Abstract
Stem cells with high proliferation, self-renewal and differentiation capacities are promising for tissue engineering approaches. Among stem cells, human tooth germ stem cells (hTGSCs) having mesenchymal stem cell characteristics are highly proliferative and able to differentiate into several cell lineages. Researchers have recently focused on transplanting stem cells with bioconductive and/or bioinductive materials that can provide cell commitment to the desired cell lineages. In the present study, effects of pluronic block copolymers (F68, F127 and P85) on in vitro myo- and neurogenic differentiation of human tooth germ stem cells (hTGSCs) were investigated. As P85 was found to exert considerable toxicity to hTGSCs even at low concentrations, it was not evaluated for further differentiation experiments. Immunocytochemical analysis, gene and protein expression studies revealed that while F68 treatment increased lineage-specific gene expression in both myo- and neuro-genically differentiated cells, F127 did not result in any remarkable difference compared to cells treated with differentiation medium. Subsequent studies are required to explore the exact mechanisms of how F68 increases the myogenic and neurogenic differentiation of hTGSCs. The present work indicates that pluronic F68 might be used in functional skeletal and neural tissue engineering applications.
Keywords: Tissue engineering, Pluronics, hTGSCs, Biopolymers, Differentiation
Introduction
Stem cells with their extensive self-renewal and differentiation potential could be used for cellular replacement therapy of various disorders including bone dehiscence, cerebral or cardiac ischemia and type 1 diabetes (Doğan et al. 2012; Watt and Huck 2013). Stem cells derived from embryonic sources or adult tissues which can be reprogrammed to pluripotent state have created new opportunities for personalized regenerative therapies. While major progress in this field has been achieved, ethical and technical problems still remain and force scientists to use alternative, safe and available cell types (Doğan et al. 2012). Hence, mesenchymal stem cells (MSCs) obtained from almost all postnatal organs such as bone marrow, adipose tissue, synovial membrane, skin and dental pulp are of great interest (da Silva Meirelles et al. 2006). Among them, human tooth germ stem cells (hTGSCs) isolated from tooth germs of young adults have been shown to exert remarkable mesenchymal characteristics and differentiate into cell lineages originated from three germ layers (Ikeda et al. 2008). Multipotency and highly proliferative properties of these stem cells may be explained by quiescent and undifferentiated state of tooth germs until the age of six (Kerkis et al. 2007). Previous studies have shown that hTGSCs are able to differentiate into osteo-, chondro-, adipo- and neurogenic cell types in in vitro culture conditions (Doğan et al. 2012; Yalvac et al. 2009). Although using appropriate cell source is the basic principle of cellular therapy, involvement of biocompatible structural components is also required to provide tissue integrity (Soleimani et al. 2010). Differentiation fate of stem cells is not only regulated by intrinsic factors but also maintained by extrinsic mechanisms including growth factors, cell–cell interactions and extracellular matrix (ECM) (Watt and Huck 2013). Direct placement of stem cells to the traumatic injured tissue lacking ECM results in inflammatory response and anoikis (Zurita et al. 2010). In this line, stem cells should be applied together with natural or synthetic scaffold systems and inductive materials that are able to support cell proliferation, growth and differentiation. Such engineered scaffolds should provide a three-dimensional structure for transplanted cells and manipulate cell differentiation toward specific cell lineage.
Pluronics, also known as poloxamers, are biocompatible synthetic polymers that are generally used in tissue engineering studies (Huang et al. 2006; Vashi et al. 2008). Pluronics are composed of poly(ethylene oxide) (PEO) and poly(propylene oxide) (PPO) units in the form of PEOX-PPOY-PEOX providing amphiphilic properties and stable interactions with biological membranes (Doğan et al. 2012). They can be applied as injectable hydrogel systems to rebuild three-dimensional (3-D) structures for stem cells used in tissue replacement therapies (Park et al. 2009). Each type of poloxamers varies depending on structural characteristics (number of subunits) (Kabanov et al. 2002). Pluronic F68 (PEO76PPO29PEO76) interacts with cellular membranes and prevents mechanical stress caused by agitation (Clincke et al. 2011). F68 was shown to exert neuroprotective role against neurotoxic molecules by protecting membrane integrity (Mina et al. 2009). F68 has also been used as a cryoprotectant agent during cryopreservation of hTGSCs (Doğan et al. 2013). Pluronic P85 (PEO26PPO40PEO26) has been used in previous studies as blockers for various transporters. P85 could be combined with drugs and enhance drug uptake and cellular accumulation (Batrakova et al. 2010). Pluronic F127 (PEO100PPO65PEO100) could be used for drug transport because of its ability to form micelles. Moreover, it has been shown that F127 interacts with cell surfaces and increases cell differentiation (Erukova et al. 2000; Vashi et al. 2008). F127 hydrogel scaffold has been shown to promote adipogenic differentiation of bone marrow derived stem cells (BMSCs) (Vashi et al. 2008; Wu et al. 2011). TGF-β1 conjugated biodegradable F127 hydrogel has been used for rabbit knee articular cartilage regeneration (Jung et al. 2010). Similarly, effective bone repair has been achieved by application of F127 combined with hydroxyapatite and biphasic calcium phosphate (Zhou et al. 2007). Besides, pluronic F68 and F127 has been used for the differentiation of hTGSCs into osteo-, chondro-, adipo- and odontogenic cell types in previous studies (Doğan et al. 2012; Tasli et al. 2013c).
In the current investigation, three different pluronics (P85, F68 and F127) were used to enhance neurogenic and myogenic differentiation potential of mesenchymal stem cells. The study is the first attempt to investigate the effects of pluronic block copolymers on neurogenic and myogenic differentiation capacities of hTGSCs.
Methods
Isolation and characterization of hTGSCs
Isolation and characterization of hTGSCs were performed as described previously (Doğan et al. 2012; Tasli et al. 2013a, c). Human tooth germ tissues were collected from the 3rd molar tooth germs of healthy young adults between 13–15 years of age. The collected tissues were harvested, minced and plated in six well plates (JET BIOFIL, Guangzhou, China). Cells were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10 % (v/v) fetal bovine serum (FBS) and 1 % (v/v) PSA (10,000 units/ml penicillin, 10,000 μg/ml streptomycin, 25 μg/ml amphotericin B) (Invitrogen, Gibco, Paisley, UK). After having reached sufficient confluence (80 %), cells were trypsinized using 0.25 % (v/v) trypsin/EDTA (Invitrogen, Gibco, UK) and seeded on a T-75 flask (TPP, Trasadingen, Switzerland). The cells were maintained at 37 °C and 5 % CO2 in a humidified incubator.
Isolated hTGSCs (passage 3) were characterized for their mesenchymal cell surface profile and differentiated into osteo-, chondro and adipogenic cell lineages as described previously (Doğan et al. 2012). Cells were trypsinized and incubated with the following phycoerythrin (PE) conjugated antibodies against CD29, CD34, CD45, CD90, CD105, CD133, CD166 and CD73 (Abcam, Cambridge, UK). Cells were washed with PBS to remove the excess primary antibodies. The flow cytometry analysis of the cells was conducted using Becton–Dickinson FACS Calibur flow cytometry system (Becton–Dickinson, San Jose, CA, USA). For differentiation experiments, cells were seeded into 24-well plates (1 × 104 cells/well) and incubated with osteo-, chondro- and adipogenic differentiation media for 10 days to visualize mesenchymal stem cell properties. Differentiation media were prepared using DMEM low glucose medium (Invitrogen, GIBCO) and changed every other day. The contents of differentiation media are shown in Table 1.
Table 1.
Differentiation media contents
| Differentiation | Content |
|---|---|
| Osteogenic medium | 100 nM dexamethasone |
| 10 mM β-glycerophosphate | |
| 0.2 mM ascorbic acid | |
| Chondrogenic medium | 1× insulin–transferrin–selenium (ITS-G) |
| 100 nM dexamethasone | |
| 100 ng/ml TGF-β | |
| 14 µg/ml ascorbic acid | |
| 1 mg/ml bovine serum albumin (BSA) | |
| Adipogenic medium | 100 nM dexamethasone |
| 5 µg/ml insulin | |
| 0.5 mM 3-isobutyl-1-methylxanthine (IBMX) | |
| 60 µM indomethacin | |
| Myogenic medium | 0.1 μM dexamethasone |
| 5 % (v/v) horse serum | |
| 50 μM hydrocortisone | |
| Neurogenic medium | 200 μM butylated hydroxyanisole |
| 5 mM KCl | |
| 2 μM valproic acid | |
| 5 μM insulin | |
| 10 ng/ml neurogenic growth factor (NGF) |
Viable cell assay
The stock solutions of F68, F127, and P85 block copolymers were prepared in PBS at a concentration of 10 % (w/v). Working solutions for each polymer were prepared at concentrations of 0.01, 0.02, 0.05, and 0.1 % (w/v) in DMEM. hTGSCs were seeded at a concentration of 5 × 103 cells/well onto 96-well plates (JET BIOFIL). The following day, cells were treated with different concentrations of F68, F127, P85 and 20 % (v/v) DMSO was used as lethal dose (positive control). Viable cells were measured by the MTS assay (CellTiter96 Aqueous One Solution, Promega, Southampton, UK) according to the manufacturer’s instructions. After incubating the cells in the presence of pluronics for 24, 48 and 72 h, 10 μl MTS reagent was added to the growth medium followed by further incubation for 2 h. Thereafter, the absorbance at 490 nm was measured by an ELISA plate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Differentiation procedure
hTGSCs were induced to differentiate into myogenic- and neurogenic-like cells in the presence of pluronics. The cells were seeded on a six well plate for RNA isolation and 48 well plate (BIOFIL, TCP, Switzerland) for immunocytochemistry analysis at a density of 5 × 104 and 1 × 104 cells/well, respectively, for each type of differentiation. Cells were incubated with defined differentiation media containing P85, F68 and F127 and pluronics were added to the cell culture for each medium renewal. For myogenic differentiation, cells were seeded on six well plates followed by the addition of myogenic differentiation medium including 5 % (v/v) horse serum, 0.1 μM dexamethasone and 50 μM hydrocortisone (Sigma, St. Louis, MO, USA) in DMEM 10 % (v/v) FBS and 1 % (v/v) PSA. Myogenic differentiation medium was replaced twice a week for 21 days as described before with slight modifications (Gang et al. 2004). For neurogenic differentiation, hTGSCs were incubated with DMEM supplemented with 10 % (v/v) FBS and 1 % (v/v) PSA containing 200 μM butylated hydroxyanisole (Sigma), 5 mM KCl, 2 μM valproic acid (Sigma), 10 ng/ml Neurogenic Growth Factor (NGF, Sigma) and 5 μM insulin. Neurogenic differentiation medium was replaced twice a week for 14 days (Tatard et al. 2007). Samples were collected for immunocytochemistry, RT-PCR and Western blot analysis at the end of differentiation procedures (21 days for myogenic differentiation and 14 days for neurogenic differentiation).
Immunostaining
Immunocytochemistry analysis was completed according to the previously described protocol (Tasli et al. 2013b). Cells were fixed with 2 % (w/v) paraformaldehyde at 4 °C for 30 min and permeabilized with 0.1 % (v/v) Triton X-100 prepared in PBS at ambient temperature for 5 min. Cells were incubated with 2 % (v/v) goat serum (Sigma) to prevent nonspecific binding of antibodies. Cells were incubated overnight at 4 °C with the following primary antibodies: For myogenic differentiation; Actin (sc-58670, Santa Cruz Biotechnology, Santa Cruz, CA, USA), α-Smooth Muscle Actin (α-SMA) (ab5694, Abcam), Desmin (ab15200, Abcam) and Myogenin (ab1835, Abcam), and for neurogenic differentiation; Enolase (sc-59536, Santa Cruz Biotechnology), Nestin (sc-20978, Santa Cruz Biotechnology), Microtubule Associated Protein 2 (MAP2) (sc-20172, Santa Cruz Biotechnology), Neurofilament (NF) (sc-20013, Santa Cruz) and Tyrosine Hydroxylase (TH) (sc-14007, Santa Cruz). Cells were washed three times with PBS and treated with secondary antibodies (Goat anti rabbit IgG Alexa Fluor 488 and Goat anti mouse IgG Alea Fluor 488, Invitrogen, Carlsbad, CA, USA) for 1 h at 4 °C. DAPI (AppliChem GmbH, Darmstadt, Germany) was used to stain the nuclei of the cells by incubating for 20 min at 4 °C. The cells were then washed three times with PBS and observed under fluorescence microscope (Nicon Eclipse TE200, Melville, NY, USA).
Western blotting
Protein expression analysis were performed according to a previously described protocol (Xu et al. 2009). Total protein was isolated from the differentiated cells by using RIPA Buffer (Santa Cruz Biotechnology) according to the manufacturer’s instructions. BCA protein assay kit (Thermo Scientific, Rockford, IL, US) was used to determine protein concentration. Protein samples were loaded to 10 % sodium dodecyl sulfate–polyacrylamide gel at a concentration of 20 µg/lane and transferred to a nitrocellulose membrane. Membranes were incubated with blocking solution containing 5 % skimmed milk prepared in TBS-T buffer. Membranes were then incubated with primary antibodies against α-SMA (ab5694, Abcam) and Desmin (ab15200, Abcam) for myogenically differentiated cells, Enolase (sc-59536, Santa Cruz) and TH (sc-14007, Santa Cruz) for neurogenically differentiated cells at 4 °C for 16 h. After washing with TBS-T for three times, membranes were incubated with secondary antibodies (HRP-conjugated) prepared in blocking buffer for 1 h. β-actin (3700S, Cell Signaling Technology, Beverly, MA, USA) antibody was used for the normalization of the data. Images were taken by using a luminometer system (ChemicDoc XRS, Biorad, Hercules, CA,USA). Average band intensities of three separate experiments were calculated using ImageJ software and normalized with respective β-actin band intensities.
Quantitative real time PCR
Total RNA was isolated by using High Pure RNA Isolation Kit (Roche, Mannheim, Germany) and cDNA synthesis was performed by using High Fidelity cDNA Synthesis Kit (Roche) according to the manufacturer’s instructions. Quantitative real time PCR (RT-PCR) was performed using Maxima SYBR Green/ROX (Fermentas, Hanover, MD, USA) for the determination of gene expression levels. Primer sequences for marker genes are shown in Table 2. Glyceraldehyde 3-phosphate dehydrogenase gene (GAPDH) was used as the house keeping gene for the normalization of the data. All RT-PCR experiments were performed using iCycler RT-PCR detection system (Bio-Rad). RT-PCR assays were conducted in a 20 µl volume including cDNA, primers and SYBR premix under the following conditions: 93 °C for 3 min, 39 cycles of 93 °C for 30 s, 61 °C for 40 s and 72 °C for 45 s, and 72 °C for 10 min. Relative quantity of targeted gene, normalized with respect to GAPDH amount, were analyzed using a formula of 2−ΔΔCt, where Ct indicates the threshold cycle.
Table 2.
The list of primers used in RT-PCR assays
| Marker | Sequences (5′–3′) |
|---|---|
| Desmin | GGAGAGCCGGATCAATCTCCCCA |
| ACGACCTCCCCATCCCGTGT | |
| Myogenin | TAAGGTGTGTAAGAGGAAGTCG |
| CCACAGACACATCTTCCACTGT | |
| α-Smooth muscle actin | AGACATCAGGGGGTGATGGT |
| ATCTTTTCCATGTCGTCCCAGTTG | |
| Nestin | GGAGTCCTGGATTTCCTTCC |
| GCCCTGACCACTCCAGTTTA | |
| Tyrosine hydroxylase | TCATCACCTGGTCACCAAGTT |
| GGTCGCCGTGCCTGTACT | |
| Neurofilament | GTGACCAAGCCCGACCTTT |
| ATTCCTCAGCGTTCTGCATGT | |
| Glyceraldehyde 3-phosphate dehydrogenase | TGGTATCGTGGAAGGACTCA |
| GCAGGGATGATGTTCTGGA |
Statistical analysis
All data are shown as the means ± standard errors. The RT-PCR data were normalized to the mRNA level of GAPDH. The statistical analysis of the results were performed using one-way ANOVA followed by the multiple-comparison Tukey’s test using GraphPad Prism 5 software. Statistical significance was determined at p < 0.05.
Results
Isolation and characterization of hTGSCs
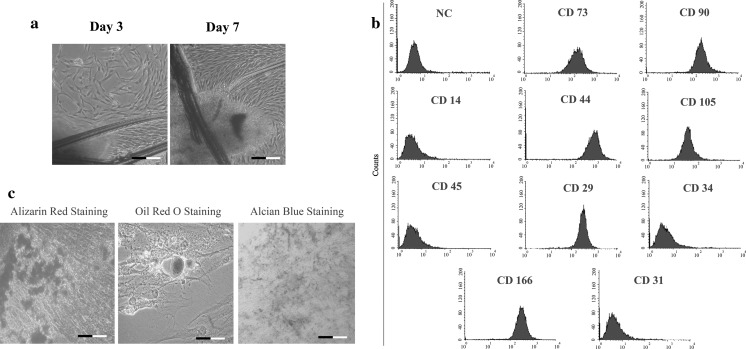
Cells displaying fibroblast-like cell morphology started to spread from human tooth germ tissues at day 3 (Fig. 1a). For the characterization of hTGSCs, they were subjected to flow cytometry analysis to show the presence of mesenchymal stem cell surface marker existence. The results revealed that while hTGSCs were stained positive for MSC cell surface markers; CD29, CD44, CD90, CD73, and CD166, they were negatively stained for hematopoietic cell markers; CD34, CD45, CD31, and CD14 (Fig. 1b). To further characterize hTGSCs, freshly isolated cells at passage 3 were differentiated into osteo-, chondro- and adipo-genic cell lineages in vitro (Fig. 1c). Alizarin red, oil red and alcian blue stainings showed successful differentiation of hTGSCs towards osteo-, adipo- and chondro-genic cell lineages, respectively.
Fig. 1.
a Isolated hTGSCs at day 3 and day 7. Cells started to spread at day 3 and became confluent at day 7. b Flow cytometry analysis of hTGSCs. Cells were positive for CD29, CD44, CD90, CD73, and CD166 and negative for CD34, CD45, CD31, and CD14. c Osteo-, adipo- and chondrogenic cell differentiation of isolated hTGSCs. Alizarin red, oil red and alcian blue staining showed that hTGSCs were differentiated to mesenchymal originated cell types. Scale bar 100 μm. NC negative control, cells not treated with antibodies in flow cytometry analysis
Cell viability
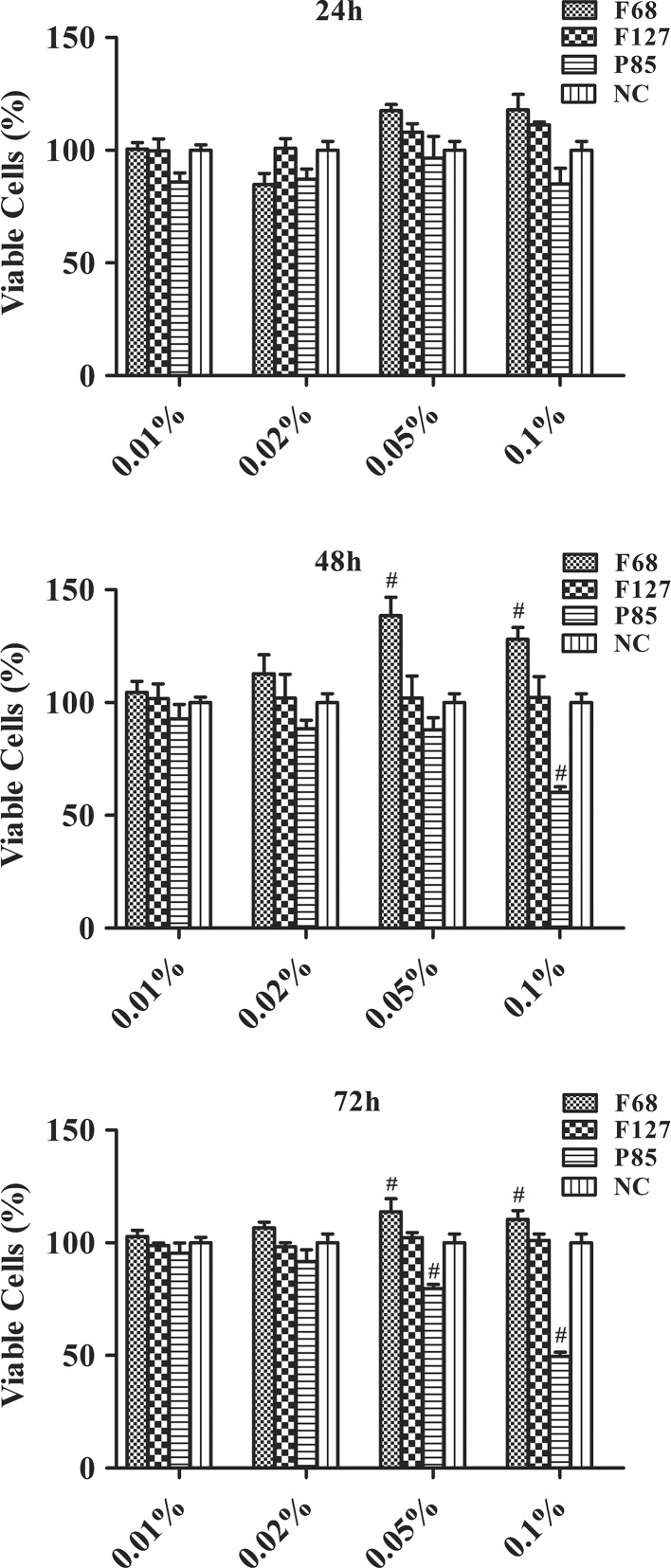
hTGSCs treated with pluronics for 24, 48 and 72 h were subjected to cell viability analysis using MTS assay. The results revealed that F68 significantly increased the cell viability at 0.05 and 0.1 % concentrations. Meanwhile, F127 did not exert subtle differences on viability of hTGSCs. P85 was shown to reduce cell viability at the same concentrations (Fig. 2).
Fig. 2.
Cell proliferation analysis. F68 increased the cell proliferation of hTGSCs, whereas P85 showed cytotoxic activity at 0.05 and 0.1 % concentrations at the end of three day incubation period. F127 did not alter cell proliferation at 24, 48 and 72 h. NC negative control (Growth medium treated group). Average values for 20 % DMSO (as lethal dose) and negative control were accepted as 0 and 100 %, respectively. # p < 0.05 versus NC
Myogenic differentiation
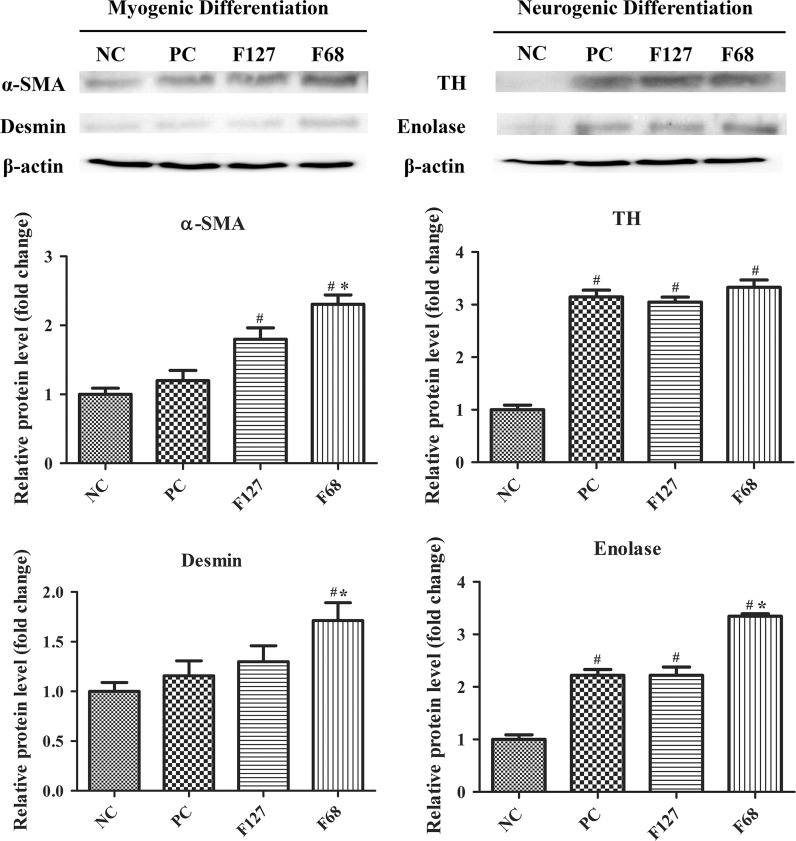
The myogenic gene expression levels were analyzed by RT-PCR to confirm the myogenic transformation. Results indicated that all myogenic markers (α-SMA, desmin and myogenin) were highly expressed in all groups except in the cells treated only by growth medium (NC, Negative control) (Fig. 3a). F68 treated cells showed significantly higher gene expression levels compared to differentiation medium treated cells (PC, Positive control), but F127 had no significant effect on myogenic gene expression. Actin, α-SMA, Desmin and MyoD were immunostained for the confirmation of myogenic differentiation of hTGSCs. Immunocytochemistry results indicate that all F68 and F127 treated groups were positively stained for Actin, α-SMA, Desmin and MyoD, demonstrating the myogenic differentiation of the cells. However, the negative control cells showed low expression levels of these marker genes (Fig. 3). Western blot analysis was conducted to confirm the effect of pluronics on myogenic protein expression in hTGSCs. In consistence with the RT-PCR assays, while F127 treatment only enhanced α-SMA protein levels, F68 treatment was found to enhance protein levels of α-SMA and Desmin compared to the other groups (Fig. 4).
Fig. 3.
Effect of pluronics on myogenic differentiation. a Myogenin, Desmin and α-Smooth Muscle Actin (α-SMA) gene expression levels. F68 increased myogenic mRNA expression levels compared to the positive and negative control groups. b Immunostaining of Actin, α-SMA, Desmin and Myogenin. Myogenic differentiation was induced in all experimental groups compared to the negative control. Scale bar 100 μm. PC positive control (myogenic differentiation medium treated group), NC negative control (growth medium treated group). *p < 0.05 versus PC, # p < 0.05 versus NC
Fig. 4.
Effect of pluronic treatment on lineage specific protein expression. F68 treatment increased both neurogenic and myogenic protein expression compared to the negative and positive control groups. TH tyrosine hydroxylase, PC positive control (differentiation medium treated groups), NC negative control (growth medium treated group). *p < 0.05 versus PC, # p < 0.05 versus NC
Neurogenic differentiation
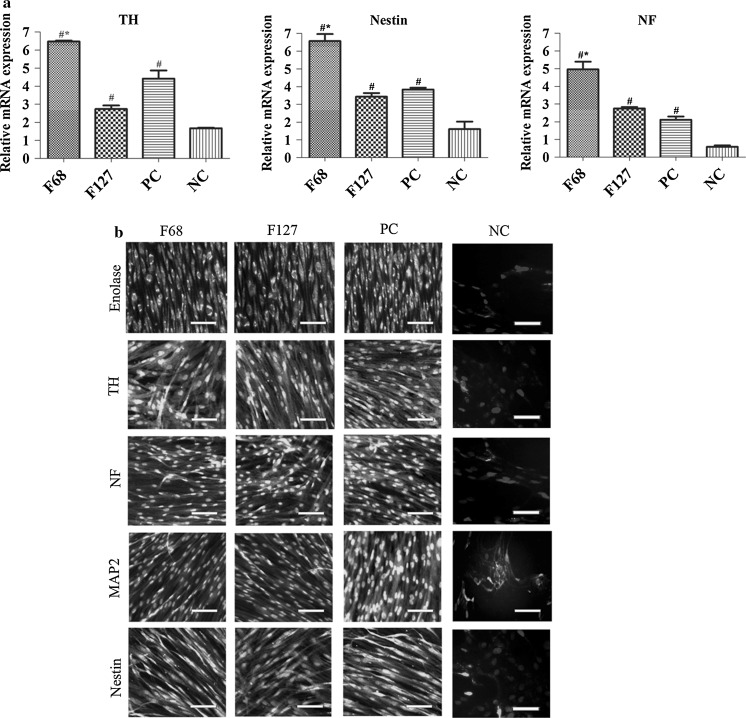
The RT-PCR results showed that F68 treated groups expressed the highest mRNA expression levels of TH, Nestin and NF compared to the positive and negative controls (Fig. 5). However F127 did not change gene expression of myogenic markers. Protein expression of Enolase, Nestin, MAP2, NF and TH showed that F68 and F127 treated groups were positively stained. Western blot assay results revealed that while pluronic treatment did not change TH protein expression levels compared to PC group, Enolase expression was found to be higher in the F68 treated cells (Fig. 4).
Fig. 5.
Effect of pluronics on neurogenic differentiation. a TH, Nestin and NF gene expressions of F68, F127 and control groups. F68 increased neurogenic gene expression levels compared to the positive and negative controls. b Immunostaining of Enolase, Nestin, MAP2, NF and TH. Neurogenic protein expression was observed in the experimental groups compared to the negative control. Scale bar 100 μm. TH tyrosine hydroxylase, NF neurofilament, MAP2 microtubule associated protein 2. PC positive control (myogenic differentiation medium treated group), NC negative control (growth medium treated group). *p < 0.05 versus PC, # p < 0.05 versus NC
Discussion
The importance of cell replacement therapy in regeneration treatments varies depending on the injured tissue. For instance, skin has the capacity to regenerate itself except for large defects such as deep burn wounds, while organs composed of terminally differentiated cells (mainly) such as myocytes and neurons are not able to repair tissues after serious myocardial infarction or neuropathological conditions, respectively. Therefore, cell therapy has recently emerged as a future solution to tissue reconstruction applications for organs with limited regeneration capacity. High proliferative, self-renewal and differentiation capacities of MSCs make them attractive for these applications. Although BMSCs are the most elucidated and primary MSCs, the difficulty of isolation procedure, reduced lifespan and differentiation capacity of BMSCs with increasing donor age have created great demand for alternative cell types such as adipose derived stem cells (ADSCs), umbilical cord blood stem cells (UCBSCs) and hTGSCs derived from third molar tooth (Kern et al. 2006). Morphological properties of MSCs derived from different tissues have been reported to be the same while their differentiation potentials to various cell lineages diverge (Wagner et al. 2005). As stem cell origin directly determines the transformation capacity into targeted cell lineage, selection of stem cells source for a specific regeneration case is vital for the functionality and effectivity of the treatment. For instance, dental pulp stem cells (DPSCs) have been proven to display better neural and epithelial differentiation potential in comparison with BMCSs (Karaöz et al. 2011), indicating higher potential of DPSCs in neuroregeneration. Apart from cell source, using differentiation regulators is also important to control differentiation procedure and increase the treatment efficiency. In the present investigation, we have evaluated whether pluronic block copolymers (F68, F127 and P85) could regulate myogenic and neurogenic differentiation of hTGSCs. According to the cell viability results, as P85 was found to be toxic to hTGSCs at defined concentrations it was not evaluated for further differentiation experiments. Similarly, we have previously reported that P85 exerted toxicity, F127 was proven to be safe and F68 was shown to increase multipotency for hTGSCs (Doğan et al. 2012; Tasli et al. 2013c). High toxicity of P85 to stem cells in comparison with F68 and F127 could be explained by inhibition of Pgp drug efflux system which results in ATP depletion and decrease in Pgp ATPase activity (Batrakova et al. 2003).
In differentiation studies, while F68 was found to increase myocytes- and neuron-like cell population derived from hTGSCs, F127 did not significantly affect cell commitment towards neurogenic and myogenic phenotypes. In consistence with the current results, we have previously shown that although F68 has increased osteo-, adipo- and chondrogenic differentiation of hTGSCs, F127 has no or little effect, indicating cell-lineage free effect of F68 on hTGSC differentiation (Doğan et al. 2012). Pluronic F68 has been shown to increase cell membrane integrity, growth and attachment of fibroblast cells, stating the potential protective effect on plasma membrane (Gigout et al. 2008). Although the exact mechanisms in which F68 increases neuro- and myogenic differentiation of human stem cells need to be explored, one possible explanation for the effects of pluronics might be the stabilization of the cell membrane during differentiation resulting in effective lineage transformation as cell membrane tension and surface area might direct cell fate and differentiation (McBeath et al. 2004; Titushkin and Cho 2006).
F127 has been used for several medical approaches including drug and gene delivery (Kedar et al. 2010), scaffold fabrication for tissue engineering practices (Lee et al. 2011), targeted therapy and diagnosis (Lin et al. 2009). Despite the multiple advantages of F127 in biomedical applications, it did not enhance in vitro myocyte and neuronal differentiation of hTGSCs in the current study.
The findings of the current study disclose promising perspectives for the tissue engineering applications of pluronic block copolymers. Given their differentiation guidance properties, pluronics would be attractive polymers to be used in muscle and neural regeneration studies. However, as the major limitation of the present study, functionality of the neuron- and myocytes-like cells derived from hTGSCs in the presence of pluronics should be conducted to assess their clinical usage. Additional efforts to investigate exact molecular mechanism of cell differentiation regulation properties of pluronics are underway. Further studies with other stem cells such as BMSCs and ADSCs are highly warranted to explore its potential use in cell therapy as a differentiation regulator and its relevance in clinical practice.
Acknowledgments
Authors would like to thanks Ayla Burçin Asutay for her assistance during flow cytometry analysis.
Footnotes
P. Neslihan Taşlı and Ayşegül Doğan have contributed equally to this work.
Contributor Information
Ayşegül Doğan, Email: aguldgn@gmail.com.
Fikrettin Şahin, Phone: +90 (216) 578 0619, Email: fsahin@yeditepe.edu.tr.
References
- Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by pluronic P85. Pharm Res. 2003;20:1581–1590. doi: 10.1023/A:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batrakova EV, Li S, Brynskikh AM, Sharma AK, Li Y, Boska M, Gong N, Mosley RL, Alakhov VY, Gendelman HE. Effects of pluronic and doxorubicin on drug uptake, cellular metabolism, apoptosis and tumor inhibition in animal models of MDR cancers. J Control Release. 2010;143:290–301. doi: 10.1016/j.jconrel.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clincke MF, Guedon E, Yen FT, Ogier V, Roitel O, Goergen JL. Effect of surfactant pluronic F-68 on CHO cell growth, metabolism, production, and glycosylation of human recombinant IFN-γ in mild operating conditions. Biotechnol Prog. 2011;27:181–190. doi: 10.1002/btpr.503. [DOI] [PubMed] [Google Scholar]
- da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- Doğan A, Yalvac ME, Sahin F, Kabanov AV, Palotas A, Rizvanov AA. Differentiation of human stem cells is promoted by amphiphilic pluronic block copolymers. Int J Nanomed. 2012;7:4849–4860. doi: 10.2147/IJN.S31949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doğan A, Yalvaç ME, Yılmaz A, Rizvanov A, Şahin F. Effect of F68 on cryopreservation of mesenchymal stem cells derived from human tooth germ. Appl Biochem Biotechnol. 2013;171:1819–1831. doi: 10.1007/s12010-013-0472-z. [DOI] [PubMed] [Google Scholar]
- Erukova VY, Krylova OO, Antonenko YN, Melik-Nubarov NS. Effect of ethylene oxide and propylene oxide block copolymers on the permeability of bilayer lipid membranes to small solutes including doxorubicin. Biochim Biophys Acta. 2000;1468:73–86. doi: 10.1016/S0005-2736(00)00244-3. [DOI] [PubMed] [Google Scholar]
- Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22:617–624. doi: 10.1634/stemcells.22-4-617. [DOI] [PubMed] [Google Scholar]
- Gigout A, Buschmann MD, Jolicoeur M. The fate of Pluronic F-68 in chondrocytes and CHO cells. Biotechnol Bioeng. 2008;100:975–987. doi: 10.1002/bit.21840. [DOI] [PubMed] [Google Scholar]
- Huang J, Chen W, Liao S, Yang C, Lin S, Wu C. Osteoblastic differentiation of rabbit mesenchymal stem cells loaded in A carrier system of Pluronic F127 and Interpore. Chang Gung Med J. 2006;29:363–370. [PubMed] [Google Scholar]
- Ikeda E, Yagi K, Kojima M, Yagyuu T, Ohshima A, Sobajima S, Tadokoro M, Katsube Y, Isoda K, Kondoh M. Multipotent cells from the human third molar: feasibility of cell-based therapy for liver disease. Differentiation. 2008;76:495–505. doi: 10.1111/j.1432-0436.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- Jung HH, Park K, Han DK. Preparation of TGF-β1-conjugated biodegradable pluronic F127 hydrogel and its application with adipose-derived stem cells. J Control Release. 2010;147:84–91. doi: 10.1016/j.jconrel.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Kabanov AV, Batrakova EV, Alakhov VY. Pluronic® block copolymers as novel polymer therapeutics for drug and gene delivery. J Control Release. 2002;82:189–212. doi: 10.1016/S0168-3659(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Karaöz E, Demircan PC, Sağlam Ö, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol. 2011;136:455–473. doi: 10.1007/s00418-011-0858-3. [DOI] [PubMed] [Google Scholar]
- Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6:714–729. doi: 10.1016/j.nano.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Kerkis I, Kerkis A, Dozortsev D, Stukart-Parsons GC, Pereira LV, Caplan AI, Cerruti HF. Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs. 2007;184:105–116. doi: 10.1159/000099617. [DOI] [PubMed] [Google Scholar]
- Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Kim J-H, Oh S-H, Kim S-J, Hah Y-S, Park B-W, Kim DR, Rho G-J, Maeng G-H, Jeon R-H. Tissue-engineered bone formation using periosteal-derived cells and polydioxanone/pluronic F127 scaffold with pre-seeded adipose tissue-derived CD146 positive endothelial-like cells. Biomaterials. 2011;32:5033–5045. doi: 10.1016/j.biomaterials.2011.03.081. [DOI] [PubMed] [Google Scholar]
- Lin J-J, Chen J-S, Huang S-J, Ko J-H, Wang Y-M, Chen T-L, Wang L-F. Folic acid–Pluronic F127 magnetic nanoparticle clusters for combined targeting, diagnosis, and therapy applications. Biomaterials. 2009;30:5114–5124. doi: 10.1016/j.biomaterials.2009.06.004. [DOI] [PubMed] [Google Scholar]
- McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/S1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- Mina EW, Lasagna-Reeves C, Glabe CG, Kayed R. Poloxamer 188 copolymer membrane sealant rescues toxicity of amyloid oligomers In Vitro. J Mol Biol. 2009;391:577–585. doi: 10.1016/j.jmb.2009.06.024. [DOI] [PubMed] [Google Scholar]
- Park KM, Lee SY, Joung YK, Na JS, Lee MC, Park KD. Thermosensitive chitosan–Pluronic hydrogel as an injectable cell delivery carrier for cartilage regeneration. Acta Biomater. 2009;5:1956–1965. doi: 10.1016/j.actbio.2009.01.040. [DOI] [PubMed] [Google Scholar]
- Soleimani M, Nadri S, Shabani I. Neurogenic differentiation of human conjunctiva mesenchymal stem cells on a nanofibrous scaffold. Int J Dev Biol. 2010;54:1295–1300. doi: 10.1387/ijdb.092999ms. [DOI] [PubMed] [Google Scholar]
- Tasli PN, Dogan A, Demirci S, Sahin F. Boron enhances odontogenic and osteogenic differentiation of human tooth germ stem cells (hTGSCs) in vitro. Biol Trace Elem Res. 2013;153:419–427. doi: 10.1007/s12011-013-9657-0. [DOI] [PubMed] [Google Scholar]
- Tasli PN, Tapsin S, Demirel S, Yalvac ME, Akyuz S, Yarat A, Sahin F. Isolation and characterization of dental pulp stem cells from a patient with Papillon–Lefevre syndrome. J Endod. 2013;39:31–38. doi: 10.1016/j.joen.2012.09.024. [DOI] [PubMed] [Google Scholar]
- Tasli PN, Yalvac ME, Sofiev N, Sahin F. Effect of F68, F127, and P85 pluronic block copolymers on odontogenic differentiation of human tooth germ stem cells. J Endod. 2013;39:1265–1271. doi: 10.1016/j.joen.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Tatard VM, D’Ippolito G, Diabira S, Valeyev A, Hackman J, McCarthy M, Bouckenooghe T, Menei P, Montero-Menei CN, Schiller PC. Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone. 2007;40:360–373. doi: 10.1016/j.bone.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Titushkin I, Cho M. Distinct membrane mechanical properties of human mesenchymal stem cells determined using laser optical tweezers. Biophys J. 2006;90:2582–2591. doi: 10.1529/biophysj.105.073775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashi AV, Keramidaris E, Abberton KM, Morrison WA, Wilson JL, O’Connor AJ, Cooper-White JJ, Thompson EW. Adipose differentiation of bone marrow-derived mesenchymal stem cells using Pluronic F-127 hydrogel in vitro. Biomaterials. 2008;29:573–579. doi: 10.1016/j.biomaterials.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402–1416. doi: 10.1016/j.exphem.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Watt FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol. 2013;14:467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- Wu H, Deng Y, Yan Y, Quan D, Si M. Adipose differentiation and adipose tissue engineering of bone marrow-derived mesenchymal stem cells using pluronic F-127 hydrogel in vitro. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2011;28:1148–1153. [PubMed] [Google Scholar]
- Xu J, Liu X, Chen J, Zacharek A, Cui X, Savant-Bhonsale S, Liu Z, Chopp M. Simvastatin enhances bone marrow stromal cell differentiation into endothelial cells via notch signaling pathway. Am J Physiol Cell Physiol. 2009;296:C535–C543. doi: 10.1152/ajpcell.00310.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalvac ME, Rizvanov AA, Kilic E, Sahin F, Mukhamedyarov MA, Islamov RR, Palotas A. Potential role of dental stem cells in the cellular therapy of cerebral ischemia. Curr Pharm Des. 2009;15:3908–3916. doi: 10.2174/138161209789649439. [DOI] [PubMed] [Google Scholar]
- Zhou AJ-J, Peel SA, Clokie CM. An evaluation of hydroxyapatite and biphasic calcium phosphate in combination with Pluronic F127 and BMP on bone repair. J Craniofac Surg. 2007;18:1264–1275. doi: 10.1097/scs.0b013e318158cb1a. [DOI] [PubMed] [Google Scholar]
- Zurita M, Otero L, Aguayo C, Bonilla C, Ferreira E, Parajon A, Vaquero J. Cell therapy for spinal cord repair: optimization of biologic scaffolds for survival and neural differentiation of human bone marrow stromal cells. Cytotherapy. 2010;12:522–537. doi: 10.3109/14653241003615164. [DOI] [PubMed] [Google Scholar]