Abstract
Wild chrysanthemum (Chrysanthemum indicum L.) is traditionally used in folk medicine as an anti-inflammatory agent. It is also used in the southwest plateau region of China to prevent ultraviolet-induced skin damage. However, the role and mechanism by which wild chrysanthemum prevents UV-induced skin damage and photoaging have never been investigated in vitro. In the present study, we found that aqueous extracts from wild chrysanthemum strongly reduced high-dose UVB-induced acute cell death of human immortalized keratinocytic HaCat cells. Wild chrysanthemum extract was also demonstrated to reduce low-dose UVB-induced expression of the photoaging-related matrix metalloproteinases MMP-2 and MMP-9. The ROS level elevated by UVB irradiation was strongly attenuated by wild chrysanthemum extract. Further study revealed that wild chrysanthemum extract reduced UVB-triggered ERK1/2 and p38 MAPK phosphorylation and their protective role, which is partially dependent on inhibiting p38 activation. These results suggest that wild chrysanthemum extract can protect the skin from UVB-induced acute skin damage and photoaging by reducing the intracellular reactive oxygen species (ROS) level and inhibiting p38 MAPK phosphorylation. The present study confirmed the protective role of wild chrysanthemum against UV-induced skin disorders in vitro and indicated the possible mechanism. Further study to identify the active components in wild chrysanthemum extract would be useful for developing new drugs for preventing and treating skin diseases, including skin cancer and photoaging, induced by UV irradiation.
Keywords: Wild chrysanthemum, Chrysanthemum indicum L, Ultraviolet, Cell death, Photoaging, Anti-oxidant
Introduction
In mammals, the skin is the largest organ of the ectodermal tissue. As the interface with the environment and the first line of defense against external factors, the skin is one of the most important parts of the body. Ultraviolet (UV) radiation is a constituent of the electromagnetic spectrum of sunlight, and excessive UV radiation harms the skin by causing acute skin inflammation, photoaging and even skin cancer. Ultraviolet B (UVB) radiation is a major component of UV that causes skin damage. Acute exposure to high doses of UVB results in acute skin damage from induced cell death (Bayerl et al. 1995). Chronic low doses of UVB exposure will cause another skin disorder, i.e., photoaging.
Skin aging is a serious problem induced by UV radiation. Intrinsic genetically programmed aging is more or less inevitable, but the skin aging induced by UV (photoaging) can be prevented in many ways. Histological changes in the extracellular matrix (ECM) result from chronic exposure to UV radiation. During the process of photoaging, ECM becomes less integrated and flexible, and this is generally the result of upregulation of metalloproteinases (MMPs) by UV radiation (Quan et al. 2009).
Reactive oxygen species (ROS) are a family of oxygen-based free radicals that contain an unpaired electron. ROS are generated by UVB radiation. UV radiation may induce the production of O2, possibly through chromophores, such as porphyrin (Ichihashi et al. 2003). The overproduction of ROS by UVB radiation contributes to many types of skin disorders, such as erythema, edema, wrinkling, photoaging, inflammation and even malignant tumors (Scharffetter-Kochanek et al. 1997, Trouba et al. 2002). The mechanisms of ROS-induced skin disorders involve DNA or protein damage (He et al. 2005), altered metabolism and dysregulated signal transduction events. Antioxidants can delay or attenuate ROS-induced cellular damage. Thus, antioxidants are considered capable of protecting the skin from UVB radiation-induced damage (Trouba et al. 2002).
The mitogen-activated protein kinase (MAPK) pathways, such as the ERK and p38 MAPK signaling pathways, play pivotal roles in almost all cell functions, including cell death (Deacon et al. 2003; Cagnol and Chambard 2009), apoptosis and necrosis (Lobner and Liot 2004). The role of MAPK in cell survival remains controversial. Some studies suggest that ERK activation enhances cell death by augmenting apoptosis, autophagy or inflammatory cell death (Cagnol and Chambard 2009). Other studies have demonstrated that MAPK activation may be a benefit to cell survival (Lin et al. 2008), and the promoting or attenuating of cell death may depend on the ERK sublocalization and the duration of its activation (Pognonec 2009). The role of p38 MAPK in cell survival is also ambiguous (Deacon et al. 2003; Gutierrez-Uzquiza et al. 2012). A previous study reported that MAPK proteins, including ERK1/2 (Chen et al. 2001), JNK1/2 (Liu et al. 2006) and p38 (Jinlian et al. 2007), are implicated in skin cancer and photoaging. There are also studies reporting that pomegranate polyphenol extract inhibits UVB-induced oxidative stress by inhibition of MAPK activation (Zaid et al. 2007).
A variety of herbs have been used in medicines and cosmetics for centuries (Korac and Khambholja 2011). The mechanisms by which they protect the skin include direct UV absorption and/or promotion of cell survival by indirect mechanisms (Korac and Khambholja 2011). Wild chrysanthemum (Chrysanthemum indicum L.) is widely used in China to cure many types of inflammatory diseases from ancient times. In the high-altitude area of southwest China, UV-induced skin disorders have a high incidence. The people living there usually use wild chrysanthemum to prevent UV-induced skin damage, including acute skin damage and skin photoaging. In the present study, we hypothesized that the protective effect against UV-induced skin damage by wild chrysanthemum may be related to its anti-oxidant properties and p38 MAPK phosphorylation inhibition.
Materials and methods
Materials
Wild chrysanthemum flowers were collected in summer in the suburbs of Kunming city, Yunnan province, China, and dried in shade for extract preparation. The dried flowers of Chrysanthemum indicum were ground to powder and extracted with sterile water for 24 h. In addition, the extract was then concentrated under reduced pressure. The concentrated extract was filtered, lyophilized, and stored at 4 °C. The yield of dried extract from starting crude materials was 10.5 %. The lyophilized powder was dissolved in sterile water and then filtered through a 0.22-μm syringe filter.
Spectrophotometric measurements
To assess whether wild chrysanthemum extract (WCE) is able to absorb UV directly, the absorbing properties of UV by wild chrysanthemum extract was measured as previously described (Wölfle et al. 2011).
Cell culture
The immortalized human keratinocyte cell line HaCat was purchased from Kunming Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Kunming, China) and was grown in Dulbecco’s modified Eagle medium (Hyclone, Beijing, China) supplemented with 10 % fetal bovine serum (FBS, Hyclone, Beijing, China).
MTT assay
The viability of the HaCat cells was determined using an MTT assay, as previously described (Lee et al. 2005). HaCat cells were plated at a density of 1 × 104 cells/well in 96-well plates. After culturing for 12 h, the medium was replaced by fresh serum-free medium (Dulbecco’s modified Eagle medium) containing different concentrations of wild chrysanthemum extract (from 50 to 6.4 mg/ml). Then, 24 h after incubation, MTT (Sigma-Aldrich, St. Louis, MO, USA) was added to the culturing medium to acquire a final concentration of 500 μg/ml. The cells were incubated for a further 2 h at 37 °C. Dark blue formazan crystals formed inside the cells were then dissolved with DMSO (Sigma-Aldrich, St. Louis, MO, USA). The absorbance was measured at 570 nm. The absorbance of the control cells were set as 100 % of viability.
UVB irradiation
UVB irradiation was conducted as previously described (Vicentini et al. 2011). In addition, 5 × 105 cells were seeded per 3.5-cm plate (Corning, Lowell, MA, USA). After culturing for 24 h, the medium was replaced with a medium containing 1 % BSA (Sigma-Aldrich, St. Louis, MO, USA) for starvation. After 24 h of starvation, the cells were irradiated with a Philips Pl-s 9w/01/2p lamp (Philips Electrical Ltd., Sywell, Northampton, England) that emitted a spectrum from 300 nm to 320 nm with a peak emission at 311 nm. The UVB dosage was monitored with a spectroradiometer (TN2340, Taina, Taiwan).
Trypan blue exclusion assay
Cells were treated with the indicated concentration of wild chrysanthemum extract or UVB irradiation. The cells were then trypsinized using 0.25 % trypsin solution (Hyclone, Beijing, China). The cells were incubated with trypan blue (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s indications (Sigma-Aldrich, St. Louis, MO, USA). The live and dead cells were counted as previously described (Xiang et al. 2012).
Hoechst 33342/PI staining
Apoptotic or necrotic cell death was determined with Hoechst 33342 (Sigma-Aldrich, St. Louis, MO, USA) staining and propidium iodide (Sigma-Aldrich, St. Louis, MO, USA) double staining. Cells after UVB irradiation were treated with 10 μg/ml Hoechst 33342 and 10 μg/ml PI for 15 min at 37 °C. Then, the cells were washed with PBS (Hyclone, Beijing, China) twice and analyzed with an Olympus FluoView FV1000 Confocal Microscope (Olympus Co., Ltd, Tokyo, Japan).
Detection of intracellular reactive oxygen species (ROS) production
The intracellular ROS levels were determined as previously described (Tsuji et al. 2011). 2′,7′-dichlorofluorescein diacetate (DCFH-DA, Beyotime, Shanghai, China) was used in the present study to detect intracellular ROS. The compound is a cell-permeable non-fluorescent probe that is de-esterified intracellularly and rapidly oxidized to highly fluorescent 2′,7′-dichlorofluorescein (DCF) in the presence of ROS. After irradiation by UVB for the indicated time, cells were incubated with DCFH-DA (5 mM) for 20 min at 37 °C, washed three times with PBS, and the fluorescence signal of DCFH (Ex = 490 nm; Em = 510 nm), the oxidation product of DCFH-DA, was analyzed with an Olympus FluoView FV1000 Confocal Microscope.
RNA isolation, cDNA synthesis and RT-PCR
After UVB irradiation, the cells were lysed using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and RNA was isolated according to the manufacturer’s instructions. The cDNA was synthesized by using a PrimeScrip RT reagent Kit (Takara, Dalian, China). Then, the MMP-2, MMP-9 and GAPDH were amplified. The primers used in the study are listed in Table 1.
Table 1.
The primer sequences used for RT-PCR
| Name | Sequences |
|---|---|
| MMP-2 Forward | GACCGCTTGGCTTCAAATCA |
| MMP-2 Reverse | CCGCATGGTCTCGATGGTAT |
| MMP-9 Forward | TCTATGGTCCTCGCCCTGAA |
| MMP-9 Reverse | CATCGTCCACCGGACTCAAA |
| GAPDH Forward | GCCGCATCTTCTTTTGCGT |
| GAPDH Reverse | GGACTGTGGTCATGAGTCCT |
Western blotting
The cells were collected, washed, and immediately lysed on ice in lysis buffer containing 50 mM HEPES (pH 7.4), 5 mM EDTA, 50 mM NaCl, 1 % Triton X-100, 50 mM NaF, 5 mg/mL aprotinin, 5 mg/mL leupeptin and 1 mM phenylmethylsulfonyl fluoride (all from Sigma-Aldrich, St. Louis, MO, USA). The proteins (30 μg) were electrophoresed on SDS–polyacrylamide gels and transferred onto PVDF membranes (Millipore, Bedford, MA, USA). The membranes were subsequently blocked with 3 % BSA (Sigma-Aldrich, St. Louis, MO, USA) and incubated with the appropriate primary and secondary antibodies. The primary antibodies including phosphor-ERK1/2 (Sc-81492), ERK2 (Sc-154), phosphor-p38 (Sc-7975-R), p38 (Sc-535), β-actin (Sc-8432) antibody and the secondary antibodies including goat anti mouse IgG-HRP (Sc-2005) and goat anti rabbit IgG-HRP (Sc-2004) were purchased from Santa Cruz (Santa Cruz, CA, USA). The dilution is 1:1000 for primary antibodies and 1:5000 for secondary antibodies. The protein bands were visualized with Super Signal chemiluminescence reagents (Pierce, Rockford, IL, USA), as previously described (Xiang et al. 2012).
Enzyme linked immunosorbent assay (ELISA)
The protein levels of MMP-2 and MMP-9 were determined using commercial ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed as previously described (Xiang et al. 2013). All data are presented as the mean ± SD. Two-sample comparisons were performed using Student’s t tests.
Results
Wild chrysanthemum extract is able to absorb UV radiation
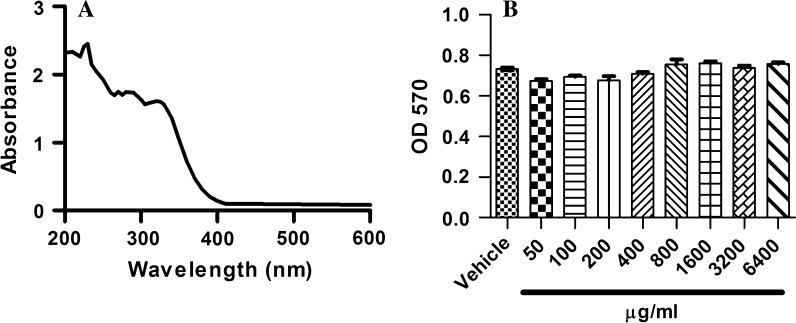
Using spectral scanning, we found that wild chrysanthemum extract (200 μg/ml) absorbed the light with wavelengths between 200 and 400 nm, which covers the UV radiation at the surface of the earth (Fig. 1a). The results indicate that topical administration was able to reduce the UV radiation to the skin.
Fig. 1.
The general features of wild chrysanthemum. a Absorption spectrum of wild chrysanthemum. b HaCat cells were incubated with different concentrations of wild chrysanthemum extract, and the cell viability was determined by MTT assay. The OD570 represented the viability of the live cells. The data in (b) represent the mean ± SD of triplicate samples, p > 0.05 (Student’s t test)
Wild chrysanthemum extract displayed no cytotoxicity to HaCat cells
The influences of wild chrysanthemum extract on the viability of HaCat are shown in Fig. 1b. The results indicate that wild chrysanthemum extract displayed almost no cytotoxicity for HaCat cells up to 6.4 mg/ml. Thus, we can conclude that it is safe to administer wild chrysanthemum extract to keratinocytes.
Wild chrysanthemum extract inhibited high-dose UVB-induced acute HaCat cell death
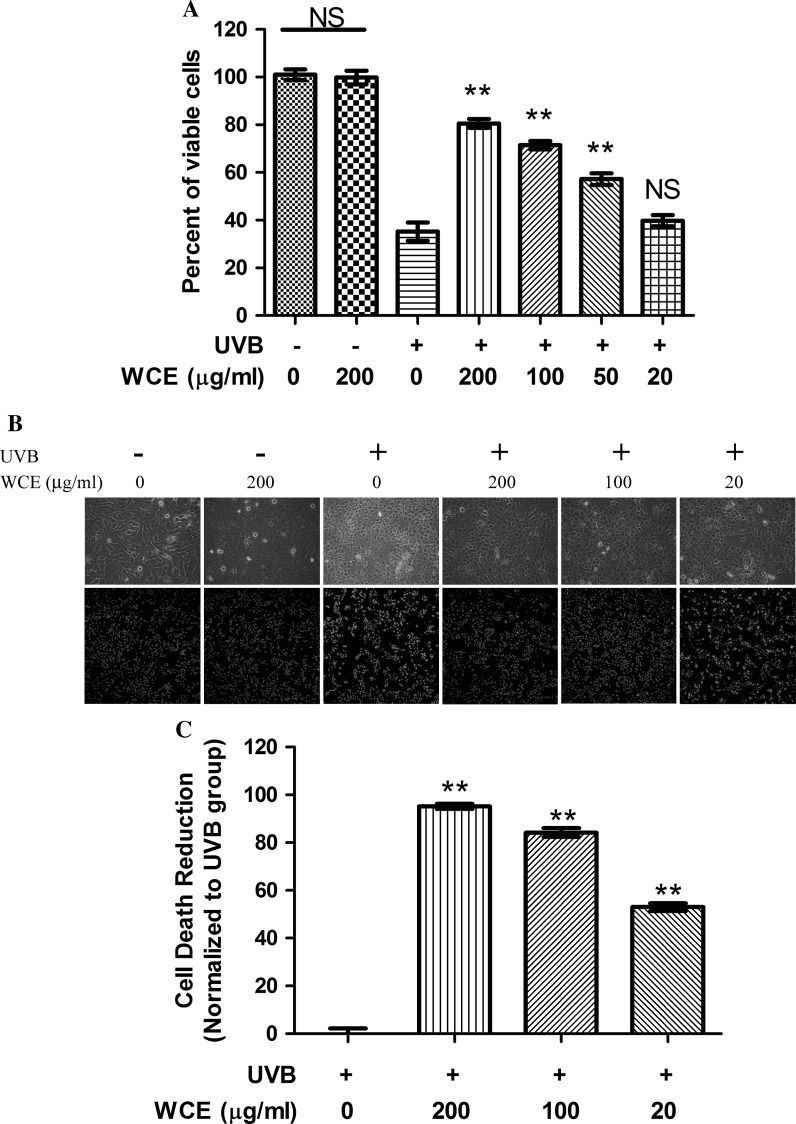
High-dose UVB irradiation induced acute cell death of keratinocytes (Caricchio et al. 2003). Here, after a dose of 120 mJ/cm2 irradiation, more than 65 % of cells were dead according to the trypan blue exclusion assay (Fig. 2a). UVB irradiation at this dose significantly increased the PI-positive cells (Fig. 2b, bottom). With phase contrast microscopy, we were able to determine that UVB irradiation markedly induced an increase in the number of cells with necrotic morphology (Fig. 2b, top). Preincubation of wild chrysanthemum extract with cells efficiently protected the cells from UVB-induced acute cell death in a dose-dependent manner. Both 200 μg/ml and 100 μg/ml wild chrysanthemum extract were able to increase the cell viability (Fig. 2a) and reduce cell death rate (Fig. 2c). The number of cells with necrotic morphology decreased. Moreover, the number of PI-positive cells also significantly decreased (Fig. 2c). However, low doses of wild chrysanthemum extract (20 μg/ml) presented little protective effect for HaCat cells against UVB irradiation.
Fig. 2.
Wild chrysanthemum extract inhibited acute cell death induced by UVB irradiation. a HaCat cells were radiated by UVB (120 mJ/cm2). To test the protective effect of Wild chrysanthemum extract, different concentrations of wild chrysanthemum extract were preincubated with HaCat cells before UVB radiation. HaCat cells that were treated under different conditions were stained with trypan blue, and the percent of viable cells was calculated. b HaCat cells treated under different conditions were photographed under an Olympus CKX 41 inverted phase contrast microscope. The cells were also stained with Hoechst/PI according to the methods described in “Materials and methods” and viewed under an Olympus FluoView FV1000 Confocal Microscope. The cells stained blue represent the living cells, and the red cells represent the dead cells. c Percentage reduction of cell death by wild chrysanthemum extract was calculated based on the Hoechst/PI staining in (b). The data in (a) and (c) represent the mean ± SD of triplicate samples; **(p < 0.01); ns (p > 0.05) (Student’s t test). The other data are representative of at least two independent experiments
Wild chrysanthemum extract attenuated the upregulation of MMPs induced by UVB
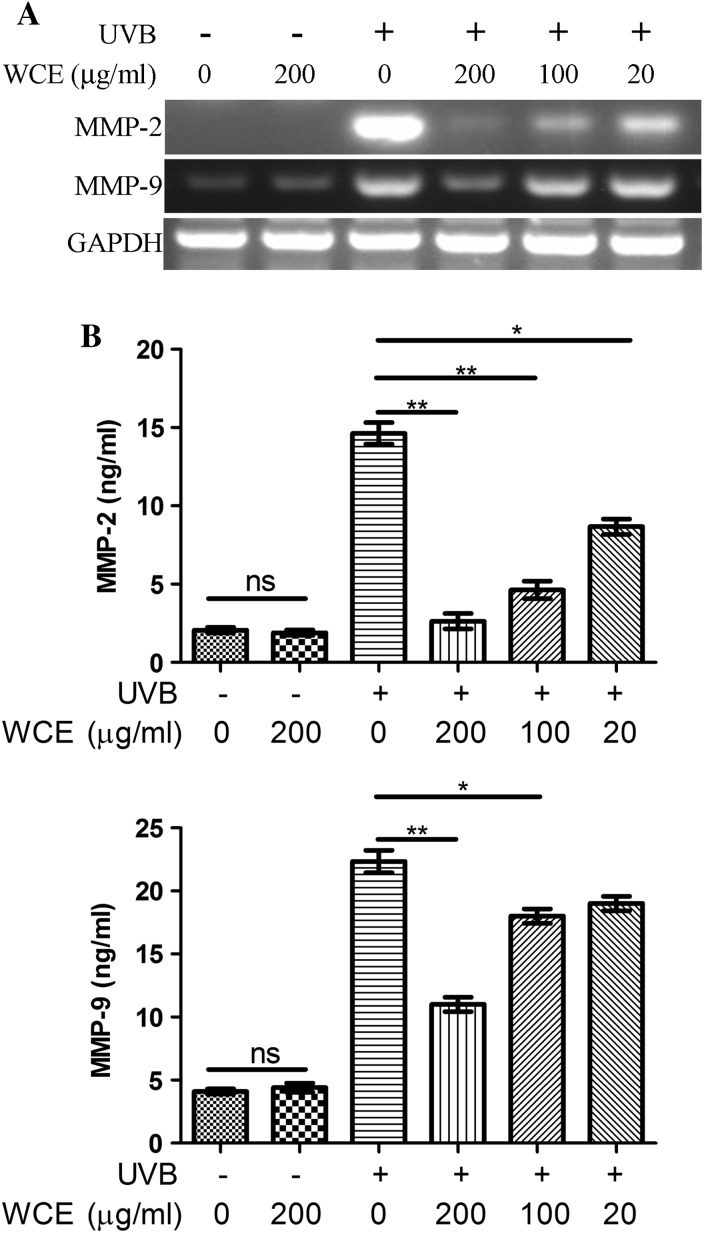
High-dose UVB-induced acute cell death of keratinocytes is fairly easily and often prevented because it may induce extreme discomfort during irradiation, which forces us to take measures to block the sunlight. However, chronic low-dose irradiation of UVB may be neglected. Moreover, it is also harmful to the human body in terms of facilitating skin aging. Thus, in the present study, we also determined the expression of the photoaging-related MMPs from low doses (30 mJ/cm2) of UVB irradiation. The results from the RT-PCR revealed that UVB induced the upregulation of MMP-2 and MMP-9 mRNA level. In addition, 200 μg/ml wild chrysanthemum extract strongly attenuated the upregulation of MMP-2 and MMP-9. Moreover, 20 μg/ml wild chrysanthemum extract slightly attenuated the upregulation of MMP-2 and MMP-9 induced by low-dose UVB irradiation (Fig. 3a). The secreted protein levels of MMP-2 and MMP-9 in the culture supernatant were also analyzed by ELISA, and the results are consistent with the results from RT-PCR (Fig. 3b). This suggests that wild chrysanthemum extract may be able to attenuate photoaging by reducing photoaging-related MMPs.
Fig. 3.
Wild chrysanthemum extract inhibited the upregulation of MMP-2 and MMP-9 induced by UVB radiation. a HaCat cells were irradiated by UVB (or preincubated with wild chrysanthemum extract) and processed for RNA isolation and semi-quantitative PCR. The cDNAs of MMP-2 and MMP-9 were amplified for 30 cycles and that of GAPDH for 20 cycles. b The MMP-2 and MMP-9 concentrations in the culture supernatant in (a) were determined with ELISA. The data in (b) represent the mean ± SD of triplicate samples; **(p < 0.01); *(p < 0.05); ns (p > 0.05) (Student’s t test). The data in (a) are representative of at least two independent experiments
Wild chrysanthemum extract reduced the ROS generation induced by UVB
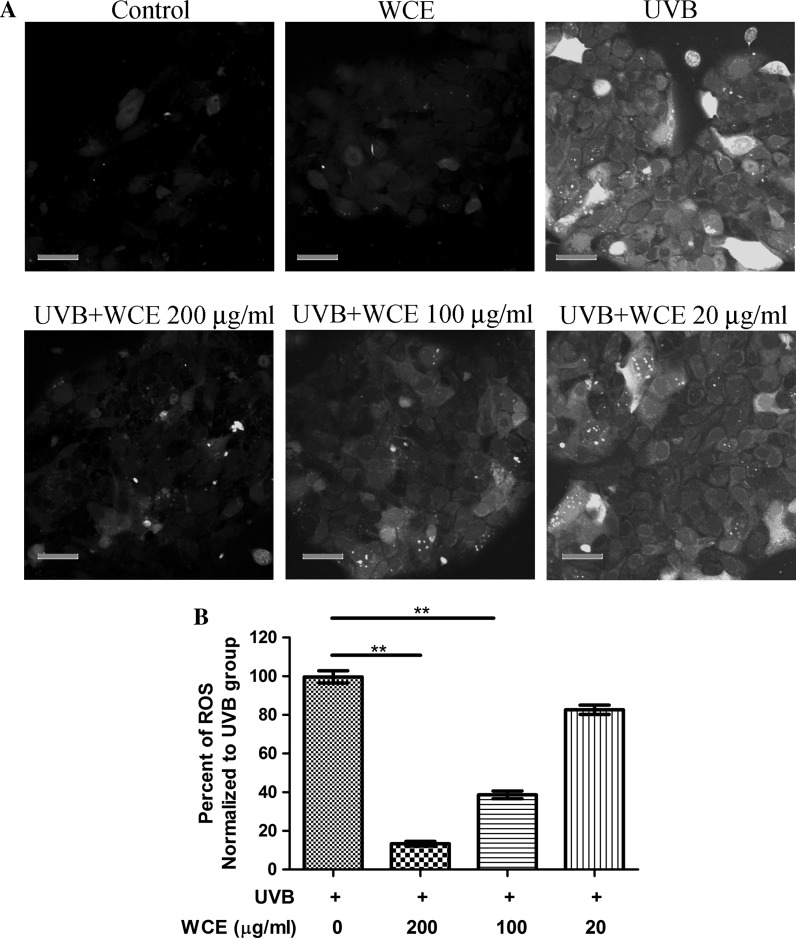
Most established UVB protective extractions from herbs or other plants are dependent on reactive oxygen species (ROS) inhibition. In the present study, we sought to test whether the protective effect exerted by wild chrysanthemum extract is related to the ROS inhibition. By using the fluorescence ROS probe DCFH-DA, it was found that UVB (120 mJ/cm2) induced the generation of ROS (Fig. 4a, top left). Preincubation of wild chrysanthemum extract with cells efficiently reduced UVB-induced ROS generation in a dose-dependent manner. 200 μg/ml wild chrysanthemum extract reduced the ROS generation by more than 80 %. 100 μg/ml wild chrysanthemum extract was also able to reduced the ROS generation by more than 50 % (Fig. 4b).
Fig. 4.
Wild chrysanthemum extract reduced the ROS generation induced by UVB. a Cells irradiated with UVB (120 mJ/cm2) were assayed for ROS generation as described in “Materials and methods”. The appearance of green fluorescence represents the intensity of the ROS generated. Scale bar 50 μm. b The fluorescence intensity in (a) was quantified with ImageJ software; the experiments were repeated three times and are presented in a bar graph. The data in (b) represent the mean ± SD of triplicate samples; **(p < 0.01) (Student’s t test)
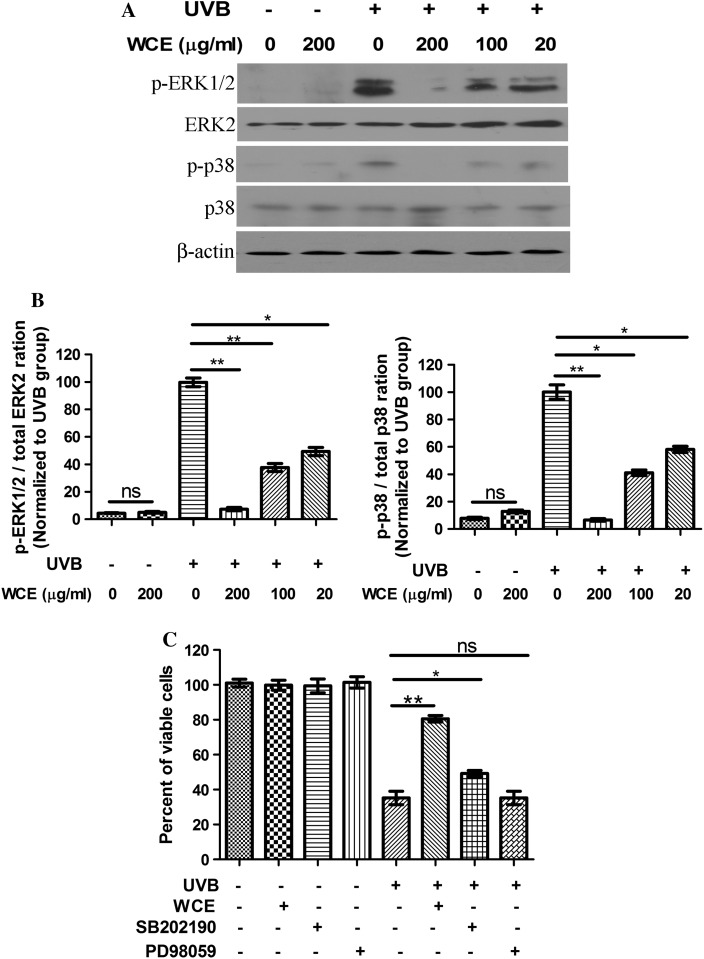
The protective effect of Wild chrysanthemum extract is partly dependent on p38 MAPK inhibition
Previous studies suggested that ERK participates in the signal transduction post UVB irradiation (Chen and Bowden 1999; Jiang et al. 2009). Thus, we analyzed whether wild chrysanthemum extract was able to influence ERK and p38 MAPK activation induced by UVB irradiation. It was demonstrated that UVB (30 mJ/cm2) irradiation induced activation of ERK1/2 and p38 MAPK, as represented by an enhanced level of phospho-ERK1/2 and phospho-p38. However, wild chrysanthemum extract preadministration attenuated ERK and p38 phosphorylation induced by UVB radiation (Fig. 5a). Both ERK1/2 and p38 phosphorylation induced by UVB radiation can be reduced by wild chrysanthemum extract (200 μg/ml) to lower than 20 % of that of the cells untreated with wild chrysanthemum extract (Fig. 5b). To explore the possible role of ERK and p38 MAPK inhibition in UVB-induced cell death and cell survival, we assessed the protective role of an ERK inhibitor (PD98059) and p38 MAPK (SB202190) in UVB-induced cell death, and we found that only SB202190 displayed a protective effect against UVB-induced cell death (Fig. 5c). Thus, we inferred that the protective effect of wild chrysanthemum extract is partly dependent on p38 MAPK inhibition.
Fig. 5.
Wild chrysanthemum extract inhibits the ERK and p38 MAPK activation induced by UVB and its protective effect is dependent on p38 activation. a Cells irradiated with UVB (30 mJ/cm2) were lysed for detection of phospho-ERK1/2, phospho-p38 MAPK, ERK2, p38 and β-actin. Wild chrysanthemum extract attenuated the ERK1/2 and p38 phosphorylation induced by UVB radiation. b The experiments in (a) were repeated three times, and the band intensity was calculated with ImageJ software and presented in a bar graph. The phospho-ERK1/2 levels were calculated and are presented in the left bar graph, and the phospho-p38 levels were calculated and presented in the right bar graph. Columns from left to right in each bar graph represent the bands from left to right in (a). c Cells were pretreated with SB202190 (2 μM) or PD98059 (50 μM) for 30 min and then irradiated with UVB (120 mJ/cm2). The cells were stained with trypan blue, and the percent of viable cells was calculated. The data in (b) and (c) represent the mean ± SD of triplicate samples; **(p < 0.01); *(p < 0.05); ns (p > 0.05) (Student’s t test)
Discussion
UV light is found in sunlight, and it constitutes approximately 10 % of the energy of sunlight. An appropriate dose of UV irradiation is good for the human body because it will promote the synthesis of vitamin D. However, excessive exposure to sunlight will cause many problems, including promoting skin cancer, inducing sunburn, damaging the immune system and facilitating photoaging. The negative effects of UV in the sunlight especially harm people who live in plateau regions as the UV radiation is much stronger in these areas. The Yunnan-Guizhou Plateau is in southwest China. Because of its high altitude and low atmospheric pressure, the level of UV radiation is quite high. Thus, people who live there suffer from the UV radiation from exposure to sunlight during their everyday life. However, it appears that they developed effective methods to protect themselves against UV-induced skin lesions by using herbal medicines. Among them, wild chrysanthemum has proven to be quite effective. They grind the flowers of wild chrysanthemum and spread them on the skin. The present study is just based on the existing usage of wild chrysanthemum to fight against UV-induced skin damage.
Wild chrysanthemum is the flower head of Chrysanthemum indicum L., of the family Asteraceae/Compositae, and the flower can be found wild in most habitats in China, Japan, India, Indochina, Portugal, and other temperate and subtropical regions. Wild chrysanthemum has long been used as Chinese traditional medicine to prevent and treat many diseases. Works from other researchers have demonstrated that the water extract of wild chrysanthemum possesses a hepatoprotective effect (Jeong et al. 2013). There has been a report demonstrating that topical application of wild chrysanthemum attenuates the development of atopic dermatitis-like skin lesions by suppressing serum IgE and some cytokines (Park et al. 2012). Wild chrysanthemum extract is also able to suppress LPS-induced inflammatory responses (Cheon et al. 2009).
Based on the folk use of wild chrysanthemum and previous studies, we hypothesized that wild chrysanthemum extract may possess the ability to protect the skin from UV-induced damage. As excessive UV exposure is harmful to human skin, sunscreen was developed to protect skin from UV (Jou et al. 2012). Traditional sunscreens can be divided into two types: physical sunscreens and chemical sunscreens. Physical sunscreens contain inorganic ingredients such as titanium dioxide or zinc oxide that can physically block out UV radiation. The chemical sunscreens contain organic compounds that absorb UV radiation, and the radiation is converted to heat. It is reported that the use of sunscreen indeed efficiently reduced the risk of melanoma (Green et al. 2011), photoaging (Seite and Fourtanier 2008) and squamous cell carcinoma (Green et al. 1999). However, people misunderstand that although sunscreens decrease the risk of UV-induced skin damage, their use may encourage increased exposure to UV and thus increase the risk of photoaging and skin cancers (Autier 2009). Sunscreens themselves have potential health risks. Some components of sunscreens may damage cells when illuminated. For these reasons, it is reasonable that plant- or animal-derived components that can protect skin from UV are safer as sunscreen components. Herbal extracts are quite promising in the development of a new generation of sunscreens or sunscreen additives. Moreover, the present study demonstrated that wild chrysanthemum extract was able to absorb UV light (Fig. 1a).
Our present study successfully proved that wild chrysanthemum was able to protect the immortalized keratinocytic cell line HaCat from high-dose UVB-induced acute cell death, which indicates that it may be useful to help preventing high-dose UVB-induced sunburn. As a matter of fact, chronic low-dose UVB irradiation-induced photoaging is much more harmful for our skin health (Kim et al. 2010). In addition, the present study demonstrated that wild chrysanthemum extract was able to reduce the UVB upregulation of MMP-2 and MMP-9, which are related to UV-induced photoaging (Park et al. 2006). These results suggest that wild chrysanthemum extract possesses the ability to inhibit UVB-induced photoaging.
ROS plays an important role both in skin physiology (Sena and Chandel 2012) and pathology (Nishigori et al. 2004). It is widely accepted that ROS participates in UV-induced skin disorders, including skin carcinoma and photoaging. Thus, an anti-oxidant is used to antagonize the generation of ROS. Studies have demonstrated that the administration of anti-oxidants protect the skin from skin cancer (Ichihashi et al. 2000) and photoaging (Lee et al. 2012). Moreover, numerous types of anti-oxidants are being used as sunscreen additives. Our study revealed that the anti-oxidant activity of wild chrysanthemum extract, which means it can also be used as a sunscreen additive to reduce the oxidative stress induced by UV. The anti-oxidative properties of wild chrysanthemum extract may be the possible mechanism basis by which it protects keratinocytes from UV-induced acute cell death and photoaging. As mentioned above, the MAPK signaling pathway plays a pivotal role in almost all cell functions, including cell death, but its role in cell survival and cell death is controversial. A previous study demonstrated that a plant extract with anti-oxidant activities is able to reduce UVB-induced ERK1/2 and p38 phosphorylation in HaCat cells (Zaid et al. 2007). In the present study, our results revealed that wild chrysanthemum extract attenuated both ERK and p38 MAPK activation. However, in the control experiments, we found that only p38 MAPK inhibition (with an inhibitor SB202190) reduced cell death induced by UVB radiation. This is consistent with the results reported by Hildesheim et al. (2004). These results suggest that the protective role of wild chrysanthemum extract may be dependent, at least partially, on the inhibition of p38 MAPK activation. Further studies are needed to elucidate the detailed mechanism by which wild chrysanthemum extract inhibits p38 activation.
In conclusion, we have demonstrated that aqueous extracts from wild chrysanthemum are able to protect skin keratinocytes from UVB-induced acute cell death and photoaging. In addition, its anti-oxidant properties may play a key role in fighting UVB-induced damage. The protective effect is partly dependent on the inhibition of UVB-induced p38 phosphorylation. The present study provides a basis for further studies to isolate and identify the effective organic compounds from the Chinese traditional herbal medicine wild chrysanthemum that can protect skin from UVB-induced skin damage and photoaging.
Acknowledgments
This work was supported by the Scientific Research Fund Project of the Yunnan Province Department of Education (2012Y149), Chinese National Natural Science Foundation (31301884, 81160302) and technological innovation projects of Chinese Academy of Sciences (022006).
Conflict of interests
The authors have declared that no competing interests exist.
Footnotes
Sujiao Sun and Ping Jiang contributed equally to this work.
References
- Autier P. Sunscreen abuse for intentional sun exposure. Br J Dermatol. 2009;161:40–45. doi: 10.1111/j.1365-2133.2009.09448.x. [DOI] [PubMed] [Google Scholar]
- Bayerl C, Taake S, Moll I, Jung EG (1995) Characterization of sunburn cells after exposure to ultraviolet light. Photodermatol Photoimmunol Photomed 11(4):149–154 [DOI] [PubMed]
- Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2009;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- Caricchio R, McPhie L, Cohen PL. Ultraviolet B radiation-induced cell death: critical role of ultraviolet dose in inflammation and lupus autoantigen redistribution. J Immunol. 2003;171:5778–5786. doi: 10.4049/jimmunol.171.11.5778. [DOI] [PubMed] [Google Scholar]
- Chen W, Bowden GT. Activation of p38 MAP kinase and ERK are required for ultraviolet-B induced c-fos gene expression in human keratinocytes. Oncogene. 1999;18:7469–7476. doi: 10.1038/sj.onc.1203210. [DOI] [PubMed] [Google Scholar]
- Chen W, Tang Q, Gonzales MS, Bowden GT. Role of p38 MAP kinases and ERK in mediating ultraviolet-B induced cyclooxygenase-2 gene expression in human keratinocytes. Oncogene. 2001;20:3921–3926. doi: 10.1038/sj.onc.1204530. [DOI] [PubMed] [Google Scholar]
- Cheon MS, Yoon T, do Lee Y, Choi G, Moon BC, Lee AY, Choo BK, Kim HK. Chrysanthemum indicum Linne extract inhibits the inflammatory response by suppressing NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol. 2009;122:473–477. doi: 10.1016/j.jep.2009.01.034. [DOI] [PubMed] [Google Scholar]
- Deacon K, Mistry P, Chernoff J, Blank JL, Patel R. p38 Mitogen-activated protein kinase mediates cell death and p21-activated kinase mediates cell survival during chemotherapeutic drug-induced mitotic arrest. Mol Biol Cell. 2003;14:2071–2087. doi: 10.1091/mbc.E02-10-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, Marks GC, Gaffney P, Battistutta D, Frost C, Lang C, Russell A. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354:723–729. doi: 10.1016/S0140-6736(98)12168-2. [DOI] [PubMed] [Google Scholar]
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29:257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Uzquiza A, Arechederra M, Bragado P, Aguirre-Ghiso JA, Porras A. p38alpha mediates cell survival in response to oxidative stress via induction of antioxidant genes: effect on the p70S6 K pathway. J Biol Chem. 2012;287:2632–2642. doi: 10.1074/jbc.M111.323709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YY, Huang JL, Block ML, Hong JS, Chignell CF (2005) Role of phagocyte oxidase in UVA-induced oxidative stress and apoptosis in keratinocytes. J Invest Dermatol 125(3):560–566 [DOI] [PubMed]
- Hildesheim J, Awwad RT, Fornace AJ., Jr p38 Mitogen-activated protein kinase inhibitor protects the epidermis against the acute damaging effects of ultraviolet irradiation by blocking apoptosis and inflammatory responses. J Invest Dermatol. 2004;122:497–502. doi: 10.1111/j.1523-1747.2004.22229.x. [DOI] [PubMed] [Google Scholar]
- Ichihashi M, Ahmed NU, Budiyanto A, Wu A, Bito T, Ueda M, Osawa T. Preventive effect of antioxidant on ultraviolet-induced skin cancer in mice. J Dermatol Sci. 2000;23:S45–S50. doi: 10.1016/S0923-1811(00)00083-9. [DOI] [PubMed] [Google Scholar]
- Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. UV-induced skin damage. Toxicology. 2003;189:21–39. doi: 10.1016/S0300-483X(03)00150-1. [DOI] [PubMed] [Google Scholar]
- Jeong SC, Kim SM, Jeong YT, Song CH. Hepatoprotective effect of water extract from Chrysanthemum indicum L. flower. Chin Med. 2013;8:7. doi: 10.1186/1749-8546-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Cao C, Lu S, Kivlin R, Wallin B, Chu W, Bi Z, Wang X, Wan Y. MEK/ERK pathway mediates UVB-induced AQP1 downregulation and water permeability impairment in human retinal pigment epithelial cells. Int J Mol Med. 2009;23:771–777. doi: 10.3892/ijmm_00000191. [DOI] [PubMed] [Google Scholar]
- Jinlian L, Yingbin Z, Chunbo W. p38 MAPK in regulating cellular responses to ultraviolet radiation. J Biomed Sci. 2007;14:303–312. doi: 10.1007/s11373-007-9148-4. [DOI] [PubMed] [Google Scholar]
- Jou PC, Feldman RJ, Tomecki KJ. UV protection and sunscreens: what to tell patients. Cleve Clin J Med. 2012;79:427–436. doi: 10.3949/ccjm.79a.11110. [DOI] [PubMed] [Google Scholar]
- Kim C, Ryu HC, Kim JH. Low-dose UVB irradiation stimulates matrix metalloproteinase-1 expression via a BLT2-linked pathway in HaCaT cells. Exp Mol Med. 2010;42:833–841. doi: 10.3858/emm.2010.42.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korac RR, Khambholja KM. Potential of herbs in skin protection from ultraviolet radiation. Pharmacogn Rev. 2011;5:164–173. doi: 10.4103/0973-7847.91114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ER, Kang YJ, Kim JH, Lee HT, Cho SG. Modulation of apoptosis in HaCaT keratinocytes via differential regulation of ERK signaling pathway by flavonoids. J Biol Chem. 2005;280:31498–31507. doi: 10.1074/jbc.M505537200. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Cho SW, Kwon YY, Kwon HS, Shin WC. Inhibitory effects of ethanol extracts from nuruk on oxidative stress, melanogenesis, and photo-aging. Mycobiology. 2012;40:117–123. doi: 10.5941/MYCO.2012.40.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E, Cavanaugh JE, Leak RK, Perez RG, Zigmond MJ. Rapid activation of ERK by 6-hydroxydopamine promotes survival of dopaminergic cells. J Neurosci Res. 2008;86:108–117. doi: 10.1002/jnr.21478. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang D, Minemoto Y, Leitges M, Rosner MR, Lin A. NF-kappaB is required for UV-induced JNK activation via induction of PKCdelta. Mol Cell. 2006;21:467–480. doi: 10.1016/j.molcel.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Lobner D, Liot G. Role of MAPK/ERK in neurotrophin-4 potentiation of necrotic neuronal death. Neurochem Res. 2004;29:2303–2309. doi: 10.1007/s11064-004-7040-4. [DOI] [PubMed] [Google Scholar]
- Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxid Redox Signal. 2004;6:561–570. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- Park CH, Lee MJ, Kim JP, Yoo ID, Chung JH. Prevention of UV radiation-induced premature skin aging in hairless mice by the novel compound Melanocin A. Photochem Photobiol. 2006;82:574–578. doi: 10.1562/2005-07-26-RA-623. [DOI] [PubMed] [Google Scholar]
- Park S, Lee JB, Kang S. Topical application of Chrysanthemum indicum L. attenuates the development of atopic dermatitis-like skin lesions by suppressing serum IgE levels, IFN-gamma, and IL-4 in Nc/Nga mice. Evid Based Complement Alternat Med. 2012;2012:821967. doi: 10.1155/2012/821967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pognonec P. ERK and cell death: overview. FEBS J. 2009;277:1. doi: 10.1111/j.1742-4658.2009.07365.x. [DOI] [PubMed] [Google Scholar]
- Quan T, Qin Z, Xia W, Shao Y, Voorhees JJ, Fisher GJ. Matrix-degrading metalloproteinases in photoaging. J Investig Dermatol Symp Proc. 2009;14:20–24. doi: 10.1038/jidsymp.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharffetter-Kochanek K, Wlaschek M, Brenneisen P, Schauen M, Blaudschun R, Wenk J (1997) UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol Chem 378(11):1247–1257 [PubMed]
- Seite S, Fourtanier AM. The benefit of daily photoprotection. J Am Acad Dermatol. 2008;58:S160–S166. doi: 10.1016/j.jaad.2007.04.036. [DOI] [PubMed] [Google Scholar]
- Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouba KJ, Hamadeh HK, Amin RP, Germolec DR. Oxidative stress and its role in skin disease. Antioxid Redox Signal. 2002;4:665–673. doi: 10.1089/15230860260220175. [DOI] [PubMed] [Google Scholar]
- Tsuji G, Takahara M, Uchi H, Takeuchi S, Mitoma C, Moroi Y, Furue M (2011) An environmental contaminant, benzo(a)pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J Dermatol Sci 62(1):42–49. doi:10.1016/j.jdermsci.2010.10.017 [DOI] [PubMed]
- Vicentini FT, He T, Shao Y, Fonseca MJ, Verri WA, Jr, Fisher GJ, Xu Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kappaB pathway. J Dermatol Sci. 2011;61:162–168. doi: 10.1016/j.jdermsci.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Wölfle U, Esser PR, Simon-Haarhaus B, Martin SF, Lademann J, Schempp CM. UVB-induced DNA damage, generation of reactive oxygen species, and inflammation are effectively attenuated by the flavonoid luteolin in vitro and in vivo. Free Radic Biol Med. 2011;50:1081–1093. doi: 10.1016/j.freeradbiomed.2011.01.027. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Gao Q, Su W, Zeng L, Wang J, Hu Y, Nie W, Ma X, Zhang Y, Lee W. Establishment, characterization and immortalization of a fibroblast cell line from the Chinese red belly toad Bombina maxima skin. Cytotechnology. 2012;64:95–105. doi: 10.1007/s10616-011-9399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Wang X, Yan C, Gao Q, Li SA, Liu J, Zhou K, Guo X, Lee W, Zhang Y. Adenosine-5′-Triphosphate (ATP) Protects Mice against Bacterial Infection by Activation of the NLRP3 Inflammasome. PLoS ONE. 2013;8:e63759. doi: 10.1371/journal.pone.0063759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaid MA, Afaq F, Syed DN, Dreher M, Mukhtar H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem Photobiol. 2007;83:882–888. doi: 10.1111/j.1751-1097.2007.00157.x. [DOI] [PubMed] [Google Scholar]