Abstract
Platelet storage and its availability for transfusion are limited to 5-6 days. Oxidative stress (OS) is one of the causes for reduced efficacy and shelf-life of platelets. The studies on platelet storage have focused on improving the storage conditions by altering platelet storage solutions, temperature, and materials. Nevertheless, the role of OS on platelet survival during storage is still unclear. Hence, this study was conducted to investigate the influence of storage on platelets. Platelets were stored for 12 days at 22°C. OS markers such as aggregation, superoxides, reactive oxygen species, glucose, pH, lipid peroxidation, protein oxidation, and antioxidant enzymes were assessed. OS increased during storage as indicated by increments in aggregation, superoxides, pH, conjugate dienes, and superoxide dismutase and decrements in glucose and catalase. Thus, platelets could endure OS till 6 days during storage, due to the antioxidant defense system. An evident increase in OS was observed from day 8 of storage, which can diminish the platelet efficacy. The present study provides an insight into the gradual changes occurring during platelet storage. This lays the foundation towards new possibilities of employing various antioxidants as additives in storage solutions.
1. Introduction
Platelets play an important role in maintaining hemostasis. Platelet transfusions are essential for treatment of patients with thrombocytopenia and are routinely used during surgery, chemotherapy, and treatment of various bleeding disorders. However, platelet storage in vitro and their availability for transfusion are limited to 5-6 days at 22°C, during which platelets lose their viability and activity [1]. Changes occur in morphology, adhesion and aggregation, membrane features, and the activation and apoptotic markers during prolonged storage [1, 2].
Banked platelets begin to lose their function, which can be due to (i) the platelet activation during preparation and storage process or (ii) the changes in pH and enzyme activation of the plasma environment or (iii) both phenomena [3]. Oxidative stress (OS) is also one of the causative factors for reduced efficacy and shelf-life of stored platelets. OS leads to decrease in platelet nitric oxide which increases platelet activation and cellular production of reactive oxygen species (ROS) [4]. But whether resulting oxidative changes trigger activation and apoptosis or activation and apoptosis trigger oxidative changes during storage is still unclear [5].
There are studies on improving the storage conditions by altering platelet storage solutions, storage materials (UPX80 [6], tri-ethylhexyl-trimellitate [7], polyolefin [8], and ELX container [9], etc.), and temperatures 4–37°C [10–21]. Few studies have reported that glucose can be a good additive for new storage formulations [22–24]. Platelet additive solutions (PAS) with different formulations have been tested and seem to be desirable over plasma for the storage of platelet concentrates [10, 25–32]. Storing platelets in additive solutions (Tyrode's buffer) diminishes the risk of infections or allergic reactions caused by platelet storage in plasma [33].
Nevertheless, platelet survival in different storage solutions is still unclear. Thus, the present study was conducted to investigate the influence of storage on platelets in Tyrode's buffer through OS markers: (i) platelet integrity: platelet aggregation, glucose, and pH; (ii) oxidative stress: superoxides, lipid peroxidation (conjugate dienes and thiobarbituric acid reactive substances (TBARS)), protein oxidation (protein carbonyls and sulfhydryls), and antioxidant enzymes (superoxide dismutase (SOD) and catalase (CAT)).
2. Experimental Procedures
Animal care and maintenance were in accordance with the ethical committee regulations.
2.1. Chemicals
Cytochrome C (Cyt C) was purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). All other chemicals were of reagent grade and organic solvents of spectral grade.
2.2. Blood Sampling
According to Rajashekharaiah et al., [34] 4-month-old male Wistar rats were used for sampling of blood. Blood was aspirated carefully from the heart into tubes with CPDA-1 (Citrate, phosphate, dextrose, and adenine) solution.
2.3. Experimental Design
Platelets isolated from blood of five different rats (n = 5) were suspended in Tyrode's buffer (NaCl-140 mM; KCl-2.7 mM; NaH2PO4-0.4 mM; NaHCO3-11.9 mM; D-glucose-11.1 mM) and stored in polypropylene tubes at 22°C for a period of 12 days, without continuous agitation. Estimation of OS was performed by assessing the biomarkers on alternate days of storage, that is, 0, 2, 4, 6, 8, 10, and 12.
2.4. Isolation of Platelets
Blood was centrifuged at 1500 rpm for 15 min at room temperature to obtain platelet-rich-plasma (PRP). The PRP was then centrifuged at 4000 rpm for 15 min at 22°C. The resulting platelet pellet was gently resuspended in Tyrode's buffer, pH 7.4 [35].
2.5. Platelet Aggregation
Platelet aggregation was measured according to modified method of Born and Cross [36]. Hundred microliters of platelet sample was added to each well of the microtiter plate and incubated at 37°C. The absorbance was recorded at 405 nm in shaking mode for 1.5–2 min. The absorbance is inversely proportional to the aggregation in platelets.
2.6. Glucose
The level of glucose in platelets during storage was assessed enzymatically by GOD-POD method as described in the Autospan Gold kit and the absorbance was taken at 546 nm [37].
2.7. pH
pH of the samples were checked using Fisher Scientific pH strips [22].
2.8. Superoxides
Superoxides in the platelets were measured according to Olas and Wachowicz [38]. Two hundred microliters of Cyt C (160 μM) was added to the platelets and incubated at 37°C. The samples were centrifuged at 3500 rpm for 5 min. Reduction of Cyt C was measured spectrophotometrically at 550 nm. To calculate the molar concentration of superoxide, extinction coefficient for Cyt C of 18,700 M−1 cm−1 was used.
2.9. Conjugate Dienes
Conjugate dienes were measured according to Olas and Wachowicz [38]. Platelet samples were transferred to ether/ethanol (1 : 3 v/v) mixture and vortexed. The mixture was centrifuged at 4000 rpm. The level of conjugate dienes was measured spectrophotometrically at 235 nm.
2.10. Thiobarbituric Acid Reactive Substances (TBARS)
TBARS were measured according to Olas et al. [39]. The platelet samples were cooled in an ice bath for 10 min. The samples were then transferred to an equal volume of 20% (v/v) cold trichloroacetic acid (TCA) in 0.6 M HCl and centrifuged at 2000 rpm for 15 min. The supernatant was mixed with 0.2 mL of 0.12 M thiobarbituric acid (TBA) in 0.26 M Tris at pH 7.0. The mixture was incubated in a boiling water bath for 15 min and the absorbance was read at 532 nm.
2.11. Protein Carbonyls
Protein carbonyls were measured according to Reznick and Packer [40]. Platelet samples were mixed with 2 mL of 10 mM dinitrophenyl hydrazine (DNPH) in 2.5 M HCl and incubated for 1 h at room temperature. After incubation, 2.5 mL of 20% TCA was added and left in ice for 10 min. The mixture was centrifuged at 3000 rpm and the supernatant was discarded. The protein pellets were washed three times with ethanol : ethyl acetate (1 : 1 v/v). The final precipitate was dissolved in 1 mL of 6 M guanidine HCl in 133 mM Tris. The absorbance was read at 370 nm. The carbonyl content was calculated using absorption coefficient of 22,000 M−1 cm−1.
2.12. Protein Sulfhydryls
The protein sulfhydryls were measured according to Habeeb [41]. Platelet samples were mixed with 1.5 mL of 0.08 M buffer (Na-PO4, 0.5 mg mL−1 of Na2-EDTA and 2% SDS, pH 8.0) and vortexed. Then 0.1 mL of 5, 5′-dithiobis-(2-nitrobenzoic acid) (DTNB) was added. This was incubated at room temperature for 15 min. The absorbance was read at 412 nm. Molar absorptivity of 13,600 M−1 cm−1 was used to calculate protein sulfhydryls.
2.13. Superoxide Dismutase (SOD, EC 1.15.1.1)
SOD in the platelet sample was measured according to Misra and Fridovich [42]. Platelet samples were added to 880 μL of carbonate buffer (0.05 M, pH 10.2, 0.1 mM EDTA). Forty microliters of epinephrine was added to the mixture and the absorbance was measured at 480 nm for 4 min. SOD activity was expressed as the amount of enzyme that inhibits oxidation of epinephrine by 50% which is equal to 1 unit.
2.14. Catalase (CAT, EC 1.11.1.6)
Catalase was measured according to Aebi [43]. Ten microliters of absolute ethanol was added to 100 μL of platelet sample and incubated in ice bath for 30 min. After incubation, 240 μL of phosphate buffer was added to the above sample. Two hundred and fifty microliters of 0.066 M H2O2 was added just before reading and the absorbance was measured at 240 nm. The molar extinction coefficient of 43.6 M−1 cm−1 was used to determine the catalase activity.
2.15. Protein
The protein concentration in the samples was determined according to Lowry et al. [44].
2.16. Statistical Analyses
Results are represented as mean ± SE. One-way ANOVA was performed between the groups for all the parameters and the results are considered significant at P < 0.05. Tukey-Kramer multiple comparison test was performed using GraphPad Prism 6 Software.
3. Results
3.1. Platelet Aggregation Increased during Storage
Aggregation increased significantly by 59% (day 2), 68% (day 8), 79% (day 10), and 74% (day 12) when compared with day 0 at P < 0.05 (Figure 1).
Figure 1.
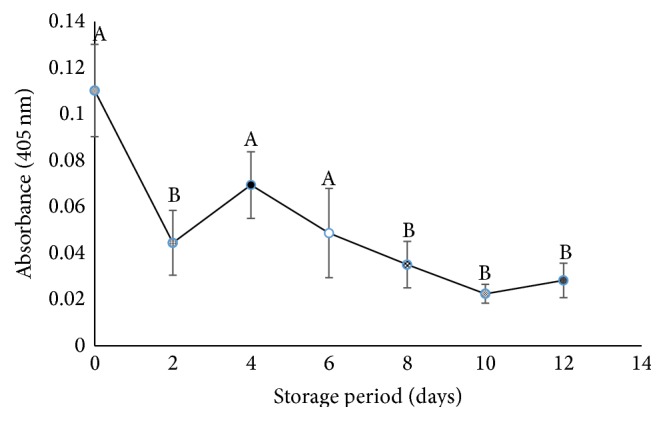
Aggregation in stored platelets. Values are mean ± SE of five animals/group. One-way ANOVA was performed between the groups followed by Tukey-Kramer multiple comparison test, using Graph Pad Prism 6 software, and represented in upper case at P < 0.05. Those not sharing the same letters are significantly different.
3.2. Superoxides Increased during Storage
Superoxides elevated significantly after day 6 of storage. There was an increase of 6-, 10-, and 3-fold on days 8, 10, and 12, respectively, against day 0 at P < 0.05. Decrement of 60% was observed on day 12 when compared with day 10 (Figure 2).
Figure 2.
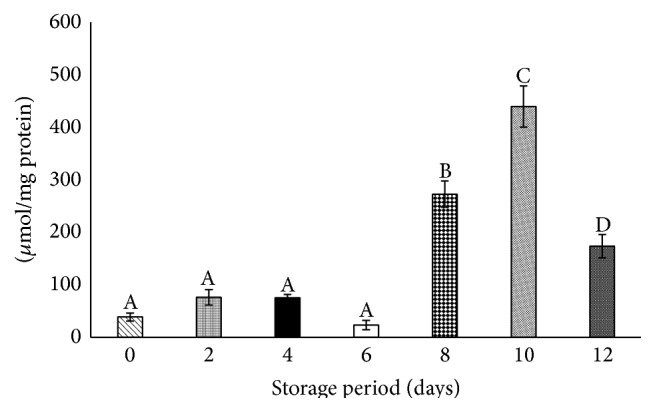
Superoxides in stored platelets. Values are mean ± SE of five animals/group. One-way ANOVA was performed between the groups followed by Tukey-Kramer multiple comparison test, using Graph Pad Prism 6 software, and represented in upper case at P < 0.05. Those not sharing the same letters are significantly different.
3.3. Glucose Decreased during Storage
Glucose levels reduced significantly (P < 0.05) throughout storage. Glucose levels decreased on day 2 by 39% and were maintained up to day 10. Decline in glucose levels by 91% was observed on day 12 against day 0 (Table 1).
Table 1.
Glucose, pH, lipid peroxidation, and protein oxidation in stored platelets.
| Storage Period (Days) | Glucose (mmol/L) | pH | Lipid peroxidation | Protein oxidation | ||
|---|---|---|---|---|---|---|
| Conjugate dienes (µmol/mg protein) | TBARS (µmol/mg Protein) | Protein carbonyls (µmol/mg protein) | Protein sulfhydryls (µmol/mg protein) | |||
| 0 | 12.22 ± 0.7a | 7.0 ± 0.0a | 23.20 ± 4.7a | 9.40 ± 3.1a | 18.45 ± 5.7a | 251.45 ± 48.0ab |
| 2 | 7.42 ± 0.6b | 7.0 ± 0.0a | 28.26 ± 11.8a | 11.31 ± 4.8a | 93.73 ± 25.0ac | 105.33 ± 30.2a |
| 4 | 5.80 ± 0.1b | 8.0 ± 0.0b | 69.64 ± 21.5a | 4.15 ± 2.3a | 185.5 ± 21.0b | 132.26 ± 15.8a |
| 6 | 5.84 ± 0.3b | 8.4 ± 0.2b | 21.52 ± 6.6a | 14.32 ± 5.4ab | 17.61 ± 5.5a | 244.57 ± 63.8ab |
| 8 | 7.33 ± 0.6b | 8.4 ± 0.2b | 96.6 ± 18.4a | 7.34 ± 1.4a | 68.37 ± 3.6ac | 392.6 ± 46.2b |
| 10 | 6.16 ± 0.1b | 9.0 ± 0.0c | 585.7 ± 41.9b | 38.4 ± 13.0b | 64.77 ± 14.7ac | 350.18 ± 37.5b |
| 12 | 1.05 ± 0.2c | 10.0 ± 0.0d | 330.9 ± 71.6c | 4.22 ± 1.0a | 146.78 ± 33.8bc | 528.18 ± 55.5c |
Values are expressed as mean ± SE of five animals/group. Changes between groups were analyzed by one-way ANOVA followed by Tukey-Kramer multiple comparison test, using GraphPad Prism 6 software. P < 0.05 was considered significant. Changes between the groups are represented in lower case. Those not sharing the same letters are significantly different.
3.4. pH Elevated with Storage
pH gradually elevated up to day 10 and by 42% on day 12 against day 0 (P < 0.05) (Table 1).
3.5. Conjugate Dienes Increased towards End of Storage
Significant increments were observed in conjugate dienes by 24- and 13-fold on days 10 and 12, respectively, with day 0 at P < 0.05 (Table 1).
3.6. TBARS Increased on Day 10
TBARS levels were maintained till day 8 but incremented by 3-fold on day 10 against day 0 (P < 0.05) (Table 1).
3.7. Protein Carbonyls Varied during Storage
Carbonyls increased by 9-fold on day 4 against day 0 at P < 0.05. Protein carbonyls declined on day 6 against day 4 and were maintained till day 10. Elevation of 6-fold was noted on day 12 against day 0 at P < 0.05 (Table 1).
3.8. Protein Sulfhydryls Increased after 8 Days of Storage
Protein sulfhydryls augmented only after day 6 and elevation of 1-fold was observed on day 12 against day 0 (P < 0.05) (Table 1).
3.9. Superoxide Dismutase Incremented after Day 6 of Storage
SOD significantly increased (P < 0.05) by 1-fold on days 6, 8, 10, and 12 with respect to day 0 (Figure 3).
Figure 3.
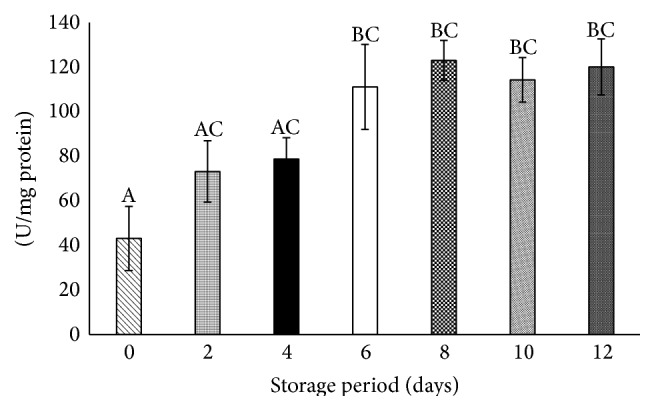
Superoxide dismutase in stored platelets. Values are mean ± SE of five animals/group. One-way ANOVA was performed between the groups followed by Tukey-Kramer multiple comparison test, using Graph Pad Prism 6 software, and represented in upper case at P < 0.05. Those not sharing the same letters are significantly different.
3.10. Catalase Declined after 8 Days of Storage
Significant decreases of 93% and 95% were observed on days 8 and 12, respectively, when compared with day 0 at P < 0.05 (Figure 4).
Figure 4.
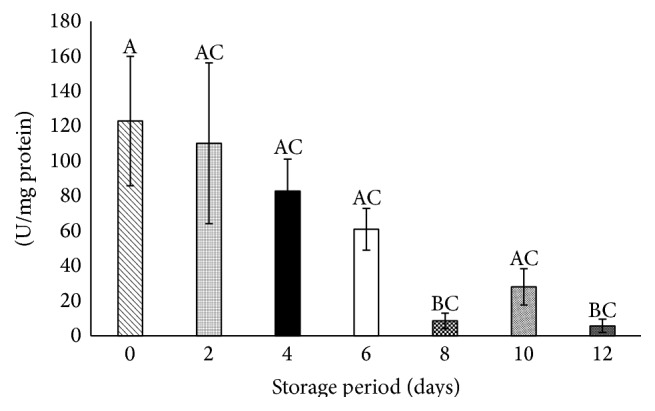
Catalase in stored platelets. Values are mean ± SE of five animals/group. One-way ANOVA was performed between the groups followed by Tukey-Kramer multiple comparison test, using Graph Pad Prism 6 software, and represented in upper case at P < 0.05. Those not sharing the same letters are significantly different.
4. Discussion
Our results showed that platelets could endure OS up to 6 days of storage and the onset of oxidative damage was noted from day 8. Platelets stored in plasma lose their functions considerably and also are exposed to infectious and allergic agents and proteolytic enzymes that can lead to premature clearance from circulation. Storing platelets in Tyrode's buffer provides optimal conditions for cell preservation and helps to control the environmental conditions during storage. Using a defined medium enhances the reproducible quality of stored platelet preparations [33]. The absence of Ca2+ in Tyrode's buffer also reduces platelet activation [45]. Platelet aggregation in our study increased in proportion to the free radical generation during storage, as observed in our results of increased superoxides during storage. The decrease in superoxides on day 12 against day 10 can be due to the decrease in number of platelets. The decrease in glucose level is directly proportional to the energy levels and platelet count [24]. The ideal pH for platelet transfusion should be between 6.4 and 7.4 at 22°C [46]. pH increased up to 10.0 by day 12 of storage. This can be due to the depletion of glucose in the medium, as glycolysis produces lactate, which reduces the pH of stored platelets [46]. The factors such as bacterial contamination, storage bag gas permeability [47], and platelet content in storage bag [48] can also affect the pH of platelets during storage.
The primary (conjugate dienes) and secondary (TBARS) products of lipid peroxidation increased at the end of storage. This can be due to elevations in superoxides and other ROS generation beyond the antioxidant defense, as poly-unsaturated fatty acids (PUFAs) are the most oxygen sensitive constituents of cells and easily suffer oxidation [49].
Reactive carbonyl groups in proteins are formed due to oxidation of arginine, lysine, threonine, or proline by the ROS in platelets [50]. The increase in carbonyls on day 4 may be due to the generation of free radicals which in turn triggers the antioxidant defenses (hormesis effect). This was elucidated in our results of day 6 where carbonyls decremented, which may be due to the activation of antioxidant defenses [51]. The elevated carbonyls on day 12 can be due to increased free radicals, overwhelming the platelet antioxidant system.
Sulfhydryls are the potential sites of reversible oxidative modification by S-glutathiolation and S-nitrosylation [52]. The augmented levels of sulfhydryls by the end of storage can be due to the activated or aggregated platelets that are known to exhibit increased surface sulfhydryls, especially protein disulfide isomerase (PDI) sulfhydryls [53]. This is in correlation with our results of increased platelet aggregation during storage.
An increase in SOD activity is the result of elevated free radicals [54]. SOD incremented by day 6 of storage and was maintained till day 12. This is in accordance with the elevation in superoxide levels as observed in our results. The level of superoxide regulates the rate of SOD activity [55]. CAT is activated at higher concentrations of H2O2 [56] and at lower concentrations, H2O2 is scavenged by glutathione peroxidase (GSH-Px) [57]. CAT activity decreased by the end of storage though there was an increase in SOD activity which can be due to the H2O2 scavenging action of GSH-Px.
5. Conclusion
Platelets could endure OS till 6 days during storage, due to the antioxidant defense system. An evident increase in OS was observed from day 8 of storage, which can diminish the platelet efficacy.
The present study provides an insight into the gradual changes occurring during platelet storage. This lays the foundation towards new possibilities of employing various antioxidants as additives in storage solutions to attenuate OS and hence enhance the stability and efficacy of stored platelets.
Acknowledgments
The authors acknowledge Dr. Leela Iyengar, Ms. Soumya Ravikumar, Mr. Carl Hsieh, and Jain University for their support.
Conflict of Interests
The authors report no conflict of interests.
References
- 1.Thon J. N., Schubert P., Devine D. V. Platelet storage lesion: a new understanding from a proteomic perspective. Transfusion Medicine Reviews. 2008;22(4):268–279. doi: 10.1016/j.tmrv.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Martín-Valmaseda E. M., Sánchez-Yagüe J., Rodríguez M. C., Gómez F. P., Llanillo M. Comparison between in vitro lipid peroxidation in fresh sheep platelets and peroxidative processes during sheep platelet ageing under storage at 4°C. Biochimica et Biophysica Acta: Biomembranes. 1999;1419(2):313–324. doi: 10.1016/s0005-2736(99)00080-2. [DOI] [PubMed] [Google Scholar]
- 3.George J. N. Platelet membrane glycoproteins: alteration during storage of human platelet concentrates. Thrombosis Research. 1976;8(5):719–724. doi: 10.1016/0049-3848(76)90253-x. [DOI] [PubMed] [Google Scholar]
- 4.Freedman J. E., Loscalzo J., Barnard M. R., Alpert C., Keaney J. F., Jr., Michelson A. D. Nitric oxide released from activated platelets inhibits platelet recruitment. The Journal of Clinical Investigation. 1997;100(2):350–356. doi: 10.1172/jci119540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goker B., Ozsavci D., Sener A., et al. Oxidative alterations during human platelet storage. Marmara Pharmaceutical Journal. 2011;15:38–42. [Google Scholar]
- 6.Kostelijk E. H., Gouwerok C. W. N., Veldman H. A., De Korte D. Comparison between a new PVC platelet storage container (UPX80) and a polyolefin container. Transfusion Medicine. 2000;10(2):131–139. doi: 10.1046/j.1365-3148.2000.00243.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh H., Chaudhary R., Ray V. Evaluation of platelet storage lesions in platelet concentrates stored for seven days. The Indian Journal of Medical Research. 2003;118:243–246. [PubMed] [Google Scholar]
- 8.Yuasa T., Ohto H., Yasunaga R., Kai T., Shirahama N., Ogata T. Improved extension of platelet storage in a polyolefin container with higher oxygen permeability. British Journal of Haematology. 2004;126(1):153–159. doi: 10.1111/j.1365-2141.2004.04994.x. [DOI] [PubMed] [Google Scholar]
- 9.Alhumaidan H., Cheves T., Holme S., Sweeney J. D. Manufacture of pooled platelets in additive solution and storage in an ELX container after an overnight warm temperature hold of platelet-rich plasma. American Journal of Clinical Pathology. 2011;136(4):638–645. doi: 10.1309/ajcpfd87thdwcsva. [DOI] [PubMed] [Google Scholar]
- 10.Chandra T., Gupta A., Kumar A. Extended shelf life of random donor platelets stored for 7 days in platelet additive solution at different temperatures. Biomedical Journal. 2014;37(4):211–217. doi: 10.4103/2319-4170.117896. [DOI] [PubMed] [Google Scholar]
- 11.Murphy S., Gardner F. H. Effect of storage temperature on maintenance of platelet viability—deleterious effect of refrigerated storage. The New England Journal of Medicine. 1969;280(20):1094–1098. doi: 10.1056/nejm196905152802004. [DOI] [PubMed] [Google Scholar]
- 12.Becker G. A., Tuccelli M., Kunicki T., et al. Studies of platelet concentrates stored at 22°C and 4°C. Transfusion. 1973;13:61–68. doi: 10.1111/j.1537-2995.1973.tb05442.x. [DOI] [PubMed] [Google Scholar]
- 13.Holme S., Vaidja K., Murphy S. Platelet storage at 22°C: effect of type of agitation on morphology, viability and function in vitro . Blood. 1978;52(2):425–435. [PubMed] [Google Scholar]
- 14.Rao A. K., Murphy S. Secretion defect in platelets stored at 4°C. Thrombosis and Haemostasis. 1982;47(3):221–225. [PubMed] [Google Scholar]
- 15.Currie L. M., Harper J. R., Allan H., Connor J. Inhibition of cytokine accumulation and bacterial growth during storage of platelet concentrates at 4°C with retention of in vitro functional activity. Transfusion. 1997;37(1):18–24. doi: 10.1046/j.1537-2995.1997.37197176946.x. [DOI] [PubMed] [Google Scholar]
- 16.Bertino A. M., Qi X. Q., Li J., Xia Y., Kuter D. J. Apoptotic markers are increased in platelets stored at 37°C. Transfusion. 2003;43(7):857–866. doi: 10.1046/j.1537-2995.2003.t01-4-00431.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi J. W., Pai S. H. Influence of storage temperature on the responsiveness of human platelets to agonists. Annals of Clinical and Laboratory Science. 2003;33(1):79–85. [PubMed] [Google Scholar]
- 18.Hoffmeister K. M., Felbinger T. W., Falet H., et al. The clearance mechanism of chilled blood platelets. Cell. 2003;112(1):87–97. doi: 10.1016/s0092-8674(02)01253-9. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J.-N., Wood J., Bergeron A. L., et al. Effects of low temperature on shear-induced platelet aggregation and activation. The Journal of Trauma—Injury, Infection & Critical Care. 2004;57(2):216–223. doi: 10.1097/01.ta.0000093366.98819.fe. [DOI] [PubMed] [Google Scholar]
- 20.Sandgren P., Shanwell A., Gulliksson H. Storage of buffy coat-derived platelets in additive solutions: in vitro effects of storage at 4°C. Transfusion. 2006;46(5):828–834. doi: 10.1111/j.1537-2995.2006.00803.x. [DOI] [PubMed] [Google Scholar]
- 21.Babic A. M., Josefsson E. C., Bergmeier W., et al. In vitro function and phagocytosis of galactosylated platelet concentrates after long-term refrigeration. Transfusion. 2007;47(3):442–451. doi: 10.1111/j.1537-2995.2007.01134.x. [DOI] [PubMed] [Google Scholar]
- 22.Amorini A. M., Tuttobene M., Lazzarino G., Denti G. Evaluation of biochemical parameters in platelet concentrates stored in glucose solution. Blood Transfusion. 2007;5(1):24–32. doi: 10.2450/2007.0019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amorini A. M., Tuttobene M., Tomasello F. M., et al. Glucose ameliorates the metabolic profile and mitochondrial function of platelet concentrates during storage in autologous plasma. Blood Transfusion. 2013;11(1):61–70. doi: 10.2450/2012.0145-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders C., Rowe G., Wilkins K., Collins P. Impact of glucose and acetate on the characteristics of the platelet storage lesion in platelets suspended in additive solutions with minimal plasma. Vox Sanguinis. 2013;105(1):1–10. doi: 10.1111/vox.12013. [DOI] [PubMed] [Google Scholar]
- 25.Holme S., Heaton W. A., Courtright M. Improved in vivo and in vitro viability of platelet concentrates stored for seven days in a platelet additive solution. British Journal of Haematology. 1987;66(2):233–238. doi: 10.1111/j.1365-2141.1987.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 26.Rock G., White J., Labow R. Storage of platelets in balanced salt solutions: a simple platelet storage medium. Transfusion. 1991;31(1):21–25. doi: 10.1046/j.1537-2995.1991.31191096179.x. [DOI] [PubMed] [Google Scholar]
- 27.De Wildt-Eggen J., Schrijver J. G., Bins M., Gulliksson H. Storage of platelets in additive solutions: effects of magnesium and/or potassium. Transfusion. 2002;42(1):76–80. doi: 10.1046/j.1537-2995.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- 28.Van der Meer P. F., Pietersz R. N. I., Reesink H. W. Storage of platelets in additive solution for up to 12 days with maintenance of good in-vitro quality. Transfusion. 2004;44(8):1204–1211. doi: 10.1111/j.1537-2995.2004.04010.x. [DOI] [PubMed] [Google Scholar]
- 29.Kerkhoffs J.-L. H., Eikenboom J. C., Schipperus M. S., et al. A multicenter randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma. Blood. 2006;108(9):3210–3215. doi: 10.1182/blood-2006-04-020131. [DOI] [PubMed] [Google Scholar]
- 30.Sweeney J., Kouttab N., Holme S., Kurtis J., Cheves T., Nelson E. Storage of platelet-rich plasma-derived platelet concentrate pools in plasma and additive solution. Transfusion. 2006;46(5):835–840. doi: 10.1111/j.1537-2995.2006.00804.x. [DOI] [PubMed] [Google Scholar]
- 31.Gupta A., Chandra T., Kumar A. In vitro function of random donor platelets stored for 7 days in composol platelet additive solution. Asian Journal of Transfusion Science. 2011;5(1):11–14. doi: 10.4103/0973-6247.75969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slichter S. J., Corson J., Jones M. K., et al. Exploratory studies of extended storage of apheresis platelets in a platelet additive solution (PAS) Blood. 2014;123(2):271–280. doi: 10.1182/blood-2013-05-501247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rock G., Swenson S. D., Adams G. A. Platelet storage in a plasma-free medium. Transfusion. 1985;25(6):551–556. doi: 10.1046/j.1537-2995.1985.25686071429.x. [DOI] [PubMed] [Google Scholar]
- 34.Rajashekharaiah V., Koshy A. A., Koushik A. K., et al. The efficacy of erythrocytes isolated from blood stored under blood bank conditions. Transfusion and Apheresis Science. 2012;47(3):359–364. doi: 10.1016/j.transci.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Carneiro A. M. D., Blakely R. D. Serotonin-, protein kinase C-, and Hic-5-associated redistribution of the platelet serotonin transporter. The Journal of Biological Chemistry. 2006;281(34):24769–24780. doi: 10.1074/jbc.m603877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Born G. V. R., Cross M. J. The aggregation of blood platelets. The Journal of physiology. 1963;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basak A. Development of a rapid and inexpensive plasma glucose estimation by two-point kinetic method based on glucose oxidase-peroxidase enzymes. Indian Journal of Clinical Biochemistry. 2007;22(1):156–160. doi: 10.1007/BF02912902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olas B., Wachowicz B. Resveratrol and vitamin C as antioxidants in blood platelets. Thrombosis Research. 2002;106(2):143–148. doi: 10.1016/s0049-3848(02)00101-9. [DOI] [PubMed] [Google Scholar]
- 39.Olas B., Nowak P., Kolodziejczyk J., Ponczek M., Wachowicz B. Protective effects of resveratrol against oxidative/nitrative modifications of plasma proteins and lipids exposed to peroxynitrite. The Journal of Nutritional Biochemistry. 2006;17(2):96–102. doi: 10.1016/j.jnutbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Reznick A. Z., Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods in Enzymology. 1994;233:357–361. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 41.Habeeb A. F. S. A. Methods in Enzymology. chapter 34. Vol. 25. Cambridge, Mass, USA: Academic Press; 1972. Reaction of protein sulfhydryl groups with Ellman's reagent; pp. 457–464. [DOI] [PubMed] [Google Scholar]
- 42.Misra H. P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. The Journal of Biological Chemistry. 1972;247(10):3170–3175. [PubMed] [Google Scholar]
- 43.Aebi H. Catalase in vitro . In: Packer L., editor. Methods in Enzymology. Vol. 105. Cambridge, Mass, USA: Academic Press; 1984. pp. 121–126. [DOI] [PubMed] [Google Scholar]
- 44.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193(1):265–275. [PubMed] [Google Scholar]
- 45.Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. The Journal of Biological Chemistry. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- 46.Dekkers D. W. C., De Cuyper I. M., Van Der Meer P. F., Verhoeven A. J., De Korte D. Influence of pH on stored human platelets. Transfusion. 2007;47(10):1889–1895. doi: 10.1111/j.1537-2995.2007.01412.x. [DOI] [PubMed] [Google Scholar]
- 47.Murphy S., Gardner F. H. Platelet storage at 22°C: role of gas transport across plastic containers in maintenance of viability. Blood. 1975;46(2):209–218. [PubMed] [Google Scholar]
- 48.Dumont L. J., VandenBroeke T. Seven-day storage of apheresis platelets: report of an in vitro study. Transfusion. 2003;43(2):143–150. doi: 10.1046/j.1537-2995.2003.00286.x. [DOI] [PubMed] [Google Scholar]
- 49.Spiteller G. Peroxyl radicals: inductors of neurodegenerative and other inflammatory diseases. Their origin and how they transform cholesterol, phospholipids, plasmalogens, polyunsaturated fatty acids, sugars, and proteins into deleterious products. Free Radical Biology and Medicine. 2006;41(3):362–387. doi: 10.1016/j.freeradbiomed.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 50.Alexandru N., Constantin A., Popov D. Carbonylation of platelet proteins occurs as consequence of oxidative stress and thrombin activation, and is stimulated by ageing and type 2 diabetes. Clinical Chemistry and Laboratory Medicine. 2008;46(4):528–536. doi: 10.1515/cclm.2008.104. [DOI] [PubMed] [Google Scholar]
- 51.Chevion S., Moran D. S., Heled Y., et al. Plasma antioxidant status and cell injury after severe physical exercise. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5119–5123. doi: 10.1073/pnas.0831097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas J. A., Mallis R. J. Aging and oxidation of reactive protein sulfhydryls. Experimental Gerontology. 2001;36(9):1519–1526. doi: 10.1016/s0531-5565(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 53.Essex D. W. The role of thiols and disulfides in platelet function. Antioxidants and Redox Signaling. 2004;6(4):736–746. doi: 10.1089/1523086041361622. [DOI] [PubMed] [Google Scholar]
- 54.Gürlek A., Tutar E., Akçil E., et al. The effects of L-carnitine treatment on left ventricular function and erythrocyte superoxide dismutase activity in patients with ischemic cardiomyopathy. European Journal of Heart Failure. 2000;2(2):189–193. doi: 10.1016/s1388-9842(00)00064-7. [DOI] [PubMed] [Google Scholar]
- 55.El-Bahr S. M. Biochemistry of free radicals and oxidative stress. Science International. 2013;1(5):111–117. doi: 10.5567/sciintl.2013.111.117. [DOI] [Google Scholar]
- 56.Kurata M., Suzuki M., Agar N. S. Antioxidant systems and erythrocyte life-span in mammals. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1993;106(3):477–487. doi: 10.1016/0305-0491(93)90121-k. [DOI] [PubMed] [Google Scholar]
- 57.Rahman K. Studies on free radicals, antioxidants and co-factors. Clinical Interventions in Aging. 2007;2(2):219–236. [PMC free article] [PubMed] [Google Scholar]


