Abstract
Bioresorbable polymers and biocorrodible metals are the latest developments in biodegradable materials used in interventional cardiology for the mechanical treatment of coronary atherosclerosis. Poly-L-lactic acid is the most frequently used bioresorbable polymer and initial evidence of feasibility, efficacy and clinical safety following deployment of polymer-based platforms was gained after completion of the first-in-man longitudinal ABSORB registries, Cohorts A and B and ABSORB Extend.
In these studies, the biologic interaction of the first-generation Absorb Bioresorbable Vascular Scaffold (BVS) (Abbott Vascular, SC, Calif., US) with the underlying vascular tissue was evaluated in vivo with multiple imaging modalities such as intravascular ultrasound (IVUS), virtual histology-IVUS, IVUS-palpography, optical coherence tomography as well as ex vivo with coronary computed tomography. Efficacy measures following this in vivo multi-imaging assessment as well as clinical safety were comparable with current generation drug-eluting stents (DES) (Abbott Vascular, SC, Calif., US) in non-complex lesions over a 3-year follow-up. Furthermore, novel properties of functional and anatomic restoration of the vessel wall during the late phases of resorption and vascular healing were observed transforming the field of mechanical treatment of atherosclerosis from delivering only acute revascularization to additionally enable late repair and subsequent restoration of a more physiologic underlying vascular tissue.
Despite the sufficient evidence and the subsequent Conformité Européenne mark approval of the first fully biodegradable scaffold (Absorb BVS) in 2012 for revascularizing non-complex lesions, the paucity of randomized comparisons of fully bioresorbable scaffolds (BRS) with metallic DES in a “real-world” clinical setting raised controversies among the interventional community for the merit of these technologies. Only recently, results from international large-scale randomized trials from the United States (U.S.), China and Japan were revealed.
Herein we provide a comprehensive overview of the ABSORB III, ABSORB China and ABSORB Japan studies demonstrating the consistent non-inferiority in clinical safety and efficacy measures of the Absorb BVS vs. current generation DES.
Introduction
The development of novel DES with more efficacious platforms have minimized risks of clinical restenosis and stent thrombosis to below 4.0% and ≈ 0.5% respectively, remaining the standard of care in interventional cardiology.1 BRS (Figure 1) appear to be appealing alternatives to metal stents in specific lesions and population subsets with potential long-term benefits attributed to full resorption and subsequent long-term functional and anatomic vessel restoration.2,3
Figure 1.
Bioresorbable scaffolds. Clinical safety and efficacy measures.
The clinical applicability of first generation BRS still remains restricted. Polymer-based scaffolds and to a lesser extend scaffolds made of biocorrodible metals have limited expansion properties and over dilation entails the risk of strut fracture or discontinuity. It is imperative for operators who implant current generation drug-eluting BRS to perform aggressive lesion preparation by adequate pre-dilatation with a near optimal size non-compliant balloon to high pressures. The accurate scaffold to artery sizing ratio of 1:1 by quantitative coronary angiography is mandatory to precisely select the most appropriate scaffold diameter. Furthermore, post-dilation should not exceed a 0.5 mm diameter overstretch of the deployed scaffold up to 18–20 atm. The use of BRS in complex lesions such as bifurcations has been only investigated in vitro with bifurcation phantom models and provisional scaffolding remains the standard approach with sequential non-compliant balloon inflations in the side branch followed by the main branch.3
Despite the encouraging preliminary observations derived from international registries such as the ABSORB Cohorts A, and B as well as the ABORB Extend, it became evident that BRS as first-generation devices need additional scaffold design improvements to optimize their mechanical performance and expand the indications of deployment from selective lesions to more complex lesion subsets.4–6 This selective pattern of indications in association with the lack of randomized comparisons with DES proved to be an obstacle for regulatory approval in the U.S. Only recently the results of international large-scale randomized trials from the U.S., China and Japan provided evidence of similar clinical safety and comparable efficacy with current generation DES.
ABSORB III randomized clinical trial
Study design
The ABSORB III was the first large-scale multicenter, randomized trial designed to support the Absorb BVS transition from the stage of pre-market approval - which allows the execution of Phase III clinical trials - to complete Food and Drug Administration (FDA) clearance for clinical use in the U.S.7,8 Two thousand and eight patients with stable or unstable angina were randomly assigned to receive in a 2:1 ratio an everolimus-eluting metallic stent (Xience) or a fully resorbable scaffold, the Absorb BVS.
The primary end-point was target-lesion failure (TLF), a composite of cardiac death, target-vessel myocardial infarction (TV-MI) or ischemia driven target lesion revascularization (ID-TLR) tested for both non-inferiority and superiority at 1-year. The treated lesions were relatively complex (Type B2), excluding highly complex subsets such as chronic total occlusions, left main disease and large bifurcations. The average lesion length was < 12 mm and the reference vessel diameter (RVD) > 2.5 mm in both arms. The only procedural characteristics different among the groups were the rates of post-dilatation which reached in the Absorb BVS vs. Xience treated vessels: 65.5% vs. 51.2%, (p < 0.001) respectively.
Results
The Absorb BVS was non-inferior to the Xience stent at 1-year meeting the primary end-point of non-inferiority. TLF occurred in 7.8% vs. 6.1% in the Absorb BVS arm vs. the Xience arm (difference, 1.7 % points; 95% confidence interval, − 0.5 to 3.9; p = 0.007 for non-inferiority and p = 0.16 for superiority) (Figure 2).
Figure 2.
The primary end-point of target-lesion failure at 1-year in the ABSORB III randomized clinical trial. Kaplan-Meier estimates of the primary end-point, which occurred in 7.8% vs. 6.1%, (p = 0.15) in the Absorb BVS vs. the Xience treated patients. Reproduced by permission N Engl J Med. 2015 Oct 12. [Epub ahead of print].
Individual components of TLF were not significantly different among both arms at 1-year. More specifically, rates of cardiac death in the Absorb BVS arm vs. Xience arm were 0.6% vs. 0.1% (p = 0.29), respectively. TV-MI event rates in the Absorb BVS treated patients vs. Xience treated patients were 6.0% vs. 4.6%, (p = 0.18) respectively as well as ID-TLR rates reaching 3.0% vs. 2.5%, (p = 0.50) respectively (Figure 3). Device thrombosis among the groups reached 1.5% in the Absorb BVS arm vs. 0.7% in the Xience arm (p = 0.13).
Figure 3.
The primary end-point of target-lesion failure individual components of cardiac death, target vessel myocardial infarction (TV-MI) and Ischemia driven target lesion revascularization (ID-TLR).
Discussion
ABSORB III is the pivotal randomized trial which compared a first generation fully absorbable scaffold with a best in class second generation metallic stent, designed to support the U.S. FDA commercial approval of the Absorb BVS. Although the trial met its primary endpoint, the delta or margin of non-inferiority set in the range of 4.5% was considered as a substantially high threshold; meanwhile this threshold complied with the guideline documents of U.S. FDA related to sufficient margins for non-inferiority trials.
It is important to note that non-inferiority is different than equivalence. This trial suggested that the new therapy is at least “no worse” than the standard of therapy based on the pre-defined marginal value in non-complex lesions. This trial did not indicate that a fully bioresorbable scaffold has equal performance to current generation DES. This was particularly evident when scaffolds were deployed in smaller vessels (RVD < 2.5 mm) where the rates of TLF at 1-year reached 19%.
Strut design (rectangular, circular, elliptical or tear-drop), and more particularly strut thickness, has been shown to be an important determinant of TLF 9; thus current generation DES are engineered with thinner struts in the range of < 90 μm. Bulky struts are prone to side-branch jailing or occlusion, delayed endothelialization in particular when overlapped scaffolds are implanted and increased mechanical stresses over the vessel wall. First generation BRS technologies have been introduced with a strut thickness similar to that of Cypher metallic stents (150 μm) to maintain their mechanical properties, as bioresorbable polymers or biocorrodible metals have inferior tensile strength compared to metallic materials. This design increases their overall crossing profile at the crimped stage (∼ 1.4 mm), restricts deliverability in tortuous anatomies, calcified lesions and small vessels (RVD < 2.5 mm).
An important observation which came across at 1-year was related to the rates of device thrombosis among the treated groups which despite were not statistically different were numerically larger in the Absorb BVS arm. Indeed the rates of device thrombosis in vessels with RVD > 2.5 mm was: 1.5% vs. 0.5%, (p = 0.13) in Absorb BVS vs. Xience treated patients. These findings were eagerly expected after the observed scaffold thrombosis rates in the “real-world” European BVS registry (GHOST-EU), which enrolled 1,189 patients and included complex percutaneous interventions such as acute coronary syndromes (ST-elevation myocardial infarction) and left main interventions. The reported cumulative incidence of definite/probable scaffold thrombosis of 2.1% at 6 months raised concerns over the appropriateness of BRS utilization in more complex lesions despite the predefined recommendation of 1-year of dual antiplatelet therapy.10
In summary, the ABORB III trial demonstrated the clinical safety and efficacy of a fully resorbable scaffold implanted in moderately complex lesions at 1-year. Although device thrombosis rates were numerically larger than the best in class metallic stent, there was no statistically significant difference among the treated groups. Obviously long-term evaluation of both cohorts will determine if the rates of late clinical events will decline in the Absorb BVS treated arm, an expectation based on the encouraging observations from first-in-man registries related to the reparative properties of the Absorb BVS.
ABSORB China randomized clinical trial
Study design
ABORB China, enrolled 480 patients randomized for elective PCI to receive in a 1:1 ratio an Absorb BVS or an Xience V stent. The trial was designed to assess in a non-inferior fashion the angiographic efficacy and clinical safety of Absorb BVS compared to the Xience V stent in order to receive regulatory approval in China.11
The primary end-point was angiographic in-segment late lumen loss (LLL) powered for non-inferiority with a margin of 0.15 mm. The treated lesions were relatively complex (Type B2), excluding highly complex subsets such as chronic total occlusions, left main disease and large bifurcations. The average lesion length was < 14 mm and the RVD > 2.5 mm in both arms. The only procedural characteristics different among the groups were the rates of post-dilatation which reached in the Absorb BVS vs. Xience treated vessels: 63.0% vs. 54.4%, (p = 0.05) respectively.
Results
The Absorb BVS was non-inferior to the Xience V stent at 1-year meeting the primary end-point of non-inferiority. In-segment LLL (in-device +5 mm proximal and distal edge vascular responses) was 0.19 ± 0.38 mm vs. 0.13 ± 0.38 mm, (Pnon-inferiority = 0.01) in the Absorb BVS vs. the Xience V stent respectively. Meanwhile it should be noted that the in-device LLL (excluding the 5 mm proximal and distal edge vascular responses) was significantly different among the Absorb BVS vs. the Xience V stent groups: 0.23 ± 0.03 mm vs. 0.10 ± 0.02 mm, (p = 0.0001) respectively. There were no definite scaffold or stent thromboses during the 1-year follow-up period. TLF rates and individual components of cardiac death, TV-MI and ID-TLR were similar among the groups at 1-year (Figure 4).
Figure 4.
Target-lesion failure and individual components of cardiac death, target vessel myocardial infarction (TV-MI) and ischemia driven target lesion revascularization (ID-TLR) in the ABSORB China trial. Reproduced by permission J Am Coll Cardiol. 2015 Oct 6. [Epub ahead of print].
ABSORB Japan randomized clinical trial
Study design
ABORB Japan, enrolled 400 patients randomized for elective PCI to receive in a 2:1 ratio an Absorb BVS or an Xience V stent. The trial was designed to assess in a non-inferior fashion the clinical safety and angiographic efficacy of Absorb BVS compared to the Xience V stent in order to receive regulatory approval in Japan.
The primary clinical endpoint was TLF at 1 year while the secondary efficacy end-point was angiographic in-segment late lumen loss (LLL) at 13 months. The treated lesions were relatively complex (Type B2), excluding highly complex subsets such as chronic total occlusions, left main disease and large bifurcations. The average lesion length was < 13 mm and the RVD > 2.5 mm in both arms.
Results
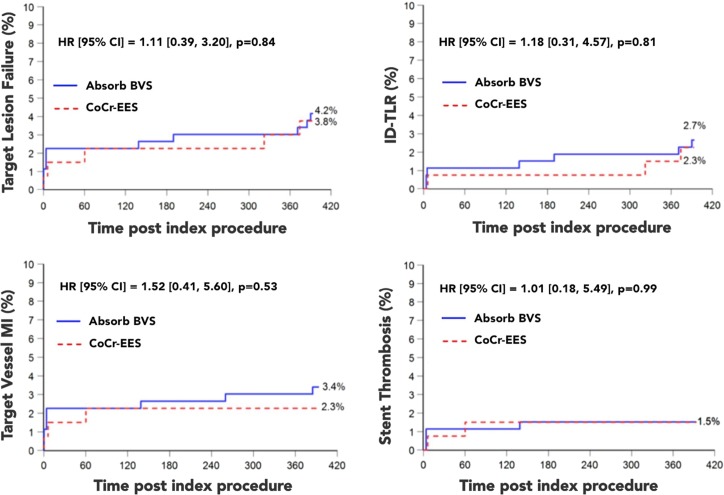
The Absorb BVS was non-inferior to the Xience V stent at 1-year meeting the primary end-point of non-inferiority. The secondary endpoint of in-segment LLL (in-device +5 mm proximal and distal edge vascular responses) did not differ among the groups and was 0.13 ± 0.30 mm vs. 0.12 ± 0.32 mm, (p = 0.74) in the Absorb BVS vs. the Xience V stent group respectively. Similarly, in-device LLL (excluding the 5 mm proximal and distal edge vascular responses) was not significantly different among the Absorb BVS vs. the Xience V stent groups: 0.19 ± 0.31 mm vs. 0.16 ± 0.33 mm, (p = 0.35) respectively. There was no difference in the rates of stent/scaffold thrombosis among the arms (Figure 5).
Figure 5.
Target-lesion failure and individual components of cardiac death and target vessel myocardial infarction (TV-MI) at 1-year, as well as stent thrombosis rates among absorb BVS and Xience V treated lesions. Reproduced by permission Eur Heart J. 2015 Sep 1. [Epub ahead of print].
Discussion
Both ABSORB China and Japan, are valuable additions to the growing body of evidence comparing a first generation fully resorbable scaffold with a best in class metallic stent. The observations are in line with ABSORB III indicating the consistent non-inferiority in clinical endpoints such as cardiac death, TV-MI, ID-TLR of the Absorb BVS when compared with the Xience stent. Meanwhile it should be noted that complex lesions were excluded from these analyses and lesions of moderate complexity were included.
Absorb BVS is a first-generation, fully bioresorbable scaffold and devices in this class are undergoing continuous developments to achieve optimal performance and biocompatibility through improved scaffold designs. The first generation Cypher DES which had a strut thickness similar to Absorb BVS (150 μm) was approved by the FDA in 2002 and despite the initial enthusiasm with the first applications of DES in reducing clinical restenosis numerous adverse late clinical events became evident such as late or very late stent thrombosis, hypersensitivity reactions, delayed endothelialization, late catch-up, impaired vasomotor function of the treated segment and neoatherosclerosis. Continuous innovation over the last 10 years enriched the armamentarium of mechanical treatment of atherosclerosis with best in class metallic devices such as the Xience stent. It is important to consider that first generation BRS were compared in a randomized fashion with latest generation metallic stents and that continuous innovations in the field of biodegradable materials and scaffold designs will allow for best in class BRS in the near future.
The merit of these technologies in interventional cardiology has not been secured yet. Despite the encouraging results derived from the aforementioned randomized trials and the promising observations from the first-in-man studies related to the functional and anatomic restoration of the vessel wall following scaffold resorption the critical component of incremental cost effectiveness ratio has not been addressed yet. BRS in Europe, Asia-Pacific and South-America are priced generally 2-times more compared to newer generation DES and the obvious question arising from the interventional community relies on the merit of a more expensive technology when there is no evident superiority over less costly devices.
Conclusions
For the time being experienced operators should handle these technologies in selected lesions and clinical subsets until future design improvements with next generation devices allow for more expanded indications. Although the concept of vascular reparation therapy in interventional cardiology for mechanically treating atherosclerosis is the unmet need; it still remains unclear if this endeavor will be achieved with bioresorbable materials.
References
- 1.Gogas BD, Maniel M, Samady H, King SB. Novel drug-eluting stents for coronary revascularization. Trends Cardiovasc Med. 2014;24(7):305–313. doi: 10.1016/j.tcm.2014.07.004. doi:10.1016/j.tcm.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Gogas BD. Bioresorbable scaffolds for percutaneous coronary interventions. Glob Cardiol Sci Pract. 2014;2014(4):409–427. doi: 10.5339/gcsp.2014.55. doi:10.5339/gcsp.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigwart U. Treatment of coronary artery disease from the inside: Light at the end of the tunnel? Glob Cardiol Sci Pract. 2015;53 doi: 10.5339/gcsp.2015.53. doi:10.5339/gcsp.2015.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MWI, Onuma Y, Garcia-Garcia HM, Mreevy R, Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): A prospective open-label trial. Lancet. 2008;371(9616):899–907. doi: 10.1016/S0140-6736(08)60415-8. doi:10.1016/S0140-6736(08)60415-8. [DOI] [PubMed] [Google Scholar]
- 5.Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hébert K, Veldhof S, Webster M, Thuesen L, Dudek D. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373(9667):897–910. doi: 10.1016/S0140-6736(09)60325-1. doi:10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 6.Abizaid A, Ribamar Costa J, Bartorelli AL, Whitbourn R, van Geuns RJ, Chevalier B, Patel T, Seth A, Stuteville M, Dorange C, Cheong W-F, Sudhir K, Serruys PW, ABSORB EXTEND investigators The ABSORB EXTEND study: Preliminary report of the twelve-month clinical outcomes in the first 512 patients enrolled. EuroIntervention. 2015;10(12):1396–1401. doi: 10.4244/EIJV10I12A243. doi:10.4244/EIJV10I12A243. [DOI] [PubMed] [Google Scholar]
- 7.Kereiakes DJ, Ellis SG, Popma JJ, Fitzgerald PJ, Samady H, Jones-Means J, Zhang Z, Cheong W-F, Su X, Ben-Yehuda O, Stone GW. Evaluation of a fully bioresorbable vascular scaffold in patients with coronary artery disease: Design of and rationale for the ABSORB III randomized trial. Am Heart J. 2015;170(4):641–651.e3. doi: 10.1016/j.ahj.2015.07.013. doi:10.1016/j.ahj.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Ellis SG, Kereiakes DJ, Metzger DC, Caputo RP, Rizik DG, Teirstein PS, Litt MR, Kini A, Kabour A, Marx SO, Popma JJ, Mreevy R, Zhang Z, Simonton C, Stone GW, ABSORB III Investigators Everolimus-eluting bioresorbable scaffolds for coronary artery disease. N Engl J Med. 2015;373(20):1905–1915. doi: 10.1056/NEJMoa1509038. doi:10.1056/NEJMoa1509038. [DOI] [PubMed] [Google Scholar]
- 9.Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schühlen H, Neumann FJ, Fleckenstein M, Pfafferott C, Seyfarth M, Schömig A. Intracoronary stenting and angiographic results: Strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103(23):2816–2821. doi: 10.1161/01.cir.103.23.2816. [DOI] [PubMed] [Google Scholar]
- 10.Capodanno D, Gori T, Nef H, Latib A, Mehilli J, Lesiak M, Caramanno G, Naber C, Di Mario C, Colombo A, Capranzano P, Wiebe J, Araszkiewicz A, Geraci S, Pyxaras S, Mattesini A, Naganuma T, Münzel T, Tamburino C. Percutaneous coronary intervention with everolimus-eluting bioresorbable vascular scaffolds in routine clinical practice: Early and midterm outcomes from the European multicentre GHOST-EU registry. EuroIntervention. 2015;10(10):1144–1153. doi: 10.4244/EIJY14M07_11. doi:10.4244/EIJY14M07_11. [DOI] [PubMed] [Google Scholar]
- 11.Gao R, Yang Y, Han Y, Huo Y, Chen J, Yu B, Su X, Li L, Kuo H-C, Ying S-W, Cheong W-F, Zhang Y, Su X, Xu B, Popma JJ, Stone GW, ABSORB China Investigators Bioresorbable vascular scaffolds versus metallic stents in patients with coronary artery disease: ABSORB China trial. J Am Coll Cardiol. 2015;66(21):2298–2309. doi: 10.1016/j.jacc.2015.09.054. doi:10.1016/j.jacc.2015.09.054. [DOI] [PubMed] [Google Scholar]