Abstract
Introduction
Colon polyps and inflammatory process play the key role in neoplasia of colorectal cancer. In recent years there have been many publications on the malignancy of hyperplastic polyp (HP) which according to the WHO classification is a non-neoplastic polyp. The aim of this study is to determine the expression of inflammatory proteins COX-2, IL-1β, TNF-α and IL-4 in the epithelium of colorectal polyps.
Material and methods
In the study, 144 colorectal polyps were analyzed. The groups of HP, classical (A) and serrated adenomas (SA) and normal mucosa (control) according to histopathological studies were selected. Immunohistochemical examinations Rusing antibodies against COX-2, IL-1β, TNF-α and IL-4 were performed. The expression of analyzed protein was evaluated using modified Remmele-Stegner scale (0-16).
Results
Statistical analysis revealed higher expression of TNF-α (16 ±3.87 vs. 1 ±5.06), IL-1β (12 ±4 vs 8 ±2.72), COX-2 (9 ±2.54 vs. 8 ±3.14) and IL-4 (12 ±3.45 vs. 4 ±3.35) in SA polyps compared to the control (p < 0.001). The HP had an increased level of expression of TNF-α (12 ±3.72 vs. 1 ±5.06, p < 0.005), COX-2 (8.5 ±1.97 vs. 8 ±3.14, p < 0.012) and IL-4 (12 ±3.46 vs. 4 ±3.35, p < 0.001). Significantly higher expression of IL-4 (12 ±2.32 vs. 4 ±3.35, p < 0.001) and IL-1β (16 ±4.32 vs. 8 ±2.72, p < 0.044) in A compared to the control were observed.
Conclusions
Expression of inflammatory factors differed between polyps. Inflammation accompanied the serrated structures which occur in polyps. The inflammatory process affects the development of colorectal polyps. The HP may predispose to malignancy.
Keywords: inflammatory cytokines, polyps
Introduction
Colorectal cancer usually originates in polyps [1]. Classification of polyps is based on the histological examination and clinical correlation. The polyps can be neoplastic or non-neoplastic. The first group is formed as a result of the increased proliferation of epithelial cells. In some lesions dysplasia might develop, which may further lead to colorectal cancer (CRC). However, up to the present day, many publication discuss the classification of neoplastic and non-neoplastic polyps. There are publications and reports on new theories on the development and classification of colorectal polyps. The aim of several studies is to determine the types of polyps which have the malignancy potential. This is a very significant problem among pathologists, oncologists and other clinicians [2, 3].
Non-neoplastic polyps are classified into hyperplastic (HP) and juvenile polyps. Neoplastic polyps include various types of adenomas. According to this division, HP is a non-neoplastic polyp, which is also confirmed by the WHO definition [4]. However, in recent years, many studies have speculated on the probability of HP malignant transformation [5, 6]. This is an important clinical aspect which may change the medical follow-up for of those patients. The frequency of HP in the population is variable. Some authors suggest that HP constitute about 40% of all colon polyps, being second after adenoma [7]. However, some studies revealed HP as the dominant lesions [8, 9]. The latest studies focus on morphologically recognized “serration” in polyps.
Currently, the most appropriate division of serrated polyps is into dysplastic and non-dysplastic polyps. Non-dysplastic polyps include HP and sessile serrated adenoma (SSA). The potentially malignant group of polyps comprises adenomas, which are divided into traditional adenomas and serrated adenomas (SA), introduced by the WHO in 2000. Dysplastic serrated polyps consist of traditional serrated adenoma and SSA with dysplasia [10, 11]. Microscopically SA is a special type of adenoma, which combines the architectural features of HP (“serration” inside the gland) and cytological features of the classic adenoma (presence of dysplasia) [12]. The classification of polyps is still developing, but there are a few facts which are unquestionable.
Many studies have revealed that polyps play an important role in the development of CRC. About 90% of premalignant lesions of the colon are polyps or precursors of polyps [13]. An important role contributing to the progression of polyps to CRC might be played by the inflammatory process in the microenvironment. In 1863 Virchow postulated a link between chronic inflammation and cancer [14]. Since then, the effects of the inflammatory process on neoplasia development in endometrial, cervical, ovarian, breast, prostate and colon cancer have been proven [15]. The microenvironment of tumors contains cells of the innate immune system. These cells secrete proinflammatory cytokines, chemokines, growth factors, and reactive oxygen species that could cause DNA damage. Interestingly, the inflammatory process affects all stages of tumor development, i.e. initiation, progression and metastasis [16, 17]. The inflammatory process is reflected by a number of cytokines and COX-2 [18]. The most frequently described cytokines in such a context are interleukin 1β (IL-1β), IL-4 and tumor necrosis factor α (TNF-α) [16]. Inflammatory cytokines are activated in a “cascade” mode. Tumor necrosis factor α begins a whole process of inflammation [19]. In classical conditions, it is produced by monocytes and macrophages under the influence of bacterial endotoxin [20]. Tumor necrosis factor α activates the monocytes and neutrophils. This factor also has cytotoxic activity against tumor cells. It also increases the release of a number of cytokines including IL-1β and IL-6. Another important cytokine is IL-4. It is secreted by activated T-cells and is associated with CRC progression [21, 22].
Another well-known factor taking part in the inflammatory process is the enzyme prostaglandin synthetase, known as COX-2. It is the target for non-steroidal anti-inflammatory drugs (NSAIDs). In addition, researchers have demonstrated that NSAIDs reduce the risk of developing CRC [23, 24]. Expression of COX-2 is regulated by IL-1β [25]. Interleukin 1β is a pro-inflammatory cytokine produced by activated macrophages. This interleukin affects the activity of cells including cell proliferation, differentiation and apoptosis.
However, despite the fact that many studies have been published on this issue, it still remains an open question as to whether the type of polyp has an impact on the occurrence and intensity of the inflammatory process or vice versa. In our study, we tried to find the answers to some these questions. The aim of our study was to determine the expression of pro-inflammatory cytokines in the most common colon polyps.
Material and methods
The study included 4,500 patients aged between 25 and 82. From those patients, 694 polyps of the colon were removed. All the material was fixed in 10% buffered formalin and processed according to the standard protocol. Next paraffin blocks were prepared. The inclusion criteria for material used in this study were the clear-cut diagnosis of mucosal changes that fit the classification criteria and the presence of sufficient material for further work. Subsequently, two independent pathologists accomplished re-verification of slides, after which 144 polyps were selected. For further studies, samples of large bowel lesions were divided into 4 subgroups: adenomas (A, 45 cases, average age 63), serrated adenomas (SA, 41 cases, average age 60.5), hyperplastic polyps (HP, 18 cases, average age 58) and normal mucosa as a control group (40 cases). After the preliminary evaluation of hematoxylin and eosin slides, the material was selected for immunohistochemical studies. We used primary antibodies against COX-2 (ab16701, Abcam, rabbit monoclonal, 1: 100), IL-1β (ab8320, Abcam, mouse monoclonal, 1: 50), TNF-α (nbp1-19532, Novus Biologicals, rabbit polyclonal, 1: 100), IL-4 (ab9622, Abcam, rabbit polyclonal, 1: 200) and for detection the EnVision system (DAKO). Antigen expression evaluation in epithelium of selected lesions was carried out using a modified Remmele-Stegner scale according to the intensity of expression and the number of cells/tissue area positively stained (with range from 1 – lowest expression to 16 – highest expression). Analysis was conducted at 20× original objective magnification for each studied antibody on 3 representative and randomly selected areas.
Statistical analysis
The statistical analysis was performed using Statistica 10.0. Normal distribution was checked using the Kolmogorov-Smirnov and Shapiro-Wilk test. As the distribution of the results differed from the normal distribution, statistical analysis was performed using nonparametric tests. The results were analyzed statistically using the nonparametric Kruskal-Wallis test at the 0.05 level of significance.
Results
COX-2 levels in epithelial cells
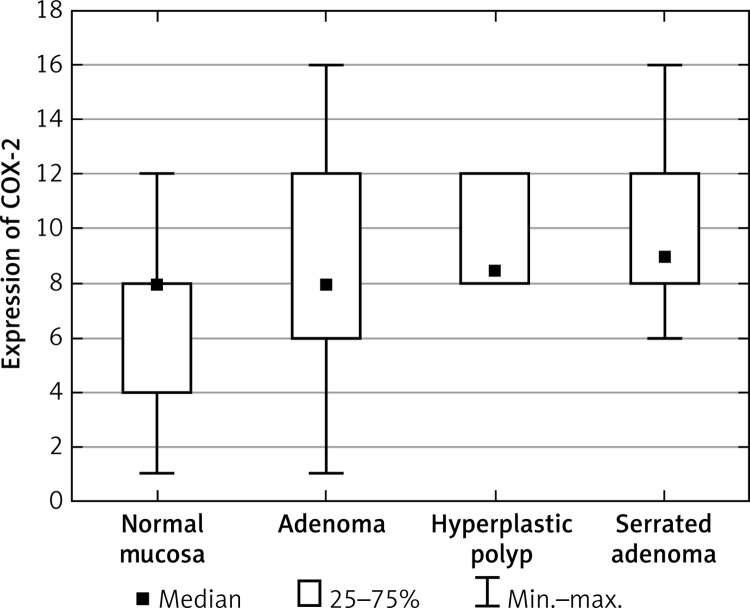
Statistical analysis demonstrated significantly higher levels of COX-2 in the group of HP patients and in the group of SA patients in comparison to the control group (Figure 1, Table I). No differences were observed regarding COX-2 level between the group of patients with adenoma and normal cells. Furthermore, in the group of patients with SA the level of COX-2 was significantly higher than in the group of patients with A. The detailed results of all groups are summarized in Table II.
Figure 1.
Average expression of COX-2 for each group of cases
Table I.
Summarized statistical significance of COX-2 for the studied groups
| Normal mucosa | Adenoma | Hyperplastic polyp | Serrated adenoma | |
|---|---|---|---|---|
| Normal mucosa | – | NS | 0.011779 | 0.000000 |
| Adenoma | NS | – | NS | 0.000002 |
| Hyperplastic polyp | 0.011779 | NS | – | NS |
| Serrated adenoma | 0.000000 | 0.000002 | NS | – |
NS – not significant.
Table II.
Summarized averages, medians and standard deviations
| Cytokine | Group | Average | Median | Standard deviation |
|---|---|---|---|---|
| COX-2 | Normal mucosa | 5.92 | 8.0 | 3.14 |
| Adenoma | 7.9 | 8.0 | 4.28 | |
| Hyperplastic polyp | 9.5 | 8.5 | 1.97 | |
| Serrated adenoma | 9.76 | 9.0 | 2.54 | |
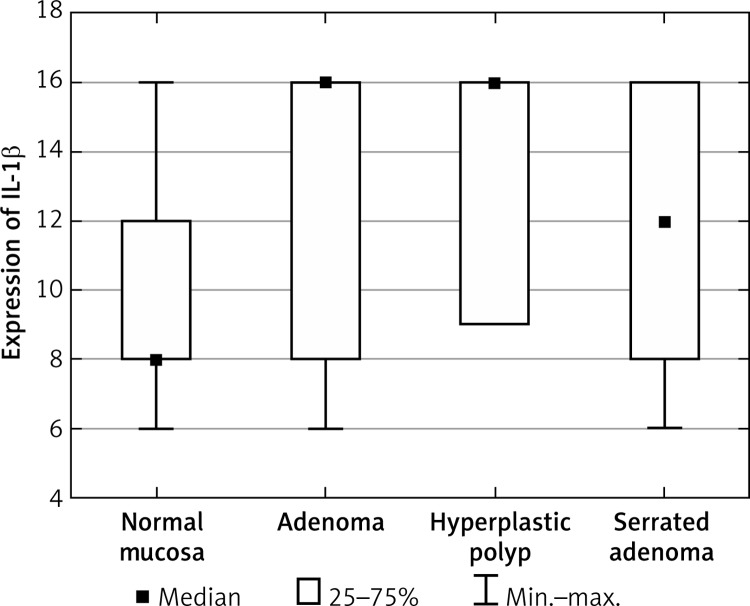
| IL-1β | Normal mucosa | 9.33 | 8.0 | 2.72 |
| Adenoma | 12.38 | 16.0 | 4.32 | |
| Hyperplastic polyp | 13.67 | 16.0 | 3.5 | |
| Serrated adenoma | 12.2 | 12.0 | 4.0 | |
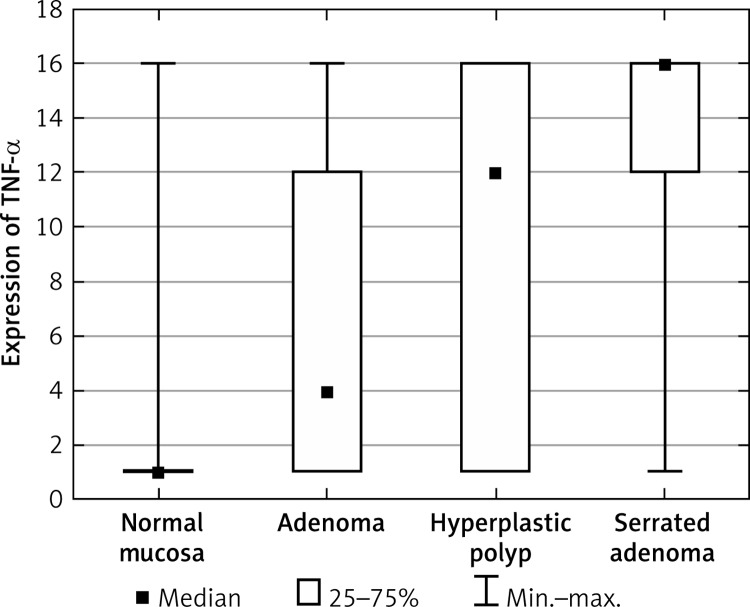
| TNF-α | Normal mucosa | 3.0 | 1.0 | 5.06 |
| Adenoma | 6.49 | 4.0 | 6.23 | |
| Hyperplastic polyp | 12.44 | 12.0 | 3.72 | |
| Serrated adenoma | 13.29 | 16.0 | 3.87 | |
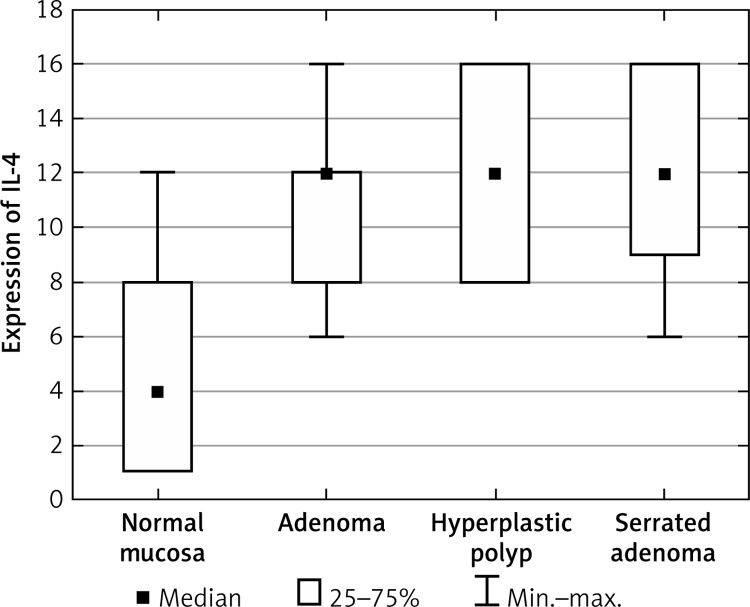
| IL-4 | Normal mucosa | 4.61 | 4.0 | 3.35 |
| Adenoma | 10.65 | 12.0 | 2.32 | |
| Hyperplastic polyp | 12.0 | 12.0 | 3.46 | |
| Serrated adenoma | 12.17 | 12.0 | 3.45 |
Cytokine IL-1β levels in epithelial cells
The level of IL-1β in epithelial cells of patients with A and SA was significantly higher than that of the controls (Figure 2, Table III). In addition, no differences were observed regarding IL-1β levels with respect to HP and normal cells. We also observed a significantly increased level in patients with SA compared to patients with A and patients with HP. No difference was observed between A and HP.
Figure 2.
Average expression of IL-1β for each group of cases
Table III.
Summarized statistical significance of IL-1β for the studied groups
| Normal mucosa | Adenoma | Hyperplastic polyp | Serrated adenoma | |
|---|---|---|---|---|
| Normal mucosa | – | 0.044136 | NS | 0.000000 |
| Adenoma | 0.044136 | – | NS | 0.006475 |
| Hyperplastic polyp | NS | NS | – | 0.014378 |
| Serrated adenoma | 0.000000 | 0.006475 | 0.014378 | – |
NS – not significant.
TNF-α levels in epithelial cells
The group of HP and SA patients exhibited an increased level of TNF-α compared to normal cells (Figure 3, Table IV). In addition, no difference was observed between A and the control group. Also the group of SA patients exhibited an increased level of TNF-α compared to A, with statistical significance.
Figure 3.
Average expression of TNF-α for each group of cases
Table IV.
Summarized statistical significance of TNF-α for the studied groups
| Normal mucosa | Adenoma | Hyperplastic polyp | Serrated adenoma | |
|---|---|---|---|---|
| Normal mucosa | – | NS | 0.004956 | 0.000001 |
| Adenoma | NS | – | NS | 0.000020 |
| Hyperplastic polyp | 0.004956 | NS | – | NS |
| Serrated adenoma | 0.000001 | 0.000020 | NS | – |
NS – not significant.
Cytokine IL-4 levels in epithelial cells
Statistical analysis showed a significantly increased level of IL-4 in the group of A, SA and HP patients compared to the control group (Figure 4, Table V). Moreover, the group of HP patients exhibited an increased level of IL-4 in comparison to A but without statistical significance.
Figure 4.
Average expression of IL-4 for each group of cases
Table V.
Summarized statistical significance of IL-4 for the studied groups
| Normal mucosa | Adenoma | Hyperplastic polyp | Serrated adenoma | |
|---|---|---|---|---|
| Normal mucosa | – | 0.000003 | 0.000044 | 0.000000 |
| Adenoma | 0.000003 | – | NS | NS |
| Hyperplastic polyp | 0.000044 | NS | – | NS |
| Serrated adenoma | 0.000000 | NS | NS | – |
NS – not significant.
Discussion
Adenomas have the most significant role in the development of colorectal cancer. Carcinogenesis occurs through two known pathways. One way is “classical”, through the adenoma. But the alternative pathway occurs through the serrated adenoma. Many studies have revealed that the inflammatory process and genetic mutations play a significant role in the process of CRC carcinogenesis. These processes probably occur already in the epithelium of adenomas. In our study, we assessed the expression of pro-inflammatory factors. They are a representative group taking part in inflammation. We determined the expression of COX-2, IL-1β, IL-4 and TNF-α respectively in the epithelia of A, SA, HP and control groups. Our study also included hyperplastic polyps, although at present there is a lot of confusion and controversy regarding their tendency to malignant transformation and potential to be the origin for CRC.
Our studies have shown elevated levels of COX-2 in hyperplastic polyps and SA. Many studies confirm and explain the reduction of occurrence of CRC in patients taking NSAIDs. Furthermore, the presence of COX-2 in both hyperplastic polyps and SA may be associated with the presence of serrated structures in polyps. And the COX-2 expression was not elevated in classical adenoma (so called non-serrated morphology). The effectiveness of NSAIDs is clearly combined with the increased expression of COX-2 in SA and HP. Comparing our study and many other studies we can conclude that the large-scale use of NSAIDs reduces the incidence of CRC [26]. Therefore the conclusion is that the spectacular effect of these drugs should solve the problem in patients with the risk of developing CRC on the base of SA or perhaps HP. However, the question is whether the beneficial effects of these drugs outweigh the side effects that might occur after the long-term of use NSAIDs. The main side effect of the use of NSAIDs, which is also the most frequently occurring and the most dangerous, is gastrointestinal bleeding of various types [27, 28]. This side effect unambiguously excludes the use of NSAIDs as a preventive method in patients with polyps because there is a potentially greater risk of complications to the patient than its benefits. In addition, our studies do not confirm the results of some other publications. Kawasaki et al. reported higher expression of COX-2 in adenomas compared to traditional serrated adenomas [29]. Asting et al. observed higher expression of prostaglandin synthetase in CRC cells [30]. The difference is the result of different populations being studied. This may be related to race, age, lifestyle, comorbidities and other environmental factors. The effect may even have a genetic basis [31].
MaihÖfner et al. observed the first elevated expression of IL-1β in CRC cells compared to the control group [32]. They also observed increased expression of COX-2 in CRC, which would explain the previously described correlation between these two inflammatory factors. Our studies showed that increased expression of IL-1β occurred in adenoma and SA. Hyperplastic polyps did not show an increase in the level of this cytokine. However, our results do not confirm the relation between COX-2 and IL-1β. This relation appeared only in serrated adenoma. Both COX-2 and IL-1β were elevated in SA.
Another important pro-inflammatory factor is TNF-α. This factor is also known as cachectin because it is associated with the formation of cachexia in patients with advanced cancer. Which is confirmed by research Gabriel et al. showing an increased expression of TNF-α in patients with poor diagnosis of CRC [28]. Elevated levels of TNF-α in CRC cells is often represented in numerous publications, but there is no reference in relation to the polyps. Our studies have shown elevated levels of TNF-α in the HP and the SA in comparison with the control group. In adenoma the level of TNF-α was not elevated. Our research shows that the previously described correlation between IL-1β and TNF-α occurred only in the case of SA. The presence of TNF-α in the polyps may be associated with a worse prognosis and with faster progression to CRC. But the presence of TNF-α in polyps may also be useful in reducing the risk of developing CRC, using the recently very popular drugs against TNF-α.
Another important cytokine is IL-4. This interleukin has an impact on inflammation and is associated with CRC progression. Galon et al. observed a significantly higher level of expression of IL-4 in cancer as compared to normal cells [19]. Kanai et al. observed increased levels of IL-4, resulting in lower levels of E-cadherin and CEA. This process leads to the inhibition of colon cancer cell-cell adhesion. They also found that IL-4 has no effect on the proliferation of CRC. Kanai et al. (also in further but not published studies) found an increased risk of liver metastases in patients with colon cancer with dominant Th2 cytokine production, among others IL-4 [29]. In our study we observed that the level of expression of IL-4 was increased in adenoma, SA and HP compared to normal tissue. High expression of IL-4 is found in all types of polyps. Additionally, higher expression of the inflammatory agent again appeared in SA compared to adenoma. Increased levels of IL-4 in all polyps may be associated with progression of polyps to CRC development. This would suggest once again the malignant nature of HP. Alternatively, increased levels of IL-4 in polyps may be associated with inhibition of the cell-cell adhesion in polyps. Finally, IL-4 participates in the development of colorectal cancer. It was confirmed by the presence of IL-4 in both premalignant lesions and CRC. In further studies, consideration should be given to the role of this cytokine in CRC neoplasia.
In conclusion, our study leads to the conclusion that the inflammatory process is not the same in all polyps of the colon. Its intensity depends on the type of polyp. The highest increase in the expression of all inflammatory factors was observed in SA. In hyperplastic polyps we observed increased levels of COX-2, TNF-α, and IL-4. These results suggest that it is associated with serrated structures that occur in these polyps. Thus, it may be assumed that the levels of inflammatory identified are associated with malignant transformation of polyps. Then we observed elevated levels of COX-2 in HP and SA, which explains the action of NSAIDs in the reduction of the risk of CRC. The adenoma showed higher expression of IL-1β and IL-4. However, IL-4 was specific, since its level was elevated in all polyps compared to the control. As previously mentioned, about 90% of premalignant lesions of the colon are in the form of polyps. Therefore based on our results the inflammatory process affects the development and progression of CRC; and therefore the precursor polyps based on our results are affected by the inflammatory process and might progress to CRC.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Watson AR, Jankowski J. Hyperplastic polyps, serrated adenomas, and the serrated polyp neoplasia pathway. Curr Colorectal Cancer Rep. 2007;3:3–9. [Google Scholar]
- 2.Klimczak A, Kempińska-Mirosławska B, Mik M, et al. Colorectal cancer mortality in Poland – analysis of regional variation. Arch Med Sci. 2014;10:63–7. doi: 10.5114/aoms.2012.28596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciria JP, Eguiguren M, Cafiero S, et al. Could preoperative short-course radiotherapy be the treatment of choice for localized advanced rectal carcinoma? Rep Pract Oncol Radiother. 2014;20:1–11. doi: 10.1016/j.rpor.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamilton SR, Vogelstein B, Kudo S. Carcinoma of the colon and rectum. Pathology and Genetics of Tumours of the Digestive System. WHO Classification of Tumours. 2000;2:105–19. [Google Scholar]
- 5.Teoh HH, Delahunt B, Isbister WH. Dysplastic and malignant areas in hyperplastic polyps of the large intestine. Pathology. 1989;21:138–42. doi: 10.3109/00313028909059551. [DOI] [PubMed] [Google Scholar]
- 6.Jass JR, Iino H, Ruszkiewicz A, et al. Neoplastic progression occurs through mutator pathways in hyperplastic polyposis of the colorectum. Gut. 2000;47:43–9. doi: 10.1136/gut.47.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liljegren A, Lindblom A, Rotstein S, Nilsson B, Rubio C, Jaramillo E. Prevalence and incidence of hyperplastic polyps and adenomas in familial colorectal cancer: correlation between the two types of colon polyps. Gut. 2003;52:1140–7. doi: 10.1136/gut.52.8.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leggett BA, Devereaux B, Biden K, Searle J, Young J, Jass J. Hyperplastic polyposis: association with colorectal cancer. Am J Surg Pathol. 2001;25:177–84. doi: 10.1097/00000478-200102000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Bauer VP, Papaconstantinou HT. Management of serrated adenomas and hyperplastic polyps. Clin Colon Rectal Surg. 2008;21:273–9. doi: 10.1055/s-0028-1089942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lash RH, Genta RM, Schuler CM. Sessile serrated adenomas: prevalence of dysplasia and carcinoma in 2139 patients. J Clin Pathol. 2010;63:681–6. doi: 10.1136/jcp.2010.075507. [DOI] [PubMed] [Google Scholar]
- 11.Torlakovic E, Skovlund E, Snover DC, Torlakovic G, Nesland JM. Morphologic reappraisal of serrated colorectal polyps. Am J Surg Pathol. 2003;27:65–81. doi: 10.1097/00000478-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Longacre TA, Fenoglio-Preiser CM. Mixed hyperplastic adenomatous polyps/serrated adenomas. A distinct form of colorectal neoplasia. Am J Surg Pathol. 1990;14:524–37. doi: 10.1097/00000478-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Balbinotti RA, Ribeiro U, Jr, Sakai P, et al. hMLH1, hMSH2 and cyclooxygenase-2 (Cox-2) in sporadic colorectal polyps. Anticancer Res. 2007;27:4465–72. [PubMed] [Google Scholar]
- 14.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 15.Morrison WB. Inflammation and cancer: a comparative view. J Vet Intern Med. 2012;26:18–31. doi: 10.1111/j.1939-1676.2011.00836.x. [DOI] [PubMed] [Google Scholar]
- 16.Goswami B, Rajappa M, Sharma M, et al. Inflammation: its role and interplay in the development of cancer, with special focus on gynecological malignancies. Int J Gynecol Cancer. 2008;18:591–9. doi: 10.1111/j.1525-1438.2007.01089.x. [DOI] [PubMed] [Google Scholar]
- 17.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2012;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiiliam CS, Mann M, Dubois RN. The role of cycloxygenases in inflammation, cancer and development. Oncogene. 1999;18:7906–16. doi: 10.1038/sj.onc.1203286. [DOI] [PubMed] [Google Scholar]
- 19.Leek RD, Landers R, Fox SB, Ng F, Hartis AL, Lewis CE. Association of tumour necrosis factor and its receptor with thymidine phosphorylase expression in invasive breast carcinoma. Br J Cancer. 1998;77:2246–51. doi: 10.1038/bjc.1998.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 21.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 22.Szylberg L, Janiczek M, Popiel A, et al. Large bowel genetic background and inflammatory processes in carcinogenesis: systematic review. Adv Clin Exp Med. 2015;4:555–63. doi: 10.17219/acem/31239. [DOI] [PubMed] [Google Scholar]
- 23.Lamont EB, Dias LE, Lauderdale DS. NSAIDs and colorectal cancer risk: do administrative data support a chemopreventive effect? J Gen Intern Med. 2007;22:1166–71. doi: 10.1007/s11606-007-0256-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–55. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Reinmuth N, Stoeltzing O, et al. Cyclooxygenase-2 is up-regulated by interleukin-1beta in human colorectal cancer cells via multiple signaling pathways. Cancer Res. 2003;63:3632–6. [PubMed] [Google Scholar]
- 26.Din FV, Theodoratou E, Farrington SM, et al. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59:1670–9. doi: 10.1136/gut.2009.203000. [DOI] [PubMed] [Google Scholar]
- 27.Carson JL, Strom BL, Soper KA, West SL, Morse ML. The association of nonsteroidal anti-inflammatory drugs with upper gastrointestinal tract bleeding. Arch Intern Med. 1987;147:85–8. [PubMed] [Google Scholar]
- 28.Gabriel SE, Jaakkimainen L, Bombardier C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs: a meta-analysis. Ann Intern Med. 1991;115:787–96. doi: 10.7326/0003-4819-115-10-787. [DOI] [PubMed] [Google Scholar]
- 29.Kawasaki T, Nosho K, Ohnishi M, et al. Cyclooxygenase-2 overexpression is common in serrated and non-serrated colorectal adenoma, but uncommon in hyperplastic polyp and sessile serrated polyp/adenoma. BMC Cancer. 2008;8:33. doi: 10.1186/1471-2407-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asting AG, Carén H, Andersson M, Lönnroth C, Lagerstedt K, Lundholm K. COX-2 gene expression in colon cancer tissue related to regulating factors and promotor methylation status. BMC Cancer. 2011;11:238. doi: 10.1186/1471-2407-11-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiedrowski M, Mroz A, Kaminski M, et al. Predictive factors of proximal advanced neoplasia in the large bowel. Arch Med Sci. 2014;10:484–9. doi: 10.5114/aoms.2013.38394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maihöfner C, Charalambous MP, Bhambra U, Lightfoot T, Geisslinger G, Gooderham NJ. Expression of cyclooxygenase-2 parallels expression of interleukin-1beta, interleukin-6 and NF-kappaB in human colorectal cancer. Carcinogenesis. 2003;24:665–71. doi: 10.1093/carcin/bgg006. [DOI] [PubMed] [Google Scholar]
- 33.Kanai T, Watanabe M, Hayashi A, et al. Regulatory effect of interleukin-4 and interleukin-13 on colon cancer cell adhesion. Br J Cancer. 2000;82:1717–23. doi: 10.1054/bjoc.2000.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]