Abstract
Gene therapy represents a feasible strategy to treat inherited monogenic diseases and intramuscular (i.m.) injection of recombinant adeno-associated viral (AAV) vector is now recognized as a convenient and safe method of gene transfer. However, this approach is hampered by immune responses directed against the vector and against the transgenic protein. We used here to reproduce this situation a mouse model where robust immune responses are induced following injection of an AAV vector coding for an immunogenic transgenic protein. We show that prophylactic oral administration of the immunogenic protein before AAV-mediated gene transfer completely prevented antibody formation and cytotoxic CD8+ T-cell response. Consistently, prophylactic oral-tolerization considerably improved long-term transgene persistence and expression. Mechanistically, inhibition of the cytotoxic immune response involved abortive proliferation of antigen-specific cytotoxic CD8+ T cells, upregulation of the PD-1 immunoregulatory molecule, downregulation of the Bcl-2 antiapoptotic factor, and their deletion in the context of AAV-mediated gene transfer. Hence, gene therapy may represent an ideal situation where oral-tolerization can be adopted before or at the same time as vector injection to efficiently prevent deleterious immune responses directed against the transgenic protein.
Introduction
The use of viral-derived vectors in gene therapy settings represents a promising strategy to treat monogenic diseases or to induce the expression of a given secreted therapeutic transgenic protein in vivo.1,2 Recombinant adeno-associated viral (AAV) vectors represent a safe and efficient way to achieve in vivo gene transfer and, depending on the serotype, can be used to transduce different target tissues.3,4 AAV vectors also demonstrated several advantages over other viral-derived vectors, as their lower immunogenicity, their ability to transduce nondividing cells, and their lower risk to induce insertional mutagenesis.1,2,3,4,5
AAV-mediated gene transfer and long-term transgene expression has been achieved in several preclinical animal models as well as in clinical trials where it was demonstrated to be safe and effective.6,7,8 However, AAV-mediated gene transfer triggers immune responses directed against the viral capsid proteins and/or against the transgenic proteins.5,7,9,10,11,12,13,14 Activation of cytotoxic CD8+ T cells mediates the destruction of transduced cells and loss of transgene expression7,9,14,15,16 and humoral immunity generates neutralizing antibodies.5,16,17,18,19 To date, administration of immunosuppressive drugs and/or careful patients selection to avoid immune responses directed notably against the transgenic protein represent the only approaches to circumvent this limitation.7,11,18,20,21,22,23,24 Yet, immune responses have been observed in clinical trials despite the use of immunosuppressive drugs.18,21 Hence, strategies aiming to circumvent immune responses following AAV vectors injection should greatly enhance long-term transgene expression and may broaden the number of patient electable for gene therapy.10,11 In our study, we evaluated an alternative approach to promote antigen-specific oral-tolerization instead of systemic immune suppression.
Oral tolerance is characterized by a specific inhibition of the immune responses directed against defined antigens administered by the oral route. Therefore, it has the advantage over drug-based immunosuppression to be antigen-specific and long-lasting. Tolerance induction represents the “default” response of the intestinal immune system. Intestinal dendritic cells, located in the lamina propria and mesenteric lymph nodes (MLNs), have been implicated in the uptake of orally derived antigens and in their tolerogenic presentation to T cells.25,26,27,28,29,30 Several mechanisms have been identified that could account for the induction of oral tolerance as anergy and/or deletion of antigen-specific T cells31,32 or induction of regulatory T cells (Tregs).25,33
Encouraging results have been obtained in clinical trials using oral tolerance to treat allergies while partial clinical responses, limited to particular subsets of individual, were observed in the context of autoimmune diseases. This suggests that the efficacy of oral-tolerization might depend on the clinical situation and on the specific immune status of each patient.34,35 One explanation may reside in the difficulty to tolerize secondary/memory immune responses suggesting that oral-tolerization may be beneficial in prophylactic rather than curative scenarios.36 Interestingly, gene therapy may offer such clinical situations by providing the possibility to tolerize individuals before vector injection.
We tested in the present study the effects of oral-tolerization in an animal model of AAV-mediated gene transfer. For that, the protein coded by the vector was first orally administrated for 7 days prior to AAV-mediated gene transfer. To stringently evaluate the capacity of this protocol to tolerize the immune system, we chose here as a protein model the highly immunogenic ovalbumin (Ova) antigen and the i.m. route. We previously showed that i.m. injection of AAV-Ova induces prominent humoral and cellular immune responses that are associated with rapid loss of transgene expression.37,38 We provide here the first proof-of-principle that oral-tolerization prior to AAV-mediated gene transfer completely abrogates humoral and cellular immune responses directed against a soluble immunogenic transgenic protein. This was associated with long-term transgene expression and with the maintenance of the secreted transgenic protein in the circulation of tolerized animals.
Results
Oral-tolerization prevents immune responses directed against the transgenic protein
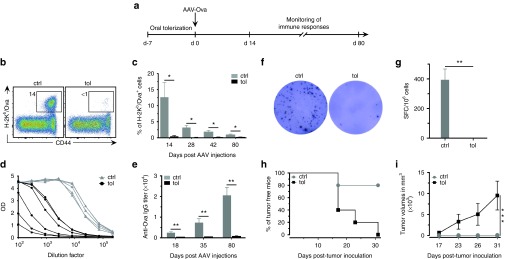
Intramuscular administration of AAV vectors can lead to a robust immune response that correlates with the elimination of the transduced cells and with the disappearance of the transgenic protein.37,39 We investigated here, in an animal model, the efficiency of oral-tolerization to prevent the immune response directed against the transgenic protein. For that, mice were orally-tolerized with the model protein ovalbumin (Ova), given at 1% in the drinking water, prior to i.m. administration of AAV-Ova at day 0. Immune responses were then monitored over time from days 14 to 80 (Figure 1a). In untolerized control animals, up to 18% of circulating CD8+ recognized Ova 14 days post-AAV-Ova transduction. In contrast, orally-tolerized mice displayed little if any cellular immune responses (Figure 1b,c). Ova-specific CD4+ and CD8+ immune responses were also undetectable by the more sensitive Enzyme-linked immunospot assay in orally-tolerized mice at day 80 (Figure 1f,g). Similarly, oral-tolerization significantly prevented the formation of anti-Ova IgG antibodies (Figure 1d,e). Of note, this oral-tolerization protocol completely blocked immune responses irrespectively of the administrated dose of AAV-Ova, i.e., 3.5 × 109, 5 × 1010, or 1011 vg (Supplementary Figure S1a–c). Also, oral feeding for only 5 days (Supplementary Figure S1d–f), or using a 10-fold lower concentration of Ova (i.e., 0.1%) (Supplementary Figure S1g), similarly resulted in complete prevention of immune responses, suggesting that robust tolerization mechanisms are induced following oral feeding.
Figure 1.
Oral-tolerization prevents immune responses directed against the transgenic protein. (a) Protocol outline. B6 mice were given 1% of Ova, ad libitum, in drinking water during 7 days and then injected with adeno-associated virus (AAV)-Ova (5 × 1010 vg/mouse) in both gastrocnemius (n = 5 per group). Immune responses were monitored between days 14 and 80 after injections. (b) Blood samples were collected 14 days post-AAV-Ova transduction and percentage of Ova-specific CD8+ T cells was determined using H-2Kb/Ova257-264 dextramers staining and flow cytometry in control (ctrl) and tolerized (tol) mice. The representative flow cytometric panels shown here represent percentage of Ova-specific CD8+ T cells in the gated CD8+ population. (c) Percentage of Ova-specific CD8+ T cells in the blood were determined at the indicated times points post AAV-Ova transduction. (d,e) Sera were collected at 80 days (d) or at the indicated time points (e) post-AAV-Ova transduction and anti-Ova IgG titers were determined by enzyme-linked immunosorbent assay. Curves represent optical density plotted against dilutions factors from data obtained at day 80 (d). The IgG titers correspond to the dilution yielding the half-maximum optical density obtained with a positive control serum used in all experiments (e). (f,g) At day 80, splenocytes were harvested and analyzed by ex vivo enzyme-linked immunospot (ELISpot) assays for their capability to secrete IFNγ after restimulation with the Ova257-264 peptide. (f) Representative ELISpot data obtained with control and tolerized mice. (g) Average number of spot forming cell per 106 splenocytes obtained in control and tolerized group of mice (n = 5/group). (h,i) 106 EG7 Ova-bearing tumor cells were injected 14 days after AAV-Ova or PBS injections (n = 5/group). Mice were monitored for tumor development (h) and tumor volume (i). Ova, ovalbumin; PBS, phosphate-buffered saline.
We then evaluated the capacity of our tolerization protocol to inhibit immune responses in less favorable conditions resulting from the use of incompletely purified AAV-preparations or from the use of self-complementary AAV vectors (scAAV). Indeed, both conditions are associated with the triggering of Toll-like receptors (TLRs) and activation of innate immune cells. To mimic these situations, we coinjected our AAV-Ova vector with contaminating CpG-ODNs, a potent TLR9 agonist. Our results demonstrate that, even in this more immunogenic situation, oral-tolerization prevented cellular and humoral immune responses directed against Ova and improved its persistence in the circulation (Supplementary Figure S2).
Next, we investigated the functionality of the cytotoxic T cells generated upon AAV-Ova transduction using a well-characterized CD8+-dependent antitumor response in vivo.38,40 For that, Ova-bearing EG7 cells were injected s.c. in tolerized or control mice 14 days after AAV-Ova injection and tumor growth was monitored overtime. Results showed that virtually all control mice rejected Ova-bearing tumor cells (Figure 1h,i). In contrast, all tolerized mice developed tumors within the first 30 days after EG7 inoculation in agreement with the absence of functional anti-Ova cytotoxic T cells response in tolerized mice.
CD8+ T cells do not infiltrate muscles in tolerized mice
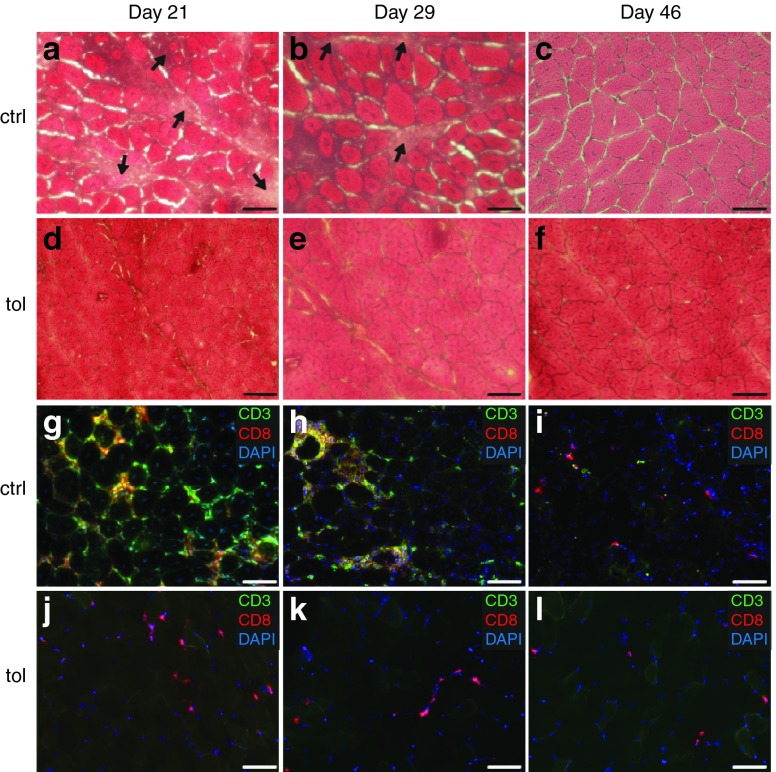
We next investigated the presence of mononuclear cells infiltration in transduced muscles sections. For that, gastrocnemius was harvested 21, 29, or 46 days after gene transfer for histological analyses. Hematoxylin and eosin staining at day 21 and 29 revealed conspicuous mononuclear cell infiltration and myofiber necrosis/regeneration evidenced by the presence of centronucleated fibers in muscle sections of untolerized mice (Figure 2a,b). Immunofluorescence confirmed the presence of cytotoxic T cells expressing both CD3 and CD8 that surrounded muscle fibers (Figure 2g,h). At the latest time point analyzed, histological muscle structure tended to normalize suggesting termination of the immune response and almost complete muscle regeneration (Figure 2c). In contrast, little if any mononuclear cell infiltration was detected in orally-tolerized mice (Figure 2d–f) and CD8+ cytotoxic T cells were virtually absent at all analyzed time points (Figure 2j–l). Only CD3-CD8+ cells, possibly corresponding to dendritic cells, were sparsely detected. Thus, oral-tolerization prior to AAV-mediated gene transfer protects transduced muscle fibers from CD8+ cytotoxic T cells.
Figure 2.
Oral-tolerization prevents muscle infiltration by CD8+ T cells. Mice were tolerized or not during 7 days with 1% Ova in drinking water and injected as before with adeno-associated virus (AAV)-Ova (5 × 1010 vg/mouse) in both gastrocnemius at day 0 (n = 6 per group). Muscles were collected at the indicated times points post-AAV-Ova injection. Muscle sections from control (a–c) and tolerized (d–f) mice were stained with hematoxylin and eosin to evaluate muscle histology and the overall level of mononuclear cell infiltration. Muscle sections from control (g–i) and tolerized (j–l) mice were analyzed by immunofluorescence after immunostaining with anti-CD3 (green) and anti-CD8 (red) antibodies and nucleus counterstaining with DAPI (blue). Scale bars represent 50 μm and arrows in (a) and (b) show area containing infiltrating cells. AAV, adeno-associated virus; ctrl, control; DAPI, 4′,6-diamidino-2-phenylindole; Ova, ovalbumin; tol, tolerized; vg, vector genome.
Oral-tolerization favors long-term transgene expression
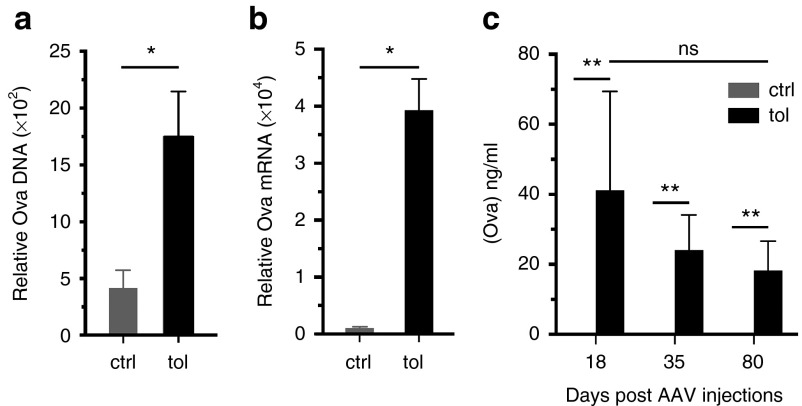
We next examined whether the control of immune responses was associated with the long-term persistence of the transgene. For that, the relative abundance of DNA and mRNA coding for Ova were quantify in transduced muscles 80 days after AAV-Ova injection. As anticipated, we observed significant higher copy numbers of the transgene and of its corresponding mRNA, in muscle samples of tolerized mice (Figure 3a,b). Concordantly, quantification of Ova in serum confirmed the stable expression of Ova in tolerized mice while Ova were under the limit of detection in control mice (Figure 3c). Hence, these results show that oral-tolerization sustains transgene persistence and expression, and allows long-term maintenance of the secreted transgenic protein.
Figure 3.
Oral-tolerization favors transgene long-term expression. AAV-Ova-injected muscles were harvested at day 80 from tolerized or control mice and transgene persistence and expression were evaluated using quantitative polymerase chain reaction. (a) Transgene persistence in transduced muscle was evaluated by quantification of Ova DNA by quantitative polymerase chain reaction. (b) Transgene expression was evaluated by quantification of the corresponding Ova mRNA by qRT-PCR. Data were normalized against the level of Eef2 mRNA (arbitrary units). (c) Mean concentrations of Ova in the sera of tolerized and control mice were determined 18, 35, and 80 days after AAV-Ova injection by enzyme-linked immunosorbent assay. AAV, adeno-associated virus; ctrl, control; Ova, ovalbumin; tol, tolerized.
Oral-tolerization induces abortive proliferation of adoptively transferred Ova-specific CD8+ T cells
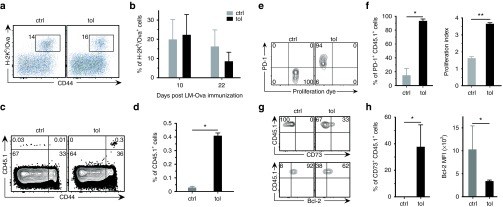
To investigate the mechanisms involved, we first analyzed the cell subsets present in the MLNs. No difference was found in the frequencies nor in the phenotypes of the analyzed subsets, that included Tregs, Bregs, and dendritic cells (Supplementary Figure S3). Also, depletion/inactivation of the vast majority of CD4+Foxp3+ Tregs at the end of the oral-tolerization protocol was not sufficient to abrogate the induced tolerance (Supplementary Figure S4). We next investigated the possible induction of anergy and/or deletion of antigen-specific T cells. As anergy can be breached by proinflammatory infections, we next infected orally-tolerized mice with a replicative Listeria monocytogenes strain expressing Ova (Lm-Ova). In contrast to what we observed following injection of the replication-defective AAV-Ova (Figure 1), mice responded well to Lm-Ova (Figure 4a,b), indicating that Ova-specific T cells are still present and are anergized rather than deleted during the initial step of oral-tolerization.
Figure 4.
Oral-tolerization induces abortive proliferation, expression of PD-1, and downregulation of Bcl-2 in adoptively transferred Ova-specific CD8+ T cells. (a,b) Mice were orally-tolerized during 7 days with 1% Ova in drinking water and then injected i.v. with 106 pfu of a live strain of Listeria monocytogenes expressing Ova (Lm-Ova) (n = 5/group). Blood samples were collected after infection to monitor the cellular levels of Ova-specific CD8+ T cells by flow cytometry. (a) Representative cytometric profiles obtained at day 10 postinfection were gated on the CD8+ population. (b) Percentage of anti-Ova CD8+ T cells 10 and 22 days postinfection are shown. (c–h) 105 Ova-specific CD8+ T cells (OT-I CD45.1+), stained with a proliferation tracking dye, were adoptively transferred into CD45.2 congenic mice. Recipient mice were then orally-tolerized during 7 days and mesenteric lymph nodes (MLNs) were harvested and analyzed by flow cytometry (n = 5/group). (c) Representative cytometric profiles, gated on the CD8+ population, and (d) percentage of CD45.1+ cells in MLNs are shown. (e) Cytometric profiles representing the level of cell proliferation (dilution of the proliferation dye) and PD-1 expression on gated CD45.1+ cells. (f) Percentage of CD45.1+PD-1+ cells in the gated CD8+ cells and their proliferation index. (g) Cytometric profiles showing the expression of CD73 (top) and Bcl-2 (bottom) on the gated CD45.1+ cells. (h) Percentage of CD45.1+CD73+ cells (left) and mean fluorescence intensity (MFI) of Bcl-2 staining (right). ctrl, control; Ova, ovalbumin; tol, tolerized.
To study more precisely the fate of anti-Ova CD8+ T cells, Ova-specific CD8+ T cells from TCR-Tg OT-I mice harboring the CD45.1 congenic marker (OT-I CD45.1+ cells) were adoptively transferred into CD45.2 recipients. Transferred cells were significantly more abundant in tolerized mice than in control animals and expressed the CD44 activation marker (Figure 4c,d). Labeling with a fluorescent dye revealed that adoptively transferred OT-I CD45.1+ cells indeed proliferated in the MLNs of tolerized mice (Figure 4e), but also acquired expression of the PD-1 exhaustion/anergic marker alongside cell division (Figure 4f). One third of transferred cells also acquired expression of CD73, a regulatory molecule involved in anergy41 (Figure 4g,h). Finally, as anergic T cell tend to die by apoptosis,42 we further analyzed the intracellular level of the antiapoptotic factor Bcl-2 and observed that transferred cells displayed reduced levels of Bcl-2 (Figure 4g,h). Taken together, these results suggest that Ova-specific T cells abortively proliferate in the MLNs upon encounter of their nominal antigen brought by the oral route.
Oral-tolerization induces deletion of Ova-specific CD8+ T cells in the context of AAV-mediated gene therapy
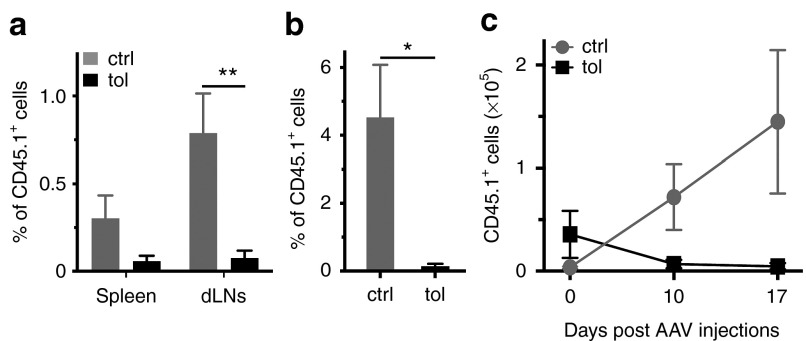
We next addressed the fate of antigen-specific cells, not only after oral-tolerization, but also following AAV-mediated gene transfer. For that, OT-I CD45.1+ cells were again injected in congenic CD45.2 recipient mice and followed after AAV-Ova i.m. injection. While previous experiments demonstrated very few transferred CD45.1+ cells in control animals before injection of AAV-Ova (Figure 4c,d), these cells were clearly visible after gene transfer in spleen, draining lymph nodes, and circulation suggesting cell rebound following antigenic stimulation (Figure 5a,b). In contrast, CD45.1+ that were more abundant in tolerized animals before gene transfer (Figure 4c,d), were almost undetectable later in spleen, draining lymph nodes, and circulation of these animals (Figure 5a,b). Lastly, we enumerated transferred cells to ascertain that CD45.1+ cell percentage faithfully reflected their absolute number. The results confirmed that the absolute number of antigen-specific CD8+CD45.1+ cells gradually increased in the peripheral lymphoid organs of untolerized mice while, in striking contrast, they completely disappeared from the lymphoid organs of tolerized mice (Figure 5c). Altogether, these data suggest that although antigen-specific CD8+ T cells divided during oral-tolerization, they acquired PD-1, downregulated Bcl-2 survival factor, and are finally deleted from the repertoire of tolerized mice upon antigen reencounter in the context of AAV-mediated muscle gene transfer.
Figure 5.
Oral-tolerization induces deletion of Ova-specific CD8+ T cells in the context of gene therapy. As in figure 4, 105 OT-I CD45.1+ cells were adoptively transferred into CD45.2 congenic recipient mice, which were then orally-tolerized during 7 days. At the end of the oral-tolerization protocol, mice were injected with AAV-Ova in both gastrocnemius (3.5 × 109 vg). Spleen, mesenteric lymph nodes (MLNs), and draining lymph nodes (dLNs) were harvested for flow cytometry analysis (n = 5/group). (a) Percentage of transferred CD45.1+ cells in the gated CD8+ population was evaluated in spleen and dLNs 10 days post-transduction. (b) In other groups of mice, percentage of transferred CD8+CD45.1+ cells was also evaluated in the blood at day 14 postinjection. (c) Total number of CD45.1+ transferred CD8+ cells was enumerated from the harvested pooled lymphoid organs (spleen, dLNs, and MLNs) before AAV-Ova injection (at day 0) and 10 and 17 days thereafter. AAV, adeno-associated virus; ctrl, control; Ova, ovalbumin; tol, tolerized; vg, vector genome.
Prophylactic oral-feeding, but not interventional oral-feeding, prevents immune responses elicited by AAV vectors
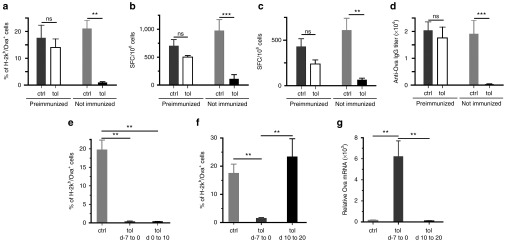
As oral-tolerization has yielded limited success in the treatment of autoimmune diseases, we next wondered whether oral-tolerization would be more efficient to prevent, rather than to suppress, immune responses. To examine this question, we applied the oral-tolerization protocol to animals preimmunized with complete Freund's adjuvant (CFA)/Ova before initiation of oral-tolerization. Results showed that oral-tolerization was not efficient to prevent immune responses elicited by AAV-Ova transduction in the preimmunized group of mice (Figure 6a–d). Similar results were obtained with animals preimmunized with Lm-Ova to elicit a memory anti-Ova immune response 35 days before the administration of the AAV-Ova vector (data not shown). Thus, oral-tolerization is very efficient to prevent primary immune responses elicited by AAV-vectors but not secondary/memory immune responses. We next directly compared prophylactic and interventional protocols by initiating antigen feeding either before, at the same time, or after vector transduction. Results showed that initiation of oral-tolerization at the same time as vector transduction was as effective as its initiation 7 days before to completely prevent the emergence of the cytotoxic CD8+ T-cell response (Figure 6e). However, expression of transgenic protein from single-stranded DNA AAV-vectors is known to require at least 7 days in vivo, suggesting that oral-tolerization may still have been initiated in this particular situation before the priming of the immune response. Concordantly, when antigen feeding was initiated 10 days after vector transduction (i.e., at a time point where immune responses become detectable), oral feeding was inefficient to suppress cytotoxic CD8+ T-cell response and to improve long-term transgene persistence (Figure 6f,g). Thus, oral-tolerization by antigen feeding is only effective when applied before or at the same time as vector transduction, two situations that are still fully compatible with the specific clinical context of gene therapy.
Figure 6.
Oral-tolerization is very efficient to prophylactically prevent immune responses, but not to suppress already primed immune responses. (a–c) Mice were preimmunized or not with CFA/Ova. Three weeks later, mice were orally-tolerized during 7 days with 1% Ova solubilized in their drinking water. At the end of the tolerization protocol, mice were transduced using 3.5 × 109 vg of AAV-Ova injected in both gastrocnemius. (a) CD8+ cellular immune responses were monitored by flow cytometry in preimmunized and non–preimmunized groups by collecting blood samples 15 days after AAV-Ova injections. Bar graph represents the percentage of Ova-specific CD8+ T cells in each indicated groups. (b,c) Eighty days after AAV-Ova injections, splenocytes were harvested and Ova-specific CD8+ (b) and CD4+ (c) immune responses were evaluated by Enzyme-linked immunospot assays. (d) Eighty days after AAV-Ova injections, sera were collected and anti-Ova IgG were titrated by enzyme-linked immunosorbent assay. (e–g) Mice were orally-tolerized during 7–10 days with 1% Ova in their drinking water either before (day −7 to 0), at the same time (day 0 to 10), or after (day 10 to 20) AAV-Ova injection. (e,f) Blood samples were collected 14 days (e) or 21 days (f) post-AAV-Ova transduction and the percentage of Ova-specific CD8+ T cells was determined using H-2Kb/Ova257-264 dextramers staining and flow cytometry in the indicated group of mice. (g) Transgene persistence and expression was evaluated by quantification of Ova mRNA by qRT-PCR in the indicated group of mice. Data are normalized against the level of Eef2 mRNA (arbitrary units). AAV, adeno-associated virus; ctrl, control; Ova, ovalbumin; tol, tolerized; vg, vector genome.
Discussion
Significant progress has been made in the field of AAV gene therapy to improve efficacy and safety of the vectors. One of the main persisting challenges is the better control of adverse immune responses to improve long-term expression of the transgene. Indeed, immune responses directed against the vector not only compromise transduction efficiency during vector reinjections, but also lead to the elimination of transduced cells by capsid-specific cytotoxic CD8+ T cells.9,12,43 The transgenic protein itself, coded by the vector, also induces immune responses that can impair gene transfer efficacy.5,16 Current strategies used in clinic to overcome this limitation include administration of immunosuppressive drugs and careful selection of the patients presenting the lower risk of developing such immune responses notably against the transgenic protein. For instance, patients enrolled in hemophilia gene therapy trials were elected based on the nature of their genetic mutation (i.e., missense rather that nonsense mutations) and based on their absence of immune responses (i.e., inhibitors formation) to protein replacement therapy.20,22,23,24 Developing strategies to inhibit these immune responses is therefore of major importance, not only to promote long-term transgenic protein expression, but also to extend gene therapy to other patients. We evaluated here an oral-tolerization strategy to specifically dampen the humoral and cellular immune responses directed against the transgenic protein coded by the AAV vector and demonstrated its efficacy to improve long-term transgene expression (Figures 1 and 3).
We used here as a model an AAV-Ova vector to elicit muscle secretion of Ova following its intramuscular administration. This experimental condition mimics clinical situations where the transgenic protein is meant to be expressed systemically such as gene therapy of hemophilic patients for instance. We chose here a highly immunogenic protein model to generate a stringent model where protocols designed to inhibit immune responses can be faithfully evaluated. We used this experimental setting to show that prophylactic oral administration of the protein of interest is able to completely prevent both humoral (Figure 1d,e) and cellular immune responses (Figure 1b,c,f,g) induced by AAV-mediated muscle gene transfer. Concordantly, the transgenic Ova protein was persistent in the serum until the latest time-points studied (Figure 3c) as was the corresponding Ova mRNA in transduced muscle (Figure 3a,b). This was in agreement with the absence of cytotoxic CD8+ T cells infiltration in the transduced muscles of orally-tolerized mice (Figure 2d–f and j–l).
Regarding the mechanism, we evaluated the potential role of Foxp3+ Tregs in the induction/maintenance of tolerance in our model. Using a robust protocol that we developed to deplete and inactivate Foxp3+ Tregs, we showed that their depletion/inactivation at the end of the oral-tolerization phase was not sufficient to abrogate orally-induced tolerance and to restore cellular and humoral immune responses (Supplementary Figure S4). This suggested, at best, a minor role of these cells in our model. However, the role of other described subsets of regulatory T cells cannot be excluded. Among the subset that have been previously implicated in oral tolerance, we analyzed the proportion CD4+LAG-3+Foxp3– Tr1 cells.44 Indeed, this subset is known to secrete IL-10, a well-known immunoregulatory cytokine, and has been previously involved in the induction of oral tolerance.45 Our results did not reveal substantial difference in the percentages of CD4+LAG-3+Foxp3– found in the mesenteric lymph nodes of tolerized animals as compared to control mice nor in their levels of LAG-3 expression (Supplementary Figure S3i and data not shown). Yet, further functional investigations, as well as the study of their antigen-specificity, and their numbers in lamina propria or Peyer's patches would be required to formally exclude their implication in our model. Th3 cells, characterized by their CD4+LAP+Foxp3– phenotype and by the production of TGF-β, represent another regulatory T-cell subset that has been associated with oral tolerization. Indeed, Th3 cells have been previously demonstrated to be elicited upon oral administration of myelin basic protein and to prevent the development of autoimmune encephalomyelitis in a mouse model.46 As we did not specifically study this subset, their role cannot be excluded in our model. This may possibly be of importance as immunoregulatory cytokines akin to IL-10 and/or TGF-β produced by Tr1 and Th3 subsets are known to considerably affect the differentiation, survival, and functions of CD8+ cytotoxic T cells.
Even if the precise mechanism involved in the regulation of the CD4+ T-cell compartment still remains to be precisely studied, we provide herein interesting data on the mechanisms involved in the regulation of CD8+ T cells compartment by oral tolerization. We show here that adoptively transferred antigen-specific CD8+ T cells gradually acquired high levels of PD-1 expression as they proliferated following initial encounter with the orally-derived antigen in MLNs (Figure 4e,f). Abortive T cells proliferation prior to tolerization has already been documented following oral-tolerization.47,48 We further followed the fate of Ova-specific CD8+ T cells upon Ova re-encounter in the context of gene therapy. We then showed that Ova-specific CD8+ T which expanded significantly in tolerized animals during the 7 days of Ova-feeding (Figures 4e,f and 5c at day 0), declined rapidly after injection of AAV-Ova while they expanded dramatically in control animals (Figure 5). Hence, CD8+ T cells that have encountered orally-derived antigen during the tolerization phase are not only incapable to respond to the same antigen in the context of AAV-mediated gene transfer but are also rapidly induced to die.
The data reported herein provide the first proof-of-principle study indicating that oral-tolerization represents a candidate approach to circumvent the bottleneck of the immune response directed toward the transgenic protein. Interestingly, the protein of interest can be given prophylactically before AAV vector injection in patients who are immunologically naive to the transgene product, provided that they have never been sensitized by protein replacement therapy. Yet, production of large quantities of proteins that would be compatible with oral administration in humans may represent a challenge. Several production systems and/or method of vectorization can however be envisioned. For instance, transgenic probiotics can be administrated orally in mice and humans without any conspicuous side effect and can possibly be used to directly deliver proteins of interest in the gastrointestinal tract.49,50,51 Beside probiotics, transgenic plants represent an alternative low cost method for the production of proteins suitable for oral ingestion. Bioproduction in comestible plants simplify the production steps, eliminate the need of extensive purification, and allow the production of glycosylated proteins that are structurally and functionally closely related to the native protein.52,53 Moreover, protein synthetized in plants are naturally bioencapsulated, ensuring their protection from gastric and proteolytic degradation and facilitate their delivery in the gut where plants are digested by the action of commensal bacteria. In addition, protein of interest can be fused to transmucosal carriers that can facilitate their delivery across intestinal epithelium. Plant-based oral tolerance has been recently illustrated in a murine model of hemophilia where prophylactic oral administration of the transplastomic plant expressing recombinant factor IX (F.IX) protein fused to the cholera toxin carrier B was demonstrated to inhibit antibodies formation in the context of protein replacement therapy.54,55,56 In the same line, the present results further illustrate the efficacy of a prophylactic oral-tolerization protocol in the context of AAV-based gene therapy to promote specific and long-term tolerance to the transgenic protein coded by the vector.
Prevention of immune sensitization, rather than control of already primed immune responses, may arguably be more efficient and may partly explain the limited success of oral-tolerization strategies in autoimmune diseases. Consistently, we showed that oral-tolerization is efficient to prevent primary immune responses elicited by AAV vectors but not secondary/memory immune responses (Figure 6a–d). Interestingly, oral-tolerization was still effective when initiated at the same time as AAV-vectors injections, possibly in relation with the known delay necessary for transgene expression when using single-stranded DNA AAV vectors. We thus believe that the present study pave the way for further investigations in larger animals of prophylactic, rather than interventional, oral-tolerization protocols to prevent immune responses against transgenic proteins and/or against the proteins of the vector. Such oral-tolerization protocol could be used in combination with oral rapamycin and/or low-dose i.v. injection of the protein of interest, that have been shown to prevent and reverse inhibitory antibodies formation elicited by i.m. injection of AAV-F.IX in an animal model of hemophilia.57 Also, given the recently characterized immunoregulatory properties of probiotics, oral administration of modified probiotics expressing the proteins of interest may further improve oral-tolerization protocols by facilitating the tolerization against multiple proteins at the same time and may thus deserve further investigation in the field of gene therapy.
Materials and Methods
Mice. Female C57BL/6Rj (B6) mice were obtained from Janvier Labs (Le Genest Saint Isle, France). T cell receptor (TCR)-transgenic OT-I mice, and congenic B6 mice expressing the CD45.1 (Ly5.1) allelic marker were obtained from Charles River Laboratories (Saint-Germain-sur-l'Arbresle, France). [OT-IxLy5.1]F1 mice were used as a source of anti-Ova TCR-transgenic CD8+ T cells expressing the CD45.1 marker. Mice were housed in a specific pathogen-free barrier facility and were between 8–12 weeks of age at beginning of the experiments.
Plasmidic constructs, preparation of recombinant AAV vectors, and muscles transduction. The plasmidic construct used for the generation of a recombinant AAV2/1 vector, coding for soluble Ova (AAV-Ova), was kindly provided by Roland W. Herzog.5,16 Recombinant AAV2/1-Ova vectors were generated using a standard helper-virus free transient transfection method and pseudotyped with AAV1 capsid proteins as described before.37,38 Genome titers, expressed by equivalent vector genomes (vg), were evaluated by dot-blot hybridization and by quantitative polymerase chain reaction. For muscle transduction, mice hind legs were shaved under anesthesia and titrated amounts of AAV-Ova vectors, (i.e., 3.5 × 109, 5 × 1010, or 1011 vg) were injected in each gastrocnemius muscles. In some experiments, mice received together with AAV-Ova (3.5 × 109 vg/mice), 10 µg of CpG ODNs 1826 (Sigma-Aldrich, Saint-Quentin Fallavier, France) to mimic situations where vector administrations are associated with TLRs activation.
Oral Ova protein administration. Oral-tolerization was induced by offering to mice, ad libitum, for 7 consecutive days, a solution of 1% Ova (Sigma-Aldrich, grade II) dissolved in their drinking water. This solution was freshly prepared each day. In some experiments, variation of this protocol was evaluated consisting of oral administration of the protein only for 5 days, or for 7 days at the reduced concentration of 0.1%. All these protocols similarly resulted in the complete prevention of cellular and humoral immune responses directed against Ova following i.m. injections of AAV-Ova vectors (data not shown).
Mice immunization. In some experiments, mice were preimmunized 21 days before oral-tolerization. For that, 100 µg of Ova was emulsified in CFA and s.c. injected in their shaved back. In other experiments, mice were immunized after oral-tolerization with a highly immunogenic vaccine consisting of a live strain of Listeria monocytogenes expressing Ova (Lm-Ova), kindly provided by G. Lauvau.58 For that, mice were injected i.v. with 106 cfu of titrated Lm-Ova preparations diluted in 100 µl of PBS as described previously.38,40
Tumor model. Mice injected 14 days before with AAV-Ova, were subcutaneously injected in their shaved flanks with 106 EL4-Ova (EG-7) tumor cells. Mice were then examined every other days and tumor development was monitored for 31 days using a digital caliper as described previously.38,40
Adoptive T-cell transfer. Ova-specific CD8+ T cells were obtained from the spleens of [OT-I x Ly5.1]F1 mice and purified by magnetic sorting using a CD8 negative isolation kit (Invitrogen, Saint-Aubin, France). More than 93% of the purified cells displayed a CD3+CD8+Vα2+Vβ5+CD45.1+CD4–CD19- phenotype as assessed by flow cytometry. For in vivo proliferation assay, cells were stained with 20 μmol/l Cell Proliferation Dye eFluor 450 (eBiosciences, Paris, France) for 10 minutes and 105 washed cells were then adoptively transferred i.v. into each B6 recipient mice.
Antibodies and flow cytometry. Fluorochrome-conjugated antibodies were all obtained from eBioscience except for antibodies to Bcl-2, CD24, CD73, CD80, CD86, and IFNγ obtained from BD Biosciences (Le Pont de Claix, France), and anti-Nrp-1 from Biolegend (London, UK). Intracellular Foxp3, Helios, Bcl-2, IL-10 staining were done using the Intracellular Fixation & Permeabilization kit (eBiosciences).38,40 PE-conjugated H-2Kb/Ova257-264 dextramers were used to detect CD8+ T cells that specifically recognize the immunodominant Ova257-264 peptide using the manufacturer's protocol (Immudex, Copenhagen, Denmark). Single-cell suspensions derived from spleen, lymph nodes, or peripheral blood were analyzed by flow cytometry using a FACSCanto-I or an LSRFortessa (BD Biosciences), and using FlowJo software (Tree Star, Ashland, OR).
Enzyme-linked immunosorbent assay and enzyme-linked immunospot assays. Quantification of soluble Ova concentration in serum and titration of anti-Ova IgG antibodies were performed by enzyme-linked immunosorbent assay as previously described.37 Anti-Ova IgG titers were defined as the dilution yielding the half-maximum optical density obtained with a positive control serum that was used throughout the all study. Titers were calculated using sigmoid curve fitting performed in Prism software (Graphpad). Enzyme-linked immunospot assays were used to quantify the numbers of Ova-specific CD8+ or CD4+ T cells secreting IFNγ upon in vitro restimulation as previously described.37 For that, 2.5 × 105 to 105 splenocytes per well were cultured overnight in RPMI medium in the presence of 10 μg/ml Ova257-264 or Ova323-339 peptides for detection of, respectively, MHC I or MHC II restricted cellular immune responses, or, with peptides SNYAKSANV, MIPQYGYL, and PQYGYLTL for detection of anti-capsid AAV1 cellular immune responses. Cultures were stopped and treated according to manufacturer's instructions (Diaclone, Besançon, France). Number of spots in each well was analyzed with an Enzyme-linked immunospot plate reader and dedicated ImmunoSpots software (C.T.L., Bonn, Germany).
Histology and immunofluorescence microscopy. Whole gastrocnemius was snap-frozen in isopentane precooled in liquid nitrogen. Transversally cryosectioned slices were either stained with hematoxylin and eosin (H&E) for histological evaluations or prepared for immunofluorescence. Briefly, muscles slices were fixed with 1% paraformaldehyde and incubated with flurochrome-conjugated anti-mouse CD8 (53–6.7) and anti-CD3 (500A2) antibodies, obtained from eBiosciences. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Slides were viewed with a Zeiss Axioplan 2 microscope, and data collected using Zeiss Apotome and Axiovision 4.1 software.
Quantification of Ova cDNA and mRNA in transduced muscles. Ova DNA and corresponding Ova mRNA were quantified from transduced muscles by real-time polymerase chain reaction using SYBR green Mastermix (Roche, Meylan, France) in a final volume of 10 μl as described previously.37,38 All quantitative polymerase chain reactions were performed with a LightCycler 480 (Roche). The relative amount of Ova DNA or Ova mRNA were determined using a standard curve obtained after serial dilutions of a plasmidic construct coding for Ova, and normalized for each sample by the amount of Eef2.
Statistical analysis. All data are shown as mean values and error bars represent standard error of the mean. Nonparametric tests were used for statistical comparison between experimental groups using one-way analysis of variance (Kruskall-Wallis tests). Unless otherwise stated, all the experiments have been repeated at least three times and illustrations shown here are representative of all experiments. Differences were considered statistically significant when P values were less than 0.05 (*), 0.01 (**), or 0.001 (***). All calculations were performed using the Prism software (Graphpad, La Jolla, CA).
SUPPLEMENTARY MATERIAL Figure S1. Evaluation of oral-tolerization protocols differing in duration and antigen dose. Figure S2. Oral-tolerization inhibits immune responses even in situations where AAV vector administration is associated with concomitant TLR9 activation. Figure S3. Oral-tolerization is not associated with obvious modification of immune cell subsets in mesenteric lymph nodes. Figure S4. Tregs depletion/inactivation is not sufficient to abrogate oral tolerance.
Acknowledgments
We would like to thank Roland W. Herzog and Grégoire Lauvau for kindly providing the plasmidic construct necessary for the preparation of viral AAV-Ova vector and the Lm-Ova vaccine, respectively. We also thank the flow cytometry facility of the Institute for Research and Innovation in Biomedicine for technical expertise, and Normandy University and INSERM for starting funds and infrastructures. R.H. is recipient of a PhD fellowship from the French Ministry of higher education and research. This work was supported by a grant from the Association Française contre les Myopathies (AFM #15909). R.H., B.C., Y.N., and G.R. performed the experiments; L.D. and L.J. provided expert technical helps and animal care; A.S. prepared the AAV vectors; R.H., O.B., and S.A. designed the experiments; R.H. and S.A. analyzed and interpreted the data; R.H., A.S., O.B., and S.A. drafted the manuscript; R.H. and S.A revised and finalized the manuscript. The authors declare no competing financial interests
Supplementary Material
References
- Wang, L and Herzog, RW (2005). AAV-mediated gene transfer for treatment of hemophilia. Curr Gene Ther 5: 349–360. [DOI] [PubMed] [Google Scholar]
- Mingozzi, F and High, KA (2011). Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet 12: 341–355. [DOI] [PubMed] [Google Scholar]
- Zincarelli, C, Soltys, S, Rengo, G and Rabinowitz, JE (2008). Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther 16: 1073–1080. [DOI] [PubMed] [Google Scholar]
- Petrs-Silva, H and Linden, R (2013). Advances in recombinant adeno-associated viral vectors for gene delivery. Curr Gene Ther 13: 335–345. [DOI] [PubMed] [Google Scholar]
- Mays, LE and Wilson, JM (2011). The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol Ther 19: 16–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire, AM, Simonelli, F, Pierce, EA, Pugh, EN Jr, Mingozzi, F, Bennicelli, J et al. (2008). Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med 358: 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno, CS, Pierce, GF, Arruda, VR, Glader, B, Ragni, M, Rasko, JJ et al. (2006). Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med 12: 342–347. [DOI] [PubMed] [Google Scholar]
- Gaudet, D, Méthot, J, Déry, S, Brisson, D, Essiembre, C, Tremblay, G et al. (2013). Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther 20: 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi, F, Maus, MV, Hui, DJ, Sabatino, DE, Murphy, SL, Rasko, JE et al. (2007). CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med 13: 419–422. [DOI] [PubMed] [Google Scholar]
- Nayak, S and Herzog, RW (2010). Progress and prospects: immune responses to viral vectors. Gene Ther 17: 295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi, F and High, KA (2011). Immune responses to AAV in clinical trials. Curr Gene Ther 11: 321–330. [DOI] [PubMed] [Google Scholar]
- Manning, WC, Zhou, S, Bland, MP, Escobedo, JA and Dwarki, V (1998). Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum Gene Ther 9: 477–485. [DOI] [PubMed] [Google Scholar]
- Fields, PA, Kowalczyk, DW, Arruda, VR, Armstrong, E, McCleland, ML, Hagstrom, JN et al. (2000). Role of vector in activation of T cell subsets in immune responses against the secreted transgene product factor IX. Mol Ther 1: 225–235. [DOI] [PubMed] [Google Scholar]
- Yuasa, K, Yoshimura, M, Urasawa, N, Ohshima, S, Howell, JM, Nakamura, A et al. (2007). Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther 14: 1249–1260. [DOI] [PubMed] [Google Scholar]
- Gao, G, Wang, Q, Calcedo, R, Mays, L, Bell, P, Wang, L et al. (2009). Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther 20: 930–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L, Dobrzynski, E, Schlachterman, A, Cao, O and Herzog, RW (2005). Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood 105: 4226–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z, Tapscott, SJ, Chamberlain, JS and Storb, R (2011). Immunity and AAV-Mediated Gene Therapy for Muscular Dystrophies in Large Animal Models and Human Trials. Front Microbiol 2: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, V, Twisk, J, Kwikkers, K, Aronica, E, Brisson, D, Methot, J et al. (2014). Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther 25: 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L, Cao, O, Swalm, B, Dobrzynski, E, Mingozzi, F and Herzog, RW (2005). Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther 12: 1453–1464. [DOI] [PubMed] [Google Scholar]
- Manno, CS, Chew, AJ, Hutchison, S, Larson, PJ, Herzog, RW, Arruda, VR et al. (2003). AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 101: 2963–2972. [DOI] [PubMed] [Google Scholar]
- Ferreira, V, Petry, H and Salmon, F (2014). Immune Responses to AAV-Vectors, the Glybera Example from Bench to Bedside. Front Immunol 5: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi, F and High, KA (2013). Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood 122: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani, AC, Tuddenham, EG, Rangarajan, S, Rosales, C, McIntosh, J, Linch, DC et al. (2011). Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365: 2357–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani, AC, Reiss, UM, Tuddenham, EG, Rosales, C, Chowdary, P, McIntosh, J et al. (2014). Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med 371: 1994–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes, JL, Siddiqui, KR, Arancibia-Cárcamo, CV, Hall, J, Sun, CM, Belkaid, Y et al. (2007). A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med 204: 1757–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, O, Jaensson, E, Persson, EK, Liu, X, Worbs, T, Agace, WW et al. (2009). Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 206: 3101–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, HL, da Cunha, AP, Quintana, F and Wu, H (2011). Oral tolerance. Immunol Rev 241: 241–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux, H, O'Garra, A, Bigler, M, Rouleau, M, Antonenko, S, de Vries, JE et al. (1997). A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389: 737–742. [DOI] [PubMed] [Google Scholar]
- Bilsborough, J, George, TC, Norment, A and Viney, JL (2003). Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology 108: 481–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goubier, A, Dubois, B, Gheit, H, Joubert, G, Villard-Truc, F, Asselin-Paturel, C et al. (2008). Plasmacytoid dendritic cells mediate oral tolerance. Immunity 29: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y, Inobe, J, Marks, R, Gonnella, P, Kuchroo, VK and Weiner, HL (1995). Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature 376: 177–180. [DOI] [PubMed] [Google Scholar]
- Friedman, A and Weiner, HL (1994). Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc Natl Acad Sci USA 91: 6688–6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X, Izikson, L, Liu, L and Weiner, HL (2001). Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol 167: 4245–4253. [DOI] [PubMed] [Google Scholar]
- Wherrett, DK, Bundy, B, Becker, DJ, DiMeglio, LA, Gitelman, SE, Goland, R et al.; Type 1 Diabetes TrialNet GAD Study Group. (2011). Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 378: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dello Iacono, I, Tripodi, S, Calvani, M, Panetta, V, Verga, MC and Miceli Sopo, S (2013). Specific oral tolerance induction with raw hen's egg in children with very severe egg allergy: a randomized controlled trial. Pediatr Allergy Immunol 24: 66–74. [DOI] [PubMed] [Google Scholar]
- Chung, Y, Chang, SY and Kang, CY (1999). Kinetic analysis of oral tolerance: memory lymphocytes are refractory to oral tolerance. J Immunol 163: 3692–3698. [PubMed] [Google Scholar]
- Adriouch, S, Franck, E, Drouot, L, Bonneau, C, Jolinon, N, Salvetti, A et al. (2011). Improved Immunological Tolerance Following Combination Therapy with CTLA-4/Ig and AAV-Mediated PD-L1/2 Muscle Gene Transfer. Front Microbiol 2: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck, E, Bonneau, C, Jean, L, Henry, JP, Lacoume, Y, Salvetti, A et al. (2012). Immunological tolerance to muscle autoantigens involves peripheral deletion of autoreactive CD8+ T cells. PLoS One 7: e36444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrzynski, E, Mingozzi, F, Liu, YL, Bendo, E, Cao, O, Wang, L et al. (2004). Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood 104: 969–977. [DOI] [PubMed] [Google Scholar]
- Hubert, S, Rissiek, B, Klages, K, Huehn, J, Sparwasser, T, Haag, F et al. (2010). Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med 207: 2561–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resta, R, Yamashita, Y and Thompson, LF (1998). Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev 161: 95–109. [DOI] [PubMed] [Google Scholar]
- Parish, IA, Rao, S, Smyth, GK, Juelich, T, Denyer, GS, Davey, GM et al. (2009). The molecular signature of CD8+ T cells undergoing deletional tolerance. Blood 113: 4575–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, WC, Paliard, X, Zhou, S, Pat Bland, M, Lee, AY, Hong, K et al. (1997). Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J Virol 71: 7960–7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani, N, Magnani, CF, Huber, S, Gianolini, ME, Pala, M, Licona-Limon, P et al. (2013). Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med 19: 739–746. [DOI] [PubMed] [Google Scholar]
- Tsuji, NM, Mizumachi, K and Kurisaki, J (2001). Interleukin-10-secreting Peyer's patch cells are responsible for active suppression in low-dose oral tolerance. Immunology 103: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y, Inobe, J, Kuchroo, VK, Baron, JL, Janeway, CA Jr and Weiner, HL (1996). Oral tolerance in myelin basic protein T-cell receptor transgenic mice: suppression of autoimmune encephalomyelitis and dose-dependent induction of regulatory cells. Proc Natl Acad Sci USA 93: 388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Junior, AB, Horta, BC, Gomes-Santos, AC, Cunha, AP, Silva Steinberg, R, Nascimento, DS et al. (2012). Oral tolerance correlates with high levels of lymphocyte activity. Cell Immunol 280: 171–181. [DOI] [PubMed] [Google Scholar]
- Sun, J, Dirden-Kramer, B, Ito, K, Ernst, PB and Van Houten, N (1999). Antigen-specific T cell activation and proliferation during oral tolerance induction. J Immunol 162: 5868–5875. [PubMed] [Google Scholar]
- Robert, S, Gysemans, C, Takiishi, T, Korf, H, Spagnuolo, I, Sebastiani, G et al. (2014). Oral delivery of glutamic acid decarboxylase (GAD)-65 and IL10 by Lactococcus lactis reverses diabetes in recent-onset NOD mice. Diabetes 63: 2876–2887. [DOI] [PubMed] [Google Scholar]
- Takiishi, T, Korf, H, Van Belle, TL, Robert, S, Grieco, FA, Caluwaerts, S et al. (2012). Reversal of autoimmune diabetes by restoration of antigen-specific tolerance using genetically modified Lactococcus lactis in mice. J Clin Invest 122: 1717–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers, P, De Smedt, T and Steidler, L (2009). Modulation of gut-associated lymphoid tissue functions with genetically modified Lactococcus lactis. Int Rev Immunol 28: 465–486. [DOI] [PubMed] [Google Scholar]
- Wang, X, Sherman, A, Liao, G, Leong, KW, Daniell, H, Terhorst, C et al. (2013). Mechanism of oral tolerance induction to therapeutic proteins. Adv Drug Deliv Rev 65: 759–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, KC, Verma, D, Singh, ND, Herzog, R and Daniell, H (2013). Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev 65: 782–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma, D, Moghimi, B, LoDuca, PA, Singh, HD, Hoffman, BE, Herzog, RW et al. (2010). Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci USA 107: 7101–7106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, A, Su, J, Lin, S, Wang, X, Herzog, RW and Daniell, H (2014). Suppression of inhibitor formation against FVIII in a murine model of hemophilia A by oral delivery of antigens bioencapsulated in plant cells. Blood 124: 1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X, Su, J, Sherman, A, Rogers, GL, Liao, G, Hoffman, BE et al. (2015). Plant-based oral tolerance to hemophilia therapy employs a complex immune regulatory response including LAP+CD4+ T cells. Blood 125: 2418–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak, S, Sarkar, D, Perrin, GQ, Moghimi, B, Hoffman, BE, Zhou, S et al. (2011). Prevention and Reversal of Antibody Responses Against Factor IX in Gene Therapy for Hemophilia B. Front Microbiol 2: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauvau, G, Vijh, S, Kong, P, Horng, T, Kerksiek, K, Serbina, N et al. (2001). Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science 294: 1735–1739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.