Abstract
Background
Menstrual irregularity is a common major complaint in women of reproductive age. It is also a known marker for underlying insulin resistance. We investigated the association between menstrual irregularity and metabolic syndrome in the general population of middle-aged women in Korea.
Methods
This cross-sectional study used data from the Korea National Health and Nutrition Examination Survey 2010–2012. A total of 2,742 subjects were included in the analysis. Participants were divided into two categories based on their menstrual cycle regularity and the relationship between metabolic syndrome and its variables was investigated by multiple logistic regression analysis.
Results
Adjusted analyses revealed significantly higher odds ratios for metabolic syndrome, high waist circumference, high triglyceride levels, and low high density lipoprotein cholesterol levels with the presence of menstrual irregularity.
Conclusion
Metabolic syndrome and its components (high waist circumference, high triglyceride levels, and low high density lipoprotein cholesterol levels) were significantly associated with menstrual irregularity in women of reproductive age.
Keywords: Menstrual Cycle, Metabolic Syndrome, Polycystic Ovary Syndrome, Insulin Resistance, Obesity
INTRODUCTION
Menstrual irregularity is a common complaint in women of reproductive age. According to classification guidelines from the International Federation of Gynecology and Obstetrics (FIGO) Menstrual Disorders Working Group, abnormal uterine bleeding is distinguished by four key characteristics: regularity, frequency, heaviness of flow, and duration. Menstrual bleeding is classified as irregular, absent, infrequent, frequent, heavy, heavy and prolonged, and light, prolonged and shortened.1)
Menstrual disorders are a known marker for underlying insulin resistance (IR).2,3,4) IR is well known to be related to type 2 diabetes mellitus (DM), hypertension (HTN), and dyslipidemia.5) Therefore, menstrual disorders are likely also associated with metabolic syndrome (MetS).
The causes of menstrual irregularity include: (1) pregnancy; (2) endocrine causes such as poorly controlled DM, polycystic ovary syndrome (PCOS), Cushing disease, thyroid dysfunction, premature ovarian failure, and late-onset congenital adrenal hyperplasia; (3) acquired conditions such as stress-related hypothalamic dysfunction, medications, exercise-induced amenorrhea, and eating disorders (both anorexia and bulimia); and (4) ovarian tumors, adrenal tumors and prolactinomas.6) Although there are many causes, our study focused on the association between menstrual irregularity and MetS.
Previous studies have reported MetS to be more prevalent in women with PCOS. However, few studies have reported on the association between menstrual irregularity and MetS in the general population. Therefore, we conducted this cross-sectional study to assess the association between MetS and menstrual irregularity in women of reproductive age in the Korean general population.
METHODS
1. Survey and Subjects
This cross-sectional study was conducted using data from Korea National Health and Nutrition Examination Survey (KNHANES) 2010–2012. The KNHANES were designed to evaluate national health and nutrition status and are conducted by the Division of Chronic Disease Surveillance under the Korea Centers for Disease Control and Prevention (KCDC). The survey consists of an interview for health status, nutritional assessment, and health examination.
For this study, 22,792 subjects were excluded among 25,534 candidates in the KNHANES 2010–2012. The exclusion criteria included men (n=11,616), age (under 19 years, over 40 years, or in a menopausal state; n=11,024), pregnancy (n=2), and missing data (n=150). Finally, a total of 2,742 subjects were included in the analysis. All participants provided written informed consent, and the institutional review board of the KCDC approved the study protocol.
2. Sociodemographic and Lifestyle Characteristics
A self-reported questionnaire was used to directly as participants about their menstrual regularity. Regular menstruation was defined as women with consistent menstrual cycles lasting less than 35 days. In contrast, irregular menstruation was defined as women with irregular menstrual cycles less or more than 35 days, regardless of the regularity.7) Occupation status, household income, educational level, and physical activity were also assessed from individual interviews conducted by trained staff. Alcohol consumption and smoking status were assessed from the self-reported questionnaire. Subjects were classified as drinkers and non-drinkers based on their alcohol drinking behaviors in the previous year. Drinkers were defined as those subjects who reported consuming at least one drink per month. Dose-related effects were not considered, which was a potential limitation of this study.7) Smoking status was categorized into current smokers and non-smokers at the time of interview. Lower income level was defined as the lowest quartile of the total participants. A higher educational level was defined as high school graduate or beyond. Subjects who exercised moderately for over 30 minutes per session more than five times per week, or those who exercised vigorously for over 20 minutes per session more than three times per week were defined as performing regular physical exercise.8)
3. Anthropometric and Biochemical Measurements
Height and body weight were measured to the nearest 0.1 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated using the formula: body weight (kg)/height2 (m2). Waist circumference (WC) was measured at the midpoint between the lower costal margin and the iliac crest during exhalation. Blood pressure was measured on the right arm in a seated position using a standard mercury sphygmomanometer (Baumanometer; WA Baum Co., New York, NY, USA) after five minutes of rest. Systolic and diastolic blood pressures were measured three times in five-minute intervals, respectively; the average of the second and third measurements was used in the analysis. Blood samples were obtained after a fasting period of at least eight hours. The levels of fasting plasma glucose (FPG), triglyceride (TG), total cholesterol, high density lipoprotein cholesterol (HDLC), and low density lipoprotein cholesterol (LDLC) were measured enzymatically using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan).
4. Metabolic Syndrome Definitions
MetS was defined according to Heart, Lung, and Blood Institute and American Heart Association guidelines as any three or more of the following: (1) FPG ≥100 mg/dL (or taking medicine for high glucose); (2) blood pressure ≥130/85 mm Hg (or taking medicine for high blood pressure); (3) TG ≥150 mg/dL (or taking medicine for high triglycerides); (4) HDLC <50 mg/dL; and (5) WC for Asians ≥80 cm (32 inch).9)
High WC (≥80 cm), high TG (≥150 mg/dL or taking medicine for high triglycerides), high FPG (≥100 mg/dL or taking medicine for high glucose), low HDLC (<50 mg/dL), and high BP (≥130/85 mm Hg or taking medicine for high blood pressure) were also defined as MetS components.
5. Statistical Analysis
SAS ver. 9.2 (SAS Institute Inc., Cary, NC, USA) was used to perform all statistical analyses. P-values less than 0.05 were considered statistically significant. Participants were divided into two categories based on menstrual cycle regularity. The baseline clinical and metabolic characteristics were expressed as mean±standard error or percentage (standard error). Differences between them based on the presence of menstrual irregularity were analyzed by chi-square test. Logistic regression models were used to calculate multivariable adjusted odd ratios (ORs) and 95% confidence intervals (CIs). There were no adjustments in the first model. The second model (model 2) was minimally adjusted for age, alcohol consumption, smoking status, and physical activity. The third model (model 3) was adjusted for the variables in model 2 plus BMI.
RESULTS
1. Baseline Characteristics according to Presence of Menstrual Irregularity
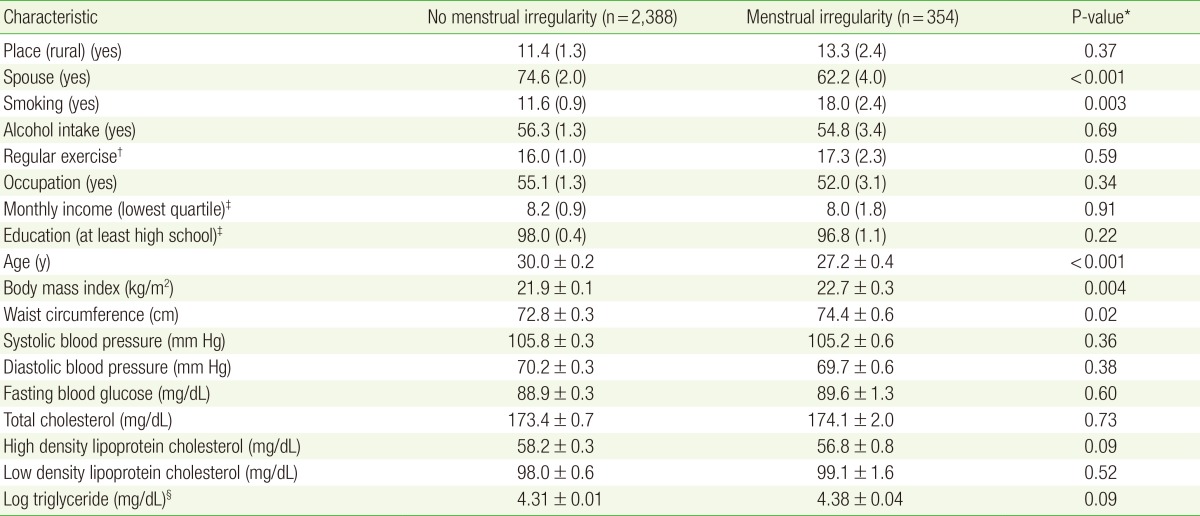
Table 1 presents the baseline clinical and metabolic characteristics of the study subjects according to the presence of menstrual irregularity. Age differed significantly between groups. Metabolic variables, including BMI and WC, also differed significantly based on the presence of menstrual irregularity. Finally, sociodemographic and lifestyle variables, including smoking status and married subjects, were also significantly different between groups based on menstrual irregularity.
Table 1. Principal clinical characteristics of subjects according to menstrual irregularity in Korean National Health and Nutrition Examination Survey 2010-2012.
Values are presented as mean±standard error (SE) or % (SE).
*Obtained by chi-square or Student t-tests. †Physical activity was defined using the International Physical Activity Questionnaire. The regular exercise group included subjects who exercised moderately more than five times a week for 30 minutes per session or those who exercised intensively more than three times a week for 20 minutes per session. ‡Household income was adjusted for the number of family members; the household income was divided into quartiles. 'Low income' and 'low education' were defined as the lowest quartile of income and ≤9 years of education, respectively. §Triglycerides levels were logarithmic transformed because of their skewed distribution.
2. Relationship between Menstrual Irregularity and Metabolic Syndrome
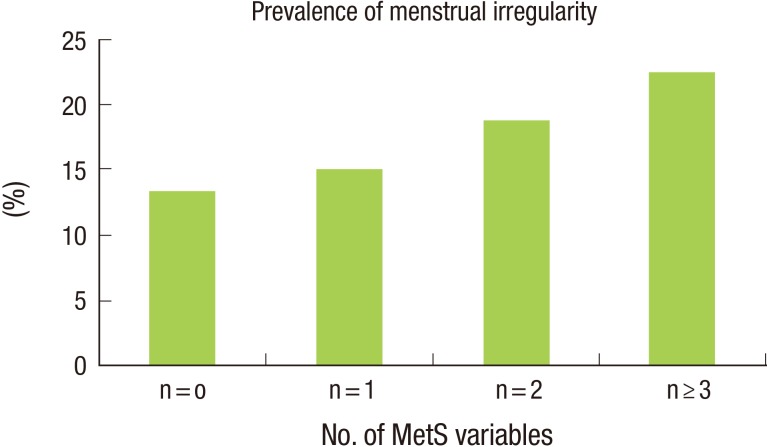
Table 2 shows the relationship between the presence of menstrual irregularity and MetS and its variables. The prevalence of MetS was significantly different according to the presence of menstrual irregularity. The prevalence of abnormal WC, TG, and HDLC levels were also significantly higher among participants with menstrual irregularity. Figure 1 shows the association between the presence of menstrual irregularity and the total number of MetS components. The presence of menstrual irregularity increased with increasing numbers of MetS components.
Table 2. Relationship between menstrual irregularity and MetS.
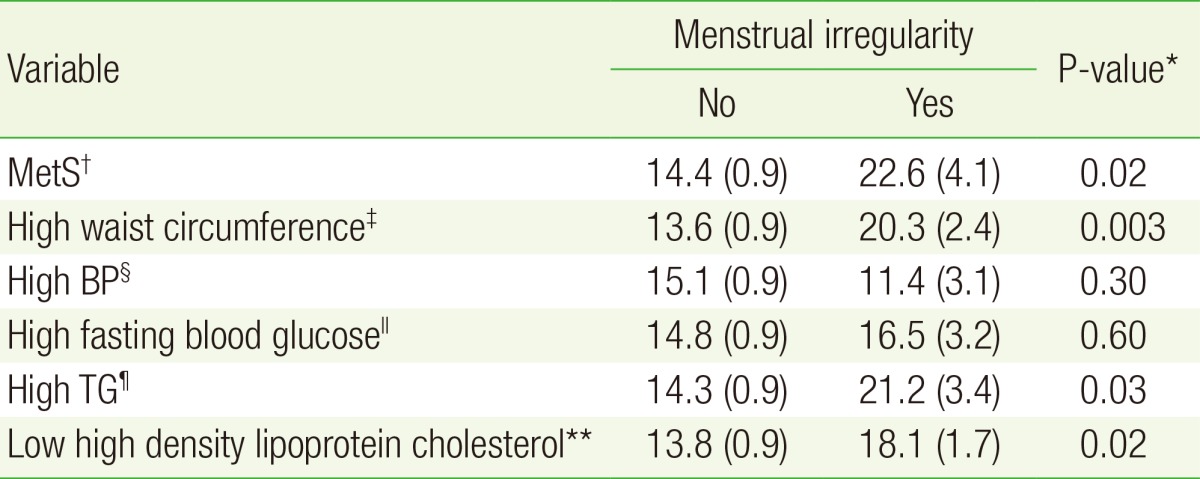
Values are presented as % (standard error).
MetS, metabolic syndrome; BP, blood pressure; TG, triglyceride.
*Obtained by chi-square or Student t-tests. †Defined according to Heart, Lung, and Blood Institute and American Heart Association guidelines. ‡For Asians ≥80 cm (32 inch). §≥130/85 mm Hg (or taking medicine for high BP). ∥≥100 mg/dL (or taking medicine for high glucose). ¶≥150 mg/dL (or taking medicine for high TG). **<50 mg/dL.
Figure 1. shows the association between the presence of menstrual irregularity and the total number of MetS components (P<0.001). MetS, metabolic syndrome.
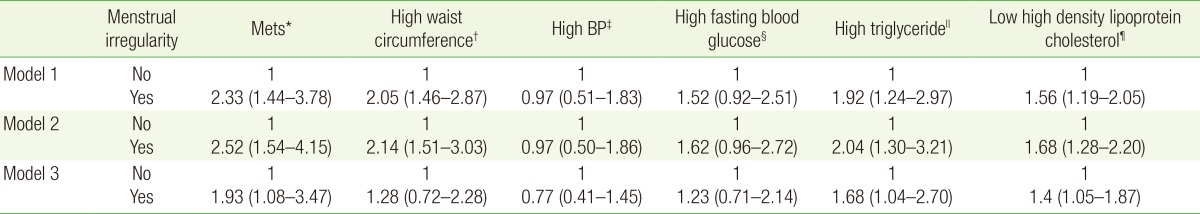
3. ORs and 95% CIs for Metabolic Syndrome Variables and Associated Traits according to the Presence of Menstrual Irregularity
Table 3 presents ORs (95% CIs) for MetS, high WC, high BP, high FPG, high TG, and low HDLC according to the presence of menstrual irregularity. ORs for MetS, high WC, high TG, and low HDLC tended to increase with the presence of menstrual irregularity in the adjusted analyses.
Table 3. ORs and 95% CIs for MetS variables and associated traits according to the presence of menstrual irregularity.
Values are presented as ORs and 95% CIs. Values are analyzed by multiple logistic regression analysis after adjusting for age, body mass index, alcohol consumption, smoking status, and physical activity. Model 1: no adjustments. Model 2: adjustment for age, alcohol consumption, smoking status, and physical activity. Model 3: adjustment for variables in model 2 plus body mass index.
OR, odds ratio; CI, confidence interval; MetS, metabolic syndrome; BP, blood pressure.
*Defined according to Heart, Lung, and Blood Institute and American Heart Association guidelines. †For Asians, ≥80 cm (32 inch). ‡≥130/85 mm Hg (or taking medicine for high BP). §≥100 mg/dL (or taking medicine for high blood glucose). ∥≥150 mg/dL (or taking medicine for high triglycerides). ¶<50 mg/dL.
DISCUSSION
The results of this cross-sectional study demonstrate that irregular menstruation is associated with MetS. Among MetS variables, high WC, high TG, and low HDLC were significantly associated with irregular menstruation. Previous studies have also reported menstrual irregularity to be related to MetS.10)
While obesity can predispose individuals to MetS, our study revealed a significant correlation between high WC and menstrual irregularity. Previous studies have shown that obese women are more likely to experience menstrual irregularity than non-obese women.11,12) Obese women have excess fat cells in which the extraglandular aromatization of androgen to estrogen occurs. These women also have lower circulating levels of sex hormone-binding globulin, which converts free androgens to estrone. This mechanism leads to development of PCOS. Patients with PCOS also frequently have IR and hyperinsulinemia.13) Obesity is most common cause of IR and hyperinsulinemia; however, despite its frequent occurrence in PCOS, obesity alone does not explain the association.14,15) PCOS and obesity appear to act synergistic on insulin.
Several studies have shown that women with PCOS have a higher prevalence of MetS than age-matched women in the general population.16,17,18) In Korea, the prevalence of MetS in women with PCOS was approximately 14.5%, nearly 3.5-fold higher than that reported for age-matched women in urban Korean populations.19,20) PCOS is the most common cause of ovulatory dysfunction and hyperandrogenism in women.21,22) It is associated with abnormal metabolic features such as IR, MetS, dyslipidemia, and increased cardiovascular risk factors.23) IR also plays an important role in the pathogenesis of PCOS.24) Androgen, which is highly associated with PCOS, may play a key role in IR. Shen et al. concluded that the degree of menstrual irregularity did not correlate with the severity of IR and metabolic disorders in women without androgen excess.25) Hyperinsulinemia is not a characteristic of hyperandrogenism in general but is uniquely associated with PCOS.15) Although subjects with PCOS were not analyzed as a separate variable on our study, it is possible that PCOS might mediate irregular menstruation.
Noticeably, high blood pressure was not significantly associated with menstrual irregularity. A previous study showed that HTN appeared to be associated with PCOS; while HTN was not associated with BMI in women with PCOS, it was associated in those without PCOS.26) This observation suggests that HTN is not significantly related to menstrual irregularity.
Our study has several limitations. First, information on menstrual cycle characteristics relied upon written questionnaires rather than menstrual diaries. Previous studies have suggested that retrospective self reports of menstrual cycle lengths are error-prone27) with variable accuracy between diary records and retrospectively recalled menstrual cycle length.28) Second, the questionnaires did not use standard criteria to define regular menstruation. According to recommendations from the FIGO, normal menstruation and the normal menstrual cycle should be defined according to the following parameters: (1) frequency of menses (24–38 days), (2) regularity of menses (variation±2–20 days), (3) duration of menstrual flow (4.5–8 days), and (4) volume of monthly blood loss (5–80 mL).1) Thus, subjects might have experienced confusion regarding their menstrual regularity, which may have led to inaccurate data. Third, another potential limitation of this study was its exclusion of users of hormonal contraceptives. Some women may have been prescribed hormonal contraceptives in response to a history of irregular menstrual cycles. Thus, the prevalence of menstrual irregularity may have been underestimated. Fourth, other causes of menstrual irregularity not related to MetS, such as pregnancy and tumors, were not excluded. Finally, we could not examine levels of hormones such as estrogen, testosterone, sex hormone-binding globulin, follicle stimulating hormone, and luteinizing hormone that could affect the associations between body composition and menstrual cycle characteristics, nor could we determine whether women with irregular or variable cycles were an- or oligoovulatory.
In conclusion, the results of our study suggest increased risk of MetS in women with menstrual irregularity. In addition, PCOS may mediate MetS. Further studies are necessary to assess the association between menstrual irregularity and MetS.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29:383–390. doi: 10.1055/s-0031-1287662. [DOI] [PubMed] [Google Scholar]
- 2.Weiss DJ, Charles MA, Dunaif A, Prior DE, Lillioja S, Knowler WC, et al. Hyperinsulinemia is associated with menstrual irregularity and altered serum androgens in Pima Indian women. Metabolism. 1994;43:803–807. doi: 10.1016/0026-0495(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 3.Dunaif A, Segal KR, Futterweit W, Dobrjansky A. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes. 1989;38:1165–1174. doi: 10.2337/diab.38.9.1165. [DOI] [PubMed] [Google Scholar]
- 4.Robinson S, Kiddy D, Gelding SV, Willis D, Niththyananthan R, Bush A, et al. The relationship of insulin insensitivity to menstrual pattern in women with hyperandrogenism and polycystic ovaries. Clin Endocrinol (Oxf) 1993;39:351–355. doi: 10.1111/j.1365-2265.1993.tb02376.x. [DOI] [PubMed] [Google Scholar]
- 5.Reaven GM. Role of insulin resistance in human disease (syndrome X): an expanded definition. Annu Rev Med. 1993;44:121–131. doi: 10.1146/annurev.me.44.020193.001005. [DOI] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics Committee on Adolescence. Diaz A, Laufer MR, Breech LL. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118:2245–2250. doi: 10.1542/peds.2006-2481. [DOI] [PubMed] [Google Scholar]
- 7.Korea Centers for Disease Control and Prevention. The Fifth Korea National Health and Nutrition Examination Survey (KNHANES V) 2010-2012. Cheongju: Korea Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 8.Chun MY. Validity and reliability of Korean version of International Physical Activity Questionnaire short form in the elderly. Korean J Fam Med. 2012;33:144–151. doi: 10.4082/kjfm.2012.33.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 10.Bouzas IC, Cader SA, Leao L, Kuschnir MC, Braga C. Menstrual cycle alterations during adolescence: early expression of metabolic syndrome and polycystic ovary syndrome. J Pediatr Adolesc Gynecol. 2014;27:335–341. doi: 10.1016/j.jpag.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Castillo-Martinez L, Lopez-Alvarenga JC, Villa AR, Gonzalez-Barranco J. Menstrual cycle length disorders in 18- to 40-y-old obese women. Nutrition. 2003;19:317–320. doi: 10.1016/s0899-9007(02)00998-x. [DOI] [PubMed] [Google Scholar]
- 12.Brown WJ, Mishra G, Kenardy J, Dobson A. Relationships between body mass index and well-being in young Australian women. Int J Obes Relat Metab Disord. 2000;24:1360–1368. doi: 10.1038/sj.ijo.0801384. [DOI] [PubMed] [Google Scholar]
- 13.Christakou CD, Diamanti-Kandarakis E. Role of androgen excess on metabolic aberrations and cardiovascular risk in women with polycystic ovary syndrome. Womens Health (Lond Engl) 2008;4:583–594. doi: 10.2217/17455057.4.6.583. [DOI] [PubMed] [Google Scholar]
- 14.Graf MJ, Richards CJ, Brown V, Meissner L, Dunaif A. The independent effects of hyperandrogenaemia, hyperinsulinaemia, and obesity on lipid and lipoprotein profiles in women. Clin Endocrinol (Oxf) 1990;33:119–131. doi: 10.1111/j.1365-2265.1990.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 15.Dunaif A, Graf M, Mandeli J, Laumas V, Dobrjansky A. Characterization of groups of hyperandrogenic women with acanthosis nigricans, impaired glucose tolerance, and/or hyperinsulinemia. J Clin Endocrinol Metab. 1987;65:499–507. doi: 10.1210/jcem-65-3-499. [DOI] [PubMed] [Google Scholar]
- 16.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 17.Weerakiet S, Bunnag P, Phakdeekitcharoen B, Wansumrith S, Chanprasertyothin S, Jultanmas R, et al. Prevalence of the metabolic syndrome in Asian women with polycystic ovary syndrome: using the International Diabetes Federation criteria. Gynecol Endocrinol. 2007;23:153–160. doi: 10.1080/09513590701214158. [DOI] [PubMed] [Google Scholar]
- 18.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–915. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 19.Park HR, Choi Y, Lee HJ, Oh JY, Hong YS, Sung YA. The metabolic syndrome in young Korean women with polycystic ovary syndrome. Diabetes Res Clin Pract. 2007;77(Suppl 1):S243–S246. doi: 10.1016/j.diabres.2007.01.065. [DOI] [PubMed] [Google Scholar]
- 20.Oh JY, Hong YS, Sung YA, Barrett-Connor E. Prevalence and factor analysis of metabolic syndrome in an urban Korean population. Diabetes Care. 2004;27:2027–2032. doi: 10.2337/diacare.27.8.2027. [DOI] [PubMed] [Google Scholar]
- 21.Setji TL, Brown AJ. Polycystic ovary syndrome: update on diagnosis and treatment. Am J Med. 2014;127:912–919. doi: 10.1016/j.amjmed.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Ecklund LC, Usadi RS. Endocrine and reproductive effects of polycystic ovarian syndrome. Obstet Gynecol Clin North Am. 2015;42:55–65. doi: 10.1016/j.ogc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 23.Teede H, Deeks A, Moran L. Polycystic ovary syndrome: a complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med. 2010;8:41. doi: 10.1186/1741-7015-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holte J. Disturbances in insulin secretion and sensitivity in women with the polycystic ovary syndrome. Baillieres Clin Endocrinol Metab. 1996;10:221–247. doi: 10.1016/s0950-351x(96)80085-1. [DOI] [PubMed] [Google Scholar]
- 25.Shen SY, Huang SY, Hsieh CH, Hsu MI, Cheng CY, Hsu CS. Clinical and biochemical characteristics of women with menstrual disturbance. Taiwan J Obstet Gynecol. 2014;53:178–182. doi: 10.1016/j.tjog.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Joham AE, Boyle JA, Zoungas S, Teede HJ. Hypertension in reproductive-aged women with polycystic ovary syndrome and association with obesity. Am J Hypertens. 2015;28:847–851. doi: 10.1093/ajh/hpu251. [DOI] [PubMed] [Google Scholar]
- 27.Small CM, Manatunga AK, Marcus M. Validity of self-reported menstrual cycle length. Ann Epidemiol. 2007;17:163–170. doi: 10.1016/j.annepidem.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Jukic AM, Weinberg CR, Wilcox AJ, McConnaughey DR, Hornsby P, Baird DD. Accuracy of reporting of menstrual cycle length. Am J Epidemiol. 2008;167:25–33. doi: 10.1093/aje/kwm265. [DOI] [PMC free article] [PubMed] [Google Scholar]