Abstract
Objectives:
Bone remodeling occurs during orthodontic treatment; this process enables tooth movement. Many factors can affect bone remodeling at the cellular level, such as nutritional supplements that can affect tooth movement. The present study was designed to evaluate the effect of dietary vitamin C on orthodontic tooth movement in rats.
Materials and Methods:
This study was carried out on 36 six-week male Wistar rats with a mean weight of 225±32 g, which were randomly allocated to two equal groups. Rats in the case group received 1wt% vitamin C in their daily water. Opening springs were placed on the incisor teeth of both case and control groups. After 17 days, rats were sacrificed; the distance between the mesio-incisal angles of these teeth was measured with a digital caliper. Histological sections were made containing incisor teeth and alveolar bone and stained by hematoxylin-eosin. The number of resorption lacunae was evaluated using light microscopy.
Results:
Our findings showed that the amount of tooth movement in the vitamin C group was significantly higher than that in the control group (P<0.001). The osteoclast counts were significantly higher in vitamin C group (P=0.036).
Conclusion:
Oral vitamin C can increase orthodontic tooth movement in rats with more osteoclast lacunae around root in the pressure area.
Keywords: Ascorbic Acid, Orthodontics, Tooth Movement, Osteoclasts, Bone Remodeling
INTRODUCTION
Bone remodeling occurring during orthodontic treatment is a process that enables movement of teeth with their surrounding supporting tissue. Factors affecting the cellular process of bone resorption and remodeling may increase or decrease tooth movement [1]. Orthodontic forces can increase secondary messengers inside the cells, which are critical for osteoblastic differentiation [2,3] and tooth movement [4,5]. The critical role of ascorbic acid (vitamin C) in osteoclast stimulation in cell culture media has been confirmed in several investigations [6,7]. Lack of vitamin C halts osteogenesis and periodontal ligament organization [8,9]. It has been shown that vitamin C deficiency during orthodontic treatment reduces the tooth movement because of its effect on tissue healing. Its main effect is on the periodontal ligament (PDL). Ascorbic acid deficiency inhibits degradation and regeneration of collagen fibers, which are important in orthodontic tooth movement [9,10]. Ascorbic acid increases the longevity and proliferation of osteoclasts and their progenitor cells [11]. Human body cannot synthetize vitamin C [12]. Normal plasma concentration of vitamin C varies from 36.1 to 79.4 μmol/L [13]. Vitamin C blood level in orthodontic patients is 17% to 75% lower than the desired level [14,15]. Vitamin C supplements are available over the counter. The present study was designed to evaluate the effect of vitamin C dietary supplementation on orthodontic tooth movement in rats.
MATERIALS AND METHODS
Sample size was determined based on the following formula. A minimum of 32 subjects was found to be adequate. Because of possible dropouts during the study, 36 rats were included.
The data for μ and σ were derived from Litton’s study [9]. This study was carried out on 36 six-week old male Wistar rats weighting 200–250 g, which were randomly allocated to two equal groups. All rats had intact incisors with normal interproximal contact and were kept in cages at a temperature of 25°C and humidity of 55% in alternating 12-hour periods of light and dark for seven days to accommodate with the new conditions. Rats with a diastema between their incisor teeth were excluded. The control group did not receive any supplemental vitamin C.
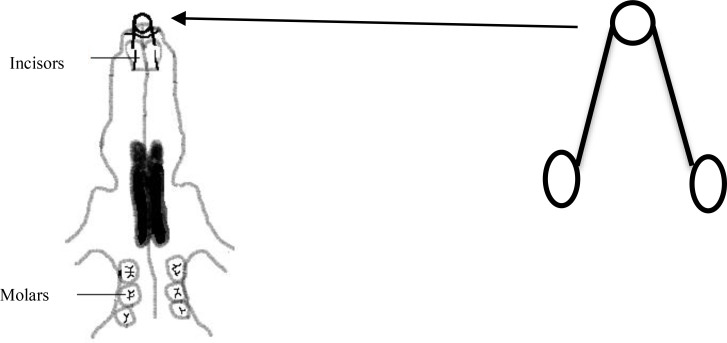
The diet of the case group contained 1wt% vitamin C [L(+)-Ascorbic acid, Acros Organics, Geel, Belgium] which was added to their water supply seven days before spring placement to provide the desired blood level at the onset of tooth movement and this level was maintained until the end of the study. The study was designed and performed according to the international guiding principles for biomedical research involving animals [16] and approved by the Research Center of Hamadan University of Medical Sciences (code #16/35/2785). Rats were anesthetized with an intra-peritoneal injection of ketamine (50mg/Kg) on the seventh day and pre-activated open springs were bonded on the maxillary incisor teeth (3 mm far from the incisal edge) using a self-etch bonding system (Transbond Plus, 3M Unitek, Monrovia, CA, USA) and flowable light-cure composite resin (Heliomolar Flow, Ivoclar Vivadent, Liechtenstein) in both the case and control groups to produce 30 g of opening force calibrated by a gauge (Fig. 1). Springs were made of 0.35 mm stainless steel wire and consisted of two 8 mm arms and a 1.5 turn helix in the middle. Rats were sacrificed by ether overdose on day 17 after bonding the spring. The distance from the mesio-incisal angle of the right incisor to that of the left incisor at the time of spring removal was measured by an expert orthodontist using Boley digital gauge (Digital caliper, Mitutoyo, Aurora, USA). Then the premaxilla was dissected, placed in fixative solution for 10 days (FineFix, Milestone, Fatebenefratelli, Italy) and then in %10 nitric acid for two days. Transverse sections with 3–5μ thickness were made using a microtome (RM 2135, Leica, Nussloch, Germany) at the coronal level of alveolar bone. The best section in each sample was stained with hematoxylin-eosin.
Fig. 1.
Opening spring and its adjustment on rat’s incisor teeth
Number of resorption lacunae in a standard field (1 × 2 mm) at the distal aspect of the incisor root (pressure side) was counted by an expert pathologist using light microscopy at ×100 magnification. Histological sections were re-measured one week later. Bland-Altman plot was applied to assess the reproducibility, and no significant differences were found between the first and second measurements. The Kolmogorov-Smirnov test was used to assess the normal distribution of data, and homogeneity of variance was evaluated using Levene’s test. T-test was also used for data analysis. P<0.05 was considered statistically significant.
RESULTS
None of the samples were excluded from the study. Table 1 shows that the amount of movement in vitamin C group was significantly higher than that in the control group (P<0.001).
Table 1.
Space between the incisors in the case and control groups (mm)
| Group | Number | Mean | Standard Deviation | Standard Error | P value |
|---|---|---|---|---|---|
| Control | 18 | 2.96 | 0.34 | 0.07 | 0.001 |
| Vitamin C | 18 | 3.61 | 0.54 | 0.08 |
Osteoclast count in each group is summarized in Table 2 and it was also significantly higher in the case group (P<0.04).
Table 2.
The number of resorption lacunae in the case and control groups
| Group | Number | Mean | Standard deviation | P |
|---|---|---|---|---|
| Control | 18 | 26.5 | 11.10 | 0.036 |
| Vitamin C | 18 | 36.68 | 14.91 |
DISCUSSION
A variety of factors may affect orthodontic tooth movement such as prostaglandins, progesterone, cAMP, IL-β1 (mediators), calcium, nitric oxide and other neurotransmitters that have been evaluated by many studies [1,4,5,17,18].
Vitamin C, commercially available in the form of a dietary supplement, is known as an important factor in bone remodeling and collagen synthesis [19] and its deficiency can cause complete arrest of osteogenesis, impair the organization of periodontal ligament and increase bone resorption [9,20].
Others have shown that vitamin C deficiency may result in lower orthodontic tooth movement via inhibition of collagen turn over [9,10, 20].
Our study demonstrated greater tooth movement and osteoclast lacunae in the experimental group who received oral vitamin C for 17 days. Litton performed a study on orthodontic tooth movement in animals with ascorbic acid deficiency and concluded that the most significant changes were observed in animals with ascorbic acid deficiency [9].
Mc Canlies et al. [20] evaluated the effect of vitamin C on the mobility and stability of incisors under the influence of orthodontic force in guinea pigs and found that the rate and the amount of separation were approximately the same in the three groups with orthodontic appliances (1 mg daily vitamin C / 0.25 mg daily vitamin C / 0 mg daily vitamin C). But after appliance removal, relapse in the latter two groups was more than in the former group.
The cellular process of osteoclast proliferation has been used as an important indicator to evaluate the extent of tooth movement [21,22]. Maximum osteoclast recruitment occurs 5–14 days after orthodontic force application [23]. On the other hand, tooth movement is a process involving bone resorption and apposition, and vitamin C can induce stem cells to differentiate into osteoblasts via the synthesis of type I collagen, interaction with integrins, activation of protein kinase pathway and phosphorylation of osteoblast-specific transcription factor [19]. Thus, it is expected that tension sites simultaneously experience osteoblastic activity. “Does this process lead to more stable tooth movement in presence of vitamin C?” Further studies are required to answer this question.
De Laurenzi et al, [24] in their study on cultured neuroectodermal cells found that ascorbic acid, in its physiological concentration, acts as a pro-oxidant and causes apoptosis of cells. Some studies reported decreased osteogenesis due to copper deficiency attributed to vitamin C intake [25,26]. According to the above-mentioned findings, it seems that more studies are warranted to better elucidate the long-term effects of vitamin C supplementation. Long-term efficacy of vitamin C supplementation to enhance orthodontic tooth movement and also the stability of tooth movement in this situation must be further investigated as well.
CONCLUSION
Vitamin C supplementation increases orthodontic tooth movement in rats.
REFERENCES
- 1-. Davidovich Z, Shanfeld JL. Cyclic AMP in alveolar bone of orthodontically treated cats. Arch Oral Biol. 1975. September; 20 (9): 567– 74. [DOI] [PubMed] [Google Scholar]
- 2-. King GJ, Keeling SD, McCoy EA, Ward TH. Measuring dental drift and orthodontic tooth movement in response to various initial forces in adult rats. Am J Orthod Dentofacial Orthop. 1991. May; 99 (5): 456– 65. [DOI] [PubMed] [Google Scholar]
- 3-. Yamasaki The role of cyclic AMP, calcium and prostaglandins in induction of Osteoclastic bone resorption in associated with experimental tooth movement. J Dent Res. 1983. August; 62 (8): 877– 81. [DOI] [PubMed] [Google Scholar]
- 4-. Shirazi M, Nilforoushan D, Alghasi H, Dehpour AR. The role of nitric oxide in orthodontic tooth movement in rats. Angle Orthod. 2002. June; 72 (3): 211– 5. [DOI] [PubMed] [Google Scholar]
- 5-. Akin E, Gurton AU, Olmez H. Effect of nitric oxide in orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2004. November; 126 (5): 608– 14. [DOI] [PubMed] [Google Scholar]
- 6-. Otsuka E, Kato Y, Hirose S, Hagiwara H. Role of ascorbic acid in the osteoclast formation: induction of osteoclast differentiation factor with formation of the extracellular collagen matrix. Endocrinology. 2000. August; 141 (8): 3006– 11. [DOI] [PubMed] [Google Scholar]
- 7-. Tsuneto M, Yamazaki H, Yoshino M, Yamada T, Hayashi S. Ascorbic acid promotes osteoclastogenesis from embryonic stem cells. Biochem Biophys Res Commun. 2005. October 7; 335 (4): 1239– 46. [DOI] [PubMed] [Google Scholar]
- 8-. Iwami-Morimoto Y, Yamaguchi K, Tanne K. Influence of dietary n-3 polyunsaturated fatty acid on experimental tooth movement in rats. Angle Orthod. 1999. August; 69 (4): 365– 71. [DOI] [PubMed] [Google Scholar]
- 9-. Litton SF. Orthodontic tooth movement during an ascorbic acid deficiency. Am J Orthod. 1974. March; 65 (3): 290– 302. [DOI] [PubMed] [Google Scholar]
- 10-. Hickory W, Nanda R. Nutritional considerations in orthodontics. Dent Clin North Am. 1981. January; 25 (1): 195– 201. [PubMed] [Google Scholar]
- 11-. Ragab AA, Lavish SA, Banks MA, Goldberg VM, Greenfield EM. Osteoclast differentiation requires ascorbic acid. J Bone Miner Res. 1998. June; 13 (6): 970– 7. [DOI] [PubMed] [Google Scholar]
- 12-. Cui J, Pan YH, Zhang Y, Jones G, Zhang S. Progressive pseudogenization: vitamin C synthesis and its loss in bats. Mol Biol Evol. 2011. February; 28 (2): 1025– 31. [DOI] [PubMed] [Google Scholar]
- 13-. Rutkowski M, Grzegorczyk K. Vitamin C in medicine: “normal concentration” in serum. Pol Merkur Lekarski. 1999. January; 6 (31): 57– 60. [PubMed] [Google Scholar]
- 14-. Cheraskin E, Ringsdorf WM., Jr. Biology of the orthodontic patient: II, lingual vitamin C test scores. Angle Orthod. 1969. October; 39 (4): 324– 5. [DOI] [PubMed] [Google Scholar]
- 15-. Cheraskin E, Ringsdorf WM., Jr. Biology of the orthodontic patient: I, plasma ascorbic acid levels. Angle Orthod. 1969. April; 39 (2): 137– 8. [DOI] [PubMed] [Google Scholar]
- 16-. Bankowski Z. International guiding principles for biomedical research involving animals. 1985. [PubMed]
- 17-. Poosti M, Basafa M, Eslami N. Progesterone effects on experimental tooth movement in rabbits. J Calif Dent Assoc. 2009. July; 37 (7): 483– 6. [PubMed] [Google Scholar]
- 18-. Kale S, Kocaderli I, Atilla P, Asan E. Comparison of the effects of 1, 25 dihydroxycholecalciferol and Prostaglandin E2 on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2004. May; 125 (5): 607– 14. [DOI] [PubMed] [Google Scholar]
- 19-. Ishikawa S, Iwasaki K, Komaki M, Ishikawa I. Role of ascorbic acid in periodontal ligament cell differentiation. J Periodontol. 2004. May; 75 (5): 709– 16. [DOI] [PubMed] [Google Scholar]
- 20-. McCanlies JM, Alexander CM, Robnett JH, Magness WB. Effect of vitamin C on the mobility and stability of guinea pig incisors under the influence of orthodontic force. Angle Orthod. 1961. October; 31 (4): 257– 263. [Google Scholar]
- 21-. Bakdash MB, Zaki HA. The impact of diet and nutrition on periodontal health. Northwest Dent. 1978. Jan-Feb; 57 (1): 5– 14. [PubMed] [Google Scholar]
- 22-. Mavragani M, Brudvik P, Selvig KA. Orthodontically Induced root and alveolar bone resorption: inhibitory effect of systemic doxycycline administration in rats. Eur J Orthod. 2005. June; 27 (3): 215– 25. [DOI] [PubMed] [Google Scholar]
- 23-. Kaku M, Kohno S, Kawata T, Fujita I, Tokimasa C, Tsutsui K, et al. Effects of vascular endothelial growth factor on osteoclast induction during tooth movement in mice. J Dent Res. 2001. October; 80 (10): 1880– 3. [DOI] [PubMed] [Google Scholar]
- 24-. De Laurenzi V, Melino G, Savini I, Annicchiarico-Petruzzelli M, Finazzi-Agrò A, Avigliano L. Cell death by oxidative stress and ascorbic acid regeneration in human neuroectodermal cell lines. Eur J Cancer. 1995; 31A (4): 463– 6. [DOI] [PubMed] [Google Scholar]
- 25-. Van den Berg GJ, Yu S, Lemmens AG, Beynen AC. Dietary ascorbic acid lowers the concentration of soluble copper in the small intestinal lumen of rats. Br J Nutr. 1994. May; 71 (5): 701– 7. [DOI] [PubMed] [Google Scholar]
- 26-. Strause L, Saltman P, Glowacki J. The effect of deficiencies of manganese and copper on osteoinduction and on resorption of bone particles in rats. Calcif Tissue Int. 1987. September; 41 (3): 145– 50. [DOI] [PubMed] [Google Scholar]