Abstract
Glaucoma is one of the leading eye diseases due to the death of retinal ganglion cells. Increasing evidence suggests that retinal Müller cells exhibit the characteristics of retinal progenitor cells and can differentiate to neurons in injured retinas under certain conditions. However, the number of ganglion cells differentiated from retinal Müller cells falls far short of therapeutic needs. This study aimed to promote the differentiation of retinal Müller cells into ganglion cells by introducing Atoh7 into the stem cells dedifferentiated from retinal Müller cells. Rat retinal Müller cells were isolated and dedifferentiated into stem cells, which were transfected with PEGFP-N1 or PEGFP-N1-Atoh7 vector, and then further induced to differentiate into ganglion cells. The proportion of ganglion cells differentiated from Atoh7-tranfected stem cells was significantly higher than that of control transfected or untransfected cells. In summary, Atoh7 promotes the differentiation of retinal Müller cells into retinal ganglion cells. This may open a new avenue for gene therapy of glaucoma by promoting optic nerve regeneration.
Keywords: Atoh7, Retinal ganglion cells, Müller cells, Progenitor cells
Introduction
Glaucoma is a group of blinding eye diseases characterized by selective and progressive death of retinal ganglion cells. In the late stage of glaucoma, about 90 % of retinal ganglion cells can be damaged. Despite the availability of numerous ways for ganglion cell protection, no treatment so far can essentially prevent ganglion cells and the optic nerve from injury. Stem cells have emerged as new approach for optic nerve regeneration (Cho et al. 2012). However, retinal stem cells exist only in the pigmented ciliary epithelium and are too few to meet the clinical need (Tropepe et al. 2000). On the other hand, the use of other stem cells, such as embryonic stem cells and neural stem cells, is greatly restricted due to ethical issues and graft rejection.
Müller cells are the major glial cell population in mammalian retina, and play important roles in the maintenance of retinal function (Bringmann et al. 2006). Extensive studies in recent years have implicated retinal Müller cells as potential retinal stem cells. A variety of factors have been shown to induce the dedifferentiation of retinal Müller cells in zebrafish, chicken and rat (Fimbel et al. 2007; Ooto et al. 2004; Abrahan et al. 2009; Das et al. 2006; Lawrence et al. 2007). The dedifferentiated retinal Müller cells exhibit the characteristics of stem cells, and continue to differentiate into neurons, including retinal ganglion cells. However, the proportions of induced ganglion cells reported in previous studies are very low. Therefore, strategies to enhance the differentiation of Müller cells into ganglion cells may hold promise for glaucoma treatment via optic nerve regeneration.
The induction and differentiation of retinal stem cells are largely regulated by the joint action of extracellular and intracellular factors. Atoh7 is an essential transcription factor for ganglion cell differentiation (Yang et al. 2003; Brown et al. 2001; Wang et al. 2001). Previous study showed that Atoh7 overexpression significantly increased the number of retinal ganglion cells differentiated from in vitro cultured retinal stem cells (Yao et al. 2007). Accordingly, we hypothesize that Atoh7 may promote the differentiation of stem cells dedifferentiated from retinal Müller cells into ganglion cells.
In this study, we cultured rat retinal Müller cells in vitro and the cells in the 3rd-4th passage were induced to dedifferentiate into stem cells with stem cell medium. Next, the stem cells were transfected with Atoh7 expression vector to induce the re-differentiation with DMEM medium containing fetal bovine serum (FBS), brain-derived neurotrophic factor (BDNF) and retinoic acid (RA).
Materials and methods
Retinal Müller cell culture and dedifferentiation
Retinal Müller cells were isolated from S/D rats as previously described (Sarthy et al. 1998). Briefly, the eyes from rats in the postnatal (PN) days 10–21 were enucleated under sterile conditions and the retina was dissected with care to avoid contamination from retinal pigment epithelia and ciliary epithelium. The remaining retinal tissues were then transferred to dissociation solution (DMEM containing 0.25 % trypsin and 0.05 % EDTA) and incubated at 37 °C for 1 h. The retina was mechanically dissociated into small aggregates and cultured in DMEM (GIBCO, Grand Island, NY, USA) containing 10 % FBS (GIBCO) at 37 °C in 5 % CO2 for 7–10 days. The culture medium was replaced every 2–3 days and floating retinal aggregates and debris were removed, leaving purified flat cell population of Müller cells attached to the bottom of 25 cm2 culture flasks (Corning, Corning, NY, USA). The cells were trypsinized and cultured in DMEM containing 10 % FBS to get further purified population every 5–7 days. To form aggregate spheres, Müller cells in the third to fourth passage were dissociated using trypsin–EDTA and cultured in stem cell medium containing DMEM/F12 (GIBCO), 10 ng/ml basic fibroblast growth factor (bFGF), 20 ng/ml epidermal growth factor (EGF) (Peprotech, Rocky Hill, NJ, USA), 1 × N2 supplement, 2.5 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (GIBCO) at a density of 1 × 105 cells/cm2 for 3–5 days. To examine the proliferation potential of stem cells dedifferentiated from Müller cells, neurospheres were exposed to 10 μg/ml of BrdU (Sigma, St. Louis, MO, USA) for 10 h to label proliferative cells. The cells were fixed using cold 4 % paraformaldehyde for immunocytochemical analysis. All animal procedures were approved by the Committee of Animal Resources at the Central South University.
Plasmid construction
The cDNA encoding Atoh7 protein was amplified by PCR with plasmid PBSII SK-Atoh7 (generously gifted by Dr. N.L. Brown) as the template and the following primers: 5′-GCGAATTCATGAAGTCGGCCTGCAAACC-3′ and 5′-GCGGATCCACGCTG GCCATGGGGAAG-3′. PCR product was subcloned into PEGFP-N1 vector (Clontech, Mountain View, CA, USA) to create PEGFP-N1-Atoh7, and the positive clones were verified by restriction enzymes analysis and DNA sequencing.
Electroporation and cell differentiation
Neurospheres dedifferentiated from Müller cells were divided into three groups: (A) neurospheres transfected by PEGFP-N1-Atoh7; (B) neurospheres transfected by PEGFP-N1; and (C) neurospheres without transfection. For electroporation, neurospheres were trypsinized and resuspended in 100 μl of the transfection solution (pH 7.4), which consisted of a mixture of sucrose (272 mM), K2HPO4 (7 mM), MgCl2 (1 mM) and 10 μg plasmid (PEGFP-N1-Atoh7 or PEGFP-N1). The cells and the plasmids were gently mixed and cooled on ice for 5-10 min, after which they were placed in an electroporation cuvette with a path length of 0.4 cm (Biosmith, San Clemente, CA, USA) and immediately pulsed using a Bio-Rad gene pulser II (Bio-Rad laboratories, Hercules, CA, USA) at 75 μF and 350 V for 3–5 min. Next, the cells were plated onto 0.01 % poly-d-lysine (Sigma, USA) coated 24-mm glass coverslips (Corning, USA) at a final concentration of 100,000 cells/well, and 1 ml of the differentiation medium supplemented with BDNF (1 ng/ml) (Peprotech), RA (1 μM) (Sigma), and 1 % FBS was added. The cells were kept at 37 °C in a 5 % CO2 incubator and the medium was changed 24 h after plating to remove debris. Thereafter, the cells were fed every 2 days by replacing one-third of the differentiation medium. Transfected cells were analyzed under fluorescence microscope (Axiovert2000, Carl Zeiss, Oberkochen, Germany) 1–14 days after transfection.
Immunocytochemistry
Immunocytochemical analysis was performed for the detection of BrdU and cell-specific markers. Briefly, cells cultured on coverslips or spheres attached onto coverslips were fixed by 4 % paraformaldehyde in 0.01 M phosphate-buffered saline (PBS) for 15 min at room temperature. The cells were permeabilized with 0.1 % Triton X-100/PBS for 10 min, blocked with 5 % goat serum for 1 h, and incubated with primary antibodies (listed in Table 1) for 1 h at room temperature. For negative controls, 0.01 M PBS was used to replace primary antibodies. After extensive washing with PBS, the cells were blocked again with 5 % goat serum for 20 min. Then the cells were incubated with Cy3- or Alexa Fluor 488-conjugated secondary antibodies (KPL, Rouses Point, NY, USA) for 1 h in the dark. Following extensive washing with PBS, 1 μg/μl DAPI (Sigma) was used to stain the nuclei. Images were taken by a fluorescence microscope or a confocal laser scanning microscope (Leica TCS SP5, Leica Microsystems GmbH, Wetzlar, Germany) and analyzed by Leica Qwin V3.1 system. Positive cells were quantified in at least 10 fields across the coverslips from three independently dissociated cultures.
Table 1.
List of primary antibodies used in this study
| Name | Host | Cell makers | Dilution | Company |
|---|---|---|---|---|
| Pax6 | Rabbit | Müller cells | 1:50 | Santa Cruz Biotechnology, Dallas, TX, USA |
| GS | Rabbit | Müller cells | 1:50 | Santa Cruz |
| Nestin | Rabbit | Retinal stem cells | 1:100 | Abcam, Cambridge, UK |
| BrdU | Mouse | Proliferation marker | 1:200 | Sigma |
| GFAP | Mouse | Glial cells | 1:200 | Sigma |
| Thy1.1 | Mouse | Ganglion cells | 1:100 | Millipore, Billerica, MA, USA |
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was isolated from cells or the retina using TRIZOL reagent (Invitrogen, Carlsbad, CA, USA). Single-stranded cDNA was synthesized using MMLV reverse transcriptase and oligo-dT primers according to the manufacturer’s instructions (Fermentas, Hanover, MD, USA). Gene-specific primers for RT-PCR analyses were designed with Primer 3 (http://primer3.sourceforge.net) and the sequences were listed in Table 2. Amplifications were performed in a GeneAmp 9700 (Applied Biosystems, Foster City, CA, USA) using the following conditions: initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 1 min, annealing between 53 and 55 °C for 30 s, and extension at 72 °C for 45 s, followed by a final extension at 72 °C for 10 min. PCR products were visualized on 1 % agarose gel. Expression levels were standardized using β-actin which served as the housekeeper gene.
Table 2.
List of primers used in this study
| Name | Sequence | Annealing temp (°C) | Size (bp) | Acc. no |
|---|---|---|---|---|
| Vimentin | Forward:5′-AAGGCACTAATGAGTCCCTGGAG-3′ | 55 | 251 | NM_031140 |
| Reverse:5′-GTTTGGAAGAGGCAGAGAAATCC-3′ | ||||
| Nestin | Forward:5′-GATCGCTCAGATCCTGGAAG-3′ | 55 | 349 | NM_012987 |
| Reverse:5′-TATAGGTGGGATGGGAGTGC-3′ | ||||
| Pax6 | Forward:5′-AGTGAATGGGCGGAGTTATG-3′ | 55 | 363 | NM_013001 |
| Reverse:5′-TACGCAAAGGTCCTGGTTTC-3′ | ||||
| Rhodopsin | Forward:5′-GTCGGCTACCACTCAGAAGG-3′ | 55 | 348 | NM_033441 |
| Reverse:5′-ACAGTCTCTGGCCAGGCTTA-3′ | ||||
| β-tubulin | Forward:5′-TGAGGCCTCCTCTCACAAGT-3′ | 55 | 393 | NM_139254 |
| Reverse:5′-TAGGGCTCTACCACGGTGTC-3′ | ||||
| Atoh7 | Forward:5′-ATGAAGTCGGCCTGCAAAC-3′ | 55 | 389 | AF_071223 |
| Reverse:5′-GGGTCTACCTGGAGCCTAGC-3′ | ||||
| β-actin | Forward:5′-GTGGGGCGCCCCAGGCACCA-3′ | 55 | 548 | XM_037235 |
| Reverse:5′-CTCCTTAATGTCACGCACGATTTC-3′ |
Statistical analysis
Data from at least three independently dissociated cultures, each measured in triplicate, were expressed as mean ± standard deviation. Quantitative differences were evaluated using the Student–Newman–Keuls (SNK-q) in SPSS12.0. P values <0.05 were considered statistically significant.
Results
Characterization of Müller cells from rat retina
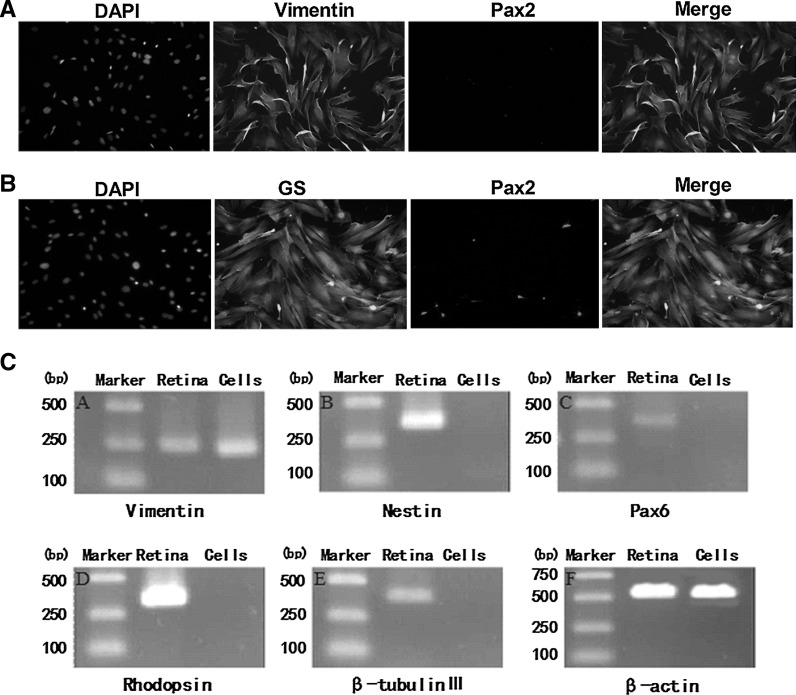
The majority of Müller cells from rat retina had abundant cytoplasm and well-defined membranes. After 7–10 days, the cells formed a complete monolayer of epithelioid cells. To determine whether the cultured cells were Müller cells, we examined Müller cell markers including Vimentin and glutamine synthetase (GS). Our results showed that most of the cells in the monolayer culture were positive for Vimentin and GS staining, but were negative for the staining of Pax2, a marker of astrocytes (Fig. 1a, b). To further ascertain the purity of Müller cell culture, we examined the expression of cell-specific transcripts. RT-PCR analysis detected the transcripts specific to Müller cells (Vimentin), retinal progenitor cells (Nestin and Pax6), rod photoreceptors (Rhodopsin), and neural cells (β-tubulin III) in the rat retina. In contrast, the cultured cells only expressed the specific transcript of Müller cells and no other cell-specific transcripts were detected. These findings suggest that the monolayer culture is enriched for Müller cells and not contaminated by other retina-derived cells (Fig. 1c).
Fig. 1.
Characterization of retinal Müller cells. a Dual staining of primary culture of retinal Müller cells at passage 3 for Vimentin and Pax2 (×100). b Dual staining of primary culture of retinal Müller cells at passage 3 for glutamine synthetase (GS) and Pax2. (×100). The nuclei were stained with DAPI. c RT-PCR analysis of the transcripts specific to Müller cells (Vimentin, panel A), retinal stem cells (Nestin and Pax6, panels B and C), photoreceptor cells (Rhodopsin, panel D), and neuronal cells (β-tubulin III, panel E) as well as β-actin (control, panel F) in rat retina (lane 2) and the primary cultured Muller cells (lane 3). Only the expression of the Müller cell-specific transcript Vimentin was detected in the primary cultured Müller cells
Dedifferentiated retinal Müller cells exhibit the characteristics of retinal stem cells
Two to three days after Müller cells were cultured in the stem cell medium, some cells underwent apoptosis; some cell processes became smaller and cell body became round; the proliferation was clonal; and a few spherical or mulberry-shaped cell spheres composed of dozens of cells appeared (Fig. 2a). At 3–5 days of culture, the cell spheres increased in number and size; cells displayed good refraction and exhibited well-defined cell boundaries at the edge of cell spheres; and the cell spheres became further rounded, resembling neurospheres (Fig. 2b). Thereafter, the cell spheres showed no significant increase in number and size. At days 7–10, the center of the cell spheres began to darken, accompanied by cell growth arrest or poor cell growth.
Fig. 2.
Dedifferentiation and characterization of retinal Müller cells. a Two to three days after retinal Müller cells were exposed to stem cell medium in vitro, a few cell spheres comprising dozens of cells were aggregated (×100); b At day five, the cells within the cell spheres displayed good refraction and well-defined cell boundaries at the edge of cell spheres; the cell spheres continued to increase in size and became round, which appeared to be neurospheres (×100); c RT-PCR analysis of retinal stem cell-specific transcript Nestin (product size 349 bp) in both cell spheres (lane 1) and retinal tissue (lane 2), demonstrating that at this stage Müller cells already acquired the characteristics of stem cells; d More than 95.07 ± 1.35 % cells within the cell spheres had positive nestin expression (red fluorescence); e 10.34 ± 3.26 % of the cells in the cell spheres showed positive GFAP expression in the cytoplasm (green fluorescence); the percentage of the cells with positive nestin and GFAP expression is shown in the upper right diagramme. f Merge of panels D and E; scale 50 μm; g Cell were administrated with BrdU for 7 days. More than 90 ± 4.12 % of the cells within the cell spheres had positive BrdU staining (red fluorescent nuclei); h BrdU-positive cells were also positive for nestin expression (green fluorescence); The percentage of the cells with positive BrdU staining and nestin expression is shown in the lower right diagramme; i Merge of panels G and H; scale 50 μm. (Color figure online)
Immunofluorescence staining showed that 95.07 ± 1.35 % of the cells within the cell spheres were positive for retinal stem cell-specific marker Nestin, suggesting that retinal Müller cells can dedifferentiate into retinal stem cells in the medium. Meanwhile, 10.34 ± 3.26 % of the cells were positively stained with glial cell-specific marker GFAP, suggesting that some retinal Müller cells still retained the characteristics of glial cells (Fig. 2d–f). Immunofluorescence staining of BrdU-labeled cell spheres showed that 90.26 ± 4.12 % of the cells within the cell spheres were BrdU positive, suggesting that newborn cell spheres have the capacity of effective proliferation (Fig. 2g–i).
RT-PCR analysis showed that the cell spheres, like the retinal tissue, could express Nestin. Since Müller cells had no Nestin expression before dedifferentiation, these findings demonstrate that Müller cells are able to acquire the phenotype of retinal stem cells under specific conditions (Fig. 2c).
Atoh7 overexpression affects phenotypes of stem cells dedifferentiated from retinal Müller cells
24 h after transfection of PEGFP-N1-Atoh7 plasmid into stem cells dedifferentiated from retinal Müller cells, scattered dead cell debris, suspended single cells and some small neurospheres were observed in the visual field, and mild green fluorescence was observed at the edge of neurospheres or in some single cells (data not shown). At 48 h, the number of positive cells increased and fluorescence intensity enhanced; green fluorescence was distributed homogeneously in the cytoplasm (Fig. 3a). RT-PCR analysis showed that at 48 h, Atoh7 expression was detected in the neurospheres but not in untransfected cells, indicating successful transfection (Fig. 3b). Three to four days after transfection, the majority of untransfected cells remained spherical. In contrast, retinal stem cells transfected with Atoh7 expression plasmid grew radially, began to differentiate, and continued to express enhanced green fluorescent protein gene (EGFP) (Fig. 3c, d).
Fig. 3.
Transfection of PEGFP-N1-Atoh7 into stem cells dedifferentiated from retinal Müller cells. a Morphology of stem cells dedifferentiated from retinal Müller cells 48 h after transfection (x 100). Left panel: observation under light microscopy; middle panel: observation under fluorescence microscopy; right panel: Merge of images obtained under light microscopy and fluorescence microscopy. b RT-PCR analysis of Atoh7 expression in transfected cells (lane 1) and untransfected cells (lane 2); bp: base pairs. c At 3–4 days after transfection, stem cells were positive for EGFP, grew radially and started to differentiate (×100); d In contrast, untransfected cells were negative for EGFP (×100)
Atoh7 overexpression affects the re-differentiation of stem cells dedifferentiated from retinal Müller cells
The three groups of neurospheres, transfected with plasmid PEGFP-N1-Atoh7, control vector PEGFP-N1 or untransfected, were induced to differentiate. At 2 h after seeding, the cell spheres in the three groups began to adhere to the coverslips. At 3 days, cells with processes around the cell spheres were significantly increased, concomitant with a gradual increase in the length of the processes. The cells grew from the center to the periphery in a radial manner (Fig. 4a). At 7–10 days, the cells reached the highest degree of differentiation, and two populations of cells with different shapes were observed. Some were flat and irregular and had short processes, suggestive of glial cells; and the others had a small cell body and one or more long processes, resembling neurons (Fig. 4b). The untransfected neurospheres were larger and the cell number was greater than neurospheres transfected with Atoh7 or control vector.
Fig. 4.
Atoh7 promotes the differentiation of retinal Müller cells into retinal ganglion cells. a Three days after induced differentiation, cells inside the neurospheres extended outwards and flattened; cell processes gradually grew in length; and the cells grew from the center to the periphery in a radial manner (×100); b Seven to ten days after induced differentiation, the cells reached the highest degree of differentiation; cells of varying sizes and shapes were observed. Some were flat and irregular with short processes, suggestive of glial cells; and the others had a small cell body and one or more long processes, resembling neurons (x 100). c Staining of DAPI, Thy1.1 and Brn3b of neurospheres; the right panel shows the merged images obtained for DAPI, Thy1.1 and Brn3b staining; d Bar chart shows the percentage of cells that were positive for both Thy1.1 and Brn3b retinal ganglion cell markers in the total differentiated cells. a–c indicates Atoh7 transfection, empty vector transfection and untransfection, respectively. e RT-PCR analysis of Thy1.1 and Brn3b expression in the total differentiated cells. a–c indicate Atoh7 transfection, empty vector transfection and untransfection, respectively
At days 7–10, the cells were stained with the antibody against ganglion cell-specific markers Thy1.1 and Brn3. Ten different visual fields in each group were selected to count the number of ganglion cells with positive Thy1.1 staining (green fluorescence) and Brn3 staining (red fluorescence), and the total number of the cells with positive DAPI staining (blue fluorescence), and the efficiency of differentiation was calculated (Fig. 4c). The cells with positive Thy1.1 and Brn3 expression accounted for 61.10 ± 1.93 % of the total cells in Atoh7 transfected neurospheres, which was significantly higher than 21.88 ± 2.05 % in the control transfected group and 21.14 ± 1.49 % in the untransfected group, Fig. 4d). Furthermore, the proportions of positive cells among three groups were compared using SNK-q test. The results showed that the proportion of ganglion cells differentiated from Atoh7 transfected neurospheres in the total differentiated cells was significantly higher than that of control transfection group (qA–B = 36.94, P < 0.01) and untransfection group (qA–C = 37.63, P < 0.01), whereas no significant difference was noted between the two control groups (qB–C = 0.70, P > 0.05) (Fig. 4d). RT-PCR analysis confirmed the upregulation of ganglion cell-specific markers Thy1.1 and Brn3 in Atoh7 transfected neurospheres (Fig. 4e). Collectively, our results indicate that Atoh7 promotes the directed differentiation of stem cells dedifferentiated from retinal Müller cells into ganglion cells.
Discussion
Glaucoma is an irreversible blindness eye disease, characterized by massive death of retinal ganglion cells. Blockade of the signaling pathway of retinal ganglion cell apoptosis, reduction of intraocular pressure, and nourishment of the optic nerve are known to be somewhat effective in prolonging the life of ganglion cells and retarding disease progression for patients with early glaucoma or progressing glaucoma (Cho et al. 2012). However, such treatment strategies are ineffective for patients with advanced glaucoma. Therefore, there is a definite need to pursue new ways to regenerate retinal ganglion cells to resist disease progression or even restore vision.
Retinal Müller cells are glial cells in the retina with proliferation potential, and offer an abundant source for cell engineering (Bringmann et al. 2006). Although there is substantial evidence that retinal Müller cells can dedifferentiate into retinal stem cells in certain conditions, it remains unclear whether they could differentiate into ganglion cells (Lawrence et al. 2007). Therefore, in the present study, we selected the stem cells dedifferentiated from rat retinal Müller cells as the target cells for gene transfection. We successfully transfected these stem cells with recombinant plasmids PEGFP-N1-Atoh7, demonstrating for the first time that Atoh7 can promote the differentiation of the stem cells dedifferentiated from Müller cells into large quantities of ganglion cells.
In this study, retina-derived cells were first cultured using the classic culture method for Müller cells. The results showed that the cultured cells displayed the general morphology of Müller cells and more than 95 % of the cells were immunopositive for Vimentin and GS, two widely accepted markers of Müller cells (Fig. 1a, b). Next, we examined the purity of Müller cell culture using the method described by Das et al. (2006). We noted that the cultured cells only expressed retinal Müller cell-specific transcript Vimentin, confirming the high purity of the cultured retinal Müller cells. In addition, the lack of expression of the stem cell-specific transcript Nestin by Müller cells at this stage shows that Müller cells, when not stimulated, do not exhibit the characteristics of stem cells.
Currently, two methods are commonly used to induce dedifferentiation of retinal Müller cells: one is to use ouabain, NMDA, or other agents to reproduce the effects of injury, and the other is to add various cell growth factors for stem cell growth. In the present study, we chose serum-free DMEM/F12 medium supplemented with EGF, FGF-2 and other cytokines to induce the dedifferentiation of retinal Müller cells. Characterization of the cell spheres dedifferentiated from retinal Müller cells showed that over 95.07 ± 1.35 % of the cells within the cell spheres had positive expression of Nestin, which was not seen in normal Müller cells. These results suggest that upon cytokine stimulation, Müller cells acquire the property of neural stem cells. Meanwhile, positive expression of GFAP, a glial cell-specific marker, was detected in the cytoplasm of 10.34 ± 3.26 % cells, indicating that some Müller cells still retained the features of glial cells. Furthermore, we found that the number of cells within the neurospheres dedifferentiated from retinal Müller cells, together with the size of the neurospheres, gradually increased over time. Immunofluorescence staining revealed that positive BrdU staining, an important indicator of proliferation, was observed in 90.26 ± 4.12 % of the cells, proving that the cells derived from dedifferentiation have the proliferative ability of stem cells.
Serum is a natural inducer of stem cell differentiation, but Kubota et al. (2006) showed that stem cells dedifferentiated from retinal Müller cells did not undergo neuronal cell differentiation when cultured in medium containing serum only. A large number of studies suggest that BDNF and RA can induce neural stem cells to differentiate into neurons and promote their maturation (Ahmed et al. 1995; Guan et al. 2001). Accordingly, to harvest more ganglion cells, a type of neurons, we chose to induce re-differentiation of the stem cells in culture medium supplemented with BDNF and RA. We found that these stem cells had the ability to further re-differentiate. Cells of various shapes were obtained as early as at days 7–10 of culture. Even without transfection with Atoh7 plasmid, the proportion of ganglion cells re-differentiated with our medium could account for 21.14 ± 1.49 % of the total differentiated cells.
Viral transduction is currently the most efficient method for transferring exogenous DNA into the cells. However, because of the inherent risk and technical difficulties associated with viral transduction, non-viral transduction methods are safer and more suitable for clinical application. Lipofection is the most common non-viral transduction method. However, our pilot study found that liposome transfection was inefficient in transfecting stem cells, with a transfection efficiency of merely 0.13 ± 0.17 % after 48 h of transfection (data not shown). This may be due to the fact that stem cells dedifferentiated from Müller cells grow in suspension, and many neural stem cells overlap with each other closely and aggregate into spheres, which may prevent liposome/DNA complexes entering the neurospheres. Moreover, liposomes had relatively high toxicity to neural stem cells, thus impairing the transfection efficiency due to excessive cell death (Kim et al. 2002).
Electroporation is a physical method to deliver foreign genes into the cells by forming pores in the cell membrane using pulsed electric field. It is generally believed that electroporation has no biological or chemical side effects, and can be applied to almost all types of cells, especially cells unamenable to liposome transfection, such as primary neurons and stem cells (Baum et al. 1994). Previous study has shown that electroporation has higher transfection efficiency than liposome-mediated transfection for stem cells (Helledie et al. 2008). Geoffroy et al. reported that the transfection efficiency of electroporation for mouse neural stem cells reached up to 30.2 ± 3.6 % (Geoffroy and Raineteau 2007). In the present study, we modified the electroporation buffer on the basis of previous studies (Aberg et al. 2001; Golzio et al. 1998), and transfected stem cells dedifferentiated from Müller cells according to the optimal voltage and capacitance values for electro-transfection of neural stem cells previously reported (Cesnulevicius et al. 2006). Our results showed that electroporation could effectively transfer exogenous genes into stem cells dedifferentiated from retinal Müller cells.
The directed induced differentiation of retinal stem cells is mainly regulated by the extracellular microenvironment factors and endogenous cytokines. Gene knockout and transgenic studies showed that transcription factor bHLH (basic helix-loop-helix) family plays an important role in regulating retinal cell differentiation (Cepko 1999). The bHLH family of vertebrates includes proneural gene Ath family, Ash family, and proneural-antagonizing Id family and Hes family (Brown et al. 1998). Ath5 is a member of the Ath family, and a homolog of the Drosophila atonal gene in the bHLH family. Atoh7 is a murine transcription factor orthologous to Ath5, and regulates the differentiation of murine retinal ganglion cells. Atoh7 begins to be specifically expressed in rat retinal stem cells from embryonic day 11, earlier than all other proneural genes. Moreover, the expression pattern of Atoh7 is consistent with the spatiotemporal pattern of retinal ganglion cell differentiation (Brown et al. 1998). Atoh7 is a key regulatory factor essential for the formation of retinal ganglion cells in vertebrates (Yang et al. 2003).
Although Atoh7 mutant mice can survive, they lack retinal ganglion cells and the optic nerve (Brown et al. 2001). Knockout of Atoh7 in mouse embryonic stem cells blocks the differentiation of about 80 % retinal ganglion cells (Wang et al. 2001). The number of retinal ganglion cells differentiated from stem cells significantly increased after ectopic expression of Atoh7 (Yao et al. 2007). These studies suggest that Atoh7 is a transcription factor essential for ganglion cell differentiation. Therefore, in the present study, we chose Atoh7 gene to transfect stem cells dedifferentiated from Müller cells in an attempt to examine whether Atoh7 can promote the differentiation into ganglion cells. The results revealed that the number of ganglion cells differentiated from stem cells transfected with empty vector exhibited no significant difference compared with untransfected stem cells, thus excluding the influence of PEGFP-N1 vector on ganglion cell differentiation. For stem cells transfected with Atoh7 expression vector, the number of the cells differentiated from neurospheres was less than that of untransfected stem cells due to the effect of electroporation and mechanical pipetting, but the proportion of ganglion cells was nearly three times as high as that of the latter. Therefore, we conclude that Atoh7 can promote the directed differentiation of stem cells dedifferentiated from Müller cells into ganglion cells.
Several limitations of this study should be pointed out. First, we only performed the experiments in vitro and further in vivo investigation are needed to evaluate whether ganglion cells we derived from Atoh7 transfected Müller cells could promote optic nerve regeneration. Second, it is known that virus infection has side effects and stem cells have the tendency of tumorigenesis. Thus we need to reduce these side effects in our future studies.
Taken together, our present study demonstrates that Atoh7 can promote the differentiation of stem cells dedifferentiated from Müller cells into ganglion cells. Nevertheless, further studies are needed to delineate whether other transcription factors are implicated in Atoh7-conferred regulation of the directed differentiation of retinal Müller cells into retinal ganglion cells and what signaling pathway is involved. Our present study lays a foundation for further investigation into the regeneration of ganglion cells and may provide a more effective way for gene therapy of glaucoma.
Acknowledgments
This study was supported by Grant from National Scientific Foundation of China (No. NSFC 81170844) and State Key Lab of Medical Genetics of China (No. 1989DA105084). We are grateful to Prof. Nadean L. Brown (University of Clicinnati College of Medicine) for kindly gifting the PBSIISK-Atoh7 plasmid. We thank Dr. Jingwei Chi, for her valuable advice and guidance; Dr. Yaping Yan, Dr. Xiaoyun Mo and Dr. Jieqiong Tan, for their helpful suggestion; Dr. Shanshan Zhang and Chunjiang Liu for technical assistance.
Footnotes
Wei-tao Song and Qi Zeng contributed equally to this work
References
- Aberg MA, Ryttsén F, Hellgren G, Lindell K, Rosengren LE, MacLennan AJ, Carlsson B, Orwar O, Eriksson PS. Selective introduction of antisense oligonucleotides into single adult CNS progenitor cells using electroporation demonstrates the requirement of STAT3 activation for CNTF-induced gliogenesis. Mol Cell Neurosci. 2001;17:426–443. doi: 10.1006/mcne.2000.0947. [DOI] [PubMed] [Google Scholar]
- Abrahan CE, Insua MF, Politi LE, German OL, Rotstein NP. Oxidative stress promotes proliferation and dedifferentiation of retina glial cells in vitro. J Neurosci Res. 2009;87:964–977. doi: 10.1002/jnr.21903. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J Neurosci. 1995;15:5765–5778. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum C, Forster P, Hegewisch-Becker S, Harbers K. An optimized electroporation protocol applicable to a wide range of cell lines. Biotechniques. 1994;17:1058–1062. [PubMed] [Google Scholar]
- Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Müller cells in the healthy and diseased retina. Prog Retin Eye Res. 2006;25:397–424. doi: 10.1016/j.preteyeres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Brown NL, Kanekar S, Vetter ML, Tucker PK, Gemza DL, Glaser T. Atoh7 encodes a murine basic helix-loop-helix transcription factor expressed during early stages of retinal neurogenesis. Development. 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- Brown NL, Patel S, Brzezinski J, Glaser T. Atoh7 is required for retinal ganglion cell and optic nerve formation. Development. 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko CL. The roles of intrinstic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr Opin Neurobiol. 1999;9:37–46. doi: 10.1016/S0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- Cesnulevicius K, Timmer M, Wesemann M, Thomas T, Barkhausen T, Grothe C. Nucleofection is the most efficient nonviral transfection method for neuronal stem cells derived from ventral mesencephali with no changes in cell composition or dopaminergic fate. Stem Cells. 2006;24:2776–2791. doi: 10.1634/stemcells.2006-0176. [DOI] [PubMed] [Google Scholar]
- Cho JH, Mao CA, Klein WH. Adult mice transplanted with embryonic retinal progenitor cells: new approach for repairing damaged optic nerves. Mol Vis. 2012;18:2658–2672. [PMC free article] [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I. Neural stem cell properties of Müller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev Biol. 2006;299:283–302. doi: 10.1016/j.ydbio.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–1724. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy CG, Raineteau O. A cre-lox approach for transient transgene expression in neural precursor cells and long-term tracking of their progeny in vitro and in vivo. BMC Dev Biol. 2007;7:45. doi: 10.1186/1471-213X-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio M, Mora MP, Raynaud C, Delteil C, Teissié J, Rols MP. Control by osmotic pressure of voltage-induced permeabilization and gene transfer in mammalian cells. Biophys J. 1998;74:3015–3022. doi: 10.1016/S0006-3495(98)78009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan K, Chang H, Rolletschek A, Wobus AM. Embryonic stem cell-derived neurogenesis: retinoic acid induction and lineage selection of neuronal cells. Cell Tissue Res. 2001;305:171–176. doi: 10.1007/s004410100416. [DOI] [PubMed] [Google Scholar]
- Helledie T, Nurcombe V, Cool SM. A simple and reliable electroporation method for human bone marrow mesenchymal stem cells. Stem Cells Del. 2008;17:837–848. doi: 10.1089/scd.2007.0209. [DOI] [PubMed] [Google Scholar]
- Kim YC, Shim JW, Oh YJ, Son H, Lee YS, Lee SH. Co-transfection with cDNA encoding the Bcl family of anti-apoptotic proteins improves the efficiency of transfection in primary fetal neural stem cells. J Neurosci Methods. 2002;117:153–158. doi: 10.1016/S0165-0270(02)00090-0. [DOI] [PubMed] [Google Scholar]
- Kubota A, Nishida K, Nakashima K, Tano Y. Conversion of mammalian Müller glia cells into a neuronal lineage by in vitro aggregate. Biochem Biophys Res Commun. 2006;351:514–520. doi: 10.1016/j.bbrc.2006.10.072. [DOI] [PubMed] [Google Scholar]
- Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, Luthert PJ, Khaw PT, Limb GA. MIO-M1 cells and similar muller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–2043. doi: 10.1634/stemcells.2006-0724. [DOI] [PubMed] [Google Scholar]
- Ooto S, Akagi T, Kageyama R, Akita J, Mandai M, Honda Y, Takahashi M. Potential for neural regeneration after neurotoxic injury in the adult mammalian retina. Proc Natl Acad Sci USA. 2004;101:13654–13659. doi: 10.1073/pnas.0402129101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Müller cell line. Invest Ophthalmol Vis Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D, Tropepe V, Coles BL, Chiasson BJ. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–2036. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- Wang SW, Kim BS, Ding K, Wang H, Sun D, Johnson RL, Klein WH, Gan L. Requirement for Atoh7 in the development of retinal ganglion cells. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ding K, Pan L, Deng M, Gan L. Atoh7 determines the competence state of retinal ganglion cell progenitors. Dev Biol. 2003;264:240–254. doi: 10.1016/j.ydbio.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Yao J, Sun X, Wang Y, Xu G, Qian J. Atoh7 promotes retinal ganglion cell expression patterns in retinal progenitor cells. Mol Vis. 2007;13:1066–1072. [PMC free article] [PubMed] [Google Scholar]