Abstract
Background/Aims
The therapeutic effect of mesalamine is considered to be dose-dependent; however, no consensus has been reached regarding the optimal doses for individual patients. This study aimed to provide new insight for dose optimization using two doses of pH-dependent release mesalamine for induction of remission of moderately active ulcerative colitis (UC).
Methods
In a multicenter, double-blind, randomized study, 110 patients with moderately active UC were assigned to two groups after treatment with a constant dose of mesalamine. Fifty-five patients were treated with a pH-dependent release formulation of 3.6 or 4.8 g/day for 8 weeks. The primary endpoint was a decrease in the UC disease activity index (UCDAI) adjusted by covariates.
Results
In the full analysis set (n=110), the mean decrease in UCDAI was 3.1 in the 3.6 g/day group and 3.4 in the 4.8 g/day group (P>0.05). In a subgroup analysis, the effectiveness of the 4.8 g/day dose was greater in particular populations, such as those who had been previously treated with a lower dose of mesalamine and those with more severe disease. The safety was comparable between the two groups.
Conclusions
The results suggest that treatment with pH-dependent release mesalamine at either 3.6 or 4.8 g/day was effective and safe for the induction of remission in patients with moderately active UC. However, the patients receiving mesalamine at 2.4 g/day but in whom the therapeutic effect is not sufficient and having more severe symptoms (UCDAI 9-10), benefit from higher doses of mesalamine compared to others.
Keywords: Asacol; pH-dependent-release mesalamine; Colitis, ulcerative; Double-blind method
INTRODUCTION
The treatment of UC centers on drug therapy, with mesalamine, steroids, immunomodulators, and biological drugs being the main treatment options. Mesalamine is an antiinflammatory drug with a localized effect on the colon, and it is widely used as first-line treatment for mild-to-moderate UC.1 There are several oral formulations of mesalamine—each of them with different release properties. Mesalamine is considered a safe drug,1,2 and no difference in the incidence of adverse events (AEs) between the different oral mesalamine formulations has been reported.3
Although the therapeutic effect of mesalamine is considered to improve with increasing dose,1,2,4 no consensus has been reached with regard to the optimal dose of mesalamine. A textbook on internal medicine states the therapeutic effect of mesalamine on UC shows a dose–response relationship of up to 4.8 g/day,5 whereas the practice guidelines published by the American College of Gastroenterology recommend that patients with mild-to-moderate extensive colitis be started on treatment with a maximum of 4.8 g/day of mesalamine.1 On the other hand, according to a systematic review by the Cochrane Collaboration,3 a daily dosage of 2.4 g appears to be a safe and effective induction therapy for patients with mildly to moderately active UC. The Cochrane Collaboration review also states that among patients with mildly active UC, a dosage of 4 to 4.8 g/day does not appear to provide any additional benefit over a dosage of 2 to 2.4 g/day. That differs from patients with moderate disease, who may benefit from an initial dose of 4.8 g/day. According to the same review, a pooled analysis of the ASCEND trials found no statistically significant difference in the clinical improvement of mild-to-moderate UC in patients administered with pH-dependent-release oral mesalamine (Asacol®) at 4.8 g/day and 2.4 g/day;6,7,8 however, a subgroup analysis found that moderate cases may benefit from 4.8 g/day. Moreover, a study that compared 4 g/day and 2.25 g/day doses of time-dependent-release mesalamine (Pentasa® tablets) in patients with moderate UC found no statistically significant difference in failure to induce remission.9
Due to there being only limited evidence regarding the benefit of exceeding 4 g/day of mesalamine in the treatment of patients with moderately active UC, the aim of the present study was to compare the efficacy and safety of oral pHdependent-release mesalamine at 3.6 g/day and 4.8 g/day in patients with moderately active UC.
METHODS
1. Study Design
This was a multicenter, randomized, double-blind, parallel group study conducted at 38 facilities in Japan from September 2012 to October 2013. Patients were randomly assigned to study groups through a central registration by dynamic allocation with biased-coin minimization. The allocation factors were severity of UC (UC disease activity index [UCDAI] of 6-8 and 9-10), inflamed areas (ulcerative proctitis and other), and prior dose of oral mesalamine (<3.6 g/day and ≥3.6 g/day). Balance within the study site was also considered. The person responsible for the allocation, who was independent from the study, prepared the study drug allocation table and randomly allocated the study drug (3.6 g/day and 4.8 g/day groups) by way of a permutation block method for sets of four patients (two patients/group). The same person sealed the study drug allocation table immediately after allocation and securely retained the table until key code breaking. The screening period was 3 to 14 days after informed consent was obtained. Eligible subjects received one of the following combinations of the study drug orally (four tablets per dosing, three times daily after each meal for 8 weeks): 4.8 g/day (four pH-dependent-release mesalamine tablets per dosing) or 3.6 g/day (three pH-dependent-release mesalamine tablets and one placebo tablet per dosing).
All procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1975 Declaration of Helsinki, as revised in 2013. Documented approval by appropriate institutional review boards was obtained for all participating sites. All subjects provided written informed consent prior to participating in the study. The trial was registered in the JapicCTI under registration no. JapicCTI-121943.
2. Subjects
Patients with moderately active UC who met all of the following inclusion criteria were enrolled in the study: UCDAI score of 6 to 10 and rectal bleeding score of ≥1, relapsingremitting-type UC, and age of 16 to 64 years at the time of informed consent. Patients with any of the following criteria were excluded: (1) daily dose of oral mesalamine <2.0 g or >4.0 g (for salazosulfapyridine, the amount equivalent to mesalamine was calculated by multiplying the dose by 0.5) within 14 days prior to screening; (2) clinical course classified as the first attack, chronic continuous, or acute fulminating; (3) comorbid intestinal stenosis, intestinal perforation, toxic megacolon, or septicemia; (4) comorbid infectious enteritis; (5) time from onset of UC ≤6 months; (6) treatment with antidiarrheal medication or laxatives within 3 days prior to screening; (7) treatment with mesalamine (enema or suppository) or corticosteroid (oral, enema, suppository, or injection) within 14 days prior to screening; (8) treatment with azathioprine or 6-mercaptopurine (oral) within 21 days prior to screening; (9) cytapheresis or endoscopic mucosal or submucosal dissection within 28 days prior to screening; (10) laparotomy, laparoscopic surgery, endoscopic enteroplasty, or surgery for hemorrhoids or perianal abscess within 56 days prior to screening; (11) use of cyclosporine or tacrolimus (oral or injection), infliximab or adalimumab (injection), or other study drugs within 84 days prior to screening; (12) history of resection of the small intestine, appendix, colon, or rectum; (13) moderate comorbid hepatic or renal disorder; (14) serious comorbid disease such as hematologic, respiratory, or circulatory disorders, psychiatric or neurological diseases, or metabolic or electrolyte abnormality; (15) treatment for malignant tumor or a follow-up duration <5 years; (16) hypersensitivity to mesalamine or salicylic acid drugs; and (17) pregnancy or lactation.
3. Study Drug
Patients were administered pH-dependent-release mesalamine (Asacol® tablets; Tillotts Pharma AG, Ziefen, Switzerland) consisting of Eudragit-S® coated pH-dependent-release mesalamine containing 400 mg of mesalamine per tablet. Eudragit-S® dissolves at a pH ≥7, and the study drug (Asacol® 400-mg tablets) is designed to release mesalamine at the terminal ileum, where pH exceeds 7. The placebo and the Asacol® tablets were indistinguishable from each other. The Asacol® and placebo tablets used in this study were supplied by Zeria Pharmaceutical Co., Ltd. (Tokyo).
The following drugs and therapies were prohibited—from the study's start to final assessment (at week 8 or discontinuation): mesalamine drugs (oral, enema, and suppository), including salazosulfapyridine; corticosteroids (oral, enema, suppository, and injection); drugs containing azathioprine or 6-mercaptopurine (oral); drugs containing cyclosporine or tacrolimus (oral and injection); drugs containing infliximab or adalimumab (injection); other study drugs; endoscopic mucosal resection or endoscopic submucosal dissection; laparotomy, laparoscopic surgery, or endoscopic enteroplasty; surgery for hemorrhoids or perianal abscess; and cytapheresis.
The following drugs were restricted—from the study's start to final assessment (at week 8 or discontinuation): antidiarrheal medications or laxatives taken 3 days prior to visit and nonsteroidal anti-inflammatory drugs taken for 3 or more consecutive days.
4. Efficacy Assessment
The primary endpoint was a decrease in UCDAI from screening to final assessment. Secondary endpoints were the proportion of patients in remission (remission proportion) and the proportion of patients in remission or showing improvement (efficacy proportion).
The UCDAI is the sum of the mucosal-appearance score (based on colonoscopy findings), stool frequency score, rectal-bleeding score, and physician's global assessment score (each score has four items ranging from 0 to 3).10 Throughout the study, each patient visited the study site every 2 weeks and in addition, separately recorded the state of rectal bleeding, stool frequency, and drug compliance in a patient diary. The stool frequency score and the rectal-bleeding score were based on patient diary entries from 3 days before the visit. Each score of the UCDAI was assessed at each visit—except for the mucosal-appearance score. Patients with a UCDAI ≤2 and a rectal-bleeding score of 0 at final assessment were defined as being in remission. Patients who did not achieve remission but whose UCDAI decreased by ≥2 at final assessment were defined as showing improvement. Efficacy was defined as either remission or improvement. Colonoscopy was performed at screening and final assessment (at week 8 or discontinuation), and the UCDAI was calculated each time.
5. Safety Assessment
Laboratory data were collected at screening, at week 4, and at final assessment (at week 8 or discontinuation); and vital signs were recorded at screening and final assessment. The presence or absence of AEs and adverse drug reactions was recorded by the study doctor at each visit. AEs were coded and tabulated using MedDRA/J terminology (Med-DRA/J Ver.16.1).
6. Statistical Analysis
As primary analysis, analysis of covariance was performed using the following allocation factors: UCDAI at screening (continuous), inflamed areas (ulcerative proctitis or other), and dose of prior oral mesalamine (<3.6 g/day or ≥3.6 g/day) as covariates. If any of the UCDAI scores were missing, the UCDAI was handled as missing and was excluded from the analysis. Moreover, if any of the UCDAI scores were missing at final assessment, the subject was considered not remitted/not improved. Secondary and subgroup analyses were performed without adjusting the allocation factors. For the safety endpoint, the frequency distribution and incidence of AEs and adverse drug reactions were compared. A significance level of 0.05 (two-sided) was used for statistical tests, and P<0.05 was considered statistically significant. A confidence level of 0.95 (two-sided) was used to calculate the CI. There was no statistical rationale for the sample size set for this study.
The safety analysis set was the population used for safety assessment and included subjects who took at least one tablet of the study drug, but it excluded patients who had goodclinical-practice noncompliance or had no safety data after the start of the study treatment. The full-analysis set was the primary analysis set, which included all subjects in the safety analysis set—except for those who had no efficacy data after the start of the study treatment or who, after the start of the study treatment, were determined not to have UC. The perprotocol set included all subjects in the full-analysis set—except for those who did not meet the inclusion criteria—who fell under the exclusion criteria, used restricted or prohibited concomitant drugs, had treatment compliance <80%, or discontinued within 3 days from the start of the study treatment. The statistical analysis plan was finalized after the blind review prior to key code breaking, and the statistical analyses were performed at Zeria Pharmaceutical Co., Ltd. All statistical calculations were performed with SAS Release 9.2 (SAS Institute Inc., Cary, NC, USA).
RESULTS
1. Subjects
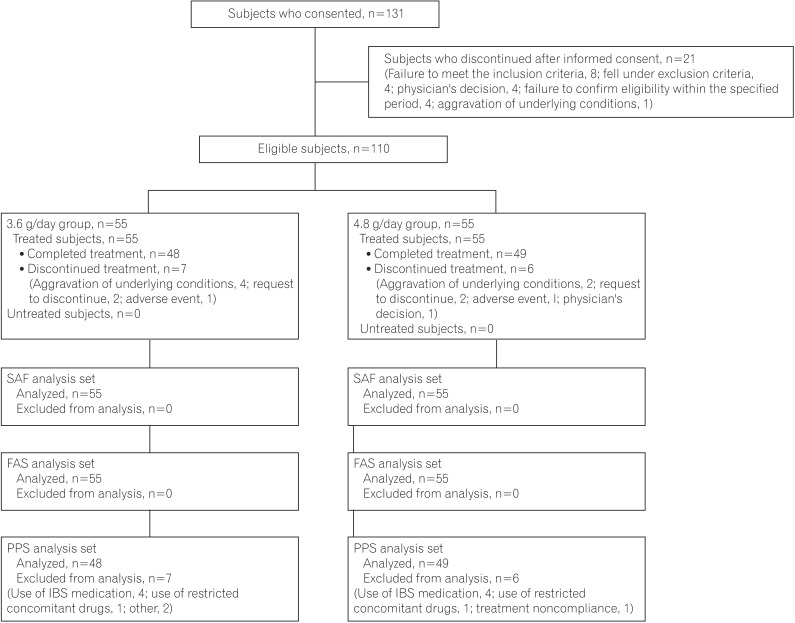
Among the 131 patients who provided informed consent, 110 eligible subjects (3.6 g/day group, 55; 4.8 g/day group, 55) received the study drug. Of those, 13 subjects (3.6 g/day group, 7; 4.8 g/day group, 6) discontinued the study. There were no between-group differences in reasons for discontinuation. The most common reason for discontinuation was aggravation of UC. The disposition of each analysis set is shown in Fig. 1. Because the analysis results of the full-analysis set and per-protocol set were similar, the analysis results of the full-analysis set are shown later.
Fig. 1. Enrollment, randomization, and analysis of the study subjects. SAF, safety analysis set; FAS, full-analysis set; PPS, per-protocol set.
Patient baseline characteristics are shown in Table 1. Most of the participants were men (62.7%), and mean age, body weight, and other characteristics were similar among the two dose groups. No adjustments were made other than the allocation factors in the efficacy analysis.
Table 1. Patient Baseline Characteristics.
| Characteristic | 3.6 g/day group (n=55) | 4.8 g/day group (n=55 ) |
|---|---|---|
| Sex | ||
| Male | 30 | 39 |
| Female | 25 | 16 |
| Age (yr) | 40.2±12.5 | 37.9±10.2 |
| Body weight (kg) | 61.4±12.1 | 62.3±10.7 |
| Duration of disease (yr) | ||
| <2 | 7 | 9 |
| ≥2 to <5 | 15 | 17 |
| ≥5 to <10 | 14 | 13 |
| ≥10 | 19 | 16 |
| Inflamed areas | ||
| Ulcerative proctitis | 11 | 9 |
| Left-sided colitis | 28 | 26 |
| Right-sided or segmental colitis | 2 | 1 |
| Extensive colitis | 14 | 19 |
| No. of normal stools | ||
| 1 | 41 | 35 |
| 2 | 8 | 13 |
| 3 | 4 | 6 |
| ≥4 | 2 | 1 |
| Time from most recent relapse (wk) | ||
| <2 | 2 | 3 |
| ≥2 to <4 | 10 | 10 |
| ≥4 to <8 | 13 | 23 |
| ≥8 to <24 | 22 | 15 |
| ≥2 | 8 | 4 |
| Severity of disease (UCDAI at initial assessment) | ||
| 6-8 | 41 | 40 |
| 9-10 | 14 | 15 |
| Dose of prior oral mesalaminea (g/day) | ||
| <3.6 | 14 | 14 |
| ≥3.6 | 41 | 41 |
Values are presented as mean±SD or n.
aOral mesalamine drugs included oral salazosulfapyridine. If salazosulfapyridine was used, the amount equivalent to mesalamine was calculated by multiplying the dose by 0.5.
UCDAI, Ulcerative Colitis Disease Activity Index.
2. Efficacy
1) UCDAI at Final Assessment
The decrease in UCDAI (primary endpoint) was 3.1 in the 3.6 g/day group and 3.4 in the 4.8 g/day group. The difference in the decrease in UCDAI between the two groups was 0.3 (95% CI, -0.7–1.3) and was not statistically significant. In the secondary analysis, the decreases in UCDAI without adjustment for covariates were 2.3 in the 3.6 g/day group (95% CI, 1.6–3.1) and 2.7 in the 4.8 g/day group (95% CI, 2.0–3.5); and a marked decrease from the time of screening was observed in both groups (Table 2).
Table 2. Decrease in Ulcerative Colitis Disease Activity Index.
| Characteristic | 3.6 g/day group (n=52) | 4.8 g/day group (n=53) | P-value |
|---|---|---|---|
| UCDAI at screening | 7.4±1.2 | 7.6±1.3 | |
| UCDAI at final assessmenta | 5.1±2.9 | 4.9±2.9 | |
| Decrease in UCDAIb (adjusted by covariates), mean (95% CI) | 3.1 (2.3–4.0) | 3.4 (2.5–4.3) | |
| Difference in decrease in UCDAIc (adjusted by covariatesd) difference (95% CI) | 0.3 (−0.7–1.3) | 0.598 | |
| Decrease in UCDAI, mean (95% CI) | 2.3 (1.6–3.1) | 2.7 (2.0–3.5) | |
| Difference in decrease in UCDAI (95% CI) | 0.4 (−0.7–1.5) | 0.494 | |
Values are mean±SD.
Missing values were excluded from the analysis.
aFinal assessment was at week 8 or at discontinuation.
bDecrease in UCDAI = UCDAI at screening – UCDAI at final assessment.
cDifference in decrease in UCDAI = decrease in UCDAI in the 4.8 g/day group – decrease in UCDAI in the 3.6 g/day group.
dCovariates were UCDAI at screening (continuous), inflamed areas (ulcerative proctitis, other), and dose of prior oral mesalamine (<3.6 g/day, ≥3.6 g/day).
UCDAI, Ulcerative Colitis Disease Activity Index.
2) Secondary Endpoints
The proportions of subjects in remission were 18.2% in the 3.6 g/day group and 25.5% in the 4.8 g/day group, and the proportions of subjects showing efficacy were 56.4% in the 3.6 g/day group and 60.0% in the 4.8 g/day group. The differences in the percentages of subjects who were in remission and showing efficacy were 7.3% (95% CI, -8.1–22.6) and 3.6% (95% CI,-14.7–22.0), respectively (Table 3).
Table 3. Remission Proportion and Efficacy Proportion.
| Characteristic | 3.6 g/day group (n=55) | 4.8 g/day group (n=55) |
|---|---|---|
| Effectivea | ||
| Remittedb | 10 | 14 |
| Improved | 21 | 19 |
| Not effective | 24 | 22 |
| Remission proportionc (%) (95% CI) | 18.2 (9.1–30.9) | 25.5 (14.7–39.0) |
| Difference in remission proportiond, difference (95% CI) | 7.3 (−8.1–22.6) | |
| Efficacy proportione (%) (95% CI) | 56.4 (42.4–69.6) | 60.0 (46.0–72.9) |
| Difference in efficacy proportionf, difference (95% CI) | 3.6 (−14.7–22.0) | |
Values are presented as n or y.
Missing values were handled as "Not effective."
aEffective was remitted and improved.
bRemitted was UCDAI ≤2 and rectal-bleeding score of 0 at final assessment.
cRemission proportion = % of patients remitted.
dDifference in remission proportion = remission proportion in the 4.8 g/day group – remission proportion in the 3.6 g/day group.
eEfficacy proportion = % of patients with effective treatment.
fDifference in efficacy proportion = efficacy proportion in the 4.8 g/day group – efficacy proportion in the 3.6 g/day group.
3) Subgroup Analysis
The difference between the 3.6 g/day and 4.8 g/day groups with respect to disease severity at screening, inflamed areas, and dose of prior oral mesalamine was investigated. In the subgroup with UCDAI scores of 6–8 at screening, the decrease in UCDAI was 2.1 in both the 3.6 g/day group and the 4.8 g/day group (95% CI, -1.2–1.2). In the subgroup with UCDAI scores of 9–10, the decreases in UCDAI were 3.1 in the 3.6 g/day group and 4.3 in the 4.8 g/day group, with a difference of 1.3 (95% CI, -1.2–3.7) between the two groups. In both subgroups, according to inflamed areas and dose of prior oral mesalamine, the decrease in UCDAI was higher in the 4.8 g/day group than in the 3.6 g/day group (Table 4).
Table 4. Subgroup Analysis According to Disease Severity, Inflamed Areas, and Dose of Prior Oral Mesalamine.
| Characteristic | 3.6 g/day group (n=52) | 4.8 g/day group (n=53) | |
|---|---|---|---|
| UCDAI at initial assessment | |||
| 6–8 | 39 | 38 | |
| Decrease in UCDAIa, mean (95% CI) | 2.1 (1.2–3.0) | 2.1 (1.3–2.8) | |
| Difference in decrease in UCDAIb, difference (95% CI) | 0.0 (−1.2–1.2) | ||
| 9–10 | 13 | 15 | |
| Decrease in UCDAI, mean (95% CI) | 3.1 (1.5–4.6) | 4.3 (2.4–6.3) | |
| Difference in decrease in UCDAI, difference (95% CI) | 1.3 (−1.2–3.7) | ||
| Decrease in UCDAI inflamed areas | |||
| Ulcerative proctitis | 11 | 9 | |
| Decrease in UCDAI, mean (95% CI) | 2.9 (1.1–4.7) | 3.1 (0.9–5.3) | |
| Difference in decrease in UCDAI, difference (95% CI) | 0.2 (−2.4–2.8) | ||
| Other | 41 | 44 | |
| Decrease in UCDAI, mean (95% CI) | 2.2 (1.3–3.1) | 2.6 (1.8–3.5) | |
| Difference in decrease in UCDAI, difference (95% CI) | 0.5 (−0.8–1.7) | ||
| Dose of prior oral mesalaminec | |||
| <3.6 g/day | 13 | 13 | |
| Decrease in UCDAI, mean (95% CI) | 3.6 (2.2–5.0) | 3.8 (2.2–5.3) | |
| Difference in decrease in UCDAI, difference (95% CI) | 0.2 (−1.8–2.2) | ||
| ≥3.6 g/day | 39 | 40 | |
| Decrease in UCDAI, Mean (95% CI) | 1.9 (1.0–2.8) | 2.4 (1.5–3.3) | |
| Difference in decrease in UCDAI, difference (95% CI) | 0.5 (−0.8–1.8) |
Final assessment was at week 8 or at discontinuation.
Missing values were excluded from the analysis.
aDecrease in UCDAI = UCDAI at screening – UCDAI at final assessment.
bDifference in decrease in UCDAI = decrease in UCDAI in the 4.8 g/day group – decrease in UCDAI in the 3.6 g/day group.
cOral mesalamine drugs included oral salazosulfapyridine. If salazosulfapyridine was used, the amount equivalent to mesalamine was calculated by multiplying the dose by 0.5.
UCDAI, Ulcerative Colitis Disease Activity Index.
Patients who received 2.0 to 4.0 g of oral mesalamine within 14 days prior to screening as a prior therapy were included in the present study; this included types of oral mesalamine other than pH-dependent-release mesalamine, such as salazosulfapyridine. (The amount equivalent to mesalamine was calculated by multiplying the dose by 0.5.)
In Table 4, in the subgroup with dose of prior oral mesalamine of <3.6 g/day, the decreases in UCDAI were 3.6 in the 3.6 g/day group and 3.8 in the 4.8 g/day group, with a difference of 0.2 (95% CI, -1.8–2.2) between the two groups. In the subgroup with dose of prior oral mesalamine of ≥3.6 g/day, the decreases in UCDAI were 1.9 in the 3.6 g/day group and 2.4 in the 4.8 g/day group, with a difference of 0.5 (95% CI, -0.8–1.8) between the two groups. There was no significant difference between the 3.6 g/day and 4.8 g/day groups in decrease in UCDAI according to dose of prior oral mesalamine (<3.6 g/day or ≥3.6 g/day).
Patients whose pH-dependent-release mesalamine dose at screening was 2.4 g/day or 3.6 g/day were analyzed to determine the effect of changing the dose. In the subgroup whose mesalamine dose at screening was 2.4 g/day, the decreases in UCDAI were 2.3 in the 3.6 g/day group and 4.5 in the 4.8 g/day group, with a difference of 2.2 (95% CI, –3.1–7.4) between the groups. In the subgroup whose mesalamine dose at screening was 3.6 g/day, the decrease in UCDAI was 2.1 in both the 3.6 g/day group and the 4.8 g/day group (Table 5).
Table 5. Subgroup Analysis According to the Prior Dose of pH-Dependent-release Mesalamine.
| Characteristic | 3.6 g/day group (n=33) | 4.8 g/day group (n=35) | |
|---|---|---|---|
| Prior dose of oral mesalamine | |||
| 2.4 g/day | 3 | 6 | |
| Decrease in UCDAIa, mean (95% CI) | 2.3 (−4.8– 9.5) | 4.5 (1.1–7.9) | |
| Difference in decrease in UCDAIb, difference (95% CI) | 2.2 (−3.1–7.4) | ||
| 3.6 g/day | 30 | 29 | |
| Decrease in UCDAI, mean (95% CI) | 2.1 (1.1–3.1) | 2.1 (1.1–3.2) | |
| Difference in decrease in UCDAI, difference (95% CI) | 0.0 (−1.4–1.5) |
Final assessment was at week 8 or at discontinuation.
Missing values were excluded from the analysis.
aDecrease in UCDAI = UCDAI at screening – UCDAI at final assessment.
bDifference in decrease in UCDAI = decrease in UCDAI in the 4.8 g/day group – decrease in UCDAI in the 3.6 g/day group.
UCDAI, Ulcerative Colitis Disease Activity Index.
3. Safety
The incidences of AEs were 43.6% (24/55 subjects) in the 3.6 g/day group and 45.5% (25/55 subjects) in the 4.8 g/day group. The incidences of adverse drug reactions were 14.5% (8/55 subjects) in the 3.6 g/day group, and 20.0% (11/55 subjects) in the 4.8 g/day group. There was no clear difference in the incidences of AEs and adverse drug reactions between the two groups.
AEs with incidences ≥5% in either group were nasopharyngitis (7.3% in the 3.6 g/day group and 12.7% in the 4.8 g/day group) and increased beta-N-acetyl-D-glucosaminidase (NAG) (12.7% in the 3.6 g/day group and 7.3% in the 4.8 g/day group). A causal relationship with the study drug was ruled out for all nasopharyngitis events; however, it could not be ruled out in many cases of increased NAG. The incidences of adverse drug reactions are shown in Table 6.
Table 6. List of Adverse Drug Reactions.
| Characteristic | 3.6 g/day group (n=55) | 4.8 g/day group (n=55) |
|---|---|---|
| All events | 8 (14.5) | 11 (20.0) |
| Abdominal discomfort | 0 (0.0) | 1 (1.8) |
| Aggravation of UC | 1 (1.8) | 0 (0.0) |
| Acute pancreatitis | 0 (0.0) | 1 (1.8) |
| Increased amylase | 0 (0.0) | 1 (1.8) |
| Increased beta-N-acetyl-D-glucosaminidase | 6 (10.9) | 4 (7.3) |
| Incresed conuated bilirubin | 0 (0.0) | 1 (1.8) |
| Incresed bilirubin | 1 (1.8) | 1 (1.8) |
| Increased urinc acid | 0 (0.0) | 1 (1.8) |
| Increased gamma-glutamyltransferase | 1 (1.8) | 0 (0.0) |
| Abnormal liver function test | 0 (0.0) | 2 (3.6) |
| Decresed white blood cell count | 0 (0.0) | 1 (1.8) |
| Incresed white blood cell count | 0 (0.0) | 1 (1.8) |
Values are presented as n (%).
Event term based on MedDRA/J Ver.16.1.
Adverse events were evaluated as related or unrelated. If assessed as related, the event was counted as an adverse drug reaction.
Regarding serious AEs, aggravation of UC occurred in one subject in the 3.6 g/day group, and acute pancreatitis and colon dysplasia occurred in one subject each in the 4.8 g/day group. Aggravation of UC and acute pancreatitis were determined to be related to the study drug. Moreover, herpes zoster oticus occurred in one subject in the 3.6 g/day group, and acute pancreatitis occurred in one subject in the 4.8 g/day group; they were determined as AEs leading to discontinuation of the study treatment.
DISCUSSION
Oral pH-dependent-release mesalamine is widely used for the treatment of UC worldwide. In Japan, pH-dependent-release mesalamine at 3.6 g/day has been approved as the maximum dose for patients with active UC; however, the use of up to 4.8 g/day is approved in many other countries. To date, no study has compared 3.6 g/day and 4.8 g/day doses of pH-dependent-release mesalamine. Thus, this study, which directly compared the therapeutic effects of those two dose levels, may provide valuable new evidence.
Because of the exploratory aspect of this study, there was no statistical rationale for the sample size, and as a result, it is difficult to draw a definite conclusion. Nevertheless, there was a marked decrease in UCDAI at final assessment in both the 3.6 g/day group and the 4.8 g/day group, and the percentages of patients showing efficacy were comparable to those found in studies performed in Japan and overseas.6,7,8,10 Therefore, both dose levels were considered to induce remission in patients with moderately active UC. However, there was no significant difference between the 3.6 g/day and 4.8 g/day groups in decrease in UCDAI at final assessment. Therefore, in Japanese patients with moderately active UC, the benefit gained from the 4.8 g/day dose compared with the 3.6 g/day dose was considered minimal. Ito et al. investigated pH-dependent-release mesalamine induction of remission at doses of 2.4 g/day and 3.6 g/day and without adjustment for covariates;10 the difference in the decrease in UCDAI at final assessment was 1.4 (1.5 in the 2.4 g/day group and 2.9 in the 3.6 g/day group). In the present study, the difference in the decrease in UCDAI was 0.4 (2.3 in the 3.6 g/day group and 2.7 in the 4.8 g/day group). Although it is not possible to simply compare the results from the two studies, the difference in the decrease in UCDAI between the 3.6 g/day and 4.8 g/day groups found in the present study was smaller than the difference between the 2.4 g/day and 3.6 g/day groups in the previous study.
The results of subgroup analysis suggested that some patients may benefit from 4.8 g/day of pH-dependent-release mesalamine. In the subgroup analysis based on the dose of oral mesalamine in prior therapy, the decrease in UCDAI in patients who had received 2.4 g/day was 2.2 points higher in the 4.8 g/day group (group with a 2.4 g dose increase) than in the 3.6 g/day group (group with a 1.2 g dose increase). Meanwhile, the decrease in UCDAI in patients who had received 3.6 g/day did not differ between the 3.6 g/day (group with no dose change) and 4.8 g/day (group with a 1.2 g dose increase) groups. Thus, the increase in dose by 1.2 g appeared to have no effect. Although the therapeutic effect of mesalamine has been proposed to improve with increasing dose,1,2 those results showed that patients receiving oral mesalamine at 2.4 g/day but in whom the therapeutic effect is not sufficient could benefit from increasing the dose up to 4.8 g/day. However, increasing the dose of oral mesalamine to 4.8 g/day may not be appropriate for patients with moderately active UC who are receiving up to 3.6 g/day. Furthermore, in the subgroup analysis based on disease severity, there was no difference in the decrease in UCDAI between the two groups in patients whose UCDAIs at screening were 6-8, whereas the decrease in UCDAIs in patients with scores of 9-10 was 1.3 points higher in the 4.8 g/day group than in the 3.6 g/day group.
Patients may be classified as severe when their UCDAIs are ≥11.11 Therefore, it seemed that patients with moderate UC whose disease severities were milder (UCDAI 6-8) were less likely to benefit from 4.8 g/day of pH-dependent-release mesalamine, and those with more-severe symptoms (UCDAI 9-10) were more likely to benefit from 4.8 g/day than from 3.6 g/day. However, because the numbers of subjects in those subgroups were small, it is not appropriate to draw a conclusion based solely on trends identified from the results of that study.
Regarding safety, there was no clear difference in incidences of AEs or adverse drug reactions between the two groups. Incidences of adverse drug reactions did not tend to be markedly higher in the 4.8 g/day group. Mesalamine is generally considered a safe drug,1,2 and 4.8 g/day of pH-dependent-release mesalamine has been reported to be a safe dose in patients with mild-to-moderate UC.7,8,9 Thus, the results from this study were in accordance with those of previous reports. However, in the present study, acute pancreatitis in one subject in the 4.8 g/day group was determined to be a serious adverse drug reaction. Pancreatitis is a known adverse reaction of mesalamine. Physicians should be aware of patients' symptoms and relevant clinical laboratory data.
Our results suggest that the safety risk of the 4.8 g/day dose is not higher than that of the 3.6 g/day dose. However, patients who did not respond sufficiently to treatment with 3.6 g/day were believed to benefit little from an increase in the dose to 4.8 g/day and may benefit more from a treatment other than oral mesalamine. Meanwhile, the results suggested that treatment with 4.8 g/day may bring benefit to particular populations, such as (1) patients who are not responding sufficiently because of the low dose of mesalamine and (2) patients with more-severe disease. However, further investigation is required to determine the optimal dose for each patient.
We must conduct more-detailed assessments of patients' backgrounds to determine which findings from the subgroup analysis of the present study can be generalized. An understanding of the limitations of mesalamine treatment based on patients' backgrounds will potentially lead to optimization of UC treatment.
A limitation of the present study was that no statistical rationale was established for the sample size of the present study, which was relatively small.
In conclusion, stand-alone treatment with pH-dependent-release mesalamine at 3.6 g/day and 4.8 g/day was effective for the induction of remission in patients with moderately active UC. The benefit from a dose exceeding 3.6 g/day—namely, 4.8 g/day—was not apparent for all patients with active UC. Safety risks were considered to be comparable between the 4.8 g/day and 3.6 g/day doses.
ACKNOWLEDGEMENTS
We thank all of the study participants, doctors, and staff who supported this study. We thank Hikaru Ito (Zeria Pharmaceutical Co., Ltd., Tokyo) for help in writing this paper and Michelle Belanger, MD, on behalf of inScience Communications for editorial support, which was funded by Zeria Pharmaceutical Co., Ltd. All named authors meet International Committee of Medical Journal Editors criteria for authorship for this manuscript; they take responsibility for the integrity of the work as a whole; and they have given final approval to the version to be published.
Footnotes
Financial support: This study was funded and supported by Zeria Pharmaceutical Co., Ltd., and Kyowa Hakko Kirin Co., Ltd. (Tokyo).
Conflict of interest: Hiroaki Ito has received consulting fees from Zeria Pharmaceutical Co., Ltd. Isamu Saida is an employee of Zeria Pharmaceutical Co., Ltd. Toshifumi Hibi has received advisory, speaker fees and grant support from Zeria Pharmaceutical Co., Ltd., speaker fees from Kyorin Pharmaceutical Co., Ltd., and consulting fees from Pfizer Japan Inc.
References
- 1.Kornbluth A, Sachar DB Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. doi: 10.1111/j.1572-0241.2004.40036.x. [DOI] [PubMed] [Google Scholar]
- 2.Carter MJ, Lobo AJ, Travis SP;, British Society. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–V16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD000543. doi: 10.1002/14651858.CD000543.pub3. [DOI] [PubMed] [Google Scholar]
- 4.Friedman S, Blumberg RS. Inflammatory bowel disease. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. 16th ed. New York: McGraw-Hill; 2004. pp. 1776–1789. [Google Scholar]
- 5.Friedman S, Blumberg RS. Inflammatory bowel disease. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J, editors. Harrison's principles of internal medicine. 18th ed. New York: McGraw-Hill; 2011. pp. 2477–2495. [Google Scholar]
- 6.Hanauer SB, Sandborn WJ, Dallaire C, et al. Delayed-release oral mesalamine 4.8 g/day (800 mg tablets) compared to 2.4 g/day (400 mg tablets) for the treatment of mildly to moderately active ulcerative colitis: The ASCEND I trial. Can J Gastroenterol. 2007;21(12):827–834. doi: 10.1155/2007/862917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanauer SB, Sandborn WJ, Kornbluth A, et al. Delayed-release oral mesalamine at 4.8 g/day (800 mg tablet) for the treatment of moderately active ulcerative colitis: The ASCEND II trial. Am J Gastroenterol. 2005;100:2478–2485. doi: 10.1111/j.1572-0241.2005.00248.x. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Regula J, Feagan BG, et al. Delayed-release oral mesalamine 4.8 g/day (800-mg) tablet is effective for patients with moderately active ulcerative colitis. Gastroenterology. 2009;137:1934–1943. doi: 10.1053/j.gastro.2009.08.069. [DOI] [PubMed] [Google Scholar]
- 9.Hiwatashi N, Suzuki Y, Mitsuyama K, et al. Clinical trial: Effects of an oral preparation of mesalazine at 4 g/day on moderately active ulcerative colitis. A phase III parallel-dosing study. J Gastroenterol. 2011;46:46–56. doi: 10.1007/s00535-010-0308-3. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Iida M, Matsumoto T, et al. Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: A double-blind, randomized study. Inflamm Bowel Dis. 2010;16:1567–1574. doi: 10.1002/ibd.21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Research Group for Intractable Inflammatory Bowel Disease (Watanabe Group) Mayo score. Disease activity index of inflammatory bowel disease. In: Hirai F, editor. Health and labour science research grants from the Japanese Ministry of Health, Labour and Welfare and research on measures for intractable disease; 2007–2009 research report. 2010. p. 640. [Google Scholar]