Abstract
Progression of many cancers is associated with tumor infiltration by mesenchymal stromal cells (MSC). Adipose stromal cells (ASC) are MSC that serve as adipocyte progenitors and endothelium-supporting cells in white adipose tissue (WAT). Clinical and animal model studies indicate that ASC mobilized from WAT are recruited by tumors. Direct evidence for ASC function in tumor microenvironment has been lacking due to unavailability of approaches to specifically inactivate these cells. Here, we investigate the effects of a proteolysis-resistant targeted hunter-killer peptide D-WAT composed of a cyclic domain CSWKYWFGEC homing to ASC and of a proapoptotic domain KLAKLAK2. Using mouse bone marrow transplantation models, we show that D-WAT treatment specifically depletes tumor stromal and perivascular cells without directly killing malignant cells or tumor-infiltrating leukocytes. In several mouse carcinoma models, targeted ASC cytoablation reduced tumor vascularity and cell proliferation resulting in hemorrhaging, necrosis, and suppressed tumor growth. We also validated a D-WAT derivative with a proapoptotic domain KFAKFAK2 that was found to have an improved cytoablative activity. Our results for the first time demonstrate that ASC, recruited as a component of tumor microenvironment, support cancer progression. We propose that drugs targeting ASC can be developed as a combination therapy complementing conventional cancer treatments.
Introduction
For a number of cancers, therapies effectively inactivating malignant and vascular cells fail to stop disease progression. This suggests that a key component of tumor microenvironment remains untargeted. Solid tumors typically contain a mixed fraction of fibroblastoid cells that infiltrate tumors.1,2 While the pool of tumor leukocytes, such as myeloid-derived suppressor cells, is maintained by hematopoietic progenitors, the cancer-associated fibroblasts are of mesenchymal origin.3,4 Accumulating evidence indicates that mesenchymal stromal cells (MSC) recruited from the bone marrow and extramedullary organs contribute to tumor stroma.1,5 MSC have trophic, vasculogenic, and immunomodulatory functions and have been shown to promote tumor growth in animal models.6
An important source of MSC capable of stimulating tumors is white adipose tissue (WAT), which is overgrown in obese individuals.1 Obesity promotes the progression of breast, prostate, and colorectal andenocarcinomas, as well as other cancers. While the mechanisms linking obesity and cancer are complex,7 our studies in mouse models have shown that recruitment of WAT-derived MSC, termed adipose stromal cells (ASC), is associated with accelerated tumor growth.8 ASC, as well as adipocytes differentiating from them and WAT-infiltrating leukocytes, contribute to the production of hormones, cytokines, and growth factors collectively termed adipokines, many of which stimulate tumor growth.7 We have hypothesized that these adipokines may be more potent when produced as paracrine factors by WAT-derived cells infiltrating tumors.9 Consistent with this proposed mechanism, animal transplantation studies showed that ASC engraftment in tumors is concomitant with potentiation of tumor vascularization and with enhanced survival and proliferation of malignant cells.8,10,11 While accumulating evidence suggests the role of ASC recruitment in tumor growth,8,12,13 no studies have been performed to inactivate ASC in order to directly test their requirement for cancer progression.
Based on our expertise in identifying receptors selectively expressed on the surface of cell populations of interest,14 we discovered Δ-decorin (ΔDCN) as a marker displayed on the surface of platelet-derived growth factor receptor β-positive (PDGFRβ+) ASC.15 A cyclic peptide termed WAT7 (amino acid sequence CSWKYWFGEC), isolated in a combinatorial library screen, was shown to bind ASC, but not MSC in other organs.15 Proapoptotic peptides designed as a fusion of a cell-targeting peptide with an amphipathic peptide KLAKLAKKLAKLAK (termed KLAKLAK2), which inactivates mitochondria upon cell internalization of the peptide-bound receptor, have been used to ablate specific cell populations in vivo.14 Recently, we reported a bimodal peptide composed of cyclic WAT7 and of KLAKLAK2 domains linked via aminohexanoic acid, termed D-WAT.16 This bimodal peptide was synthesized with all amino acids as D-enantiomers to enable proteolysis resistance. We demonstrated that D-WAT depleted ASC in mice, which expectedly resulted in adipose tissue growth suppression.
Here, we tested whether ASC depletion could inhibit experimental cancer (Figure 1a). By targeting ASC with D-WAT, as well as with its potentiated derivative, in four different mouse cancer models, we show that ASC-derived tumor stromal cells are rate limiting for tumor growth. We conclude that ASC promote proliferation and survival of cancer cells by supporting functionality of tumor vasculature and introduce a principally new anticancer treatment strategy.
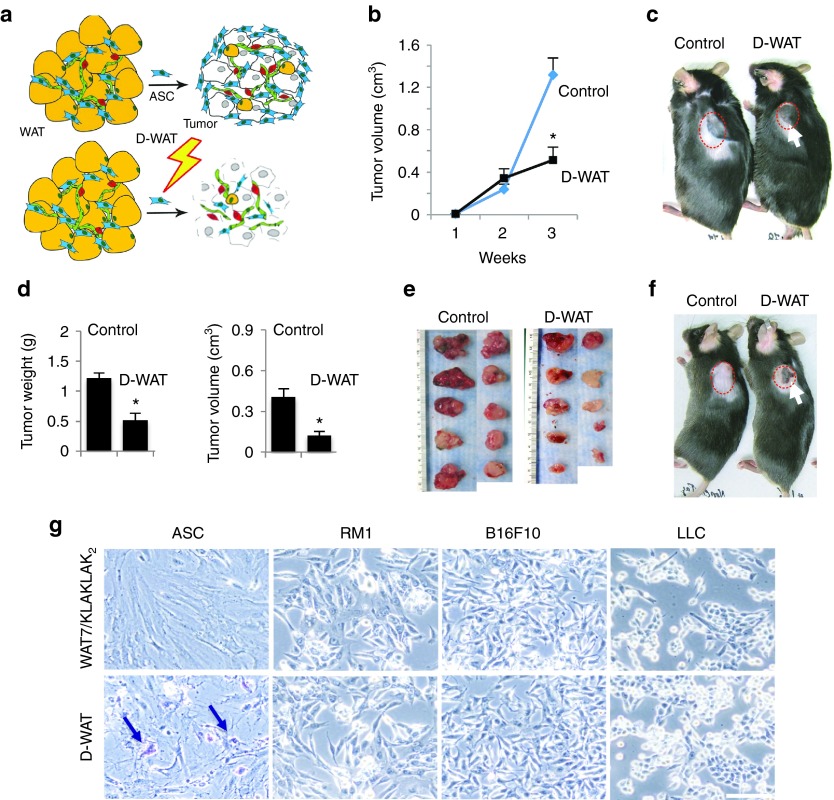
Figure 1.
D-WAT treatment inhibits tumor growth in mouse models. (a) Strategy for adipose stromal cells (ASC) depletion in cancer. White adipose tissue (WAT) contains adipocytes (yellow), endothelium (green), leukocytes (red), and adipose stromal cells (ASC, blue). Tumors (gray) recruit ASC, which support cancer progression. ASC targeting (bolt) is expected to compromise tumor stroma and suppress cancer progression. (b) Growth of E0771 breast adenocarcinoma orthotopically grafted into control and D-WAT-treated chimeric GFP/RFP mice (n = 10/group). (c) Representative RM1 prostate adenocarcinoma grafts grown in obese mice treated with vehicle (control) or D-WAT (n = 10/group). Note tumor ulceration (arrow) in D-WAT-treated mice. (d) Quantification of RM1 tumor weight and volume from (c) demonstrating D-WAT effect. (e) Tumors from mice in (c) at week 3 postgrafting. (f) Mice raised on regular chow and grafted with RM1 prostate adenocarcinoma were treated with vehicle (control) or D-WAT. (g) Cells treated with 0.05 mmol/l mixture of uncoupled WAT7 and KLAKLAK2 or D-WAT for 2 hours and stained with Trypan Blue showing specific killing of ASC. Arrows indicate dead cells. In all graphs, plotted is mean ± standard error of the mean; *P < 0.05 versus control. Scale bar, 100 µm. Experiments were performed at least twice with similar results.
Results
D-WAT treatment inhibits tumor growth in mouse models
We inspected the effect of D-WAT experimental monotherapy on the growth of tumor grafts in immunocompetent C57BL/6 mice. Animals were raised on high-fat diet to induce diet-induced obesity (DIO). While female mice are relatively DIO-resistant, male mice undergo profound DIO and harbor increased ASC numbers in expanded WAT, which promotes tumor growth.8 Upon grafting mice with isogeneic carcinoma cells, mice were metronomically subcutaneously (SC)-injected with 14.5 mg/kg D-WAT into the lower flanks three times a week for a period of 3 weeks, based on the established regimen.16 Despite not being profoundly obese, female mice orthotopically grafted with EO771 breast adenocarcinoma displayed a suppression of tumor growth compared to sham-injected mice (Figure 1b). Treatment of male mice with SC-grafted B16F10 melanoma, Lewis lung carcinoma (LLC), and RM1 prostate adenocarcinoma gave similar results. Three weeks after grafting, when most sham-injected mice had ulcer-free tumors approaching 1 cm in diameter, D-WAT-treated mice had smaller and/or ulcerated tumors (Figure 1c). There was a significant difference in both tumor volume and weight between treated and control mice (Figure 1d). Tumors resected from D-WAT-treated mice were typically whiter than control, indicating decreased blood perfusion (Figure 1e). We also performed D-WAT treatments in mice raised on regular diet. Tumor ulceration was also observed (Figure 1f). Consistent with dependence of accelerated tumor growth on increased ASC abundance in obesity,8 the tumor-inhibitory effect of D-WAT in lean mice was less pronounced. Cell culture experiments demonstrated that D-WAT did not directly affect viability of LLC, B16F10, or RM1 cells and specifically induced apoptosis of ASC (Figure 1g), while a control KLAKLAK2 fusion peptide with scrambled WAT7 sequence had only low effect on ASC viability measured using the CellTiter-Blue assay (Supplementary Figure S1).
D-WAT treatment specifically depletes PDGFRβ+ stromal cells from tumors
To confirm in vivo ASC specificity of D-WAT, we treated DIO mice that had been irradiated with 900 cGy 2 weeks prior to SC tumor cell inoculation. Tumors were more ulcerated and grew slower in D-WAT treated mice (Figure 2a) suggesting that D-WAT antitumor effects are not mediated by hematopoietic progenitors killed by this dose of radiation. We also used a previously characterized model8 based on two syngeneic mouse strains, in which one (host) ubiquitously expresses green fluorescent protein (GFP) and the other (donor)—red fluorescent protein (RFP). The chimeric GFP/RFP mice generated through RFP marrow transplantation into lethally irradiated GFP mice make it possible to distinguish hematopoietic cells (RFP+) from extramedullary (GFP+) cells. We performed collagenase digestion of tumors 3 weeks postinoculation into control and D-WAT-treated GFP/RFP mice and cultured the cell suspension ex vivo for 1 day. Analysis of adherent cells identified GFP-RFP- malignant cells, GFP+RFP- cells with multigonal morphology typical of ASC or endothelial cells, and round GFP-RFP+ leukocytes (Figure 2b). Cell quantification revealed that D-WAT treatment selectively reduced the frequency of GFP+ cells, while RFP+ leukocyte frequency was not affected (Figure 2c). Because ASC constitute a large proportion of adherent GFP+ cells in tumors of DIO mice,8 these data provide additional evidence that D-WAT targets ASC in vivo.
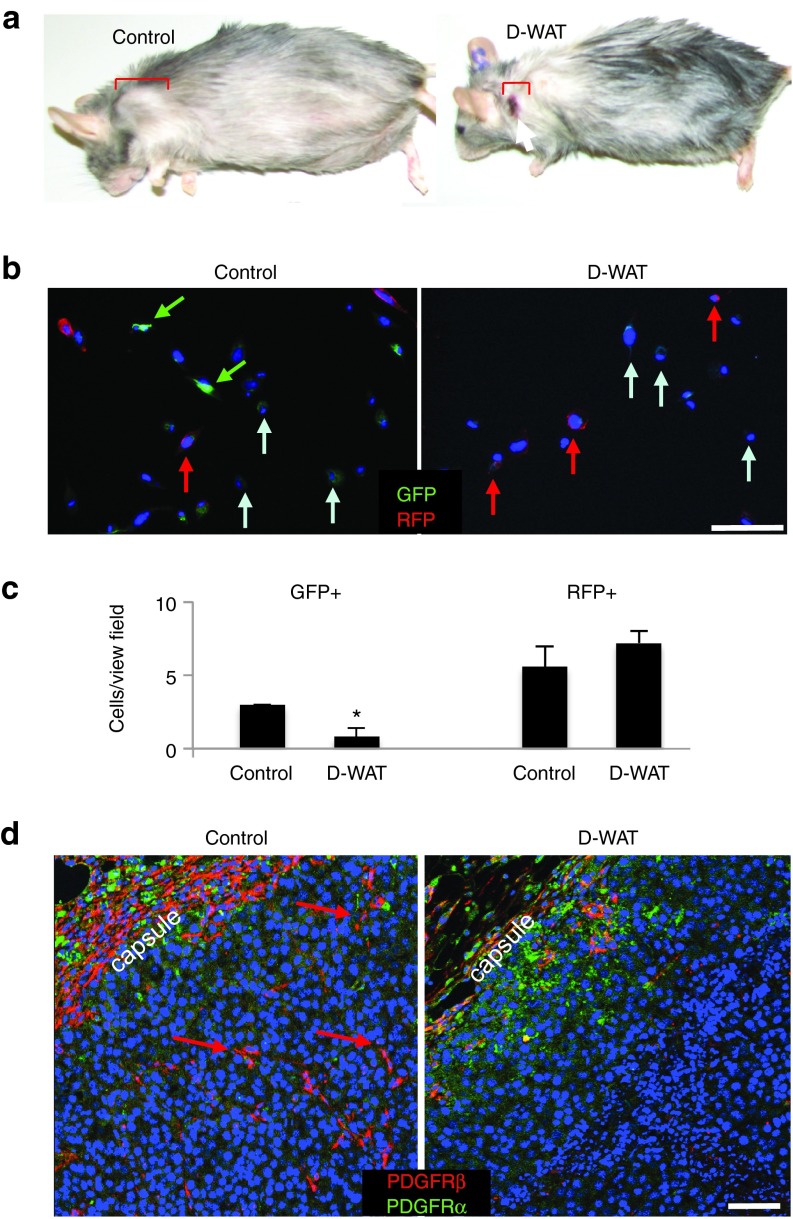
Figure 2.
D-WAT treatment specifically deletes PDGFRβ+ stromal cells from tumors. (a) Representative Lewis lung carcinoma grafts grown in lethally irradiated obese GFP mice treated with vehicle (control) or D-WAT. (b) Cells adherent 1 day postplating of cell suspension from equal volume of E0771 tumors from control and D-WAT-treated chimeric GFP/RFP mice (Figure 1b). Green/red fluorescence channel merging demonstrates host stromal cell recovery (GFP+) reduced for treated mice. In contrast, leukocytes (RFP+) are recovered from tumors of both control and treated mice. (c) Based on the analysis of 10 view fields (20×) data from (b) was quantified and plotted as mean ± SEM; *P < 0.05 versus control. (d) Sections of B16F10 grafts grown in obese mice treated with vehicle (control) or D-WAT subjected to immunofluorescence with antibodies specific for mouse PDGFRβ (red) and PDGFRα (green). Note that PDGFRβ+ stromal cells (arrows) are absent upon D-WAT treatment. Nuclei are blue. Scale bar, 100 µm. Experiments were performed at least twice with similar results.
We also performed immunofluorescence analysis of sections from tumors grown in control and D-WAT-treated mice (Figure 1b) to confirm the selectivity of cell depletion. In WAT, D-WAT selectively targets ASC expressing PDGFRβ, and upon their depletion the population of PDGFRα+ ASC is expanded.16 We, therefore, used antibodies against PDGFRα and PDGFRβ to investigate both of these mesenchymal stromal populations in tumors. D-WAT treatment resulted in markedly reduced abundance of PDGFRβ+ stromal cells in both peripheral and internal tumor areas (Figure 2d). In contrast, PDGFRα+ cells, observed only at the tumor periphery, were not visibly affected by D-WAT treatment (Figure 2d). These data indicate that D-WAT selectively depletes PDGFRβ+ ASC in tumors.
D-WAT and its potentiated derivative cause tumor hemorrhage and necrosis
Previously, a cytotoxic peptide KFAKFAKKFAKFAK (termed KFAKFAK2) targeted to tumors has shown efficacy in mouse models.17 We used a proteolysis-resistant all-D-enantiomer of WAT7-KFAKFAK2 peptide for a side-by-side comparison with D-WAT. Upon tumor cell inoculation, we performed metronomic injections of identical doses of all-D D-WAT or WAT7-KFAKFAK2 peptides; control mice were injected with saline. At week 3 time point, when tumors approached 1 cm in diameter for sham-injected mice, peptide-treated mice had significantly smaller tumors (Figure 3a). WAT7-KFAKFAK2 treatment had a significantly stronger inhibitory effect on tumor growth than D-WAT (Figure 3a). To investigate the underlying cause of ASC depletion effect on tumor growth, we analyzed tumor sections stained with hematoxylin/eosin (H/E). While in control mice tumors were mainly populated by viable malignant cells (violet H/E staining), tumor composition was markedly different in tumors upon both D-WAT and WAT7-KFAKFAK2 treatment (Figure 3b). Each peptide induced hemorrhaging identified as red blood cell-infiltrated areas. Vast areas of pale acellular areas indicative of necrosis were also observed in tumors upon treatment with either D-WAT or WAT7-KFAKFAK2. We also tested peptide WAT7-KFAKFAK2 in lean BALB/C mice orthotopically grafted with breast carcinoma 4T1.2 cells. As in obese mice, peptide treatment induced tumor necrosis and ulceration (Supplementary Figure S2).
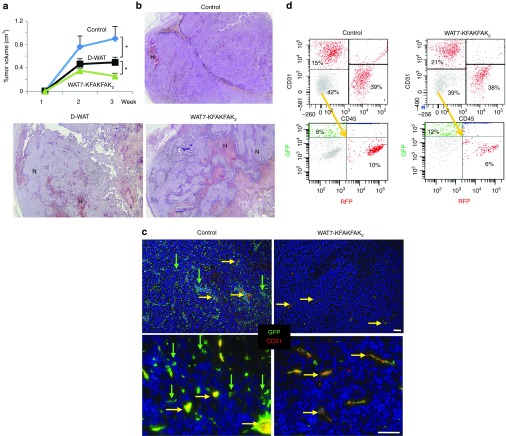
Figure 3.
D-WAT and its potentiated derivative cause tumor hemorrhage and necrosis. (a) B16F10 grafts were grown in obese mice treated with vehicle (control), D-WAT, or WAT7-KFAKFAK2 (n = 10/group). Tumor growth rate is plotted as mean tumor volume ± SEM; *P < 0.05 versus control. (b) Hematoxylin/eosin staining of tumor sections representative for each group in (a) reveals necrosis (N) and hemorrhage (H) induced by both peptides. (c) EO771 grafts were grown in GFP mice treated with vehicle (control) or WAT7-KFAKFAK2. Immunofluorescence section analysis with antibodies specific for CD31 (red) and GFP (green) reveals blood vessels (yellow) and reduced infiltration of host stromal cells (green) in treated mice. Nuclei are blue. Scale bar, 100 µm. (d) Flow cytometric analysis of cell suspension from RM1 tumors grown in mice untreated or treated with WAT7-KFAKFAK2. In C57BL/6-Tg(Pdgfra-cre)1Clc x B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J progeny used as tumor host, PDGFRβ+ ASC, leukocytes and endothelial cells are RFP+GFP-, while PDGFRα+ ASC are RFP-GFP+. Note that treatment does not affect endothelial (CD31+) and hematopoietic (CD45+) cell frequency among total viable cells. Gating of CD31-/CD45- cells into no-color malignant cells (grey), RFP+ cells, and GFP+ cells shows that PDGFRβ+ ASC (RFP+) are depleted while PDGFRα+ ASC (GFP+) are enriched by treatment. Experiments were performed at least twice with similar results.
To confirm that WAT7-KFAKFAK2 inhibits tumor growth through the same mechanism as D-WAT, we analyzed peptide-treated mice ubiquitously expressing GFP that had been grafted with tumors. There was a clear reduction in the frequency of GFP+ cells upon WAT7-KFAKFAK2 treatment, consistent with the peptide depleting stromal cells (Figure 3c). Immunofluorescence analysis of the sections with CD31 antibodies revealed that the majority of GFP+ cells remaining in tumors of peptide-treated mice were endothelial and composed of vessels with lumens that were typically less open than those in control tumors (Figure 3c). In contrast, GFP+ stromal cells negative for CD31, which were abundant in control tumors, were depleted upon peptide treatment (Figure 3c).
We also quantified the effect of WAT7-KFAKFAK2 treatment on tumor composition by flow cytometry (Figure 3d). Analysis was performed in a transgenic lineage tracing mouse model based on RFP and GFP reporters.18 RM1 cells were orthotopically grafted into male progeny of C57BL/6-Tg(Pdgfra-cre)1Clc and B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J. In these mice, the majority of host cells, including PDGFRβ+ ASC, as well as all hematopoietic and endothelial cells, express loxP-flanked RFP (tdTomato) which blocks the expression of downstream GFP. In contrast, PDGFRα+ ASC expressing the Cre recombinase driven by PDGFRα promoter, as well as their derivatives, become indelibly green due to loxP-flanked RFP excision and induction of GFP. Upon treatment, there was no reduction in the tumor frequency of RFP+ endothelial (CD31+) and hematopoietic (CD45+) cells. Analysis of CD31−/CD45− cells (containing no-color malignant cells and mesenchymal stroma) confirmed that that there was a decrease in the frequency of PDGFRβ+ ASC (RFP+), while the frequency of PDGFRα+ ASC (GFP+) was elevated. Combined, these data indicate that WAT7-KFAKFAK2 and D-WAT deplete tumor PDGFRβ+ stromal cells.
ASC-depleting peptides inhibit tumor vasculature and cell proliferation
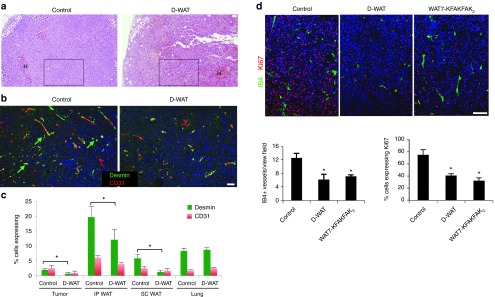
Finally, we wished to determine the mechanism underlying tumor hemorrhage and necrosis observed upon peptide-mediated ASC depletion. Because ASC excess in tumors has been associated with promoted vascularization, a requisite of tumor cell proliferation and survival,8 we hypothesized that ASC depletion impairs functionality of tumor vasculature. To test this hypothesis, we analyzed tumor sections from control and D-WAT-treated mice. For this analysis, we compared tumors comparable in size from treated and control animals. We selected areas that did not display profound hemorrhaging and necrosis and that were populated by viable malignant cells (Figure 4a). Immunofluorescence analysis with CD31 antibodies revealed a change in vasculature organization in tumors upon treatment, although the frequency of CD31+ vessels was not reduced (Figure 4b). Because ASC contribute to the peri-endothelial niche in both WAT and tumors,19 we also analyzed the expression of a pericyte marker desmin. A significant reduction in the frequency of viable desmin+ cells was observed upon D-WAT treatment for both intraperitoneal (IP) WAT, SC WAT and tumors, while pericytes in the lung were not affected (Figure 4c). We also performed a side-by-side comparison of tumor vascularization and proliferation in mice treated with D-WAT and WAT7-KFAKFAK2. Each compound caused a decrease in the frequency of microvessels stained with isolectin B4 (Figure 4d). Frequency of proliferating cells identified based on nuclear Ki-67 expression was also reduced by both ASC-depleting agents (Figure 4d). Combined these data indicate that ASC depletion compromises tumor growth, at least in part, through inhibition of vascularization, without which tumor cell proliferation and survival is compromised.
Figure 4.
Adipose stromal cells-depleting peptides inhibit tumor vasculature and cell proliferation. (a) Hematoxylin/eosin staining of Lewis lung carcinoma graft sections from Figure 2a. (b) Immunofluorescence analysis of magnified insets indicated in (a) with antibodies specific for CD31 (red) and desmin (green). (c) Quantification of data in (b) plotted as mean % of CD31+ or desmin+ cells (among nucleated cells) ± standard error of the mean (SEM); *P < 0.05 versus control (n = 10/group). Frequency of desmin+ cells is reduced in tumors of D-WAT-treated mice, as well as in white adipose tissue (WAT) depots (positive control), but not in the lung (negative control). (d) Immunofluorescence of B16F10 sections from (Figure 3b) with antibodies specific for Ki-67 (red) and IB4 (green). Plots below show data quantified as mean number of IB4+ vessels/20× view field (N = 20) or % Ki-67+ cells (among nucleated cells) ± SEM; *P < 0.05 versus control (n = 10/group). Nuclei are blue. Scale bar, 100 µm. Experiments were performed at least twice with similar results.
Discussion
Organization and function of the complex tumor stromal component remains poorly understood.1,20 Mesenchymal stroma is believed to influence distinct stages of cancer progression and chemoresistance through complex mechanisms.6 MSC secrete cytokines driving the epithelial-mesenchymal transition, as well as a plethora of other angiogenic, immunosuppressive, antiapoptotic, and mitogenic factors. Their activity promotes tumor vascularization and is responsible for deposition of extracellular matrix and tumor desmoplasia. MSC can also mute antitumor immune response through their effect on T-cells and tumor-associated macrophages, which are also key players in cancer progression.2
Our previous studies have revealed that ASC, the WAT-derived MSC, represent a cell population that may underlie the link between obesity and cancer.8,12,13 Using animal models, we have demonstrated that ASC infiltrate tumors where they contribute to the populations of pericytes and intratumoral adipocytes. Our studies have suggested that ASC-derived tumor stroma promotes cancer by engaging in vascularization, which stimulates malignant cell survival and proliferation. Despite these implications, the role of MSC in general, or of ASC specifically, in cancer has not yet been proven in vivo due to the lack of genetic or pharmacological tools to specifically inactivate them. Here, we for the first time directly tested whether ASC are rate limiting for tumor growth. In addition to testing the previously characterized hunter-killer peptide D-WAT,16 we developed the WAT7-KFAKFAK2 peptide as a new ASC-targeting compound with an improved targeted cytotoxic activity. Neither D-WAT nor WAT7-KFAKFAK2 caused detectable systemic toxicity upon a single injection dose of up to 30 mg/kg. No appetite suppression or other adverse effects was detected upon a cumulative metronomic injection dose of 174 mg/mouse over 3 weeks (data not shown).
Tests of D-WAT or WAT7-KFAKFAK2 as a monotherapy in several mouse cancer models demonstrated specific depletion of PDGFRβ+ mesenchymal stroma from WAT and tumors upon treatment. Our data indicate that ASC play an important role in vascularization of growing tumors by significantly contributing to the pool of PDGFRβ+ stromal/perivascular cells. Interestingly, our results show that PDGFRα+ MSC, which serve as progenitors of myofibroblasts in other organs and of beige adipocytes in WAT,16 do not significantly contribute to tumor microenvironment. Depletion of PDGFRβ+ ASC resulted in an effect opposite to that induced by ectopic ASC administration12,13 and by excessive ASC recruitment from WAT surplus in obese mice.8 The effect of intratumoral ASC depletion on the vessel phenotype suggests that limitation of vascular patency underlies the reduced tumor cell proliferation and survival observed in treated animals.
Combined, our results demonstrate the supportive role of ASC in tumor growth, thus establishing a mechanistic link between obesity and cancer. This and other recent studies8,12,13,21 suggest ASC as a potential drug target. In mouse cancer models, pericytes have been shown to suppress metastatic dissemination, while ASC have been reported to have prometastatic effects.5,10,11 This suggests that in tumor stroma, in addition to supporting vasculature, ASC have other important cancer-promoting functions. Future studies will determine whether ASC depletion can improve the efficacy of cancer treatment. Experimental targeted therapeutics based on KLAKLAK2 and KFAKFAK2 have entered clinical trials.14,17 The potential of compounds developed here to be used as a combination cancer therapy remains to be tested. We propose that mesenchymal stroma targeting with compounds described here, or with their derivatives, may improve the efficacy of conventional chemotherapy and radiotherapy.
Materials and Methods
Experimental animals. Animal studies were approved by the Institutional Animal Care and Use Committee of UTHealth. C57BL/6, BALB/c, C57BL/6-Tg(UBC-GFP)30Scha/J (GFP mice), B6.Cg-Tg(ACTB-mRFP1)1F1Hadj/J (RFP mice), C57BL/6-Tg(Pdgfra-cre)1Clc, B6.129(Cg)-Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J strains were from Jackson Laboratory (Bar Harbor, ME). DIO was modeled by feeding mice 58 kcal% (fat) diet (Jackson D12331) for 4 months postweaning. For tumor grafting, 1 × 105 cells were injected with a 21-gauge needle subcutaneously (SC) onto lower back or into the mammary fat pad (EO771). Tumor size was measured with a caliper and volume was calculated as length × width2 × 0.52. Where indicated, GFP DIO mice were irradiated with 900 cGy and grafted with tumor cells the same day. Where indicated, lethally irradiated GFP DIO mice received RFP bone marrow transplants as described8 and the chimeric GFP/RFP mice were grafted with tumor cells 1 month later.
Peptides. Proteolysis-resistant WAT7-KLAKLAK2 (D-WAT) and WAT7-KFAKFAK2 were made as “all-D-amino acid” peptides with the targeting and apoptotic domains linked by aminohexanoic acid linker NH-(CH2)5-CO. Peptides were synthesized as acetate salt, cysteine-cyclized, chromatographically purified to 99% and quality-controlled (mass spectroscopy) by Ambiopharm (North Augusta, SC). Peptides were dissolved in phosphate-buffered saline to 10 mmol/l and aliquots were stored frozen until dilution in phosphate-buffered saline, filtration, and use. For peptide administration, the metronomic SC injection protocol was used as described16 starting the day after tumor grafting.
Cell lines and primary cell culture. Cells were cultured in α-minimum essential medium supplemented with 10% fetal bovine serum and penicillin–streptomycin. Cell lines B16F10, and LLC were from the American Type Culture Collection (Manassas, VA) E0771 was from F. Sirotnak, 4T1.2 was from R. Anderson, and RM1 was from T. Thompson. Primary cells were isolated and cultured as described.8,15 Freshly isolated ASC were briefly cultured to remove nonadherent cells. Trypan blue exclusion assay was used to determine cell viability in peptide-treated ASC and tumor cell lines. Cells were treated with 0.05 mmol/l of D-WAT or WAT7-KLAKLAK2 in 0.5 ml culture medium for 2 hours when cell density reached 80% confluency. Cells were stained with 0.4% Trypan Blue solution (MP Biomedicals, Santa Ana, CA) for 10 minutes to visualize live and dead cells. The CellTiter-Blue assay (Promega, San Luis Obispo, CA) was used to quantify D-WAT effect on ASC proliferation/survival. Scrambled peptide CWWGSFYEKC-NH-(CH2)5-CO-KLAKLAK2 was used as a negative control. Adherent ASC were detached with trypsin and plated onto 96-well plate at 5,000 cells/well (in 100 µl medium). Adherent ASC were treated with 0.05 mmol/l D-WAT or scrambled peptide for 3 hours. The peptide-treated ASC were incubated at 37 °C, 5% CO2. The effect of peptide treatment was assessed on day 0, 1, 3, and 5. At each time-point, 20 µl of CellTiter-Blue reagent was added to each well, and cells were incubated for additional 6 hours, after which fluorescence was measured at (560ex/590em).
Immunofluorescence. Sections of formalin-fixed paraffin-embedded tissues were analyzed by immunofluorescence as described.8,15 Antigen retrieval, washing with 0.2% Triton X-100 and blocking in Serum-Free Protein Block (Dako, Carpinteria, CA) was followed by incubation with primary antibodies (4 °C, 12 hours) and secondary antibodies (RT, 1 hour) in phosphate-buffered saline containing 0.05% Tween 20. Cells plated on 24-well plate were fixed with 4% paraformaldehyde, permeabilized with Triton-X 100, blocked with serum-free protein block and followed by incubation with primary and secondary antibodies, as above. Antibodies used: rabbit anti-Ki-67 (Thermo Scientific, Grand Island, NY, 1:100); rabbit anti-desmin ab15200 (Abcam, 1:100); goat and rabbit anti-CD31 (Santa Cruz Biotechnology, Dallas, TX; 1:100); rabbit anti-PDGFRβ ab32570 (Abcam, 1:100); rat anti-PDGFRα ab51875 (Abcam, Cambridge, MA; 1:100) and goat anti-GFP (GeneTex, Irvine, CA; 1:100). Biotinylated Griffonia simplicifolia isolectin B4 (IB4) was from Vector Labs (Burlingame, CA; 1:50) and used with Alexa 488-conjugated streptavidin. Secondary antibodies were donkey Alexa 488-conjugated (1:150) IgG from Invitrogen and Cy3-conjugated (1:300) IgG from Jackson ImmunoResearch (West Grove, PA). Nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI). For quantifications, at least 10 random 10× or 20× magnification fields were blindly scored and/or measured using microscope grid. IB4+ vessel counts were blindly scored by two independent investigators. Tissue images were acquired with an Olympus BX51 upright fluorescence microscope; cell images—with an Olympus IX70 (Olympus, Center Valley, PA) inverted fluorescence microscope. MagnaFire software and Amira 5.4 software (VSG, Burlington, MA) were used for data capture and analysis.
Flow cytometry. Cells were analyzed using FACSAria II and FACS Diva software (BD Biosciences, San Jose, CA). Tumor tissue cell suspensions were pregated to exclude debris, cell clumps, as well as dead cells based on DAPI staining. Gating was performed as described8 based on tdTomato and GFP fluorescence and using the following IgG clones: anti-CD31 (MEC 13.3), anti-CD45 (30-F11), and the corresponding isotype controls (BD Biosciences).
Statistics. Microsoft Excel was used to graph data as mean ± SEM and to calculate P-values using homoscedastic Student's t-test. For multigroup analysis of variance, analysis of variance was used.
SUPPLEMENTARY MATERIAL Figure S1. D-WAT but not scrambled hunter killer peptide kills ASC. Figure S2. ASC depletion effect in non-obese mouse tumor model.
Acknowledgments
We thank Dhanashree Bibikar, Thanh Vu, and Zhanguo Gao for technical assistance. This work was supported in part by Cancer Prevention and Research Institute of Texas Awards RP100400 and RP110776. Kolonin and The University of Texas Health Science Center at Houston have research-related financial interests in Astrotide, Inc.
Supplementary Material
References
- Kolonin, MG, Evans, KW, Mani, SA and Gomer, RH (2012). Alternative origins of stroma in normal organs and disease. Stem Cell Res 8: 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D and Coussens, LM (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21: 309–322. [DOI] [PubMed] [Google Scholar]
- Karnoub, AE, Dash, AB, Vo, AP, Sullivan, A, Brooks, MW, Bell, GW et al. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449: 557–563. [DOI] [PubMed] [Google Scholar]
- Bhowmick, NA, Neilson, EG and Moses, HL (2004). Stromal fibroblasts in cancer initiation and progression. Nature 432: 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, Y, Kim, JK, Shiozawa, Y, Wang, J, Mishra, A, Joseph, J et al. (2013). Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun 4: 1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantrain, CF, Feron, O, Marbaix, E and DeClerck, YA (2008). Bone marrow microenvironment and tumor progression. Cancer Microenviron 1: 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J, Morley, TS, Kim, M, Clegg, DJ and Scherer, PE (2014). Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol 10: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y, Daquinag, AC, Amaya-Manzanares, F, Sirin, O, Tseng, C and Kolonin, MG (2012). Stromal progenitor cells from endogenous adipose tissue contribute to pericytes and adipocytes that populate the tumor microenvironment. Cancer Res 72: 5198–5208. [DOI] [PubMed] [Google Scholar]
- Sirin, O and Kolonin, MG (2013). Treatment of obesity as a potential complementary approach to cancer therapy. Drug Discov Today 18: 567–573. [DOI] [PubMed] [Google Scholar]
- Orecchioni, S, Gregato, G, Martin-Padura, I, Reggiani, F, Braidotti, P, Mancuso, P et al. (2013). Complementary populations of human adipose CD34+ progenitor cells promote growth, angiogenesis, and metastasis of breast cancer. Cancer Res 73: 5880–5891. [DOI] [PubMed] [Google Scholar]
- Rowan, BG, Gimble, JM, Sheng, M, Anbalagan, M, Jones, RK, Frazier, TP et al. (2014). Human adipose tissue-derived stromal/stem cells promote migration and early metastasis of triple negative breast cancer xenografts. PLoS One 9: e89595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y, Daquinag, A, Traktuev, DO, Amaya-Manzanares, F, Simmons, PJ, March, KL et al. (2009). White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res 69: 5259–5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopp, AH, Zhang, Y, Solley, T, Amaya-Manzanares, F, Marini, F, Andreeff, M et al. (2012). Omental adipose tissue-derived stromal cells promote vascularization and growth of endometrial tumors. Clin Cancer Res 18: 771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonin, MG, Saha, PK, Chan, L, Pasqualini, R and Arap, W (2004). Reversal of obesity by targeted ablation of adipose tissue. Nat Med 10: 625–632. [DOI] [PubMed] [Google Scholar]
- Daquinag, AC, Zhang, Y, Amaya-Manzanares, F, Simmons, PJ and Kolonin, MG (2011). An isoform of decorin is a resistin receptor on the surface of adipose progenitor cells. Cell Stem Cell 9: 74–86. [DOI] [PubMed] [Google Scholar]
- Daquinag, AC, Tseng, C, Salameh, A, Zhang, Y, Amaya-Manzanares, F, Dadbin, A et al. (2015). Depletion of white adipocyte progenitors induces beige adipocyte differentiation and suppresses obesity development. Cell Death Differ 22: 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callery, EC, Hansel, W and Bartlewski, PM (2010). The effects of a low therapeutic dose of βCG fragment-lytic peptide conjugates on ovarian function and gonadotropin secretion in ewes: a randomized controlled trial. Reprod Biol 10: 195–213. [DOI] [PubMed] [Google Scholar]
- Berry, R and Rodeheffer, MS (2013). Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol 15: 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traktuev, DO, Merfeld-Clauss, S, Li, J, Kolonin, M, Arap, W, Pasqualini, R et al. (2008). A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res 102: 77–85. [DOI] [PubMed] [Google Scholar]
- San Martin, R, Barron, DA, Tuxhorn, JA, Ressler, SJ, Hayward, SW, Shen, X et al. (2014). Recruitment of CD34(+) fibroblasts in tumor-associated reactive stroma: the reactive microvasculature hypothesis. Am J Pathol 184: 1860–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowicka, A, Marini, FC, Solley, TN, Elizondo, PB, Zhang, Y, Sharp, HJ et al. (2013). Human omental-derived adipose stem cells increase ovarian cancer proliferation, migration, and chemoresistance. PLoS One 8: e81859. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.