Abstract
Due to their high chemical stability, lithium titanate (Li2TiO3) nanoparticles (LTT NPs) now are projected to be transferred into different nanotechnology areas like nano pharmacology and nano medicine. With the increased applications of LTT NPs for numerous purposes, the concerns about their potential human toxicity effects and their environmental impact are also increased. However, toxicity data for LTT NPs related to human health are very limited. Therefore we aimed to investigate toxicity potentials of various concentrations (0–1,000 ppm) of LTT NPs (<100 nm) in cultured primary rat hepatocytes. Cell viability was detected by [3-(4,5-dimethyl-thiazol-2-yl) 2,5-diphenyltetrazolium bromide] (MTT) assay and lactate dehydrogenase (LDH) release, while total antioxidant capacity (TAC) and total oxidative stress (TOS) levels were determined to evaluate the oxidative injury. DNA damage was analyzed by scoring liver micronuclei rates and by determining 8-oxo-2-deoxyguanosine (8-OH-dG) levels. The results of MTT and LDH assays showed that higher concentrations of dispersed LTT NPs (500 and 1,000 ppm) decreased cell viability. Also, LTT NPs increased TOS (300, 500 and 1,000 ppm) levels and decreased TAC (300, 500 and 1,000 ppm) levels in cultured hepatocytes. The results of genotoxicity tests revealed that LTT NPs did not cause significant increases of micronucleated hepatocytes and 8-OH-dG as compared to control culture. In conclusion, the obtained results showed for the first time that LTT NPs had dose dependent effects on oxidative damage and cytotoxicity but not genotoxicity in cultured primary rat hepatocytes for the first time.
Keywords: Lithium titanate nanoparticles, Genotoxicity, Cytotoxicity, Rat Hepatocytes, Oxidative stress
Introduction
Nanotechnology has emerged to be one of the most powerful technology creating approaches in the past half a century (Zhang et al. 2011). Today, nanotechnology has spawned the development of a veritable plethora of novel nanoparticles for diverse applications ranging from solar energy capture to cosmetics and drug delivery (Riehemann et al. 2009; Lin et al. 2010; Cai and Xu 2011). With frequent exposure to dispersed nanoparticles from composite products or workplaces, there is an increased chance for nanoparticles or nano composites to enter human body and to relocate in metabolism-active organs (Song et al. 2009). Thus, studying the toxicity of nanomaterials is of importance to provide guidance to occupational health and safety (Li et al. 2008; Lanone et al. 2009; Turkez et al. 2013a). The discussion about safety concerns associated with small particles is ongoing for many decades and is to a large extent related to potential risks following inhalation, oral, parenteral or dermal exposure. (Schmid et al. 2009). Especially ultrafine or nanoparticles are in the focus of the debate, e.g. ECETOC (2005), usually meant to have one or more size dimensions between 0.1 and 100 nm.
In the last twenty years, many efforts were made to investigate the toxicity of micro sized natural and man-made mutagens in human life and the ability of therapeutic substances on reducing the toxicity of various toxicants (Turkez et al. 2005; Geyikoglu and Turkez 2005; Turkez et al. 2005; Turkez and Sisman 2007; Turkez et al. 2007; Sisman and Turkez 2010). But the toxic effects of nano-sized particles were not fully detailed except for some titanium, tungsten, cadmium and zinc oxide nanoparticles (Zhang et al. 2013). However, the recent report indicated that there was a lack of systematic assessment of the DNA damaging and carcinogenic potential of NPs in spite of their extensive use in nanotechnological applications (Turkez et al. 2013b).
Lithium titanate (Li2TiO3) nanoparticles (LTT NPs) are a powder at room temperature and are one of the most attractive tritium breeders for breeding blanket in fusion reactor from viewpoints of low tritium inventory, high chemical stability and so on (Tsuchiya et al. 2001). LTT NPs have excellent properties such as best chemical stability in air, most perfect tritium release characteristics at low temperature, low activation and reasonable lithium atom density. They are also used as an additive in porcelain enamels and ceramic insulating bodies (Xiangwei et al. 2008).
In the last years, titanate containing nanomaterials have attracted a dramatic and exponentially increasing interest; in particular for their potential applications in the biomedical field (Ciofani et al. 2010; Davis et al. 2010; Wataha et al. 2010). Thus, possible health impact of LTT NPs upon introduction into the body is of great interest. With the increased applications of LTT NPs, the concerns about their potential human toxicity effects and their environmental impact were also increased. Cytotoxicity, inflammation and increased oxidative stress through reactive oxygen species (ROS) formation are prominently discussed to be relevant factors regarding the safety of small particles down to the nano-range (Knaapen et al. 2004; Unfried et al. 2007; Lewinski et al. 2008; Turkez 2008, 2011). It has been reported that different sizes and morphologies of nanoparticles have the potential to influence the interaction with many kinds of biomolecules including proteins, enzymes and DNA (Grandjean-Laquerriere et al. 2005; Ramesh et al. 2007; Xu et al. 2012). Moreover, the liver was considered a target site for NP toxicity, due to NP accumulation within it after ingestion, inhalation or absorption (Wang et al. 2011). However, recorded hepatotoxicity data for LTT NPs relating to human health are very scarce.
Since this is considered to be of particular importance for the investigation of mechanisms of ROS formation and oxidative stress, in the present study, specific measurements were performed in cultured rat primary hepatocytes as in vitro model system for assessing the impact of LTT NPs on human and environmental health. Different aqueous LTT NPs concentrations (0, 5, 10, 20, 50, 75, 100, 150, 300, 500 and 1,000 ppm) were investigated in vitro. All samples were evaluated for their ability to cause genotoxicity, cytotoxicity and ROS generation in cultures. Cytotoxicity was detected by the MTT [3-(4,5-dimethyl-thiazol-2-yl) 2,5-diphenyltetrazolium bromide] assay and lactate dehydrogenase (LDH) release, while total antioxidant capacity (TAC) and total oxidative stress (TOS) levels were determined to evaluate the oxidative injury. Genotoxicity was assessed by scoring LMN (liver micronucleus) formations and by determining 8-OH-dG levels. The results should serve as screening elements for the potential hazard of LTT NPs in a weight-of-evidence approach.
Materials and methods
Synthesis and characterization of LTT NPs (~50 nm, <100 nm)
In the literature: for the preparation of lithium based oxide ceramic powders, several techniques such as solid-state reaction, solution combustion process, hydrothermal and sol–gel process have been available. Among these techniques, the sol–gel technique is attractive due to its easy manipulation of the samples, simplicity, safety, low cost, easy control of chemical components. The sol–gel method involves the dispersion of metallic salts in solutions. The sol is later solidified through stages of stiffening and polymerization to give a gel (gelation). The gel so obtained is thoroughly washed with distilled water or alcohol, filtered, dried and finally heated to high temperatures to obtain the required material. Therefore, in the present study, we synthesized Li2TiO3 powders via sol–gel route (Poulter and Pryde 1968; Han et al. 1995; Fehr and Schmidbauer 2007; Ilıcan et al. 2008; Kataoka et al. 2009; Ghodsi and Absalan 2010; Lu et al. 2012).
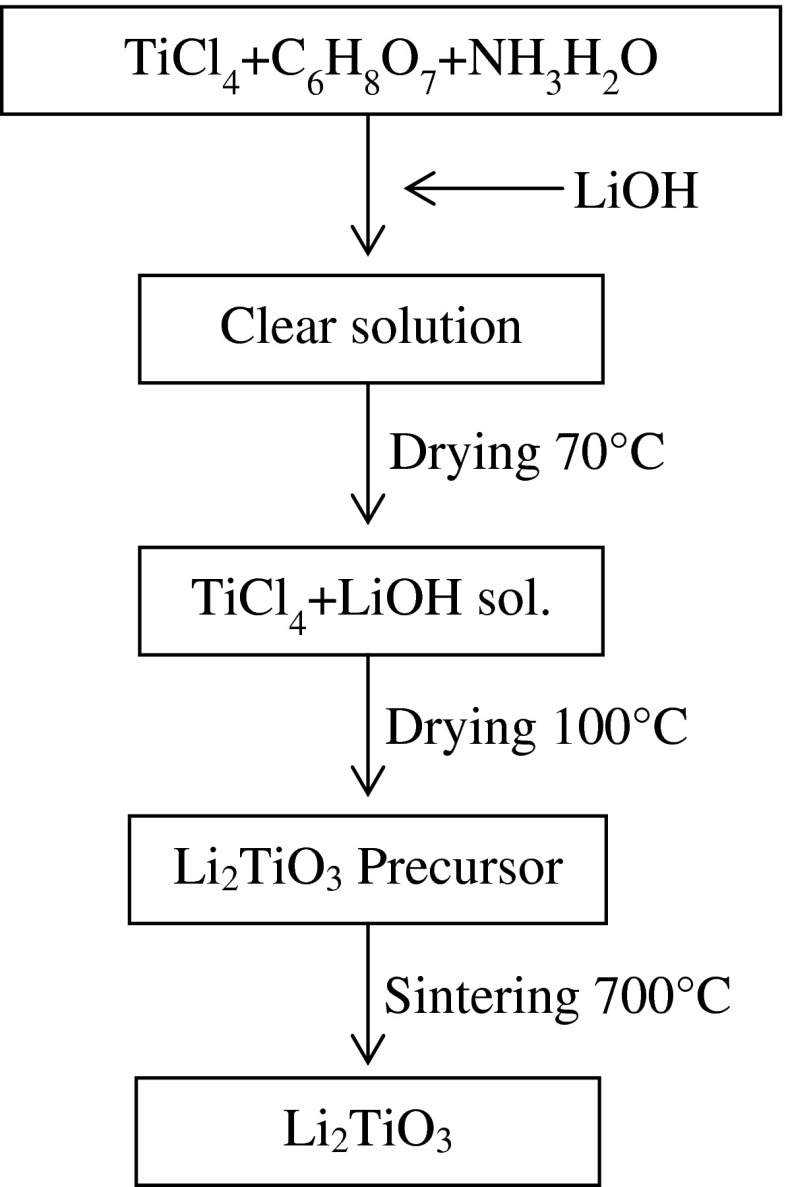
To synthesize Li2TiO3 powders high purity TiCl4 (Sigma, St. Louis, MO, USA), HCl (Merck, Darmstadt, Germany), LiOH (Sigma), C6H8O7 (Sigma) and NH3H2O (Merck) are used as starting materials. Because of TiCl4 is very evaporative, TiCl4-HCl solutions were prepared with dissolving TiCl4 in 3 mol/L HCl. The molar ratio of LiOH: TiCl4: C6H8O7 was adjusted to 4:2:1. NH3H2O was used to set the pH of the solution to 7. The obtained solutions were stirred continuously and heated at 70 °C until formation of the gel. Then the gel was dried at 150 °C. At the end of this procedure the powders were calcined at 700 °C for 5h for obtaining white color Li2TiO3 powders. The experimental procedure is shown in Fig. 1.
Fig. 1.
Schematic diagram for synthesis procedure of Li2TiO3 by sol–gel technique (Jian-Wen et al. 2004)
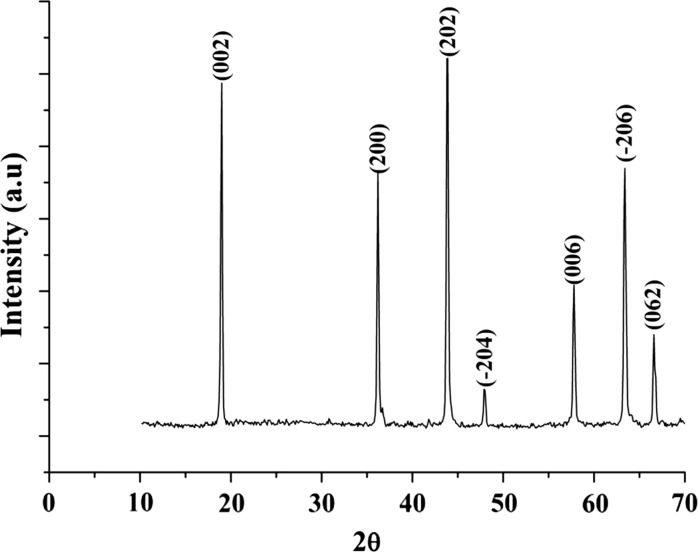
Crystal structure for the synthesized sample was carry out by X-ray diffraction technique (Fig. 2). According to this analysis, synthesized samples have a monoclinic crystal structure with space group C2/c (JPCDS Card No: 33-0831). The lattice parameter of the unit cell was calculated: a = 4.77 Å, b = 8.77 Å, c = 9.81 Å and β = 80,386°. Our results are in harmony with another study (Hoshino et al. 2007). The average particle size of the sample was calculated 51.2 nm using the Scherrer formula: d = Kλ × (β cosθ)−1 where d is the mean crystallite size, K is the grain shape dependent constant 0.89, λ is the wavelength of the incident beam in nm, θ is the Bragg reflection angle, and β is the line broadening at half the maximum intensity in radians (Farrukh et al. 2012).
Fig. 2.
XRD pattern of Li2TiO3 sample sintered at 700 °C
Animals
Male rats of Sprague–Dawley strain (from Medical Experimental Research Center, Atatürk University, Erzurum, Turkey), of 200–300 g body weight, were used throughout the present studies. They were allowed water and standard laboratory chow ad libitum and were maintained under standard light, temperature, and relative humidity conditions. The study protocol was approved by the local ethical committee. All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals.
Hepatocyte isolation
Rats were sacrificed by CO2 overdose, and the livers were removed immediately. Isolated hepatocytes from rats were prepared by the collagenase perfusion technique. The liver was perfused through the hepatic portal vein with calcium-free Hanks balanced salt solution (Sigma, St Louis, MO, USA) to remove blood for about 10 min at a flow rate of 2.5 mL/min. As soon as the liver became grayish brown in color, a second buffer solution containing collagenase (Hank’s balanced salt supplemented with 4 mM calcium chloride (Sigma) and 0.5 mg collagenase/mL (Sigma, St Louis, MO, USA) was perfused at the same rate until the liver appeared to have broken up. After treatment the liver was minced into 3- to 4-mm pieces with a sterile scalpel. Following mechanical dissociation, the cells were filtered through a gauze and centrifuged at 1,350 rpm for 5 min. Then, the hepatocytes were collected in medium containing bovine serum albumin (Sigma) and bovine insulin (Sigma-Aldrich). The cell suspension was filtered through a gauze again and allowed to sediment for 20 min to eliminate cell debris, blood, and sinusoidal cells. The cells were then washed three times by centrifugation at 50 g, tested by Trypan blue dye (Sigma-Aldrich) exclusion for viability (always in the range of 82–93 %).
Cultivation
The hepatocytes were suspended in a mixture of 75 % Eagle’s minimum essential medium (Sigma-Aldrich) and 25 % medium 199 (Gibco, Grand Island, NY, USA), supplemented with 10 % fetal calf serum containing (Sigma-Aldrich) streptomycin (50 µg), penicillin (5 µg), bovine insulin (5 µg), bovine serum albumin (1 mg) and NaHCO3 (2.2 mg). For the experimental procedure, hepatocytes were plated in multi-well tissue culture plates (3 × 105 cells in a well area of 3.8 cm2; 8 × 105 cells in a well area of 9.6 cm2). The medium was changed 3–4 h later. The effect of LTT NPs was studied after 72 h of exposure in cultures maintained with a medium deprived of fetal calf serum but supplemented with hydrocortisone hemisuccinate (7 × 10−7M). Hepatocytes were cultured for an additional 8 h before treatment.
Treatments
After 8 h of plating, when primary hepatocytes got adhered and attained their epithelial morphology, culture medium was aspirated and replaced with an equal volume of the medium supplemented with different concentrations of aqueous LTT NPs (5, 10, 20, 50, 75, 100, 150, 300, 500 and 1,000 ppm) followed by incubation in CO2 incubator for 72 h (n = 6). Mitomycin C (MMC; C15H18N4O5; Sigma, at 10−7M) applied group was considered as positive control (Control+).
MTT assay
Viability of cells was assessed by measuring the formazan formation from 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) spectrophotometrically test. MTT solution was prepared at 1 mg/mL in PBS and was filtered through a 0.2 µm filter. Then, 50 µL of MTT plus 200 µL of DMEM without phenol red were added into the wells. Cells were incubated for 4 h at 37 °C with 5 % CO2, 95 % air and complete humidity. After 4 h, the MTT solution was removed and replaced with 200 µL of DMSO and 25 µL Sørenson’s glycine buffer (glycine 0.1 M, NaCl 0.1 M, pH 10.5 with 0.1 NaOH). The plate was further incubated for 5 min at room temperature, and the optical density (OD) of the wells was determined using a plate reader at a test wavelength of 570 nm and a reference wavelength of 630 nm.
LDH assay
Lactate dehydrogenase (LDH) activity was measured in the culture medium as an index of cytotoxicity, employing an LDH kit (Bayer Diagnostics®, Puteaux, France) adapted to the auto analyzer (ADVIA 1650, Siemens Healthcare Diagnostics, Malvern, PA, USA). Enzyme activity was expressed as the extracellular LDH activity percentage of the total activity in the plates.
Total antioxidant capacity and total oxidant status assays
The automated Trolox equivalent TAC and total oxidant status (TOS) assays were carried out in the culture medium by commercially available kits (Rel Assay Diagnostics®, Gaziantep, Turkey). The major advantage of TAC test is to measure the antioxidant capacity of all antioxidants in a biological sample and not just the antioxidant capacity of a single compound. In this test, antioxidants in the sample reduce dark blue-green colored ABTS radical to colorless reduced ABTS form. The change of absorbance at 660 nm is related with total antioxidant level of the sample. The assay is calibrated with a stable antioxidant standard solution that is traditionally named as Trolox Equivalent that is a vitamin E analog.
Since the measurement of different oxidant molecules separately is not practical and their oxidant effects are additive, the TOS of a sample is measured and this is named total peroxide (TP), serum oxidation activity (SOA), reactive oxygen metabolites (ROM) or some other synonyms. In the TOS assay performed here, oxidants present in the sample oxidize the ferrous ion–chelation complex to ferric ion. The oxidation reaction is prolonged by enhancer molecules, which are abundantly present in the reaction medium. The ferric ion forms a colored complex with a chromogen in an acidic medium. The color intensity, which can be measured spectrophotometrically, is related to the total amount of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide and the results are expressed in terms of micromolar hydrogen peroxide equivalent per liter (μmol H2O2 Equiv./L).
LMN assay
Liver micronucleus assay (LMN) was performed according to the protocol previously described by Suzuki et al. (2009). Immediately prior to evaluation, 10–20 μL of hepatocyte suspension (10,000–20,000 cells) was mixed with an equal volume of acridine orange (AO)–DAPI (4′,6-diamidino-2-phenylindole dihydrochloride) stain solution (AO, 0.5 mg/mL; DAPI, 10 μg/mL) for fluorescent staining. Approximately 10–20 μL of the mixture was dropped onto a glass slide and covered with a cover glass. Samples of well-isolated hepatocytes were evaluated with the aid of a fluorescence microscope counting the number of MNHEPs in 2000 hepatocytes for each animal. MNHEPs were defined as hepatocytes with round or distinct MNs that stained like the nucleus, with a diameter 1/4 or less than that of the nucleus, and were confirmed by focusing up and down, taking into account hepatocyte thickness by one observer.
Nucleic acid oxidation
8-hydroxy-2′-deoxyguanosine assay kits were purchased from Cayman Chemical (Ann Arbor, MI, USA) for determining 8-OH-dG levels in cultures. Since it is a competitive assay that can be used for the quantification of 8-OHdG in homogenates and recognizes both free 8-OHdG and DNA-incorporated 8-OH-dG, many researches are performed using this protocol. This assay depends on the competition between 8-OHdG and 8-OHdG-acetylcholinesterase (AChE) conjugate (8-OH-dGTracer) for a limited amount of 8-OHdG monoclonal antibodies. All procedures were carried out in accordance with the provider’s manual.
Statistical analysis
The experimental data were analyzed using one-way analysis of variance (ANOVA) and Fischer’s least significant difference (LSD) tests to determine whether any treatment significantly differed from the controls or each other. Results presented as mean ± SD values and the level of 0.05 was regarded as statistically significant.
Results
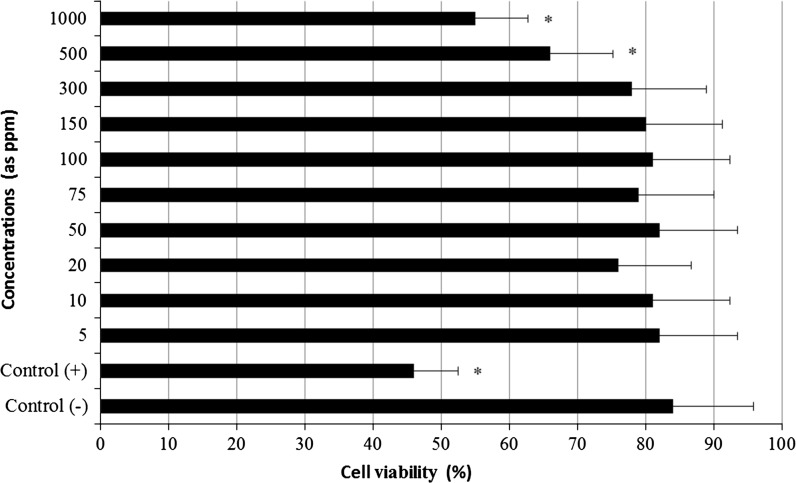
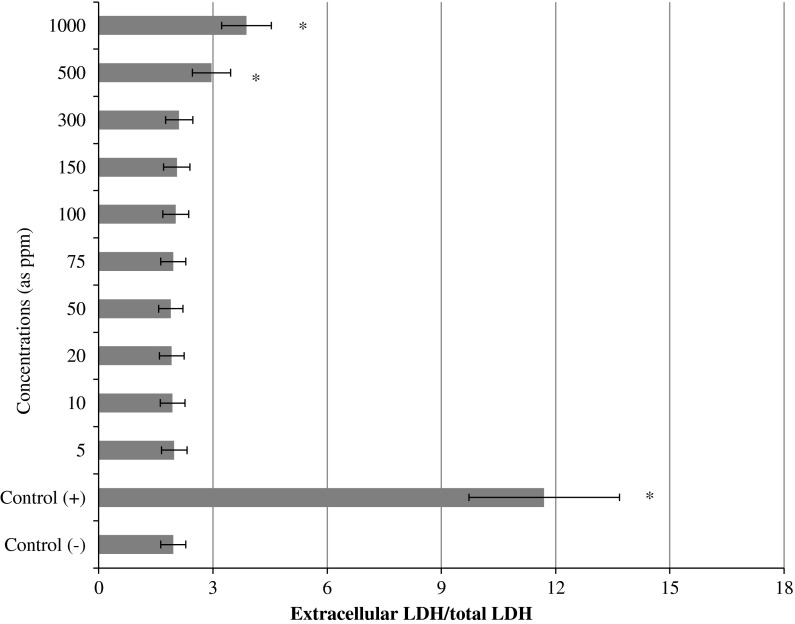
The results of cell viability measured by MTT assay are shown in Fig. 3. When assayed in vitro on the hepatocyte cells using the MTT assay, the value for the Mitomycin C (MMC; 10−7M)-treated cells (as control+) was approximately 2.4 fold lower than that for the control−. Likewise the higher concentrations of LTT NPs (500 and 1,000 ppm) caused significant (p < 0.05) decreases of the cell viability. However, the hepatocyte cells exposed to lower doses than 500 ppm of LTT NPs did not show any significant change in cell viability during 72 h as determined by the MTT assay. In addition, no cytotoxicity was observed for control− cells. MMC-induced hepatic damage was clearly evidenced by six fold increases in the activity of LDH compared with the observations of the negative controls (Fig. 4). LDH was not affected by low doses of LTT NPs alone. But the increases of the levels of enzyme reached statistical significances at 500 and 1,000 ppm.
Fig. 3.
MTT reduction [3-(4,5-dimethyl-thiazol-2-yl) 2,5-diphenyltetrazolium bromide] in cultured rat hepatocytes maintained in the presence of different LTT NPs concentrations for 72 h. Each individual hepatocyte culture without LTT NPs was studied as a negative control group (Control−). MMC alone added group was considered as a positive control (Control+). Values are mean ± standard deviation (n = 6). Asterisk symbol presents significant differences at the p < 0.05 level from the control− group
Fig. 4.
Extracellular level of lactate dehydrogenase (LDH) in cultured rat hepatocytes maintained in the presence of different LTT NPs concentrations for 72 h. Asterisk symbol presents significant differences at the p < 0.05 level from the control− group
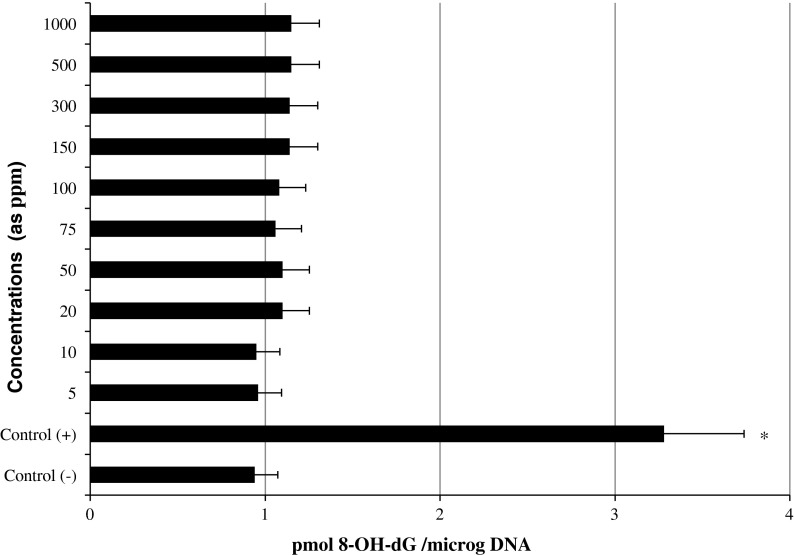
The levels of 8-oxo-2-deoxyguanosine (8-OH-dG) in cultured rat hepatocytes of controls and experimental groups are shown in Fig. 5. Firstly, the levels of 8-OH-dG, a sensitive marker of oxidative DNA damage, were quantified with regard to MMC treatment. It was observed that MMC significantly increased 8-OH-dG concentrations in the hepatocyte cultures after 72 h. On the contrary 8-OH-dG levels did not increase in the hepatocyte cells that were treated with different ppm of LTT NPs.
Fig. 5.
8-oxo-2-deoxyguanosine (8-OH-dG) adducts in cultured rat hepatocytes maintained in the presence of different LTT NPs concentrations for 72 h. Asterisk symbol presents significant differences at the p < 0.05 level from the control− group
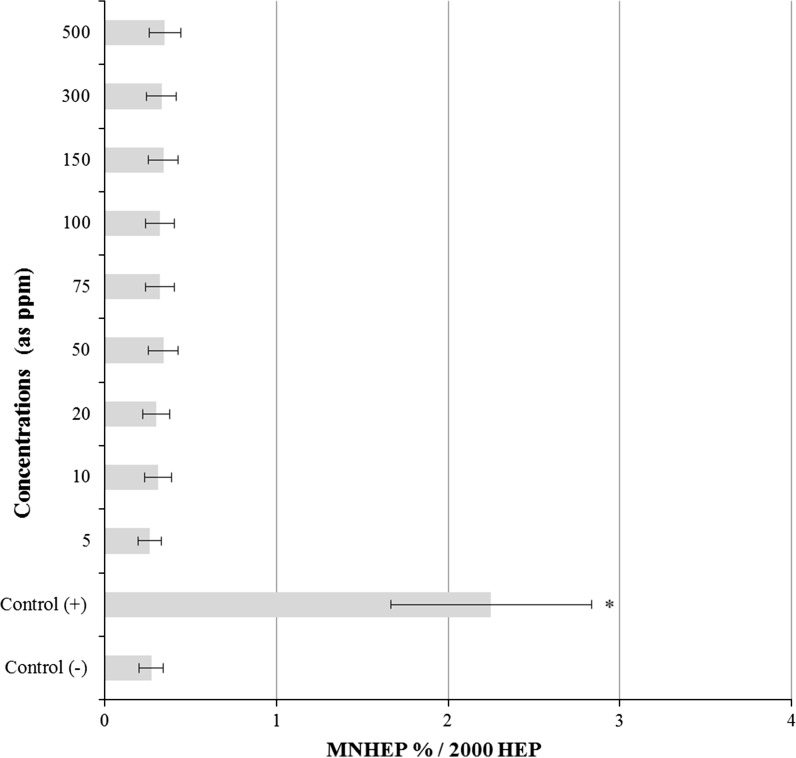
The results of the observed LMN rates in primary rat hepatocyte cells after 72 h LTT NPs treatment are presented in Fig. 6. LMN analyses showed statistically significant differences (p < 0.05) between control− and LTT NPs applied cultures. But the highest concentration of LTT NPs (1,000 ppm) caused sterility of the cultures due to its cytotoxic activity.
Fig. 6.
Results of liver MN assay in cultured rat hepatocytes maintained in the presence of LTT NPs for 72 h. HEP hepatocyte, MNHEPs Number of micronucleated hepatocytes. Abbreviations are as in Fig. 3. Asterisk symbol presents significant differences at the p < 0.05 level from the control− group
Table 1 shows the effects of LTT NPs on oxidant status in cultured rat hepatocytes which were determined by TAC and TOS analysis. As shown in Table 1, the TAC value decreased with the addition of MMC while TOS value increased. In contrast to this, the treatments with 5, 10, 20, 50, 75, 100 and 150 ppm of LTT NPs did not alter the both the TAC and TOS levels. However nanomaterials applications at higher doses (300, 500 and 1,000 ppm) changed the TAC and TOS levels. Thus, LTT NPs had dose dependent effects on oxidative damage in hepatocytes in vitro.
Table 1.
Extracellular TAC and TOS levels in cultured rat hepatocytes maintained with different concentrations of LTT NPs for 72 h. Abbreviations are as in Fig. 3. Asterisk symbol presents significant differences at the p < 0.05 level from the control− group
| Treatment | Parameters | |
|---|---|---|
| TAC (mmol trolox equiv./l) | TOS (µmol H2O2 equiv./l) | |
| Control− | 3.9 ± 0.9 | 9.1 ± 2.9 |
| Control+ | 2.1 ± 0.7* | 16.5 ± 3.4* |
| 5 ppm | 3.6 ± 0.9 | 9.2 ± 4.6 |
| 10 ppm | 4.0 ± 0.7 | 9.4 ± 3.7 |
| 20 ppm | 3.8 ± 0.9 | 9.1 ± 4.0 |
| 50 ppm | 4.1 ± 1.0 | 9.2 ± 4.1 |
| 100 ppm | 3.6 ± 1.0 | 9.5 ± 3.9 |
| 150 ppm | 3.6 ± 0.8 | 9.5 ± 4.3 |
| 300 ppm | 3.0 ± 1.0* | 10.6 ± 4.6* |
| 500 ppm | 2.8 ± 0.9* | 11.7 ± 4.6* |
| 1,000 ppm | 2.7 ± 1.0* | 12.5 ± 4.5* |
Discussion
In the present investigation it was aimed to evaluate the cytotoxicity, genotoxicity and oxidative damage in cultured healthy rat hepatocyte cells in response to different concentrations of LTT NPs. Trends in cytotoxicity as observed for the different concentrations of these NPs were overall highly similar using the two independent assays (MTT and LDH) in cultured rat hepatocytes. A particular contrast was observed for the highest concentrations (500 and 1,000 ppm) of LTT NPs which induced a pronounced LDH release and decrease of MTT. As a matter of fact, it was concluded that TiO2-based nano materials including titanate nanotubes had a strong dose-dependent effect on cell proliferation and cell death (Magrez et al. 2009). Rothen-Rutishauser et al. (2007) reported that the size of nano-particles or their aggregates can be considered a potential determinant for uptake and subsequent macrophage responses that could explain for the observed differences in LDH release at the highest concentration of NPs (Albrecht et al. 2009). Since particles in the fine size range may induce more pronounced responses than in the nano-range (Rothen-Rutishauser et al. 2007), the “ultrafine hypothesis” has been challenged previously by experimental studies (Schins et al. 2004). Albrecht et al. (2009) found that cytotoxicity as observed at high concentrations did not necessarily represent a compound-related effect, and may at least partly be due to physical coverage of the cells by the test NPs.
A complete understanding of how LTT NPs interact with hepatocytes, especially at the molecular level, is still unclear. Our findings demonstrated that direct exposure of hepatocytes to LTT NPs induced the intracellular ROS generation, reduced the TAC, and subsequently caused dysfunction of these cells. In accordance with our findings, it was reported that engineered nanomaterials either metals (like silver), carbon, or metal oxides (like zinc oxide, magnesium oxide, and titanium oxide) induce toxicity and oxidative stress by generating free radicals (Turkez and Geyikoglu 2007; Anreddy et al. 2013). Mechanisms involved in the reduction of cell viability through oxidative stress by LTT NPs are not yet determined. However, NPs have been shown to change intracellular calcium concentrations, activate transcription factors and modulate cytokine production via generation of free radicals. And these radicals were found to be generated from the surface of NP when both the oxidants and free radicals are bound to the particle surface. Again, ROS were reported to be induced endogenously where the mitochondrion is a major cell target for NP-induced oxidative stress. Once NPs enter in the mitochondria, they produce ROS by impairing electron transport chain, structural damage, activation of NADPH-like enzyme system, and depolarization of the mitochondrial membrane (Sioutas et al. 2005; Xia et al. 2006; Manke et al. 2013).
Oxidative stress is associated with protein and lipid oxidation, ultimately leading to a profound alteration in mitochondrial function (Pan et al. 2009). Any change in the mitochondrial membrane permeability is known to be an early event in apoptosis (Liu and Sun 2010). It is known that ROS activates multiple signaling pathways, including mitogen activated protein kinase (MAPK) family and p53 expression signal transduction cascades (Simbula et al. 2007). P53, Bax and Bcl-2 are also generally thought to be involved in mitochondria-dependent apoptosis (Liu and Sun 2010). P-JNK, p–c-Jun, p-p53, Bax, and cleaved caspase-3 were significantly increased in human umbilical vein endothelial cells (HUVECs) after exposure to silica nanoparticles, while Bcl-2 was dramatically suppressed. ROS scavenger could markedly inhibit JNK, c-Jun and p53 activity, indicating that ROS may be upstream effectors of JNK and p53 (Liu and Sun, 2010). Also, Hsin et al. (2008) found that nano silver-induced apoptosis was mediated by ROS via JNK and p53 activation. ROS could also directly activate p53, most probably by the induction of DNA damage (Simbula et al. 2007; Turkez and Togar 2010; Turkez and Geyikoglu 2010; Turkez et al. 2012). Based on the results of Liu and Sun (2010), the following silica nanoparticles-mediated signaling pathways for apoptosis related events were proposed as ROS production (JNK/c-Jun phosphorylation), P53 activation, Bax upregulation and Bcl-2 down regulation.
Studies of Oesterling et al. (2008), Schanen et al. (2009) and Liu and Sun (2010) showed that higher dose exposure to nanomaterials including silica nanoparticles, titanium dioxide, zinc oxide, and alumina nanoparticles led to pro-inflammatory and pro-coagulant responses in endothelial cells. It is an accepted view point that ROS are involved in many of the processes underlying endothelial activation (e.g., the up regulation of adhesion molecules and chemokines, increased expression of tissue factor). Many of the key signal transduction molecules involved in endothelial activation, such as various MAPKs and the transcription factors NF-kB, are known to be redox sensitive (Alom-Ruiz et al. 2008). To assess the biological effect of different concentrations of LTT NPs on rat hepatocyte cells, cell viability was determined. In the present study, MTT and LDH assays showed that the higher concentrations of LTT NPs (500 and 1,000 ppm) decreased cell viability. Also, Liu and Sun (2010) found that exposure to high concentrations (100–200 ppm) of silica nanoparticles caused an increase of cytotoxicity in HUVECs. The LDH release was also increased by silica nanoparticles only at the highest concentration, indicating that exposure to high concentrations of silica nanoparticles can affect cell-membrane integrity and lead to cell death by inducing apoptosis and/or necrosis. Liu and Sun (2010) showed that intracellular ROS generation of HUVECs by silica nanoparticles was gradually increased in a time- and dose-dependent manner, suggesting that oxidative stress occurred not at once, but continuously during cell culture. ROS played a central role in silica nanoparticles-mediated apoptosis. Disturbance of membrane integrity has been recently suggested as possibly one of the mechanisms for cytotoxic action in nanoparticles (Kim et al. 2009).
Up to date many in vitro investigations conducted have revealed interactions between the NPs and the DNA per se but have not considered genotoxicity mechanisms that originate from intercellular processes (Klien and Godniccvar 2012). The genotoxicity of TiO2 NPs has been investigated with a variety of genetic end points in animals and cultured mammalian cells (Nakagawa et al. 1997; Turkez and Geyikoglu 2007; Xu et al. 2009), although it remained controversial (Lu et al. 1998). Our results also indicated that LTT NPs did not induce genotoxic damage. In fact, the results of the study by Bhattacharya et al. (2009) showed that titanium NPs were located in the cytosol near the nucleus but were not found inside the nucleus, in mitochondria or ribosomes. Then it was revealed that these NPs induce oxidative stress but not DNA-breakage in human lung cells. In accordance with this finding, Kang et al. (2008) suggested that nano-TiO2 induced ROS generation in cultured human lymphocytes, thereby activating p53-mediated DNA damage checkpoint signals.
In summary, data from the current study showed that exposure to LTT NPs at high concentrations caused ROS generation and decreased TAC in cultured primary rat hepatocytes. Also, it caused decreased cell viability that was detected by increased MTT and LDH release. But exposure to LTT NPs even at high concentrations did not cause genotoxic damages (by LMN rates and 8-OH-dG level) in hepatocytes. At the highest concentration (1,000 ppm) LTT NPs caused sterility of the cultures due to its cytotoxic activity. Moreover, our overall findings suggested that exposure to LTT NPs could cause cytotoxicity and oxidative damage on rat hepatocyte cells in a dose dependent manner without damaging DNA.
References
- Albrecht C, Scherbart AM, van Berlo D, Braunbarth CM, Schins RPF, Scheel J. Evaluation of cytotoxic effects and oxidative stress with hydroxyapatite dispersions of different physicochemical properties in rat NR8383 cells and primary macrophages. Toxicol In Vitro. 2009;23:520–530. doi: 10.1016/j.tiv.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Alom-Ruiz SP, Anilkumar N, Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal. 2008;10:1089–1100. doi: 10.1089/ars.2007.2007. [DOI] [PubMed] [Google Scholar]
- Anreddy RN, Yellu NR, Devarakonda KR. Oxidative biomarkers to assess the nanoparticle-induced oxidative stress. Methods Mol Biol. 2013;1028:205–219. doi: 10.1007/978-1-62703-475-3_13. [DOI] [PubMed] [Google Scholar]
- Bhattacharya K, Davoren M, Boertz J, Schins RP, Hoffmann E, Dopp E. Titanium dioxide nanoparticles induce oxidative stress and DNA-adduct formation but not DNA-breakage in human lung cells. Part Fibre Toxicol. 2009;6:17. doi: 10.1186/1743-8977-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai XJ, Xu YY. Nanomaterials in controlled drug release. Cytotechnol. 2011;63:319–323. doi: 10.1007/s10616-011-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani G, Danti S, D’Alessandro D, Moscato S, Petrini M, Menciassi A. Barium titanate nanoparticles: highly cytocompatible dispersions in glycol-chitosan and doxorubicin complexes for cancer therapy. Nanoscale Res Lett. 2010;5:1093–1101. doi: 10.1007/s11671-010-9607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RR, Hobbs DT, Kahshaba R, Sehkar P, Seta FN, Messer RL, Lewis JB, Wataha JC. Titanate particles as agents to deliver gold compounds to fibroblasts and monocytes. J Biomed Mater Res A. 2010;93:864–869. doi: 10.1002/jbm.a.32407. [DOI] [PubMed] [Google Scholar]
- ECETOC (2005) Workshop on Testing Strategies to Establish the Safety of Nanomaterials. November 2005, Barcelona, Workshop Report No: 7
- Farrukh MA, Tan P, Adnan R. Influence of reaction parameters on the synthesis of surfactant-assisted tin oxide nanoparticles. Turk J Chem. 2012;36:303–314. [Google Scholar]
- Fehr T, Schmidbauer E. Electrical conductivity of Li2TiO3 ceramics. Solid State Ion. 2007;178:35–41. doi: 10.1016/j.ssi.2006.11.002. [DOI] [Google Scholar]
- Geyikoglu F, Turkez H. Protective effect of sodium selenite on genotoxicity to human whole blood cultures induced by aflatoxin B-1. Brazil Arch Biol Technol. 2005;48:905–910. doi: 10.1590/S1516-89132005000800006. [DOI] [Google Scholar]
- Ghodsi FE, Absalan H. Comparative study of ZNO thin films prepared by different sol–gel route. Acta Phys Pol, A. 2010;118:659–664. [Google Scholar]
- Grandjean-Laquerriere A, Laquerriere P, Guenounou M, Laurent-Maquin D, Phillips TM. Importance of the surface area ratio on cytokines production by human monocytes in vitro induced by various hydroxyapatite particles. Biomater. 2005;26:2361–2369. doi: 10.1016/j.biomaterials.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Han SD, Campet G, Huang SY, Shasrty MCR, Portier J, Lassegues JC, Dweik HS. A new method for the preparation of fine-grained SnO2 and WO3 powders: influence of the crystallite size on the electrochemical insertion of Li + in SnO2 and WO3 electrodes. Active Passive Electron Component. 1995;18:39–51. doi: 10.1155/1995/79465. [DOI] [Google Scholar]
- Hoshino T, Tanaka K, Makita J, Hashimoto T. Investigation of phase transition in Li2TiO3 by high temperature X-ray diffraction. J Nuclear Mat. 2007;367:1052–1056. doi: 10.1016/j.jnucmat.2007.03.179. [DOI] [Google Scholar]
- Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179:130–139. doi: 10.1016/j.toxlet.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Ilıcan S, Caglar Y, Caglar M. Preparation and characterization of ZnO thin films deposited by sol–gel spin coating method. J Optoelectron Adv Mater. 2008;10:2578–2583. [Google Scholar]
- Jian-wen Y, Hui Z, Haryun Z, Yarryang D, Jian L, Xuan Z. Synthesis and electrochemical properties of nanocrystalline Li[Li1/3Ti3/5O4] by complex sol–gel method. Trans Nonferrous Met Soc China. 2004;14:1012–1016. [Google Scholar]
- Kang SJ, Kim BM, Lee YJ, Chung HW. Titanium dioxide nanoparticles trigger p53-mediated damage response in peripheral blood lymphocytes. Environ Mol Mutagen. 2008;49:399–405. doi: 10.1002/em.20399. [DOI] [PubMed] [Google Scholar]
- Kataoka K, Yasuhiko T, Norihito K, Hideaki N, Junji A, Yasushi I, Ken-ichi O. Crystal growth and structure refinement of monoclinic Li2TiO3. Mater Res Bull. 2009;44:168–172. doi: 10.1016/j.materresbull.2008.03.015. [DOI] [Google Scholar]
- Kim S, Choi JE, Choi J, Chung KH, Park K, Yi J. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro. 2009;23:1076–1084. doi: 10.1016/j.tiv.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Klien K, Godniccvar J. Genotoxicity of metal nanoparticles: focus on in vivo studies. Arh Hig Rada Toksikol. 2012;63:133–145. doi: 10.2478/10004-1254-63-2012-2213. [DOI] [PubMed] [Google Scholar]
- Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: Mechanisms. Int J Cancer. 2004;109:799–809. doi: 10.1002/ijc.11708. [DOI] [PubMed] [Google Scholar]
- Lanone S, Rogerieux F, Geys J, Dupont A, Maillot-Marechal E, Boczkowski J. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part Fibre Toxicol. 2009;6:14–19. doi: 10.1186/1743-8977-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:26–49. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- Li SQ, Zhu RR, Zhu H, Xue M, Sun XY, Yao SD, Wang SL. Nanotoxicity of TiO2 nanoparticles to erythrocyte in vitro. Food Chem Toxicol. 2008;46:3626–3631. doi: 10.1016/j.fct.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Lin LN, Liu Q, Song L, Liu FF, Sha JX. Recent advances in nanotechnology based drug delivery to the brain. Cytotechnol. 2010;62:377–380. doi: 10.1007/s10616-010-9295-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sun J. Endothelial cells dysfunction induced by silica nanoparticles through oxidative stress via JNK/P53 and NF-kB pathways. Biomater. 2010;31:8198–8209. doi: 10.1016/j.biomaterials.2010.07.069. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Ho IC, Lee TC. Induction of sister chromatid exchanges and micronuclei by titanium dioxide in Chinese hamster ovary-K1cells. Mutat Res. 1998;414:15–20. doi: 10.1016/S1383-5718(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Lu J, Caiyun N, Qing P, Yadong L. Single crystalline lithium titanate nanostructure with enhanced rate performance for lithium ion battery. J Power Sources. 2012;202:246–252. doi: 10.1016/j.jpowsour.2011.11.032. [DOI] [Google Scholar]
- Magrez A, Horváth L, Smajda R, Salicio V, Pasquier N, Forró L, Schwaller B. Cellular toxicity of TiO2-based nanofilaments. ACS Nano. 2009;3:2274–2280. doi: 10.1021/nn9002067. [DOI] [PubMed] [Google Scholar]
- Manke A, Wang L, Rojanasakul Y (2013) Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int 2013:942916, doi: 10.1155/2013/942916 [DOI] [PMC free article] [PubMed]
- Nakagawa Y, Wakuri S, Sakamoto K, Tanaka N. The photo genotoxicity of titanium dioxide particles. Mutat Res. 1997;394:125–132. doi: 10.1016/S1383-5718(97)00126-5. [DOI] [PubMed] [Google Scholar]
- Oesterling E, Chopra N, Gavalas V, Arzuaga X, Lim EJ, Sultana R. Alumina nanoparticles induce expression of endothelial cell adhesion molecules. Toxicol Lett. 2008;178:160–166. doi: 10.1016/j.toxlet.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Pan Y, Leifert A, Ruau D, Neuss S, Bornemann J, Schmid G. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small. 2009;5:2067–2076. doi: 10.1002/smll.200900466. [DOI] [PubMed] [Google Scholar]
- Poulter KF, Pryde JA. Chemisorption of hydrogen on rhenium. Brit J Appl Phys Ser. 1968;1:169. [Google Scholar]
- Ramesh M, Turner LF, Yadav R, Rajan TV, Vella AT, Kuhn LT. Effects of the physico-chemical nature of two biomimetic crystals on the innate immune response. Int Immunopharmacol. 2007;7:1617–1629. doi: 10.1016/j.intimp.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Riehemann K, Schneider SW, Luger TA, Godin B, Ferrari M, Fuchs H. Nanomedicine-challenge and perspectives. Angew Chem Int Ed Engl. 2009;48:872–897. doi: 10.1002/anie.200802585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothen-Rutishauser B, Mühlfeld C, Blank F, Musso C, Gehr P. Translocation of particles and inflammatory responses after exposure to fine particles and nanoparticles in an epithelial airway model. Part Fibre Toxicol. 2007;4:9. doi: 10.1186/1743-8977-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schanen BC, Karakoti AS, Seal S, Drake DR, Warren WL, Self WT. Exposure to titanium dioxide nanomaterials provokes inflammation of an in vitro human immune construct. ACS Nano. 2009;3:2523–2532. doi: 10.1021/nn900403h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schins RP, Lightbody JH, Borm PJ, Shi T, Donaldson K, Stone V. Inflammatory effects of coarse and fine particulate matter in relation to chemical and biological constituents. Toxicol Appl Pharmacol. 2004;195:1–11. doi: 10.1016/j.taap.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Schmid O, Moller W, Semmler-Behnke M, Ferron GA, Karg E, Lipka J, Schulz H, Kreyling WG, Stoeger T. Dosimetry and toxicology of inhaled ultrafine particles. Biomarkers. 2009;14:67–73. doi: 10.1080/13547500902965617. [DOI] [PubMed] [Google Scholar]
- Simbula G, Columbano A, Ledda-Columbano GM, Sanna L, Deidda M, Diana A. Increased ROS generation and p53 activation in alpha-lipoic acidinduced apoptosis of hepatoma cells. Apoptosis. 2007;12:113–123. doi: 10.1007/s10495-006-0487-9. [DOI] [PubMed] [Google Scholar]
- Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric Ultrafine Particles (UFPs) and implications in epidemiologic research. Environ Health Perspect. 2005;113:947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisman T, Turkez H. Toxicologic evaluation of imazalil with particular reference to genotoxic and teratogenic potentials. Toxicol Ind Health. 2010;26:641–648. doi: 10.1177/0748233710375951. [DOI] [PubMed] [Google Scholar]
- Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34:559–567. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Takasawa H, Kobayashi K, Terashima Y, Shimada Y, Ogawa I, Tanaka J, Imamura T, Miyazaki A, Hayashi M (2009) Evaluation of a liver micronucleus assay with 12 chemicals using young rats (II): a study by the Collaborative Study Group for the Micronucleus Test/Japanese Environmental Mutagen Society-Mammalian Mutagenicity Study Group. Mutagen 24:9–16 [DOI] [PubMed]
- Tsuchiya K, Nakamichi M, Nagao Y, Enoeda M, Osaki T, Tanaka S, Kawamura H. In-situ tritium recovery experiments of blanket in-pile mockup with Li2TiO3 pebble bed in Japan. J Nuclear Sci Technol. 2001;38:996–999. doi: 10.1080/18811248.2001.9715128. [DOI] [Google Scholar]
- Turkez H. Effects of boric acid and borax on titanium dioxide genotoxicity. J Appl Toxicol. 2008;28:658–664. doi: 10.1002/jat.1318. [DOI] [PubMed] [Google Scholar]
- Turkez H. The role of ascorbic acid on titanium dioxide-induced genetic damage assessed by the comet assay and cytogenetic tests. Exp Toxicol Pathol. 2011;63:453–457. doi: 10.1016/j.etp.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F. An in vitro blood culture for evaluating the genotoxicity of titanium dioxide: the responses of antioxidant enzymes. Toxicol Ind Health. 2007;23:19–23. doi: 10.1177/0748233707076764. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F. Boric acid: a potential chemoprotective agent against aflatoxin b1 toxicity in human blood. Cytotechnol. 2010;62:157–165. doi: 10.1007/s10616-010-9272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkez H, Sisman T. Anti-genotoxic effect of hydrated sodium calcium aluminosilicate on genotoxicity to human lymphocytes induced by aflatoxin B1. Toxicol Ind Health. 2007;23:83–89. doi: 10.1177/0748233707076738. [DOI] [PubMed] [Google Scholar]
- Turkez H, Togar B. The genotoxic and oxidative damage potential of olanzapine in vitro. Toxicol Ind Health. 2010;26:583–588. doi: 10.1177/0748233710373090. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Keles MS. Biochemical response to colloidal bismuth subcitrate: dose-time effect. Biol Trace Elem Res. 2005;105:151–158. doi: 10.1385/BTER:105:1-3:151. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Tatar A, Keleş S, Ozkan A. Effects of some boron compounds on peripheral human blood. Z Naturforsch C. 2007;62:889–896. doi: 10.1515/znc-2007-11-1218. [DOI] [PubMed] [Google Scholar]
- Turkez H, Geyikoglu F, Tatar A, Keles MS, Kaplan I. The effects of some boron compounds against heavy metal toxicity in human blood. Exp Toxicol Pathol. 2012;64:93–101. doi: 10.1016/j.etp.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Turkez H, Cakmak B, Celik K. Evaluation of the potential in vivo genotoxicity of Tungsten (VI) oxide nanopowder for human health. Key Engineering Mater. 2013;543:89–92. doi: 10.4028/www.scientific.net/KEM.543.89. [DOI] [Google Scholar]
- Turkez H, Celik K, Cakmak B. Biosafety evaluation of nanoparticles in view of genotoxicity and carcinogenicity studies: a systematic review. Key Eng Mater. 2013;543:200–203. doi: 10.4028/www.scientific.net/KEM.543.200. [DOI] [Google Scholar]
- Unfried K, Albrecht C, Klotz LO, von Mikecz A, Grether-Beck S, Schins RPF. Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicol. 2007;1:52–71. doi: 10.1080/00222930701314932. [DOI] [Google Scholar]
- Wang Y, Aker WG, Hwang HM, Yedjou CG, Yu H, Tchounwou PB. A study of the mechanism of in vitro cytotoxicity of metal oxide nanoparticles using catfish primary hepatocytes and human HepG2 cells. Sci Total Environ. 2011;409:4753–4762. doi: 10.1016/j.scitotenv.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wataha JC, Hobbs DT, Wong JJ, Dogan S, Zhang H, Chung KH, Elvington MC. Titanates deliver metal ions to human monocytes. J Mater Sci Mater Med. 2010;21:1289–1295. doi: 10.1007/s10856-009-3941-8. [DOI] [PubMed] [Google Scholar]
- Xia T, Kovochich M, Brant J. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- Xiangwei W, Zhaoyin W, Bin L, Xiaogang X. Sol–gel synthesis and sintering of nano-size Li2TiO3 powder. Material Lett. 2008;62:837–839. doi: 10.1016/j.matlet.2007.06.073. [DOI] [Google Scholar]
- Xu A, Chai Y, Hei TK. Genotoxic responses to titanium dioxide nanoparticles and fullerene in gptdelta transgenic MEF cells. Part Fibre Toxicol. 2009;6:3. doi: 10.1186/1743-8977-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Zhang YL, Song C, Wu LL, Gao HW. Interactions of hydroxyapatite with proteins and its toxicological effect to zebrafish embryos development. PLoS ONE. 2012;7:e32818. doi: 10.1371/journal.pone.0032818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Li ZH, Mao XZ, Wang WC. Advances in bone repair with nanobiomaterials: mini-review. Cytotechnol. 2011;63:437–443. doi: 10.1007/s10616-011-9367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Song W, Guo J, Zhang J, Sun Z, Li L, Ding F, Gao M. Cytotoxicity of different sized TiO2 nanoparticles in mouse macrophages. Toxicol Ind Health. 2013;29:523–533. doi: 10.1177/0748233712442708. [DOI] [PubMed] [Google Scholar]