Abstract
Traumatic brain injury (TBI) is followed by a state of metabolic dysfunction, affecting the ability of neurons to use energy and support brain plasticity; there is no effective therapy to counteract the TBI pathology. Brain-derived neurotrophic factor (BDNF) has an exceptional capacity to support metabolism and plasticity, which highly contrasts with its poor pharmacological profile. We evaluated the action of a flavonoid derivative 7,8-dihydroxyflavone (7,8-DHF), a BDNF receptor (TrkB) agonist with the pharmacological profile congruent for potential human therapies. Treatment with 7,8-DHF (5 mg/kg, ip, daily for 7 days) was effective to ameliorate the effects of TBI on plasticity markers (CREB phosphorylation, GAP-43 and syntaxin-3 levels) and memory function in Barnes maze test. Treatment with 7,8-DHF restored the decrease in protein and phenotypic expression of TrkB phosphorylation after TBI. In turn, intrahippocampal injections of K252a, a TrkB antagonist, counteracted the 7,8-DHF induced TrkB signaling activation and memory improvement in TBI, suggesting the pivotal role of TrkB signaling in cognitive performance after brain injury. A potential action of 7,8-DHF on cell energy homeostasis was corroborated by the normalization in levels of PGC-1α, TFAM, COII, AMPK and SIRT1 in animals subjected to TBI. Results suggest a potential mechanism by which 7,8-DHF counteracts TBI pathology via activation of the TrkB receptor and engaging the interplay between cell energy management and synaptic plasticity. Since metabolic dysfunction is an important risk factor for the development of neurological and psychiatric disorders, these results set a precedent for the therapeutic use of 7,8-DHF in a larger context.
Keywords: Cognition; 7,8-dihydroxyflavone; Metabolism; Plasticity; Traumatic Brain Injury
1. Introduction
Traumatic brain injury (TBI) is associated with lowered capacity of the brain for plasticity and with long-term disturbances in cognitive function [1]. Most of the attempts to develop pharmaceutical approaches to reduce the sequel of TBI have yielded unsuccessful results [2] mostly based on the multifactorial character of the TBI pathology. Therefore, approaches empowered with the capacity to normalize several weaknesses affected by the TBI pathology likely have advantages over confined approaches. The therapeutic potential of brain-derived neurotrophic factor (BDNF) is strong based on its pleiotropic capacity [3]; however, limitations associated with the delivery of BDNF into the brain, its poor pharmacokinetic profile and short half life [4] have rendered therapeutic intentions impractical.
The extraordinary capacity of BDNF to support several aspects of brain plasticity and function, suggests that agents that stimulate the TrkB receptor, without the poor pharmacokinetics of BDNF, can be ideal therapeutic agent to combat the TBI pathology. BDNF and TrkB signaling are reduced in the TBI pathology [5], thereby reducing neuronal function and making neurons more vulnerable to secondary challenges. 7,8-dihydroxyflavone (7,8-DHF) is a member of the flavonoid family of compounds present in fruits and vegetables, which acts as a small molecule TrkB agonist [4]. The binding of 7,8-DHF to the cysteine cluster 2 and leucine-rich region in the extracellular domain of the TrkB receptor provokes TrkB receptor dimerization and autophosphorylation, which leads to activation of downstream signaling cascades similar to BDNF. In particular, 7,8-DHF has demonstrated neuroprotective effects against oxidative stress incurred from glutamate toxicity [6], decreases infarct volumes in stroke, and reduces toxicity in an animal model of Parkinson′s disease [4]. These features portray 7,8-DHF as an ideal candidate to be used therapeutically to counteract the effects of the TBI pathology.
Patients suffering from mild or moderate concussive injury experience abnormalities in the control of brain metabolism [7, 8] and increased risk for secondary brain injury [9, 10]. A growing line of research indicates that approaches leading to promote energy homeostasis are a productive strategy to support brain function [11], and to counteract the pathogenesis of TBI [12]. The peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1α) is a transcriptional regulator of various transcription factors important to maintain mitochondrial homeostasis [13], including nuclear respiratory factors (NRFs). The NRFs activate the mitochondrial transcription factor A (TFAM) that regulates mitochondrial DNA (mtDNA) transcription and replication [14, 15]. The adenosine monophosphate-activated protein kinase (AMPK) acts as an energy sensor in the cell and regulates PGC-1α [16]. In turn, PGC-1α can also influence neuronal plasticity by acting on BDNF [17]. BDNF supports a range of metabolic events, which are crucial for neuronal function [18, 19], and they are compromised in the TBI pathology. Therefore, we sought to activate the TrkB receptor using the safety profile of 7,8-DHF to counteract energy dysfunction, enhance plasticity, and improve behavioral performance after TBI.
2. Materials and methods
2.1. Animals and experimental design
Male Sprague–Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) approximately 2 months old were housed in polyacrylic cages and maintained under standard housing conditions (room temperature 22–24°C) with 12 h light/dark cycle. All animals had free access to food and drinking water. After acclimatization for 1 week on standard rat chow, rats were trained on the Barnes maze test for 5 days to learn the task, and then subjected to the either sham or fluid percussion injury (FPI). All animals received intraperitoneal injections (1ml/kg) of either 7,8-dihydroxyflavone (7,8-DHF; 5 mg/kg, i.p.) or vehicle (VEH; 30% DMSO in PBS) once daily for 7 consecutive days, starting from the day of injury till 2 h prior to the memory retention behavior. 7,8-DHF (TCI America, OR, USA) was dissolved in phosphate-buffered saline (PBS) containing 30% dimethylsulfoxide (DMSO) and prepared fresh daily. The dose of 7,8-DHF was chosen on the basis of previous in vivo studies [4, 20, 21], which demonstrated that systemic administration of 7,8-DHF leads to central TrkB activation, and related behavioral changes. Memory retention was tested by Barnes maze after 1 week of injury and animals were sacrificed immediately by decapitation for tissue collection (Fig. 1). Based on the injury (Sham vs. FPI) and treatment (VEH vs. 7,8-DHF), there were four experimental groups (n = 7 per group): (I) sham plus vehicle (Sham/VEH); (II) sham plus 7,8-dihydroxyflavone (Sham/7,8-DHF); (III) fluid percussion injury plus vehicle (FPI/VEH); (IV) fluid percussion injury plus 7,8-dihydroxyflavone (FPI/7,8-DHF).
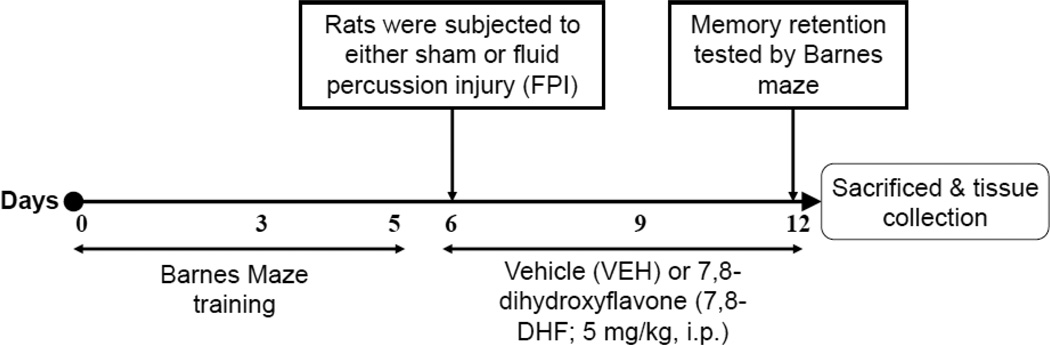
Fig. 1.
Schematic timeline representing experimental design: Rats were subjected to 5 days training on the Barnes maze test, followed by either sham or fluid percussion injury (FPI). All animals received intraperitoneal injection (1ml/kg) of either vehicle (VEH; 30% DMSO in PBS) or 7,8-dihydroxyflavone (7,8-DHF; 5 mg/kg, i.p.) once daily for 7 consecutive days, starting from the day of injury till 2 h prior to the memory retention test. Memory retention was tested by Barnes maze at 7 days of injury and animals were sacrificed immediately for tissue collection.
In order to validate that the effects of 7,8 DHF occurred via the trkB receptor, a separate set of animals were given a single unilateral intrahippocampal injection of K252a (a TrkB antagonist) bound to fluorescence latex microspheres (Lumaflour Corp., FL, USA), or microsphere vehicle as a control (n=7 per group). The treatments were as follows: microspheres vehicle injected group followed by FPI and 7,8-DHF (VEH/FPI/7,8-DHF) and K252a injected group followed by FPI and 7,8-DHF (K252a/FPI/7,8-DHF). FPI and 7,8-DHF (5 mg/kg, ip) treatments were given beginning on the third day following the intrahippocampal injection. Memory retention was tested by Barnes maze 1 week post FPI, and animals were sacrificed immediately following the test via decapitation.
All experiments were performed in accordance with the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of California at Los Angeles (UCLA) Chancellor’s Animal Research Committee (ARC). The suffering and number of animals used were minimized.
2.2. Administration of K252a into the hippocampus
The microspheres were coated with K252a (46.8 ng/µl sterile water) by passive absorption, according to previously described methods [22, 23]. The concentration of K252a was chosen based on its effective blockade for BDNF receptor TrkB [23, 24]. Prior to injection,, 2–5% isoflurane anesthesia was administered to the rats using a Mobile Laboratory Animal Anesthesia System (VetEquip Inc., CA, USA). Rats were positioned in a stereotaxic apparatus to secure the sight for the injection. Vehicle or K252a imbedded in microspheres was injected directly into the left hippocampus (3.8 mm posterior to bregma, 2.6 mm lateral to midline and 3.7 mm vertical from skull) using a Hamilton syringe in a volume of 2 µl over 15 min. After the injection, the skull was sutured and rats were placed in a heated recovery chamber before being returned to their home cages.
2.3. Fluid percussion injury
The injury was performed as previously described [25]. In brief, animals were anesthetized by 2–5% isoflurane mixed with 100% O2 using a Mobile Laboratory Animal Anesthesia System (VetEquip Inc., CA, USA). A 3.0-mm-diameter craniotomy was made over the left parietal cortex, 3.0 mm posterior to bregma and 6.0 mm lateral (left) to the midline with a high-speed drill (Dremel, WI, USA). A plastic injury cap was placed over the craniotomy with silicone adhesive and dental cement. When the dental cement hardened, the cap was filled with 0.9% saline solution. Anesthesia was discontinued and the injury cap was attached to the fluid percussion device. At the first sign of hind-limb withdrawal to a paw pinch, a moderate fluid percussion pulse (2.7 atm) was administered to the epidural space. Immediately upon responding to a paw pinch, anesthesia was restored and the skull was sutured. Neomycin was applied on the suture and the rats were placed in a heated recovery chamber before being returned to their cages. Sham animals underwent an identical preparation with the exception of the lesion.
2.4. Barnes maze test
All rats were tested on the Barnes maze before and after experimental exposure to assess the cognitive functions [26]. In brief, our maze was manufactured from acrylic plastic to form a disk 1.5 cm thick and 115 cm in diameter, with 18 evenly spaced 7 cm holes at its edges. The disk was brightly illuminated by four overhead halogen lamps to provide an aversive stimulus. Animals were trained to locate a dark escape chamber hidden underneath a hole positioned around the perimeter of a disk. All trials were recorded simultaneously by a video camera installed directly overhead at the centre of the maze. Animals were trained with two trials per day for five consecutive days before being subjected to the experimental conditions. A trial was started by placing the animal in the centre of the maze covered under a cylindrical start chamber; after a 10 s delay, the start chamber was raised. A training session ended after the animal had entered the escape chamber or when a pre-determined time (5 min) had elapsed, whichever came first. Memory retention was tested during Barnes maze trials performed one week after injury. All surfaces were routinely cleaned before and after each trial to eliminate possible olfactory cues from preceding animals.
2.5. Tissue collection
After the memory test the animals were killed immediately by decapitation and the fresh brains were dissected out, frozen in dry ice and stored at −70°C until use.
2.6. Immunobloting
The hippocampal tissues from the left hemisphere were homogenized in a lysis buffer containing 137 mM NaCl, 20 mM Tris–HCl pH 8.0, 1% NP40, 10% glycerol, 1 mM phenylmethylsulfonylfluoride (PMSF), 10 µg/ml aprotinin, 0.1 mM benzethonium chloride, 0.5 mM sodium vanadate. The homogenates were then centrifuged, the supernatants were collected and total protein concentration was determined according to MicroBCA procedure (Pierce, IL, USA), using bovine serum albumin (BSA) as standard.
Briefly, protein samples were separated by electrophoresis on a 10% polyacrylamide gel and electrotransferred to a PVDF membrane (Millipore, MA, USA). Non-specific binding sites were blocked in Tris-buffered saline (TBS), pH 7.6, containing 5% non-fat dry milk. Membranes were rinsed in buffer (0.05% Tween-20 in TBS) and then incubated with anti-actin or anti-BDNF, anti-pTrkB, anti-TrkB, anti-AMPK, anticytochrome c oxidase II (COII), anti-mtTFA (TFAM), anti-GAP-43, anti-syntaxin-3 (1:500; Santa Cruz Biotechnology, CA, USA), anti-PGC-1α, anti-SIRT1, anti-pCREB, anti-CREB, (1:1000, Millipore, MA, USA), anti-pAMPK (1:1000; Cell signaling technology, MA, USA) followed by anti-rabbit or anti-goat or anti-mouse IgG horseradish peroxidase-conjugate (1:10,000; Santa Cruz Biotechnology, CA, USA). After rinsing with buffer, the immunocomplexes were visualized by chemiluminescence using the ECL kit (Amersham Pharmacia Biotech Inc., NJ, USA) according to the manufacturer's instructions. The film signals were digitally scanned and then quantified using ImageJ software. Actin was used as an internal control for western blot such that data were standardized according to actin values.
2.7. Immunohistochemistry
Additional rats (n=4) from each group were anesthetized with isoflurane, then intracardially perfused with PBS (pH 7.4) followed by 4% paraformaldehyde in phosphate buffer (pH 7.4) and 30% sucrose in paraformaldehyde. Tissues were flash frozen on dry ice and stored at −80°C. For immunofluorescence staining, serial coronal brain sections (30 µm) were cut on a cryostat and collected into 0.01M phosphate buffered saline (PBS). After washing, free-floating sections were heated to 80°C for 30 min in 10 mM sodium citrate buffer (pH 8.5) to promote antigen binding and allowed to return to room temperature. Non-specific binding was blocked by incubating the sections with 0.01M PBS solution containing 0.5% BSA and 0.5% Triton X-100 for 2 h at room temperature. Sections were then incubated with rabbit polyclonal anti-pTrkB receptor (Y816) primary antibody at a dilution of 0.3 µg/mL, (a kind gift from Dr. Moses Chao) or mouse monoclonal anti-GAP-43 primary antibody (1:250; Santa Cruz Biotechnology, CA, USA), in 0.01 M PBS solution containing 0.2% BSA and 0.6% Triton X-100 at 4°C overnight. After thorough washing with 0.01M PBS, the sections were incubated with rabbit or mouse secondary antibody (Cy3; 1:250; FITC; 1:250, Jackson Immunoresearch; West Grove, PA, USA) for 1.5 h at room temperature. Sections were mounted using Prolong Gold antifade reagent (Life technologies, New York, NY, USA). The staining was visualized under Zeiss microscope (Zeiss Imager.Z1; Gottingen, DE) using the Axiovision software (Carl Zeiss Vision, version 4.6).
For brightfield staining, serial coronal brain sections (30 µm) were cut on a cryostat, collected free-floating in 0.01M PBS. Tissue sections were processed for localization of syntaxin-3 according to immunohistochemical procedures described previously [27]. After washing in 0.01M PBS, sections were incubated with goat polyclonal anti-syntaxin-3 (1:250; Santa Cruz Biotechnology, CA, USA) antibody overnight at 4°C. Following incubation, sections were washed with 0.01M PBS and incubated for 1.5 h in biotinylated secondary antibody at room temperature. Antibody binding was visualized using the avidin–biotin– horseradish peroxidase complex (Vectastain ABC Kit, Vector Laboratories, CA, USA) and subsequently enhanced by DAB-nickel (DAB Peroxidase Substrate kit Vector Laboratories, CA, USA) incubation. The rinsed sections were then mounted on gelatin-coated glass slides, dehydrated, cleared, and coverslipped. The staining was visualized under Zeiss microscope (Zeiss Imager.Z1; Gottingen, DE) using the Axiovision software (Carl Zeiss Vision, version 4.6).
2.8. Statistical Analysis
The results are represented as mean ± standard error of the mean (SEM). Protein results are expressed as percentage of Sham/VEH group and statistical analysis was performed by two-way ANOVA [(injury: Sham vs. FPI) and (treatment: VEH vs. 7,8-DHF)]. Post-hoc analyses were conducted using Bonferroni’s multiple comparison tests to determine the significance of difference among various groups. The results of K252a experiments were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparison tests. A level of 5% probability was considered as statistically significant. Pearson correlation analysis was performed on individual samples to evaluate the association between variables.
3. Results
3.1. 7,8-DHF reduces cognitive dysfunction after TBI
All animals were tested for spatial learning in the Barnes maze for 5 days before being subjected to the experimental conditions. All groups showed a decrease in latency time to find the escape hole from first to last day of training, and showed similar latency time on last day (day 5), suggesting the rats were in the same cognitive condition before starting the experiments (data not shown).
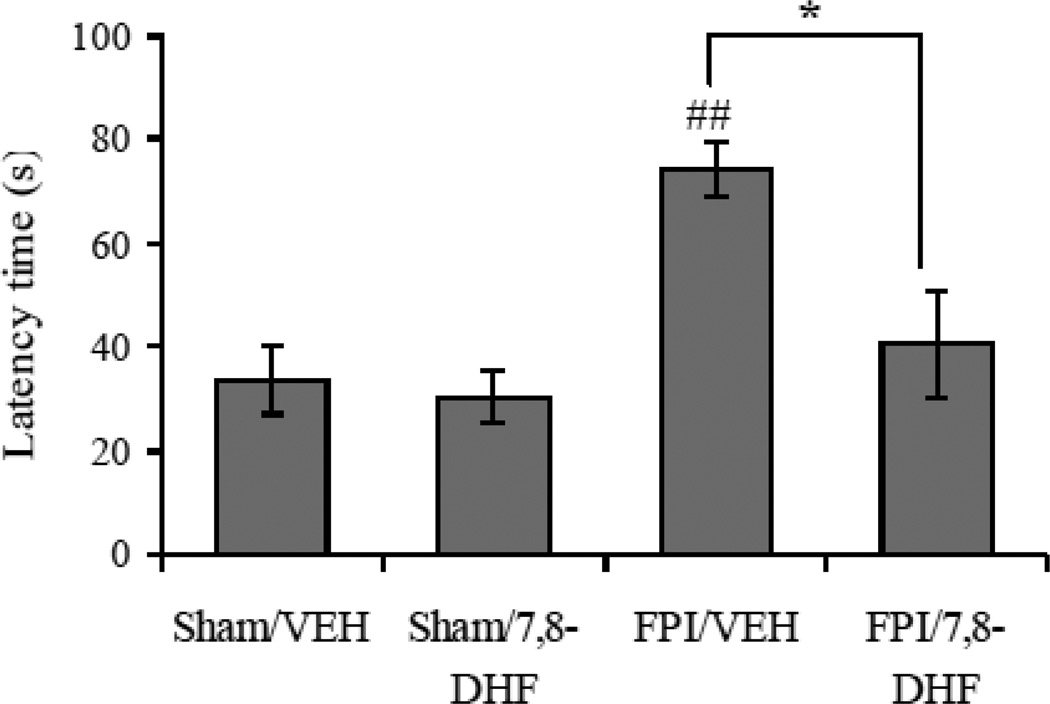
One week after injury, a Barnes maze test was performed for 1 day to evaluate memory retention in vehicle and 7,8-DHF treated animals. Two-way ANOVA analysis showed a significant effect of injury (F1,24 = 12.71 p<0.01) and treatment (F1,24 =6.79 p<0.05), and an interaction between injury vs. treatment (F1,24 =4.51 p<0.05) for the latency time in Barnes maze. The post-hoc analysis showed a significant increase in latency time in animals subjected to the TBI, which is indicative of memory impairment (FPI/VEH vs. Sham/VEH, p<0.01). In turn, the treatment with 7,8-DHF in TBI animals resulted in a significant reduction in latency time (FPI/7,8-DHF vs. FPI/VEH, p<0.05), whereas 7,8-DHF per se did not affect the cognitive state of animals (Sham/7,8-DHF vs. Sham/VEH, p>0.05). These results indicate that 7,8-DHF confers protection against TBI induced memory deficits (Fig. 2). The vehicle used for solubilizing 7,8 DHF was (30% DMSO in PBS), which may have some independent effect on memory, however we observed no differences in behavioral outcomes between (30% DMSO in PBS) and PBS injected animals (data not shown).
Fig. 2.
Comparison of latency times in Barnes maze test in vehicle (VEH) and 7,8-dihydroxyflavone (7,8-DHF; 5 mg/kg, ip) treated rats, subjected to either sham or fluid percussion injury (FPI). Values are expressed as mean ± SEM. ##P<0.01 vs. Sham/VEH; *P<0.05 vs. FPI/VEH; ANOVA (two-way) with Bonferroni's comparisons post-hoc test.
3.2. 7,8-DHF treatment restores activation of TrkB signaling in TBI
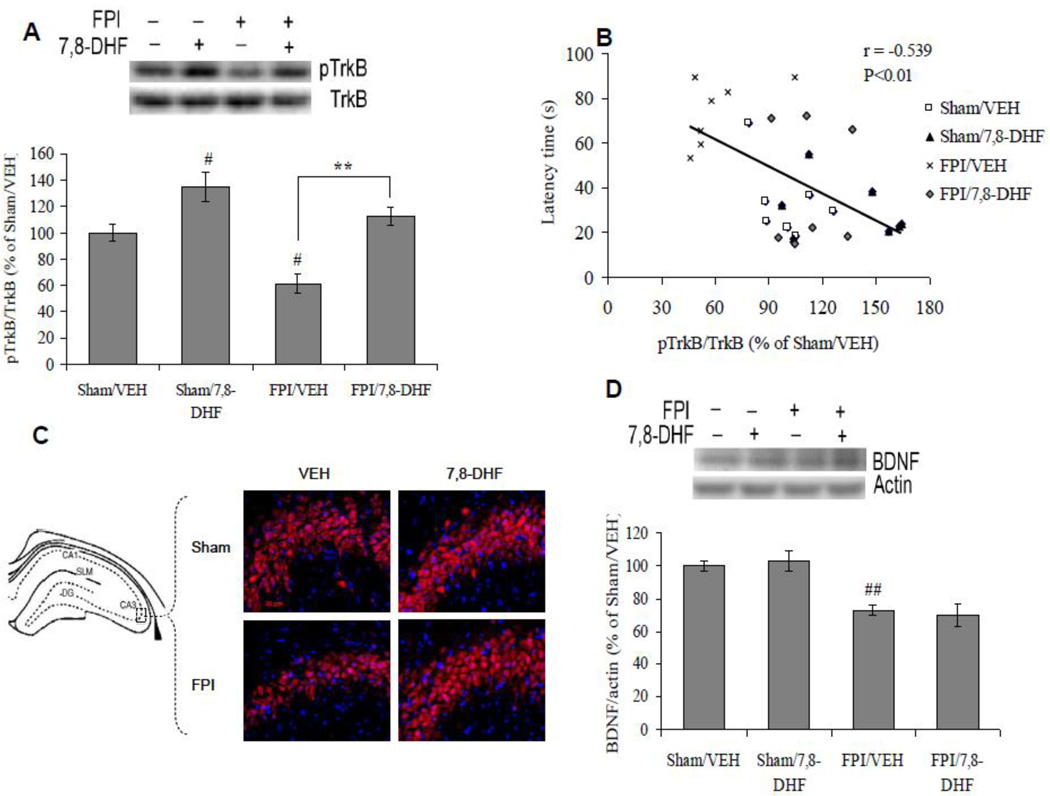
We quantified the levels of BDNF and its receptor TrkB phosphorylation in animals treated with 7,8-DHF with or without TBI. The injury (F1,24 =14.28 p<0.01) and treatment (F1,24 =28.12 p<0.01) had significant effects on the phosphorylation of TrkB, as observed by ANOVA (two-way) analysis. Post-hoc analyses showed that treatment with 7,8-DHF enhanced TrkB phosphorylation in sham animals (Sham/7,8-DHF vs. Sham/VEH, p<0.05). TBI reduced the phosphorylation of TrkB (FPI/VEH vs. Sham/VEH, p<0.05), which was ameliorated by the treatment with 7,8-DHF (FPI/7,8-DHF vs. FPI/VEH, p<0.01), arguing in favor of the therapeutic potential of 7,8-DHF (Fig. 3A). TrkB phosphorylation changed in inverse proportion to the latency time in Barnes maze (r = −0.539, p<0.01), suggesting an association between TrkB signaling and memory performance (Fig. 3B). Additionally, we analyzed the effects of FPI and 7,8-DHF on the phenotypic expression of hippocampal TrkB phosphorylation using immunofluorescence staining. FPI showed a qualitative decrease in the labeling for phosphorylated TrkB receptor, particularly in the CA3 subfield. In turn, the treatment with 7,8-DHF showed a qualitative increase in pTrkB expression in both sham and FPI animals (Fig. 3C).
Fig. 3.
(A) Levels of TrkB phosphorylation, (B) correlation analysis of TrkB phosphorylation with latency time in Barnes maze test and (D) levels of BDNF in vehicle (VEH) and 7,8-dihydroxyflavone (7,8-DHF; 5 mg/kg, ip) treated rats, subjected to either sham or fluid percussion injury (FPI). Data are expressed as percentage of Sham/VEH (mean ± SEM). #P<0.05, ##P<0.01 vs. Sham/VEH; **P<0.01 vs. FPI/VEH; ANOVA (two-way) with Bonferroni's comparisons post-hoc test. (C) Immunofluorescence staining of pTrkB (Cy3) in hippocampal coronal sections from vehicle and 7,8-DHF (5 mg/kg, ip) treated groups, subjected to either sham or FPI (scale bar: 20 µm).
We quantified the effects of 7,8-DHF treatment on BDNF levels based on the capacity of 7,8-DHF to work as a BDNF analog on TrkB receptor activation. Two-way ANOVA analysis showed a significant effect of injury (F1,24 =34.17 p<0.01) on BDNF levels. Post-hoc test showed a significant decrease in BDNF levels in animals subjected to TBI (FPI/VEH vs. Sham/VEH, p<0.01). The treatment with 7,8-DHF did not affect the levels of BDNF in sham (Sham/7,8-DHF Vs Sham/VEH, p>0.05) or in animals subjected to TBI (FPI/7,8-DHF Vs FPI/VEH, p>0.05; Fig. 3D).
3.3. Intrahippocampal K252a counteracts the beneficial effect of 7,8-DHF in TBI
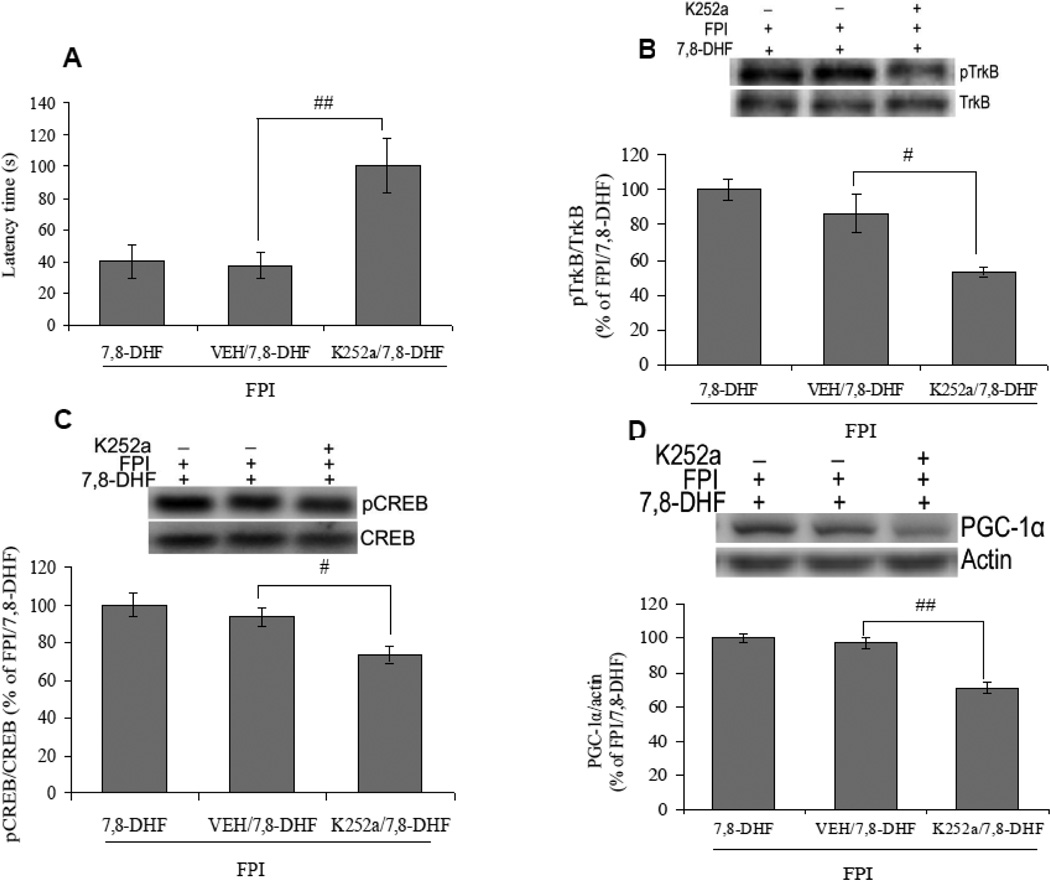
Since 7,8-DHF is as selective TrkB agonist, therefore, we have studied the effect of a TrkB antagonist, K252a on memory functions and TrkB activation in 7,8-DHF treated TBI animals, to determine whether the activation of TrkB signaling is the underlying basis for the regulation of cognitive performance. Results showed that intrahippocampal injection of K252a counteracted the memory improving action of 7,8-DHF in TBI as evidenced by an increased latency time on Barnes maze in K252apretreated TBI animals, subjected to the 7,8-DHF treatment (K252a/FPI/7,8-DHF vs. VEH/FPI/7,8-DHF, p<0.01; Fig. 4A). According to our results, K252a also ameliorated the 7,8-DHF induced TrkB phosphorylation in animals subjected to TBI (K252a/FPI/7,8-DHF vs. VEH/FPI/7,8-DHF, p<0.05; Fig. 4B). We assessed the effect of K252a on TrkB downstream signaling molecules such as CREB and PGC-1α. The data show that intrahippocampal injection of K252a counteracted the effects of 7,8-DHF on CREB phosphorylation and levels of PGC-1α in animals subjected to TBI (K252a/FPI/7,8-DHF vs. VEH/FPI/7,8-DHF, p<0.05; Fig. 4C and 4D). These results provide evidence for a role of TrkB signaling for the action of 7,8-DHF on CREB and PGC-1α, and a possible role in rescuing a memory deficit.
Fig. 4.
Effects of K252a (46.8 ng/µl), a TrkB antagonist, on 7,8-dihydroxyflavone (7,8-DHF; 5 mg/kg, ip) induced changes in (A) latency times in Barnes maze and (B) TrkB phosphorylation in rats subjected to the fluid percussion injury (FPI). K252a coated fluorescence latex microspheres were prepared, to inject K252a directly into the left hippocampus and normal fluorescence latex microspheres were used as a vehicle. Data for TrkB phosphorylation are expressed as percentage of FPI/7,8-DHF (mean ± SEM). #P<0.05, ##P<0.01 vs. VEH/FPI/7,8-DHF; ANOVA (one-way) with Bonferroni's comparisons post-hoc test.
3.4. 7,8-DHF treatment counteracts the reduction of molecules associated with brain plasticity in TBI
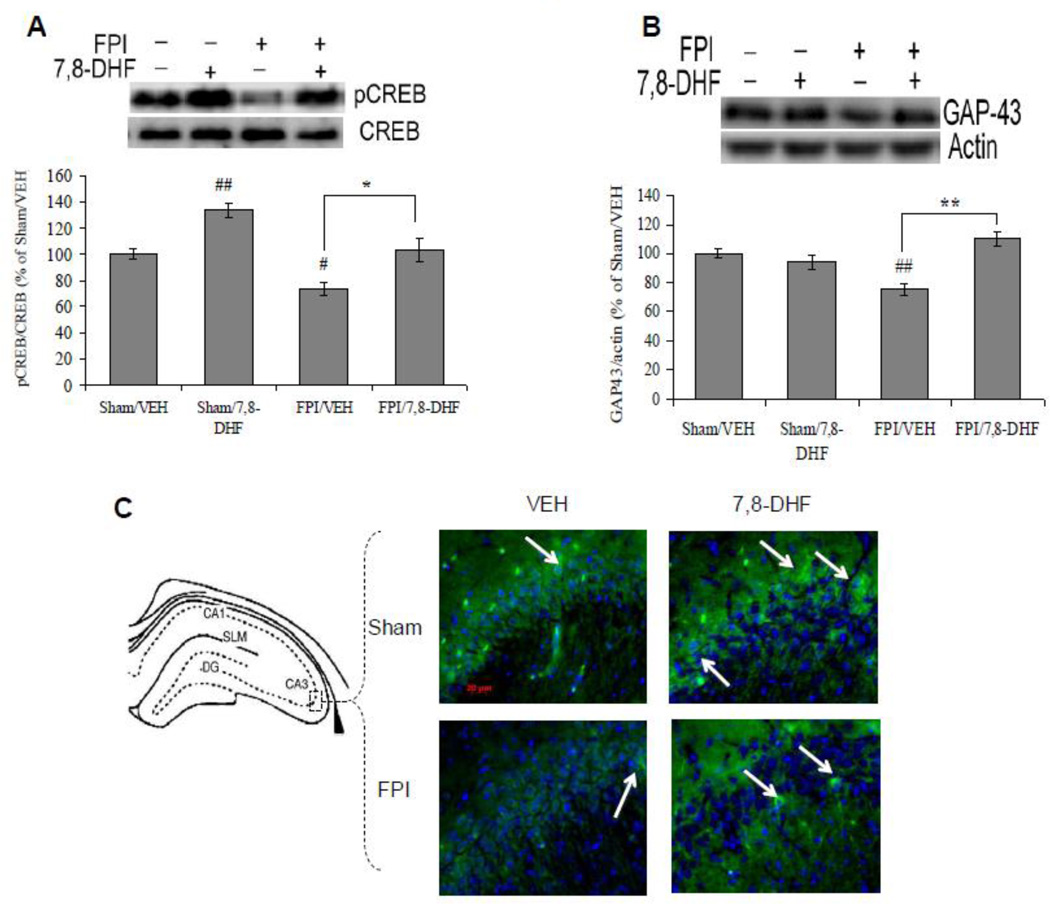
We assessed cAMP-response element binding (CREB) protein, a family of transcription factors that play a major role in synaptic plasticity and cognitive functions [28]. The phosphorylation of CREB changed significantly with injury (F1,24 =21.79 p<0.01) and treatment (F1,24 =27.30 p<0.01) according to ANOVA (two-way) analysis. Post-hoc analysis showed that the 7,8-DHF elevated the phosphorylation of CREB in sham animals (Sham/7,8-DHF vs. Sham/VEH, p<0.01). TBI reduced the levels of CREB phosphorylation (FPI/VEH vs. Sham/VEH, p<0.05), whereas the treatment with 7,8-DHF restored the levels of CREB phosphorylation in animals subjected to TBI (FPI/7,8-DHF vs. FPI/VEH, p<0.05) such that they were not different from Sham/VEH controls (Fig. 5A).
Fig. 5.
(A) Levels of CREB Phosphorylation, (B) levels of GAP-43 and (D) syntaxin-3 in vehicle (VEH) and 7,8-dihydroxyflavone (7,8-DHF; 5 mg/kg, ip) treated rats, subjected to either sham or fluid percussion injury (FPI). Data are expressed as percentage of Sham/VEH (mean ± SEM). #P<0.05, ##P<0.01 vs. Sham/VEH; *P<0.05, **P<0.01 vs. FPI/VEH; ANOVA (two-way) with Bonferroni's comparisons post-hoc test. (C) Immunofluorescence staining of GAP-43 (FITC) in hippocampal coronal sections from vehicle and 7,8-DHF (5 mg/kg, ip) treated groups, subjected to either sham or FPI (scale bar: 20 µm). (E) Brightfield staining of syntaxin-3 in hippocampal coronal sections from Sham/VEH, FPI/VEH and FPI/7,8-DHF (5 mg/kg, ip) groups (scale bars: 100 and 50 µm).
We assessed the levels of GAP-43, a growth associated protein, which provides an index of growth or plasticity as it is expressed at high levels during development and axonal regeneration [29]. Two-way ANOVA revealed a significant effect of injury (F1,24 = 12.33, p<0.01) and treatment (F1,24 = 24.15, p<0.01) for the levels GAP-43. The posthoc test showed that TBI reduced the levels of GAP-43 (FPI/VEH vs. Sham/VEH, p<0.01), whereas the treatment with 7,8-DHF counteracted the effect of TBI on GAP-43 (FPI/7,8-DHF vs. FPI/VEH, p<0.01; Fig. 5B). There was a marked qualitative reduction in GAP-43 immunoreactivity within the pyramidal cells of CA3 subregion in TBI, which was restored after the treatment with 7,8-DHF (Figure 5C).
We have also assessed the levels of syntaxin-3, a protein responsible for transporting vesicles, which participates in exocytosis and also plays important role in the growth of neuritis [30]. The levels of syntaxin-3 changed significantly with injury (F1,24 =5.67 p<0.05) and treatment (F1,24 =13.93 p<0.01, two-way ANOVA). Post-hoc analyses showed a significant decrease in the levels of syntaxin-3 in animals subjected to the TBI (FPI/VEH vs. Sham/VEH, p<0.05). The 7,8-DHF per se did not affect the levels of syntaxin-3 (Sham/7,8-DHF vs. Sham/VEH, p>0.05), while the treatment with 7,8-DHF restored the levels of synatxin-3 in TBI (FPI/7,8-DHF vs. FPI/VEH, p<0.01; Fig. 5D). Further, we have analyzed the effects of FPI and 7,8-DHF on the phenotypic expression of syntaxin-3 using immunohistochemistry. Syntaxin-3 staining was predominantly distributed in the apical and basal dendrites of CA1 pyramidal cells and in the granule cells of the dentate gyrus (DG) of the hippocampal formation. FPI resulted in reduced syntaxin-3 staining in CA1 and DG, which was counteracted by the treatment with 7,8-DHF (Fig. 5E).
3.5. 7,8-DHF treatment normalizes alterations in molecules associated with mitochondrial biogenesis after TBI
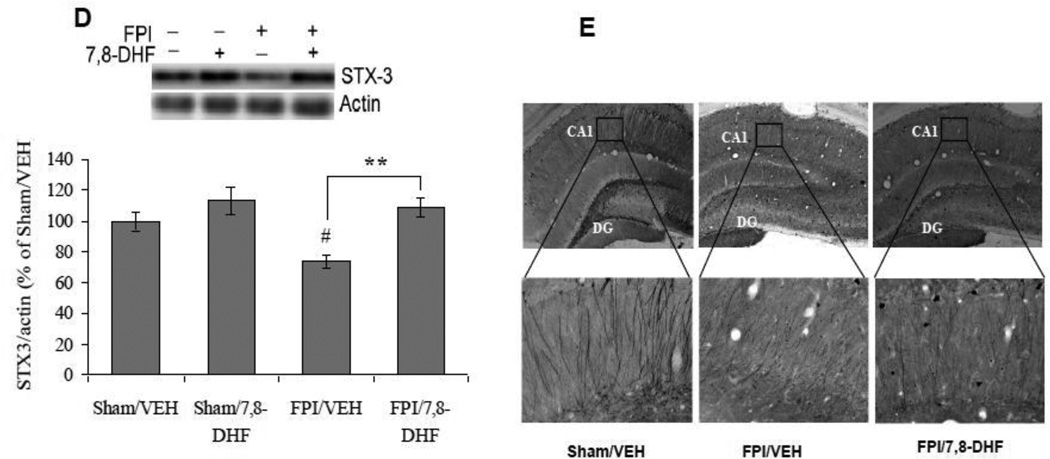
We examined the levels of PGC-1α, which is a member of a family of transcription co-activators that plays a central role in mitochondrial biogenesis. Two-way ANOVA analysis revealed a significant effect of injury (F1,24 =6.15 p<0.05) and treatment (F1,24 =14.74 p<0.01), as well as their interaction (F1,24 =29.53 p<0.01) on PGC-1α. The post-hoc analysis showed that TBI negatively influences the levels of PGC-1α (FPI/VEH vs. Sham/VEH, p<0.01). The treatment with 7,8-DHF counteracted the effect of brain trauma on levels of PGC-1α (FPI/7,8-DHF vs. FPI/VEH, p<0.01; Fig. 6A). We also observed that the levels of PGC-1α were negatively correlated with latency time in Barnes maze test (r = −0.527, p<0.01) suggesting that an increased PGC-1α may contribute to improvement in memory function (Fig. 6B).
Fig. 6.
(A) Protein levels of PGC-1α, (B) correlation analysis of PGC-1α with latency time in Barnes maze test, (c) TFAM levels, (D) COII levels and (E) correlation analysis of COII with latency time in Barnes maze test in vehicle (VEH) and 7,8-dihydroxyflavone (7,8-DHF; 5 mg/kg, ip) treated rats, subjected to either sham or fluid percussion injury (FPI). Data are expressed as percentage of Sham/VEH (mean ± SEM). ##P<0.01 vs. Sham/VEH; *P<0.05, **P<0.01 vs. FPI/VEH; ANOVA (two-way) with Bonferroni's comparisons post-hoc test.
To analyze the role of TrkB activation on the maintenance of mitochondrial protein function after TBI, we quantified the mitochondrial transcription factor A (TFAM). A significant interaction between treatment vs. injury (F1,24 = 22.64 p < 0.01) was observed for the levels of TFAM using two-way ANOVA. The post-hoc test performed for multiple comparisons showed that TBI resulted in a significant reduction in the levels of TFAM (FPI/VEH vs. Sham/VEH, p<0.01). In turn, the treatment with 7,8-DHF, significantly ameliorated the impact of TBI on TFAM protein levels (FPI/7,8-DHF vs. FPI/VEH, p<0.01; Fig. 6C).
We assessed the levels of mitochondrial cytochrome oxidase II (COII), which provides an index of mitochondrial function/mass and plays an important role in mitochondrial oxidative phosphorylation. A significant effect of injury (F1,24 = 24.90 p<0.01) and treatment (F1,24 = 7.90 p<0.05) as well as a significant interaction between injury vs. treatment (F1,24 = 4.59 p<0.05) were observed on COII levels using two-way ANOVA. The post-hoc analysis showed that COII levels were reduced after brain injury (FPI/VEH vs. Sham/VEH, p<0.01), while the treatment with 7,8-DHF maintained the levels of COII after TBI (FPI/7,8-DHF vs. FPI/VEH, p<0.05; Fig. 6D). The negative correlation between latency time and level of COII (r = −0.663, p<0.01) suggests that memory function tested in the Barnes maze may rely on the levels of mitochondrial proteins (Fig. 6E).
3.6. 7,8-DHF counteracted alterations in markers of energy management following TBI
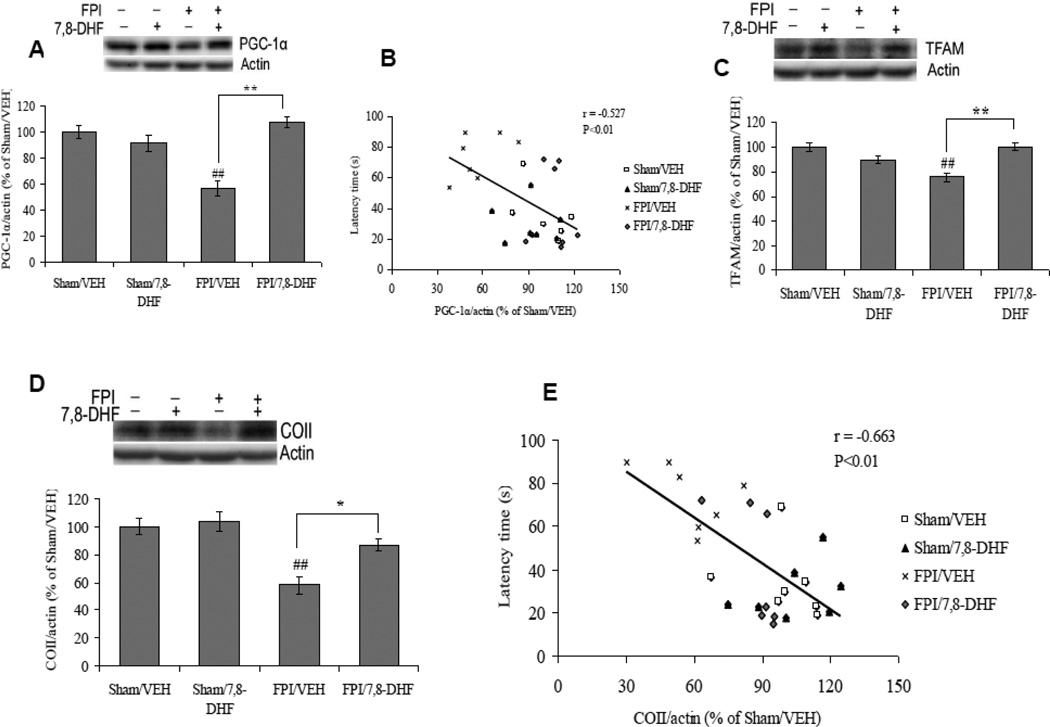
We evaluated the effects of FPI and 7,8-DHF on AMP activated protein kinase (AMPK), a protein that control the balance and transduction of cellular energy. A twoway ANOVA analysis (injury vs. treatment) indicated a significant effect of injury (F1,24 = 272.35 p<0.01) and treatment (F1,24 = 104.90 p<0.01), and an interaction between 7,8-DHF vs. injury (F1,24 = 53.81 p<0.01) for AMPK phosphorylation. The Bonferroni posthoc test for multiple comparisons showed that TBI reduced the levels of AMPK phosphorylation (FPI/VEH vs. Sham/VEH, p<0.01), while the 7,8-DHF treatment appeared to protect the brain against the effects of the injury as the levels of AMPK phosphorylation was significantly high in 7,8-DHF treated group subjected to FPI (FPI/7,8-DHF vs. FPI/VEH, p<0.05). The treatment with 7,8-DHF also elevated levels of AMPK phosphorylation in sham animals (Sham/7,8-DHF vs. Sham/VEH, p<0.01; Fig. 7A).
Fig. 7.
(A) Phosphorylation of AMPK and (B) levels of SIRT1 in vehicle (VEH) and 7,8-dihydroxyflavone (7,8-DHF; 5 mg/kg, ip) treated rats, subjected to either sham or fluid percussion injury (FPI). Data are expressed as percentage of Sham/VEH (mean ± SEM). ##P<0.01 vs. Sham/VEH; *P<0.05, **P<0.01 vs. FPI/VEH; ANOVA (two-way) with Bonferroni's comparisons post-hoc test.
Based on the demonstrated role of sirtuin 1 (SIRT1) in cell energy metabolism, we have evaluated the effects of FPI and 7,8-DHF on SIRT1. Two-way ANOVA analysis showed a significant effect of injury (F1,24 =30.49 p<0.01) and treatment (F1,24 =11.64 p<0.01), and significant interaction between injury vs. treatment (F1,24 =8.21 p<0.01). Levels of SIRT1 were reduced after TBI in comparison to the sham group (FPI/VEH vs. Sham/VEH, p<0.01). Treatment with 7,8-DHF ameliorated the effect of TBI on SIRT1 as evidenced by a significant increase in SIRT1 level in 7,8-DHF animals subjected to TBI (FPI/7,8-DHF vs. FPI/VEH, p<0.01; Fig. 7B).
4. Discussion
We assessed the capacity of the TrkB receptor agonist 7,8-DHF to counteract the effects of TBI on cognitive function. According to our results, systemic administration of 7,8-DHF significantly attenuated disrupted memory function by activating hippocampal TrkB receptor, evidenced by the counteracting action of K252a, a TrkB antagonist, on 7,8-DHF induced memory improvement and TrkB activation in TBI. In addition, the 7,8-DHF treatment was effective to ameliorate the effects of TBI on CREB phosphorylation, GAP-43, and syntaxin-3 levels. These effects engaged molecular systems related to energy homeostasis (AMPK and SIRT1) and mitochondrial biogenesis (PGC-1α, TFAM and COII), indicating that activation of cellular energy metabolism may be an important step for the action of 7,8-DHF on plasticity. These findings emphasize the potential of 7,8-DHF as an efficacious and non-invasive therapeutic agent to downgrade the TBI pathology. Our results also indicate that dysfunction in the BDNF system is a crucial step by which TBI compromises the capacity of cell to metabolize energy necessary for normal cognitive processing.
4.1. BDNF-TrkB signaling system and cognitive functions
BDNF-TrkB signaling is critically important for cell functioning such that dysfunction of TrkB has been implicated in various neurodegenerative diseases and psychiatric disorders [31]. The capacity of BDNF-TrkB signaling to engage metabolism and plasticity makes it a suitable therapeutic target for counteracting the pathology associated with TBI. 7,8-DHF is a member of the flavonoid family, which, similar to BDNF has a high binding affinity for the TrkB receptor. Furthermore, 7,8-DHF crosses the blood brain barrier and has a safe pharmacokinetic profile, making it an excellent therapeutic agent [4, 32]. According to our results, TBI reduces the levels of molecular systems related to BDNF, including phosphorylation of the TrkB receptor. In turn, the treatment with 7,8-DHF was able to attenuate the effect of TBI on the activation and phenotypic expression of TrkB without effecting endogenous BDNF levels, which indicates that systemic administration of 7,8-DHF directly activates TrkB. In addition, our findings show that 7,8-DHF rescues hippocampal-dependent cognitive deficit in TBI, which is negatively correlated with TrkB phosphorylation. Together these data provide an indication for the importance of TrkB activation in maintaining cognitive functions during TBI, which is in agreement with previous reports showing that systemic 7,8-DHF can ameliorate the memory deficit through restoring TrkB dysfunction in transgenic mouse model of Alzheimer’s disease [21]. Furthermore, our results showing the obstructive ability of K252a against memory improvements and TrkB signaling activation following 7,8-DHF treatment corroborate the pivotal role of TrkB signaling on cognitive recovery after TBI.
4.2. Mitochondrial biogenesis
TBI occurrence compromises molecular systems involved in mitochondrial biogenesis via influencing transcription factors that are important for maintaining neuronal plasticity. In particular, PGC-1α is a co-transcriptional regulator that activates various transcription factors, including nuclear respiratory factors (NRFs). In turn, the NRFs activate the mitochondrial transcription factor TFAM, which stimulates mtDNA transcription. Our results show that treatment with 7,8-DHF restored levels of PGC-1α and TFAM, which were reduced after TBI, emphasizing the role of TrkB activation in maintaining a homeostatic balance during energy crisis. Our findings showing that latency time in Barnes maze was reduced in proportion to increased in PGC-1α, support the action of PGC-1α on maintaining behavioral performance.
PGC-1α is a potent coactivator of a plethora of transcription factors that influence cellular energy and mitochondrial functions by activating the mitochondrial proteins such as cytochrome C oxidase II (COII). COII is a protein subunit of the terminal and highly regulated enzyme complex IV of the electron transport chain in mitochondria [27]. COII plays a key role in controlling ATP production as it helps to establish a transmembrane difference of proton electrochemical potential in the electron transport chain of mitochondria. We observed a significant decline in COII levels after TBI reflecting the action of TBI on energy depression. The fact that COII levels were restored by 7,8-DHF treatment supports the concept that 7,8-DHF is beneficial for the maintenance of cellular energy. Similar to our results for PGC-1α, we observed that latency time in the Barnes maze was inversely proportional to COII levels, emphasizing the role of cell energy on maintaining cognitive performance.
4.3. Energy management system, brain plasticity and neuronal signaling
Brain energy metabolism is central to all cellular processes that maintain neuronal functionality and has the capacity to modulate synaptic plasticity and behavior [33]. It has been reported that BDNF stimulates energy metabolism in developing cortical neurons during energy demand [18] and regulates mitochondrial oxidative efficiency by engaging complex I [19]. ATP and NAD are small molecules involved in all energy transactions in cells, and can be sensed by regulatory proteins, such as AMP-activated protein kinase (AMPK, which senses the AMP/ATP ratio) and sirtuins (which require NAD to deacetylate protein substrates), which both have the capacity to activate PGC-1α [34]. PGC-1α is involved in the transcription of genes promoting mitochondrial biogenesis as well as the mitochondrial anti-oxidant system to protect neurons against oxidative damage [35, 36]. Mitochondrial biogenesis constitutes a critical aspect of the AMPK response to energy deficient states, demonstrated to occur through PGC-1α [16]. SIRT1 deacetylase increases mitochondrial density in neurons by requisite activation of the transcriptional activity of PGC-1α [37]. In neurons, the AMPK-SIRT1-PGC-1α signaling pathway plays a critical role in energy metabolism by regulating mitochondrial function [16]. Of these, AMPK is a serine-threonine kinase, which has the ability to sense low energy levels and to activate or inhibit molecules to re-establish the proper energy balance of the cell. According to our results, TBI reduced the activation of AMPK, an indicator of a disturbance in energy homeostasis. In turn, the activation of AMPK with 7,8-DHF advocates that TrkB signaling may activate mechanisms to conserve ATP levels in the hippocampus. A mechanistic association between AMPK and SIRT1 seems to exist as NAD, a critical substrate for SIRT1 function, also activates AMPK in a dosedependent manner [38]. The function of SIRT1 has been associated with the control of cellular homeostasis and energy metabolism [39, 40], and our findings that TBI reduces SIRT1 levels seem to reflect the action of TBI on energy management. The fact that the treatment with 7,8-DHF normalized SIRT1 levels emphasizes the involvement of TrkB activation in maintaining energy homeostasis.
SIRT1 (mammalian Sir2 homologue) has been shown to modulate synaptic plasticity and memory formation via post-transcriptional regulation of CREB [41]. It has also been reported that AMPK regulates cAMP-response element binding (CREB) proteins [42], which is a family of transcription factors, playing a major role in synaptic plasticity and cognitive functions [28]. TrkB signaling is crucial for the action of CREB on synaptic plasticity and learning and memory [43]. This implies that the effects of 7,8-DHF on increasing CREB activation may be significant for modulation of synaptic plasticity. CREB is a potent activator of PGC-1α [44], which emphasizes the double function of CREB as a synaptic activator and energy modulator. Furthermore, according to our results, TBI compromised the molecular systems important for the maintenance of synaptic plasticity and repair such as GAP-43 and syntaxin-3. It is reported that synaptic dysfunctions in hippocampal CA1 such as reduced basal transmission and long-term potentiation leads to behavioral deficits in mouse model of Alzheimer’s disease [45]. Therefore, the reduced immunoreactivity of syntaxin-3 in dendrites of CA1 pyramidal cells after FPI might be associated with cognitive dysfunctions. GAP-43 is an intracellular membrane-associated phosphoprotein, present in growth cones and pre-synaptic terminals important for formation of neuronal connections, and synaptic remodeling following traumatic insult [29]. In addition, GAP-43 has also been suggested as an important mediator for the neurotrophic effects of BDNF on neuronal survival and plasticity [46]. The treatment with 7,8-DHF counteracted the TBI induced reductions in GAP-43 and syntaxin-3 at protein and phenotypic expression levels, suggesting an action of TrkB activation on synaptic plasticity and growth. Based on these results, it appears that TrkB signaling is pivotal for engaging signals related to synaptic plasticity and energy management.
In summary, our results show that TBI disturbs pathways associated with energy homeostasis and synaptic plasticity, and that 7,8-DHF counteracted these disturbances. The action of 7,8-DHF is associated with key molecular events of mitochondrial biogenesis and synaptic plasticity with important implications for cognitive function (Fig. 8). The overall results provide insight to understand how metabolic and plasticity signals interact with each other to influence long-term neuronal resilience against neurological and psychiatric disorders. The results also portray 7,8-DHF as an effective therapeutic agent to counteract crucial aspects of the broad TBI pathology.
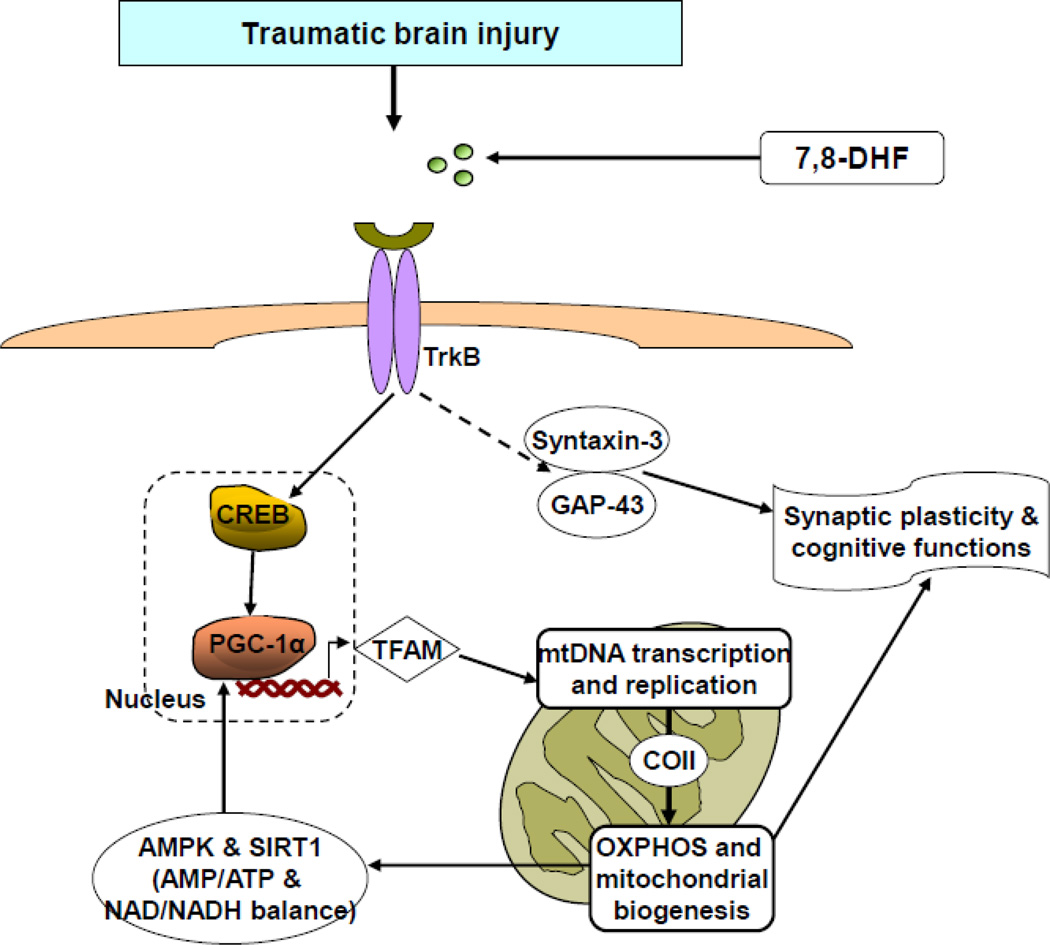
Fig. 8.
Proposed mechanism by which activation of BDNF receptor TrkB using 7,8-dihydroxyflavone (7,8-DHF), a small-molecule TrkB agonist, improves brain plasticity and mental health following traumatic brain injury (TBI). 7,8-DHF is a member of the flavonoid family that crosses the blood–brain barrier and activates the TrkB receptor similar to the BDNF. The treatment with 7,8-DHF restores the TBI induced disruption of TrkB signaling, which plays a pivotal action on mitochondrial biogenesis through metabolic activators such as PGC-1α. PGC-1α improves mitochondrial function by activating mitochondrial DNA (mtDNA) transcription, and mitochondrial proliferation through mitochondrial transcription factor A (TFAM). The mtDNA transcription leads to the synthesis of mitochondrial proteins including cytochrome c oxidase (COII), which is a key player for ATP production by oxidative phosphorylation (OXPHOS) in mitochondria. ATP and NAD are small molecules involved in all energy transactions in cells, and can be sensed by regulatory proteins, such as AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) respectively, which both have the capacity to activate PGC-1α. The alteration in AMPK-SIRT1-PGC-1α signaling pathway plays a critical role to regulate mitochondrial energy system, and is important for maintaining cognitive function and synaptic plasticity via CREB. Regulation of synaptic function by 7,8-DHF can also be mediated by syntaxin-3 and GAP-43. Overall, the diagram illustrates a mechanism by which the interplay between energy management and plasticity regulates neuronal function under homeostatic and injured conditions.
Supplementary Material
Highlights.
7,8-DHF counteracts behavioral and molecular dysfunction in the TBI pathology.
7,8-DHF normalizes events interfacing cell metabolism and plasticity.
Pivotal role of TrkB signaling on 7,8-DHF effects on cognitive recovery after TBI.
Potential therapeutic action of 7,8-DHF reducing cognitive failure in TBI pathology.
Acknowledgements
This work was supported by National Institutes of Health Grant NS050465.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no conflict of interest for any of the contributing authors.
References
- 1.McAllister TW. Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry. 2008;7:3–10. doi: 10.1002/j.2051-5545.2008.tb00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiong Y, Mahmood A, Chopp M. Emerging treatments for traumatic brain injury. Expert Opin Emerg Drugs. 2009;14:67–84. doi: 10.1517/14728210902769601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang SW, Liu X, Yepes M, Shepherd KR, Miller GW, Liu Y, Wilson WD, Xiao G, Blanchi B, Sun YE, Ye K. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci U S A. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaplan GB, Vasterling JJ, Vedak PC. Brain-derived neurotrophic factor in traumatic brain injury, post-traumatic stress disorder, and their comorbid conditions: role in pathogenesis and treatment. Behav Pharmacol. 2010;21:427–437. doi: 10.1097/FBP.0b013e32833d8bc9. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Chua KW, Chua CC, Yu H, Pei A, Chua BH, Hamdy RC, Xu X, Liu CF. Antioxidant activity of 7,8-dihydroxyflavone provides neuroprotection against glutamate-induced toxicity. Neurosci Lett. 2011;499:181–185. doi: 10.1016/j.neulet.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 7.Kato T, Nakayama N, Yasokawa Y, Okumura A, Shinoda J, Iwama T. Statistical image analysis of cerebral glucose metabolism in patients with cognitive impairment following diffuse traumatic brain injury. J Neurotrauma. 2007;24:919–926. doi: 10.1089/neu.2006.0203. [DOI] [PubMed] [Google Scholar]
- 8.Eakins J. Blood glucose control in the trauma patient. J Diabetes Sci Technol. 2009;3:1373–1376. doi: 10.1177/193229680900300617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu-DeRyke X, Collingridge DS, Orme J, Roller D, Zurasky J, Rhoney DH. Clinical impact of early hyperglycemia during acute phase of traumatic brain injury. Neurocrit Care. 2009;11:151–157. doi: 10.1007/s12028-009-9228-6. [DOI] [PubMed] [Google Scholar]
- 10.Griesdale DE, Tremblay MH, McEwen J, Chittock DR. Glucose control and mortality in patients with severe traumatic brain injury. Neurocrit Care. 2009;11:311–316. doi: 10.1007/s12028-009-9249-1. [DOI] [PubMed] [Google Scholar]
- 11.Anglin RE, Rosebush PI, Noseworthy MD, Tarnopolsky M, Mazurek MF. Psychiatric symptoms correlate with metabolic indices in the hippocampus and cingulate in patients with mitochondrial disorders. Transl Psychiatry. 2012;2:e187. doi: 10.1038/tp.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lifshitz J, Sullivan PG, Hovda DA, Wieloch T, McIntosh TK. Mitochondrial damage and dysfunction in traumatic brain injury. Mitochondrion. 2004;4:705–713. doi: 10.1016/j.mito.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 13.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 14.Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta. 2012;1819:921–929. doi: 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, Rustin P, Gustafsson CM, Larsson NG. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 16.Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng A, Wan R, Yang JL, Kamimura N, Son TG, Ouyang X, Luo Y, Okun E, Mattson MP. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burkhalter J, Fiumelli H, Allaman I, Chatton JY, Martin JL. Brain-derived neurotrophic factor stimulates energy metabolism in developing cortical neurons. J Neurosci. 2003;23:8212–8220. doi: 10.1523/JNEUROSCI.23-23-08212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markham A, Cameron I, Franklin P, Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20:1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- 20.Andero R, Daviu N, Escorihuela RM, Nadal R, Armario A. 7,8-dihydroxyflavone, a TrkB receptor agonist, blocks long-term spatial memory impairment caused by immobilization stress in rats. Hippocampus. 2012;22:399–408. doi: 10.1002/hipo.20906. [DOI] [PubMed] [Google Scholar]
- 21.Devi L, Ohno M. 7,8-dihydroxyflavone, a small-molecule TrkB agonist, reverses memory deficits and BACE1 elevation in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2012;37:434–444. doi: 10.1038/npp.2011.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riddle DR, Katz LC, Lo DC. Focal delivery of neurotrophins into the central nervous system using fluorescent latex microspheres. Biotechniques. 1997;23:928–934. 936–927. doi: 10.2144/97235rr02. [DOI] [PubMed] [Google Scholar]
- 23.Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between BDNF and signal transduction modulators in the regulation of the effects of exercise on synapticplasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Real CC, Ferreira AF, Chaves-Kirsten GP, Torrão AS, Pires RS, Britto LR. BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson's disease. Neuroscience. 2013;237:118–129. doi: 10.1016/j.neuroscience.2013.01.060. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Ying Z, Gomez-Pinilla F. A pyrazole curcumin derivative restores membrane homeostasis disrupted after brain trauma. Exp Neurol. 2010;226:191–199. doi: 10.1016/j.expneurol.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 27.Harris LK, Black RT, Golden KM, Reeves TM, Povlishock JT, Phillips LL. Traumatic brain injury-induced changes in gene expression and functional activity of mitochondrial cytochrome C oxidase. J Neurotrauma. 2001;18:993–1009. doi: 10.1089/08977150152693692. [DOI] [PubMed] [Google Scholar]
- 28.Benito E, Barco A. CREB's control of intrinsic and synaptic plasticity: implications for CREB-dependent memory models. Trends Neurosci. 2010;33:230–240. doi: 10.1016/j.tins.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 30.Martín-Martín B, Nabokina SM, Lazo PA, Mollinedo F. Co-expression of several human syntaxin genes in neutrophils and differentiating HL-60 cells: variant isoforms and detection of syntaxin 1. J Leukoc Biol. 1999;65:397–406. doi: 10.1002/jlb.65.3.397. [DOI] [PubMed] [Google Scholar]
- 31.Gupta VK, You Y, Gupta VB, Klistorner A, Graham SL. TrkB Receptor Signalling: Implications in Neurodegenerative, Psychiatric and Proliferative Disorders. Int J Mol Sci. 2013;14:10122–10142. doi: 10.3390/ijms140510122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X, Chan CB, Jang SW, Pradoldej S, Huang J, He K, Phun LH, France S, Xiao G, Jia Y, Luo HR, Ye K. A Synthetic 7,8-Dihydroxyflavone Derivative Promotes Neurogenesis and Exhibits Potent Antidepressant Effect. J Med Chem. 2010 doi: 10.1021/jm101206p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Head E, Gharib AM, Yuan W, Ingersoll RT, Hagen TM, Cotman CW, Ames BN. Memory loss in old rats is associated with brain mitochondrial decay and RNA/DNA oxidation: partial reversal by feeding acetyl-L-carnitine and/or R-alpha - lipoic acid. Proc Natl Acad Sci U S A. 2002;99:2356–2361. doi: 10.1073/pnas.261709299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC. Roles of Oxidative Stress, Apoptosis, PGC-1α and Mitochondrial Biogenesis in Cerebral Ischemia. Int J Mol Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 37.Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, Kaasik A. PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem. 2009;284:21379–21385. doi: 10.1074/jbc.M109.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafaeloff-Phail R, Ding L, Conner L, Yeh WK, McClure D, Guo H, Emerson K, Brooks H. Biochemical regulation of mammalian AMP-activated protein kinase activity by NAD and NADH. J Biol Chem. 2004;279:52934–52939. doi: 10.1074/jbc.M409574200. [DOI] [PubMed] [Google Scholar]
- 39.Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 2002;298:2390–2392. doi: 10.1126/science.1077650. [DOI] [PubMed] [Google Scholar]
- 40.Hallows WC, Lee S, Denu JM. Sirtuins deacetylate and activate mammalian acetyl-CoA synthetases. Proc Natl Acad Sci U S A. 2006;103:10230–10235. doi: 10.1073/pnas.0604392103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomson DM, Herway ST, Fillmore N, Kim H, Brown JD, Barrow JR, Winder WW. AMP-activated protein kinase phosphorylates transcription factors of the CREB family. J Appl Physiol. 2008;104:429–438. doi: 10.1152/japplphysiol.00900.2007. [DOI] [PubMed] [Google Scholar]
- 43.Alonso M, Vianna MR, Izquierdo I, Medina JH. Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cell Mol Neurobiol. 2002;22:663–674. doi: 10.1023/a:1021848706159. [DOI] [PubMed] [Google Scholar]
- 44.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–183. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 45.Kimura R, Ohno M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol Dis. 2009;33:229–235. doi: 10.1016/j.nbd.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta SK, Mishra R, Kusum S, Spedding M, Meiri KF, Gressens P, Mani S. GAP-43 is essential for the neurotrophic effects of BDNF and positive AMPA receptor modulator S18986. Cell Death Differ. 2009;16:624–637. doi: 10.1038/cdd.2008.188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.