 2006;35:571–577. doi: 10.1207/s15374424jccp3504_8. [DOI] [PubMed] [Google Scholar]
- Galambos NL, Barker ET, Krahn HJ. Depression, self-esteem, and anger in emerging adulthood: Seven-year trajectories. Developmental Psychology. 2006;42:350–365. doi: 10.1037/0012-1649.42.2.350. 2006-03514-013. [DOI] [PubMed] [Google Scholar]
- Grant BF, Harford TC. Comorbidity between DSM-IV alcohol use disorders and major depression: Results of a national survey. Drug Alcohol Dependence. 1995;39:197–206. doi: 10.1016/0376-8716(95)01160-4. 0376871695011604. [DOI] [PubMed] [Google Scholar]
- Hoza B, Gerdes AC, Hinshaw SP, Arnold LE, Pelham WE, Jr, Molina BS, Wigal T. Self-perceptions of competence in children with ADHD and comparison children. Journal of Consulting and Clinical Psychology. 2004;72:382–391. doi: 10.1037/0022-006X.72.3.382. [DOI] [PubMed] [Google Scholar]
- Hoza B, Murray-Close D, Arnold LE, Hinshaw SP, Hechtman L. Time-dependent changes in positively biased self-perceptions of children with attention-deficit/hyperactivity disorder: A developmental psychopathology perspective. Developmental Psychopathology. 2010;22:375–390. doi: 10.1017/S095457941000012X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner TE, Walker RL, Pettit JW, Perez M, Cukrowicz KC. Evidenced-based assessment of depression in adults. Psychological Assessment. 2005;17:267–277. doi: 10.1037/1040-3590.17.3.267. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Gardner CO, Kendler KS, Prescott CA. The temporal relationship of the onsets of alcohol dependence and major depression: Using a genetically informative study design. Psychological Medicine. 2006;36:1153–1162. doi: 10.1017/S0033291706007860. [DOI] [PubMed] [Google Scholar]
- Kuriyan AB, Pelham WE, Jr, Molina BS, Waschbusch DA, Gnagy EM, Sibley MH, Kent KM. Young adult educational and vocational outcomes of children diagnosed with ADHD. Journal of Abnormal Child Psychology. 2013;41:27–41. doi: 10.1007/s10802-012-9658-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford JE, Erath S, Yu T, Pettit GS, Dodge KA, Bates JE. The developmental course of illicit substance use from age 12 to 22: Links with depressive, anxiety, and behavior disorders at age 18. Journal of Child Psychology and Psychiatry. 2008;49:877–885. doi: 10.1111/j.1469-7610.2008.01915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Solomon A, Seeley JR, Zeiss A. Clinical implications of “subthreshold” depressive symptoms. Journal of Abnormal Psychology. 2000;109(2):345–351. [PubMed] [Google Scholar]
- Little RJA. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83(404):1198–1202. [Google Scholar]
- Mehta PD, West SG. Putting the individual back into individual growth curves. Psychological Methods. 2000;5:23–43. doi: 10.1037//1082-989x.5.1.23. [DOI] [PubMed] [Google Scholar]
- Meinzer MC, Lewinsohn PM, Pettit JW, Seeley JR, Gau JM, Chronis-Tuscano A, Waxmonsky JG. Attention-deficit/hyperactivity disorder in adolescence predicts onset of major depressive disorder through early adulthood. Depression and Anxiety. 2013;30:546–553. doi: 10.1002/da.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer MC, Pettit JW, Viswesvaran C. The co-occurrence of attention-deficit/hyperactivity disorder and unipolar depression in children and adolescents: A meta-analytic review. Clinical Psychology Review. 2014;34:569–607. doi: 10.1016/j.cpr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Molina BSG, Hinshaw SP, Swanson JM, Arnold LE, Vitiello B, Jensen PS, Houck PR. The MTA at 8 years: Prospective follow-up of children treated for combined-type ADHD in a multisite study. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:484–500. doi: 10.1097/CHI.0b013e31819c23d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Cheong J, Marshal MP, Gnagy EM, Curran PJ. Childhood attention-deficit/hyperactivity disorder (ADHD) and growth in adolescent alcohol use: The roles of functional impairments, ADHD symptom persistence, and parental knowledge. Journal of Abnormal Psychology. 2012;121:922–935. doi: 10.1037/a0028260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina BSG, Pelham WE, Marshal MR, Curran PJ, Gnagy EM, Thompson AL, Cheong JW. Developmental influences on the associations among childhood ADHD, antisocial behaviors, and alcohol use. Alcoholism-Clinical and Experimental Research. 2007;31:285a–285a. [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. 6. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, Milich R. Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE, Hoza B. Intensive treatment: A summer treatment program for children with ADHD. In: Hibbs ED, Jensen PS, editors. Psychosocial treatments for child and adolescent disorders: Empirically based strategies for clinical practice. New York: American Psychological Association; 1996. [Google Scholar]
- Pettit JW, Roberts RE, Lewinsohn PM, Seeley JR, Yaroslavsky I. Developmental relations between perceived social support and depressive symptoms through emerging adulthood: Blood is thicker than water. Journal of Family Psychology. 2011;25:127–136. doi: 10.1037/A0022320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Safren SA, Otto MW, Sprich S, Winett CL, Wilens TE, Biederman J. Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behaviour Research and Therapy. 2005;43:831–842. doi: 10.1016/j.brat.2004.07.001. S0005-7967(04)00136-6. [DOI] [PubMed] [Google Scholar]
- Safren SA, Sprich S, Mimiaga MJ, Surman C, Knouse L, Groves M, Otto MW. Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: A randomized controlled trial. Journal of the American Medical Association. 2010;304:875–880. doi: 10.1001/jama.2010.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley MH, Kuriyan AB, Evans SW, Waxmonsky JG, Smith BH. Pharmacological and psychosocial treatments for adolescents with ADHD: An updated systematic review of the literature. Clinical Psychology Review. 2014;34:218–232. doi: 10.1016/j.cpr.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Sibley MH, Pelham WE, Derefinko KJ, Kuriyan AB, Sanchez F, Graziano PA. A pilot trial of Supporting Teens’ Academic Needs Daily (STAND): A parent-adolescent collaborative intervention for ADHD. Journal of Psychopathology and Behavioral Assessment. 2013;35:436–449. doi: 10.1007/s10862-013-9353-6. [DOI] [Google Scholar]
- Sibley MH, Pelham WE, Molina BS, Gnagy EM, Waxmonsky JG, Waschbusch DA, Kuriyan AB. When diagnosing ADHD in young adults emphasize informant reports, DSM items, and impairment. Journal of Consulting and Clinical Psychology. 2012;80:1052–1061. doi: 10.1037/a0029098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Marks DJ, Wasserstein J, Mitchell K, Abikoff H, Alvir JM, Kofman MD. Efficacy of meta-cognitive therapy for adult ADHD. American Journal of Psychiatry. 2010;167:958–968. doi: 10.1176/appi.ajp.2009.09081123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoolmiller M, Kim HK, Capaldi DM. The course of depressive symptoms in men from early adolescence to young adulthood: Identifying latent trajectories and early predictors. Journal of Abnormal Psychology. 2005;114:331–345. doi: 10.1037/0021-843X.114.3.331. 2005-09257-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxmonsky JG, Waschbusch DA, Babinski DE, Humphrey HH, Alfonso A, Crum KI, Pelham WE. Does pharmacological treatment of ADHD in adultsenhance parenting performance? Results of a double-blind randomized trial. Cns Drugs. 2014;28:665–677. doi: 10.1007/s40263-014-0165-3. [DOI] [PubMed] [Google Scholar]
- Wigal T, Brams M, Gasior M, Gao J, Squires L, Giblin J 316 Study Group. Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behavioral and Brain Functions. 2010;6:1–14. doi: 10.1186/1744-9081-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wymbs B, Molina B, Pelham W, Cheong J, Gnagy E, Belendiuk K, Waschbusch D. Risk of intimate partner violence among young adult males with childhood ADHD. Journal of Attention Disorders. 2012;16:373–383. doi: 10.1177/1087054710389987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaroslavsky I, Pettit JW, Lewinsohn PM, Seeley JR, Roberts RE. Heterogeneous trajectories of depressive symptoms: Adolescent predictors and adult outcomes. Journal of Affective Disorders. 2013;148:391–399. doi: 10.1016/j.jad.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]