Abstract
Background
Laparoscopic gastrectomy requires a reverse-Trendelenburg position and prolonged pneumoperitoneum and it could cause significant changes in cerebral homeostasis and lead to cognitive dysfunction. We compared changes in regional cerebral oxygen saturation (rSO2), early postoperative cognitive function and hemodynamic variables in patients undergoing laparoscopic gastrectomy with those patients that underwent conventional open gastrectomy.
Methods
Sixty patients were enrolled in this study and the patients were distributed to receive either laparoscopic gastrectomy (laparoscopy group, n = 30) or open conventional gastrectomy (open group, n = 30). rSO2, end-tidal carbon dioxide tension, hemodynamic variables and arterial blood gas analysis were monitored during the operation. The enrolled patients underwent the mini-mental state examination 1 day before and 5 days after surgery for evaluation of early postoperative cognitive function.
Results
Compared to baseline value, rSO2 and end-tidal carbon dioxide tension increased significantly in the laparoscopy group after pneumoperitoneum, whereas no change was observed in the open group. No patient experienced cerebral oxygen desaturation or postoperative cognitive dysfunction. Changes in mean arterial pressure over time were significantly different between the groups (P < 0.001).
Conclusions
Both laparoscopic and open gastrectomy did not induce cerebral desaturation or early postoperative cognitive dysfunction in patients under desflurane anesthesia. However, rSO2 values during surgery favoured laparoscopic surgery, which was possibly related to increased cerebral blood flow due to increased carbon dioxide tension and the effect of a reverse Trendelenburg position.
Keywords: Cerebral oxygen saturation, Laparoscopic gastrectomy, Postoperative cognitive function
Introduction
Postoperative cognitive impairment is an important issue because of its effects on health related quality of life and health economics. Although many benefits, such as, less postoperative pain, faster recovery, shorter hospital stay, and others, have been reported for laparoscopic procedures as compared with conventional open procedures, limited information is available regarding effects of cerebral function. Gameiro et al. [1] inferred more beneficial neuropsychological outcomes after laparoscopic colon surgery in terms of postoperative mood and neuropsychological and serum biochemical parameters than after open colon surgery. However, Gipson et al. [2] found significant decreases in cerebral oxygenation, which have been shown to be correlated with neurocognitive changes, following 10–12 mmHg of pneumoperitoneum in some patients.
Laparoscopic gastrectomy requires a reverse-Trendelenburg position and prolonged pneumoperitoneum for a good surgical view and smooth surgical manipulation. However, since elevated thoracoabdominal pressure disturbs cerebral venous drainage and CO2 absorption dilates cerebral vessels and increases cerebral blood volume [3], it could cause significant changes in cerebral homeostasis and lead to cognitive dysfunction. Furthermore, the 20° reverse-Trendelenburg position has been reported to decrease the concentration of cerebral oxy-hemoglobin and total hemoglobin during laparoscopic cholecystectomy [4]. In addition, such changes in intracranial pressure, cerebral perfusion, and oxygenation lead to longer recovery and impaired cognitive function [5]. In this study, we hypothesized that there might be a changes in regional cerebral oxygen saturation (rSO2) during laparoscopic gastrectomy and subsequent changes in postoperative cognitive function as compared with conventional open gastrectomy. Therefore, we compared changes in rSO2, hemodynamic variables, arterial blood gas analysis and early postoperative cognitive function, as measured by the mini-mental state examination (MMSE), in patients that underwent laparoscopic gastrectomy and compared these with changes in patients that underwent conventional open gastrectomy.
Materials and Methods
After obtaining approval from the Institutional Review Board of our institution, written informed consent was obtained from eligible participants. Sixty adult patients, American Society of Anesthesiologists physical status class I or II, aged 25 to 70 years undergoing gastrectomy were enrolled in this study. Between May 2012 and November 2013, patients who underwent laparoscopic or open gastrectomy were enrolled in this prospective study as a sequential manner until the 30 subjects were recruited in each group. Patients with morbid obesity (body mass index > 30 kg/m2) or cerebrovascular disease, severe chronic obstructive pulmonary disease, active airway infection or history of obstructive airway reaction were excluded. The patients were distributed to one of two groups either laparoscopic gastrectomy (laparoscopy group, n = 30) or open conventional gastrectomy (open group, n = 30) according to previous decisions made by a medical team, and therefore, they were not randomized. The enrolled patients underwent MMSE 1 day before (baseline) and 5 days after surgery. MMSE was accessed by the senior trainee who did not know about the patient's group. Postoperative cognitive dysfunction was defined as a reduction of MMSE scores more than 2 points of baseline.
All patients received glycopyrrolate (0.2 mg) and midazolam (0.05 mg/kg) intramuscularly as premedication 1 h before anesthesia induction. On arrival at the operation room, a pulse oximeter, non-invasive blood pressure, and an electrocardiograph were applied. Anesthesia was induced with propofol (1.5–2.0 ml/kg), rocuronium (0.6 mg/kg), and alfentanil (15 µg/kg). After tracheal intubation, anesthesia was maintained with desflurane at 5–7 vol% in 60% oxygen in air to maintain a bispectral index (BIS) score between 40 and 60 (BIS vista monitor revision 3.0; Aspect Medical Systems, Norwod, MA, USA). After anesthetic induction, a 20 G radial arterial catheter was inserted for continuous arterial monitoring and sampling. Esophageal temperature was measured during operative procedures, and rSO2 was monitored using cerebral oximeter sensors (INVOS 5100; Somanetics Corporation, Troy, MI, USA) on both frontotemporal areas.
Lungs were ventilated at a tidal volume of 8 ml/kg at a respiratory rate of 8–14 breaths/min to maintain an end-tidal carbon dioxide concentration (ETCO2) of 30–35 mmHg. No external positive end-expiratory pressure (PEEP) was applied. Immediately after CO2 insufflation, patient position was changed from supine to a 20° reverse-Trendelenburg position. Intra-abdominal pressure was maintained at 12–15 mmHg during laparoscopy.
Hemodynamic variables, ETCO2 and rSO2 were monitored continuously and recorded before anesthesia induction (pre-IND), 10 min after anesthesia induction (IND, baseline value), and immediately, 10, 20, 30, 40, and 50 min after CO2 insufflation in the laparoscopy group or opening the peritoneum in the open group (T0, T10, T20, T30, T40, and T50, respectively), and at the end of surgery (END). Cerebral desaturation was defined as a reduction of rSO2 < 75% of baseline for ≥15 seconds. Arterial blood gas parameters were measured at 10 min after anesthesia induction in the supine position (IND), at 30 minutes after pneumoperitoneum or peritoneal opening (T30), at the end of surgery (END), and at 30 minutes after arrival in the postanesthetic care unit.
To calculate sample sizes, we used previously reported mean and standard deviation (SD), which were 70.7% and 6.2, respectively, at 30 min after pneumoperitoneum in the Trendelenburg position [6]. To detect the mean intergroup difference of 5% in rSO2 values, 25f patients per group were required for an α-error of 0.05 and a power of 80%. Considering possible dropouts, 30 patients were recruited in per group.
SPSS 17.0 (SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. Normal distribution of data was tested using Kolmogorov-Smirnov tests. The Chi-square test or the independent t-test was used to compare data between the two groups or subgroup analysis of MMSE (≥ 60 vs. < 60 yr) as appropriate. Hemodynamic variables, ETCO2, and rSO2 at each time point were compared using t-test. Changes in hemodynamic variables, ETCO2, and rSO2 over time between the groups were compared using repeated measures ANOVA and, if Mauchly's sphericity test statistic was significant, Greenhouse-Geisser (if ɛ > 0.75) or Huynh-Feldt (if ɛ < 0.75) correction was used. Post-hoc comparison within the group was performed using Bonferroni's test [7]. Values are expressed as means ± SD, median [Q1–Q3] or as numbers of patients, and P values of < 0.05 were considered statistically significant.
Results
Thirty patients were initially enrolled in each group, but two patients in the open group were excluded from the analysis due to changes in operative plan due to diffuse dissemination of cancerous tissue or the incidental detection of another lesion.
Patients' characteristics and peri-operative data are listed in Table 1. No intergroup differences were observed between patients' characteristics. Fluid balances during operative procedures were significantly different in the two groups. Total infused volume of crystalloid (P = 0.049), colloid (P = 0.004), and urine output (P = 0.009) were significantly larger in the open group. Estimated blood loss was also significantly larger in the open group (P = 0.010).
Table 1. Patient Characteristics and Perioperative Data.
| Variables | Laparoscopy (n = 30) |
Open (n = 28) |
P value |
|---|---|---|---|
| Age (yr) | 56 ± 10 | 57 ± 11 | 0.796 |
| Weight (kg) | 64 ± 10 | 64 ± 12 | 0.977 |
| Height (cm) | 166 ± 7 | 167 ± 6 | 0.518 |
| Gender (M/F) | 23 / 7 | 23/5 | 0.607 |
| Hypertension (n) | 10 | 15 | 0.185 |
| Diabetes mellitus (n) | 4 | 5 | 0.454 |
| Operation time (min) | 203 [147–232] | 213 [191–250] | 0.151 |
| Pneumoperitoneum time (min) | 118 [78–180] | - | |
| Total infused fluid | |||
| Crystalloid (ml) | 1000 [700–1300] | 1200 [900–1500] | 0.049 |
| Colloid (ml) | 600 [450–700] | 700 [600–1000] | 0.004 |
| Urine output (ml) | 128 [89–203] | 200[130–350] | 0.009 |
| Estimated blood loss (ml) | 300 [175–500] | 500 [350–500] | 0.010 |
| Pre-induction variables | |||
| Mean arterial pressure (mmHg) | 102 ± 11 | 107 ± 14 | 0.114 |
| Heart rate (beats/min) | 68 ± 15 | 71 ± 14 | 0.358 |
| Regional cerebral oxygen saturation (%) | 68 ± 6 | 65 ± 8 | 0.168 |
Values are means ± SD, medians [Q1–Q3] or number of patients.
MMSE scores at preoperatively and at 5 days postoperatively were similar between groups (P = 0.939 and P = 0.562) and education levels were similar between groups (P = 0.897) (Table 2). Twenty five of the recruited patients were over 60 years old and MMSE scores were significantly different for young (age < 60 yr) and old (age ≥ 60 yr) patients preoperatively (P = 0.001) and postoperatively (P = 0.005) with differences of education levels (P = 0.036) (Table 3). No case of postoperative cognitive dysfunction (defined as a reduction of baseline score > 2 points) was encountered.
Table 2. Mini-mental State Examination and Education Levels between Laparoscopy and Open Group.
| Variables | Laparoscopy (n = 30) |
Open (n = 28) |
P value |
|---|---|---|---|
| Mini-mental state examination | |||
| Preoperative | 28 [26–29] | 28 [26–30] | 0.939 |
| Postoperative 5 day | 29 [27–30] | 29 [27–30] | 0.562 |
| Education level | |||
| 0–6 yr of schooling | 8 | 6 | 0.897 |
| 7–9 yr of schooling | 6 | 6 | |
| Over 10 yr of schooling | 16 | 16 |
Values are median [Q1–Q3] or number of patients.
Table 3. Mini-mental State Examination and Education Levels between Young and Old Aged.
| Variables | Young (n = 33) |
Old (n = 25) |
P value |
|---|---|---|---|
| Mini-mental state examination | |||
| Preoperative | 28 [27–29] | 27 [25–28] | 0.001 |
| Postoperative 5 day | 29 [28–30] | 27 [27–29] | 0.005 |
| Education level | |||
| 0–6 yr of schooling | 5 | 9 | 0.036 |
| 7–9 yr of schooling | 5 | 7 | |
| Over 10 yr of schooling | 23 | 9 |
Values are median [Q1–Q3] or number of patients. Young: age < 60 years, Old: age ≥ 60 years.
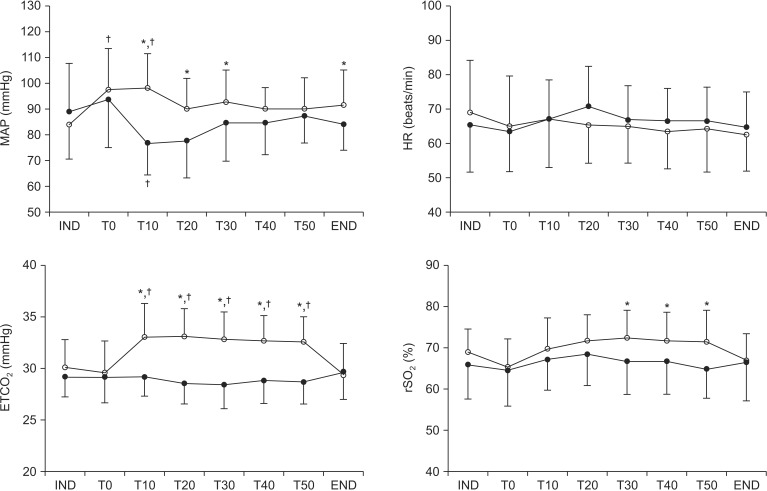
Changes in hemodynamic variables, ETCO2, and rSO2 are illustrated in Fig. 1. All variables had normal distributions (all P > 0.05). Mean arterial blood pressure (MAP) was significantly higher in the laparoscopy group than in open group at T0, 10, 20, 30 and the end of surgery. In MAP, Mauchly's test was not significant (χ2[27] = 54.1, P = 0.17) and the change in MAP over time differed significantly between the groups (F[7, 364] = 4.2, P < 0.01). As compared with baseline (IND), MAP decreased after peritoneal opening in the open group and increased after pneumoperitoneum in the laparoscopy group. ETCO2 was significantly higher in the laparoscopy group than open group at T10-50. In ETCO2, Mauchly's test was significant (χ2[27] = 0.00, P < 0.01) and the change in ETCO2 over time differed significantly between the groups (F[3.8, 195] = 16.6, P < 0.01). Compared to baseline values, ETCO2 was significantly increased after peumoperitoneum in the laparoscopy group. rSO2 was significantly higher in the laparoscopy group than in open group at T30, 40, and 50. In rSO2, Mauchly's test was significant (χ2[27] = 0.00, P < 0.01) and the change in rSO2 over time differed significantly between the groups (F[4.1, 212] = 4.0, P = 0.003). No patient experienced cerebral oxygen desaturation (≤ 75% of baseline) at any time.
Fig. 1. Changes in mean arterial pressure, heart rate, end-tidal carbon dioxide tension, and regional cerebral oxygen saturation in the laparoscopy (○) and open (●) groups. Error bars are SDs. IND: 10 min after anesthesia induction, T0: immediately after CO2 insufflation in the laparoscopy group or peritoneal opening in the open group, T10–50: 10–50 min after CO2 insufflation in the laparoscopy group or peritoneal opening in the open group, END: end of operation, MAP: Mean arterial blood pressure, HR: heart rate. *P < 0.05 vs. open group, †P < 0.05 vs. IND within the group.
Arterial blood gas analysis results are summarized in Table 4. Higher PaCO2 and HCO3– (P = 0.001 and P = 0.037, respectively) with lower pH (P < 0.001) were obtained in the laparoscopy group than in the open group at 30 min after pneumoperitoneum. Hematocrit was higher in the laparoscopy group than in the open group at 30 min after pneumoperitoneum (P = 0.037).
Table 4. Arterial Blood Gas Analysis.
| IND | T30 | END | PACU | ||
|---|---|---|---|---|---|
| pH | Laparoscopy | 7.48 ± 0.04 | 7.42 ± 0.04* | 7.44 ± 0.05 | 7.40 ± 0.06 |
| Open | 7.48 ± 0.04 | 7.47 ± 0.04 | 7.44 ± 0.04 | 7.39 ± 0.05 | |
| PaCO2 (mmHg) | Laparoscopy | 35 ± 3 | 40 ± 4* | 36 ± 4 | 40 ± 6 |
| Open | 34 ± 4 | 35 ± 6 | 36 ± 4 | 40 ± 8 | |
| PaO2 (mmHg) | Laparoscopy | 273 ± 53 | 270 ± 50 | 291 ± 46 | 182 ± 58 |
| Open | 280 ± 45 | 243 ± 62 | 267 ± 45 | 186 ± 75 | |
| HCO3- (mmol/L) | Laparoscopy | 26 ± 3 | 27 ± 3* | 25 ± 2 | 25 ± 2 |
| Open | 26 ± 2 | 25 ± 2 | 25 ± 2 | 24 ± 2 | |
| Lactate (mmol/L) | Laparoscopy | 0.8± 0.5 | 1.0 ± 0.6 | 1.5 ± 0.9 | 1.7 ± 0.9 |
| Open | 0.8 ± 0.3 | 1.0 ± 0.4 | 1.5 ± 1.0 | 1.7 ± 1.3 | |
| Hematocrit (%) | Laparoscopy | 39 ± 4 | 39 ± 5* | 35 ± 5 | 39 ± 5 |
| Open | 37 ± 6 | 36 ± 5 | 33 ± 5 | 37 ± 5 |
Values are means ± SD. IND: 10 min after anesthesia induction, T30: 30 min after CO2 insufflation in the laparoscopy group or peritoneal opening in the open group, END: end of operation, PACU: 30 minutes after arriving in the post-anesthetic care unit. *P < 0.05 vs. open group.
Discussion
This study demonstrated laparoscopic surgery in the reverse Trendelenburg position shows higher rSO2 levels, ETCO2 levels, and higher MAP during pneumoperitoneum, and higher postoperative hematocrit levels than conventional open gastrectomy. However, neither modality was associated with postoperative cognitive dysfunction.
Mild head elevation of less than 30 degree had been known to reduce intracranial pressure, increase cerebral perfusion pressure, and maintain cardiac output with little change in MAP [8,9]. In addition, an elevated PaCO2 levels is known to increase cerebral blood flow and decrease cerebral metabolic rate for oxygen [10,11]. In this study, the higher rSO2 observed in the laparoscopy group could be explained in part by higher cerebral blood flow due to elevated ETCO2 and PaCO2 levels during pneumoperitoneum in the reverse Trendelenburg position [12].
rSO2 estimates brain tissue oxygenation by detecting the relative absorptions of non-oxygenated and oxygenated hemoglobin, and thus, reflects changes in the balance between oxygen delivery and consumption in the brain [13]. Kitajima et al. [4] demonstrated that cerebral oxyhemoglobin and total hemoglobin, measured by near infrared laser spectroscopy, decrease significantly in the head up position and remained low after a return to the supine position and at the end of laparoscopic cholecystectomy surgery. Although we did not measure values for cerebral oxy- and total hemoglobin directly, the consistent rSO2 values obtained indicated that no deleterious reduction in oxyhemoglobin occurred in our patients in either group. In addition, hematocrit was significantly higher in the laparoscopy group than in the open group (39% ± 5% vs. 36% ± 5%, P = 0.037) and estimated blood loss during operation was lower.
Previous reports have demonstrated that pneumoperitoneum induces hemodynamic changes [14,15]. Neuroendocrine changes induced by pneumoperitoneum lead to MAP increases [16]. We found an increase in MAP during pneumoperitoneum and a decrease during peritoneal opening in the open group, which may have contributed to the higher rSO2 levels observed in the laparoscopy group.
The risk of cognitive impairment has been associated with several perioperative factors [17,18,19], for example, general versus regional anesthesia, major versus minor surgery, and old age have been reported increase the risk of postoperative cognitive dysfunction [17,18,19,20,21]. In a previous study in 18056 participants reported that MMSE is varies within the population by age and education level [22]. We also found significantly lower MMSE scores in patient aged ≥ 60 years with low education levels, regardless of the operative procedure used. rSO2 is closely correlated with cognitive decline after major abdominal surgery in the elderly [23]. In the present study, we included patient's ages under 70 years without an uncontrolled co-morbidity, and no patient experienced cerebral oxygen desaturation or cognitive dysfunction, which does not concur with previous reports, in which the incidence of postoperative cognitive dysfunction was found to be 36%–41% in older patients [24,25]. This discrepancy suggests that underlying co-morbidity rather than age per se influences postoperative cognitive dysfunction. In a comparison of the effects of anesthetic agents on early postoperative cognitive dysfunction [25,26,27], desflurane was favoured to sevoflurane and propofol. In the present study, we selected desflurane for anesthetic maintenance and this might have contributed to our result.
In the present study, we did not investigate the effects of pneumoperitoneum and of reverse Trendelenburg position on cerebral blood flow, which would have allowed use to determine their independent hemodynamic and cerebral effects. In addition, we excluded patients at high-risk group of cognitive impairment, such as, those aged > 70 years, those with an uncontrolled comorbidity, and the obesity, which restricts the generalization of our findings. The present study was not a randomized and double-blinded study because patients were treated with laparoscopy or laparotomy according to the decision of the medical team, which was one of limitations of the study.
In conclusion, both open and laparoscopic gastrectomy did not induce cerebral desaturation or early postoperative cognitive dysfunction in patients under desflurane anesthesia. However, rSO2 values during surgery favoured laparoscopic surgery, which was possibly related to increased cerebral blood flow due to increased PaCO2 and positional effects.
References
- 1.Gameiro M, Eichler W, Schwandner O, Bouchard R, Schön J, Schmucker P, et al. Patient mood and neuropsychological outcome after laparoscopic and conventional colectomy. Surg Innov. 2008;15:171–178. doi: 10.1177/1553350608320554. [DOI] [PubMed] [Google Scholar]
- 2.Gipson CL, Johnson GA, Fisher R, Stewart A, Giles G, Johnson JO, et al. Changes in cerebral oximetry during peritoneal insufflations for laparoscopic procedures. J Minim Access Surg. 2006;2:67–72. doi: 10.4103/0972-9941.26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke SJ, Paterson-Brown S. Association between laparoscopic abdominal surgery and postoperative symptoms of raised intracranial pressure. Surg Endosc. 2001;15:723–725. doi: 10.1007/s00464-001-0004-8. [DOI] [PubMed] [Google Scholar]
- 4.Kitajima T, Okuda Y, Yamaguchi S, Takanishi T, Kumagai M, Ido K. Response of cerebral oxygen metabolism in the head-up position during laparoscopic cholecystectomy. Surg Laparosc Endosc. 1998;8:449–452. [PubMed] [Google Scholar]
- 5.Zhou X, Wu MC, Wang YL, Song XY, Ling NJ, Yang JZ, et al. Mannitol improves cerebral oxygen content and postoperative recovery after prolonged retroperitoneal laparoscopy. Surg Endosc. 2013;27:1166–1171. doi: 10.1007/s00464-012-2569-9. [DOI] [PubMed] [Google Scholar]
- 6.Park EY, Koo BN, Min KT, Nam SH. The effect of pneumoperitoneum in the steep Trendelenburg position on cerebral oxygenation. Acta Anaesthesiol Scand. 2009;53:895–899. doi: 10.1111/j.1399-6576.2009.01991.x. [DOI] [PubMed] [Google Scholar]
- 7.Pandit JJ. The analysis of variance in anaesthetic research: statistics, biography and history. Anaesthesia. 2010;65:1212–1220. doi: 10.1111/j.1365-2044.2010.06542.x. [DOI] [PubMed] [Google Scholar]
- 8.Ng I, Lim J, Wong HB. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54:593–597. discussion 598. doi: 10.1227/01.neu.0000108639.16783.39. [DOI] [PubMed] [Google Scholar]
- 9.Feldman Z, Kanter MJ, Robertson CS, Contant CF, Hayes C, Sheinberg MA, et al. Effect of head elevation on intracranial pressure, cerebral perfusion pressure and cerebral blood flow in head injured patients. J Neurosurg. 1992;76:207–211. doi: 10.3171/jns.1992.76.2.0207. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Kanno I, Ibaraki M, Hatazawa J, Miura S. Changes in human cerebral blood flow and cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab. 2003;23:665–670. doi: 10.1097/01.WCB.0000067721.64998.F5. [DOI] [PubMed] [Google Scholar]
- 11.Prough DS, Rogers AT, Stump DA, Mills SA, Gravlee GP, Taylor C. Hypercarbia depresses cerebral oxygen consumption during cardiopulmonary bypass. Stroke. 1990;21:1162–1166. doi: 10.1161/01.str.21.8.1162. [DOI] [PubMed] [Google Scholar]
- 12.Choi SH, Lee SJ, Rha KH, Shin SK, Oh YJ. The effect of pneumoperitoneum and Trendelenburg position on acute cerebral blood flow-carbon dioxide reactivity under sevoflurane anaesthesia. Anaesthesia. 2008;63:1314–1318. doi: 10.1111/j.1365-2044.2008.05636.x. [DOI] [PubMed] [Google Scholar]
- 13.Tobias JD, Johnson GA, Rehman S, Fisher R, Caron N. Cerebral oxygenation monitoring using near infrared spectroscopy during one-lung ventilation in adults. J Minim Access Surg. 2008;4:104–107. doi: 10.4103/0972-9941.45206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joshi GP, Hein HA, Mascarenhas WL, Ramsay MA, Bayer O, Klotz P. Continuous transesophageal echo-Doppler assessment of hemodynamic function during laparoscopic cholecystectomy. J Clin Anesth. 2005;17:117–121. doi: 10.1016/j.jclinane.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 15.Struthers AD, Cuschieri A. Cardiovascular consequences of laparoscopic surgery. Lancet. 1998;352:568–570. doi: 10.1016/S0140-6736(97)11478-7. [DOI] [PubMed] [Google Scholar]
- 16.O'Leary E, Hubbard K, Tormey W, Cunningham AJ. Laparoscopic cholecystectomy: haemodynamic and neuroendocrine responses after pneumoperitoneum and changes in position. Br J Anaesth. 1996;76:640–644. doi: 10.1093/bja/76.5.640. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Yan J, Han X. Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Exp Ther Med. 2013;5:489–494. doi: 10.3892/etm.2012.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen LS. Postoperative cognitive dysfunction: incidence and prevention. Best Pract Res Clin Anaesthesiol. 2006;20:315–330. doi: 10.1016/j.bpa.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen LS, Johnson T, Kuipers HM, Kristensen D, Siersma VD, Vila P, et al. Does anesthesia cause postoperative cognitive dysfunction? A randomized study of regional versus general anaesthesia in 438 elderly patients. Acta Anaesthesiol Scand. 2003;47:260–266. doi: 10.1034/j.1399-6576.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 21.Tzabar Y, Asbury AJ, Millar K. Cognitive failures after general anaesthesia for day-case surgery. Br J Anaesth. 1996;76:194–197. doi: 10.1093/bja/76.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269:2386–2391. [PubMed] [Google Scholar]
- 23.Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Danelli G, et al. Continuous monitoring of cerebral oxygen saturation in elderly patients undergoing major abdominal surgery minimizes brain exposure to potential hypoxia. Anesth Analg. 2005;101:740–747. doi: 10.1213/01.ane.0000166974.96219.cd. [DOI] [PubMed] [Google Scholar]
- 24.Monk TG, Weldon BC, Garvan CW, Dede DE, van der, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 25.Rörtgen D, Kloos J, Fries M, Grottke O, Rex S, Rossaint R, et al. Comparison of early cognitive function and recovery after desflurane or sevoflurane anaesthesia in the elderly: a double-blinded randomized controlled trial. Br J Anaesth. 2010;104:167–174. doi: 10.1093/bja/aep369. [DOI] [PubMed] [Google Scholar]
- 26.Royse CF, Andrews DT, Newman SN, Stygall J, Williams Z, Pang J, et al. The influence of propofol or desflurane on postoperative cognitive dysfunction in patients undergoing coronary artery bypass surgery. Anaesthesia. 2011;66:455–464. doi: 10.1111/j.1365-2044.2011.06704.x. [DOI] [PubMed] [Google Scholar]
- 27.Xu D, Yang W, Zhao G. Effect of propofol and inhalation anesthesia on postoperative cognitive dysfunction in the elderly: a meta-analysis. Nan Fang Yi Ke Da Xue Xue Bao. 2012;32:1623–1627. [PubMed] [Google Scholar]