Summary
The anti‐inflammatory role of heme oxygenase‐1 (HO‐1) has been studied extensively in many disease models including asthma. Many cell types are anti‐inflammatory targets of HO‐1, such as dendritic cells and regulatory T cells. In contrast to previous reports that HO‐1 had limited effects on basophils, which participate in T helper type 2 immune responses and antigen‐induced allergic airway inflammation, we demonstrated in this study, for the first time, that the up‐regulation of HO‐1 significantly suppressed the maturation of mouse basophils, decreased the expression of CD40, CD80, MHC‐II and activation marker CD200R on basophils, blocked DQ‐ovalbumin uptake and promoted basophil apoptosis both in vitro and in vivo, leading to the inhibition of T helper type 2 polarization. These effects of HO‐1 were mimicked by exogenous carbon monoxide, which is one of the catalytic products of HO‐1. Furthermore, adoptive transfer of HO‐1‐modified basophils reduced ovalbumin‐induced allergic airway inflammation. The above effects of HO‐1 can be reversed by the HO‐1 inhibitor Sn‐protoporphyrin IX. Moreover, conditional depletion of basophils accompanying hemin treatment further attenuated airway inflammation compared with the hemin group, indicating that the protective role of HO‐1 may involve multiple immune cells. Collectively, our findings demonstrated that HO‐1 exerted its anti‐inflammatory function through suppression of basophil maturation and activation, but promotion of basophil apoptosis, providing a possible novel therapeutic target in allergic asthma.
Keywords: allergic airway inflammation, basophil, heme oxygenase‐1, T helper type 2 response
Abbreviations
- APC
allophycocyanin
- BMBs
bone marrow‐derived basophils
- CO
carbon monoxide
- DT
diphtheria toxin
- HO‐1
heme oxygenase‐1
- IL‐4
interleukin‐4
- MLNs
mediastinal lymph nodes
- OVA‐sIgE
ovalbumin‐specific immunoglobulin E
- PE
phycoerythrin
- Sn‐PP
Sn‐protoporphyin
- Tg
transgenic
- Th2
T helper type 2
Introduction
Heme oxygenase (HO) is a rate‐limiting enzyme in heme catabolism, catalysing the decomposition of heme to equal amounts of carbon monoxide (CO), biliverdin and free iron, whereas HO‐1, an inducible isoform of HO, is regarded as a protective heat‐shock protein1 and has important anti‐inflammatory functions.2, 3, 4, 5 A large number of studies have shown that HO‐1 played extensive protective roles in inflammatory diseases such as asthma,4, 6, 7, 8, 9 inflammatory bowel disease10, 11, 12, 13 and uveitis.14, 15 In addition, HO‐1 exerts its effects on multiple immune cells, such as promoting maturation, differentiation and function of dendritic cells,16, 17 inhibiting the antigen‐presenting function of dendritic cells,18 inducing tolerance of dendritic cells19 and suppressing degranulation of mast cells.20, 21 In previous studies, HO‐1 was found to enhance the function of CD4+ CD25+ regulatory T cells.13, 22 Recent studies have shown that the expression of HO‐1 is significantly up‐regulated in T helper type 2 (Th2) ‐mediated allergic diseases23, 24, 25, 26 and further induction of HO‐1 expression could significantly inhibit allergic inflammation.4, 6, 22, 27, 28
Recently, there has been great progress in research into the biological functions of basophils in allergic diseases. It has been shown that FceRIα + CD49b+ c‐Kit− basophils act as antigen‐presenting cells by taking up and processing antigens in helminth infection or after papain injection.29, 30, 31, 32 In addition, basophils express MHC class II and co‐stimulatory molecules, as well as secreting interleukin‐4 (IL‐4) and thymic stromal lymphopoietin,30, 31, 32, 33 which are critical checkpoints in the development and regulation of Th2 cell immunity. Moreover, depletion of circulating basophils can significantly inhibit Th2 immune responses in animal models of parasite infection and allergic dermatitis.29, 34 The important role of basophils in allergic airway inflammation has also been identified in our previous study.35 Moreover, it has been reported that both hemin and exogenous CO significantly raised cGMP levels in basophils and inhibited IgG anti‐Fcε (anti‐IgE)‐induced activation of basophils.36 As CO is one of the catalytic products of HO‐1, the involvement of HO‐1 in regulating the behaviours of basophils needs to be further investigated.
Our hypothesis is that HO‐1 expressed by basophils can suppress allergic airway inflammation by regulating basophil functions. In this study, we demonstrated that bone‐marrow‐derived basophils (BMBs) express HO‐1; furthermore, up‐regulated HO‐1 expression inhibits the maturation and activation of basophils, but promotes their apoptosis, suggesting a novel effect of HO‐1 to regulate basophil functions and attenuate Th2 immune responses.
Materials and methods
Mouse sensitization, challenge and hemin or Sn‐protoporphyrin IX treatment
Six‐ to 8‐week‐old BALB/c mice (Shanghai Laboratory Animal Company, Shanghai, China), DO11.10 mice (The Jackson Laboratory, Bar Harbor, ME, USA) and Bas‐TRECK transgenic (Tg) mice (a kind gift from Riken Yokohama Branch Centre for Integrative Medical Sciences) were used for the experiments. All mice were maintained in the specified pathogen‐free facilities in the Research Centre for Experimental Medicine of Ruijin Hospital and all animal procedures were approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. An asthmatic mouse model was established as described previously.35 Mice received intraperitoneal injections of 100 μg (0·2 ml) of ovalbumin (OVA; Sigma‐Aldrich, St Louis, MO) dissolved in aluminium hydroxide adjuvant suspension on days 0 and 14. On day 14, 50 μl normal saline containing 2 mg/ml OVA was administered intranasally under isoflurane inhalation anaesthesia. Then, the mice were challenged on days 23, 24 and 25 with intranasal administration of 50 μl normal saline containing 2 mg/ml OVA to initiate allergic airway inflammation (OVA group). The control group was sensitized with 0·2 ml aluminium hydroxide adjuvant suspension and challenged with PBS instead of OVA. All mice were killed on day 26. Hemin (Sigma‐Aldrich) or Sn‐protoporphyrin IX (SnPP) (Frontier Scientific Inc., Logan, UT) was administered as described previously.20 Ovalbumin‐sensitized and ‐challenged mice received an intraperitoneal. injection of 75 μmol/kg hemin or hemin plus 75 μmol/kg SnPP on days –2, –1, 12, 13, 21, 22 and 25 in hemin and hemin plus SnPP groups, respectively. Hemin or SnPP was dissolved in 0·1 mol/l NaOH, diluted with PBS and titrated to pH 7·5 with 0·2 mol/l HCl. A total volume of 0·2 ml was used for intraperitoneal. injection. To examine basophil antigen uptake, OVA immunized animals were anaesthetized with isoflurane and given 30 μg DQ‐OVA (Invitrogen, Carlsbad, CA) in 50 μl PBS intranasally on day 23, and killed 4 hr later.
Tissue harvest and preparation of single‐cell suspension
Twenty‐four hours after the last OVA challenge, mice were killed by CO2 inhalation, a blood sample was collected for OVA‐specific IgE (OVA‐sIgE) detection. The lung was perfused by heart puncture with 10 ml of cold PBS. The inferior lobe of the right lung was fixed in buffered 4% formaldehyde solution for histology analysis, and the other parts were minced and digested by collagenase IV (Sigma‐Aldrich) in an incubator at 37° with 5% CO2 for 45 min, and then passed through a 70‐μm cell strainer to obtain a single‐cell suspension. Mediastinal lymph nodes (MLNs) were harvested in RPMI‐1640 medium. Single‐cell suspensions were prepared by grinding lymph nodes against a 70‐μm cell strainer and treated with erythrocyte lysis buffer to remove red blood cells, and further used for basophil purification and detection. To detect the Th1/Th2 cell population in MLN, cells were plated in 24‐well plates at a density of 5 × 106 cells/ml and were stimulated with 500 μg/ml OVA for 5 days. Experiments were performed three times using five animals of each group.
Enzyme‐linked immunosorbent assay
An ELISA for OVA‐sIgE was performed as described before.20 Briefly, 100 μl serum (diluted 1 : 10) or standard mouse OVA‐sIgE (Serotec, Kidlington, UK) were added into 0·01% OVA pre‐coated plates followed by adding 100 μl horseradish peroxidase‐conjugated goat anti‐mouse IgE antibody (1 : 2000; Serotec) for 1 hr. Then, 100 μl 3,3′,5,5′‐tetramethylbenzidine reagent was added for 20 min (Jingmei, Yanchen, Jiangshu, China). The reaction was stopped by 100 μl 1 mol/l sulphuric acid. The absorbance at 450 nm in each well was obtained using a plate‐reader (Bio‐Rad, Hercules, CA) and serum OVA‐sIgE concentration was calculated against the standard curve. The concentration of IL‐4 in serum and supernatants was also examined by ELISA according to the manufacturer's protocols (BioLegend, San Diego, CA).
Flow cytometery
Basophils and surface expression of co‐stimulatory molecules were detected by flow cytometry. Cultured BMBs or lung‐tissue‐derived basophils were blocked with anti‐FcγRII/III (eBioscience, San Diego, CA), then phycoerythrin (PE) Cy7‐anti‐FcεRIα, allophycocyanin (APC) ‐anti‐c‐kit (BioLegend) and FITC‐anti‐CD49b (eBioscience) were used to identify basophils. The PE‐labelled anti‐CD40, anti‐CD80 and anti‐CD1Ad antibodies (BioLegend) were added to determine the surface expression of co‐stimulatory molecules; PE‐anti‐CD200R (BioLegend) was used to examine basophil activation. Briefly, single cells from lung tissues or BMBs were first treated for 30 min at 4° with 10 μg/ml anti‐FcγRII/III followed by treatment for 1 hr at 4° with fluorescence‐labelled anti‐mouse FcεRIα, c‐Kit and CD49b antibodies or together with one of following fluorescence‐labelled anti‐mouse CD40, CD80, MHC II, HO‐1 and CD200R in 100 μl staining buffer (5% fetal bovine serum in PBS). After being washed twice, samples were analysed on a FACS Calibur (BD Biosciences, San Jose, CA, USA).
To detect DQ‐OVA uptake by basophils, lung derived single‐cell suspensions from DQ‐OVA‐challenged mice or DQ‐OVA‐cultured BMBs treated with and without hemin or hemin plus SnPP were stained with PE Cy7‐anti‐FcεRIα, APC‐anti‐c‐kit and PE‐anti‐CD49b antibodies to examine the uptake of DQ‐OVA. Apoptosis of basophils was detected using Annexin V‐FITC Apoptosis Detection Kit (eBioscience) according to the manufacturer's protocols. Single cells from lung tissues and BMBs were stained PE Cy7‐anti‐FcεRIα, APC‐anti‐CD49b and FITC‐anti‐Annexin V in staining buffer. Propidium Iodide (PI) was added 10 min before analysis by flow cytometry. Flow cytometry results were analysed using cellquest software (BD Pharmingen, San Diego, CA).
Lung histology
The lungs were fixed in 4% buffered formaldehyde overnight and embedded in paraffin. Lung sections of 5 μm were stained with haematoxylin and eosin and examined using light microscopy (Olympus, Tokyo, Japan).
BMB culture and HO‐1 or CO‐releasing molecule interference
Culture of BMB was performed as described previously.33 Briefly, bone marrow cells from normal mice were cultured for 10 days with 10 ng/ml IL‐3 (R&D Systems, Minneapolis, MN) in complete RPMI‐1640 medium supplemented with 10% fetal bovine serum, 50 μmol/l 2‐mercaptoethanol, 2 mmol/l l‐glutamine, 100 U/ml penicillin and 100 U/ml of streptomycin. To study the effect of HO‐1 on basophil maturation, hemin was added at concentrations of 10, 20 and 30 μmol/l to induce HO‐1 expression. In the hemin plus SnPP group, 30 μmol/l hemin plus 30 μmol/l SnPP were used to inhibit HO‐1 enzyme activity, respectively. All media were changed every 48 hr. Maturation of basophils was determined by the percentage of basophils after bone marrow culture according to the surface markers CD49b, c‐kit and FcεRIα. To further study the HO‐1 affecting the expression of basophil surface markers, cultured BMBs were treated for an additional 48 hr with or without 30 μmol/l of hemin or hemin plus 30 μmol/l SnPP. The concentration of bilirubin in BMBs cultured medium was calculated by spectrophotometry at a wavelength of 438 nm. To investigate whether CO, one of the main catalytic products of HO‐1, exerted a similar role on basophils, basophils were treated with an exogenous CO‐releasing molecule (Sigma‐Aldrich) at concentrations of 5, 25 and 50 μmol/l.
Th2 cell polarization
Splenic naive T cells from DO11.10 mice were purified using a MagCellect kit from R&D Systems according to the manufacturer's instructions. Th2 cell polarization were performed as described elsewhere.32, 33 Briefly, 1 × 106 naive T cells were co‐cultured with 0·5 × 106 sorted and hemin or hemin plus SnPP pre‐treated BMBs in 48‐well plates in the presence of 20 U/ml IL‐2 (R&D Systems), 10 ng/ml IL‐3, 100 μg/ml Dinitrophenyl (DNP)‐OVA (Biosearch Technologies, Novato, CA), and 10 μg/ml anti‐DNP IgE (Sigma‐Aldrich). As control, 1 × 106 naive T cells alone were used. Fresh complete RPMI‐1640 medium with 10 U/ml IL‐2 was added at day 3. After 5 days of culture, cells were stimulated by 20 ng/ml PMA (Sigma‐Aldrich) and 1 μg/ml ionomycin (Sigma‐Aldrich) for a further 6 hr. Two hours before collection, Brefeldin A (eBioscience) was added into the culture medium at the ratio of 1 : 1000. The collected cells were surface stained with FITC‐anti‐CD4 antibody (BD Pharmingen). After washing, fixing and permeabilizing, cells were intracellularly stained with PE‐anti‐IL‐4 and APC‐anti‐interferon‐γ antibodies (eBioscience), and examined with flow cytometry to detect the Th1/Th2 subsets.
Immunocytochemistry of HO‐1 expression in basophils
To further confirm the HO‐1 expression in basophils, sorted BMBs were further treated with of hemin or hemin plus SnPP for 48 hr in the presence of 10 ng/ml IL‐3. Basophils were collected and were washed twice. Cytospin was performed. After fixation and permeabilization, basophils were intracellularly stained with rabbit anti‐mouse HO‐1 primary antibody (Sigma‐Aldrich), followed by further staining of horseradish peroxidase‐conjugated anti‐rabbit IgG (Sigma‐Aldrich). Expression of HO‐1 was visualized by adding DAB and stained for counting using haematoxylin (Sigma‐Aldrich). Slides were examined with an Olympus microscope (×40 magnification).
RT‐PCR detection of HO‐1 mRNA
The expression of HO‐1 mRNA was detected by real‐time PCR. Briefly, total RNA was extracted from FACS‐purified BMBs using an RNeasy Mini kit (Qiagen, Valencia, CA). First‐strand cDNA synthesis was accomplished with an Omniscript Reverse Transcriptase Kit (Qiagen). The expression of HO‐1 and β‐actin was amplified by real‐time PCR using primer pairs (HO‐1 sense, 5′‐CCC ACC AAG TTC AAA CAG CTC‐3′, and HO‐1 antisense, 5′‐AGG AAG GCG GTC TTA GCC‐3′; β‐actin sense, 5′‐ATG CCA ACA CAG TGC TGT CT‐3′, and antisense, 5′‐AAG CAC TTG CGG TGC ACG AT‐3′). The real‐time PCR was performed using an RT2 Real‐time PCR Master Mix (SABiosciences, Frederick, MD) for 40 cycles at 95° for 15 seconds, 55° for 40 seconds and 72° for 30 seconds. The mRNA level of HO‐1 in each sample was normalized to β‐actin mRNA and quantified using the 2−ΔΔCt method. The result was expressed as fold difference between hemin, hemin+SnPP treatment groups and control groups.
Basophil conditional depletion and adoptive transfer
To confirm the HO‐1 protective role in basophil‐mediated OVA‐induced allergic airway inflammation, diphtheria toxin (DT) ‐mediated basophil‐specific depletion and basophil adoptive transfer were performed as described. Bas‐TRECK Tg mice were given intraperitoneal injection of 750 ng/20 g bodyweight DT (Sigma‐Aldrich) in 200 μl PBS daily for two consecutive days before OVA sensitization and challenge to conditionally deplete basophils. The control mice were treated with PBS. The mice treated with DT had a transient depletion of basophils, whereas the majority of mast cells remained unaffected during the treatment.37 Flow cytometry was performed to detect the percentage of basophils in peripheral blood and bone marrow. Bas‐TRECK Tg mice were immunized with OVA and treated by hemin as described above, and divided into five groups including a control group (PBS treatment and aluminium hydroxide sensitization and PBS challenge), OVA group (PBS treatment and OVA sensitization and challenge), basophil depletion group (DT treatment and OVA sensitization and challenge), hemin group (PBS+hemin treatment and OVA sensitization and challenge) and hemin+DT group (DT+hemin treatment and OVA sensitization and challenge). Mice were killed on day 26.
For adoptive transfer of basophils, cultured BMBs from normal BALB/c mice were purified by FACSAria according to its cell surface markers (FcεR1+ CD49b+ c‐kit− CD11c− cells) and confirmed morphologically using Wright–Giemsa staining. Purified basophils were treated with 30 μmol/l hemin, SnPP or hemin plus SnPP for 48 hr and were further treated with 500 μg/ml OVA, 100 μg/ml DNP‐OVA (Biosearch Technologies), and 10 μg/ml anti‐DNP IgE (Sigma‐Aldrich) for 4 hr to activate basophils. The basophils were washed four times with PBS and re‐suspended in PBS at 2·5 × 106/ml. The adoptive transfer model was established as described previously.9 Briefly, 2·5 × 105 basophils treated with hemin, SnPP or hemin plus SnPP or 2·5 × 105 FcεR1− CD49b− CD11c− cells (control group) in 200 μl PBS were delivered to naive BALB/c mice via intraperitoneal injection on day 0 and day 14. On days 21, 22 and 23 after adoptive transfer, BALB/c mice were challenged with 100 μg OVA in 50 μl normal saline intranasally under isoflurane inhalation anaesthesia. Mice undergoing a PBS tail vein injection on day 0 and intranasal challenge with normal saline were regarded as the control group. Mice were killed on day 24.
Statistical analysis
Experiments were repeated more than three times in vivo and in vitro. Five mice were used in each group for in vivo experiments and more than three samples were calculated in each group of the in vitro experiments. Data were presented as mean ± SEM and analysed with SPSS 16.0 software (SPSS Inc, Chicago, IL, USA). The comparison among groups was performed with an independent‐samples t‐test or analysis of variance. P < 0·05 was considered statistically significant.
Results
Hemin interference induced expression of HO‐1 in basophils and increased bilirubin production
HO‐1 is an inducible enzyme in various cell types in response to stress such as oxidative stress, heavy metals, cytokines, etc. According to others and our previous studies, as the substrate of HO‐1, hemin can significantly induce HO‐1 expression and enhance its activity, whereas SnPP can also increase HO‐1 expression but significantly inhibits HO‐1 activity. These two chemicals were widely used in many experiments. Therefore, we examined the inducible expression of HO‐1 in BMBs derived from normal BALB/c mice treated with hemin or hemin plus SnPP. Cultured BMBs were sorted by flow cytometry, the purity was > 95%. Sort‐purified BMBs were further treated with or without hemin and hemin plus SnPP for 48 hr in an IL‐3 environment. Real‐time PCR revealed that both hemin and hemin plus SnPP treatment significantly enhanced HO‐1 mRNA expression (Fig. 1a left). Flow cytometry analysis showed that 47% cultured BMBs expressed HO‐1, but > 91% and 71% BMBs were cells positive for HO‐1 after treatment with hemin and hemin plus SnPP, respectively (gated on the FcεRIα + CD49b+c‐kit− subset) (Fig. 1a middle and b). Next, we investigated whether hemin promoted HO‐1 activity; the concentration of bilirubin, which is one of the main metabolites of HO‐1, was detected after different doses of hemin were added to cultured BMBs. As showed in Fig. 1(a), treatment with different doses of hemin significantly increased bilirubin levels in medium after 48 hr compared with controls, especially in 30 μmol/l hemin treatment (Fig. 1a right). Immunocytochemistry staining further confirmed HO‐1 expression in sorted BMBs (Fig. 1c). These results indicated that hemin can induce HO‐1 expression, and increase its activity to produce catalytic products such as bilirubin.
Figure 1.
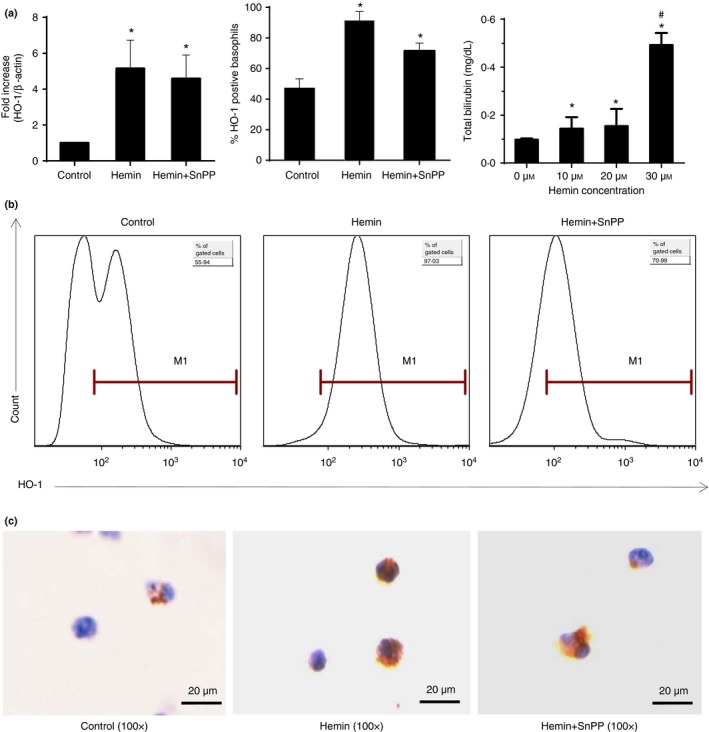
Hemin interference induced expression of HO‐1 in basophils and increased bilirubin production. Cultured and FACS‐purified bone‐marrow‐derived basophils BMBs from BALB/c mice were further treated with 30 μmol/l of hemin or hemin plus SnPP for 48 hr in the presence of 10 ng/ml interleukin‐3 (IL‐3). Cells without hemin and SnPP treatment were control groups. The concentration of bilirubin was determined after 48 hr of culture. RT‐PCR, flow cytometry and immunocytochemistry were performed for detecting HO‐1 expression in basophils. (a) Left, HO‐1 expression from purified basophils by RT‐PCR, the level of HO‐1 mRNA was normalized to β‐actin. Data are presented as fold change in hemin and hemin plus SnPP groups compared with the control group (*P < 0·05, versus control group); Middle, the percentage of HO‐1 positive basophils (*P < 0·05, versus control group); Right, the concentration of bilirubin in medium (*P < 0·05, versus 0 μmol/l group, #P < 0·01 30 μmol/l group versus 10 μmol/l group and 20 μmol/l group). (b) Intracellular staining for HO‐1 in cultured BMBs (gated on Fcε RI α + CD49b+ c‐kit− subset). (c) Immunocytochemistry staining for HO‐1 in purified basophils, scale bar, 20 μm (DAB stain, 100×). Data are representative of three independent experiments.
HO‐1 and exogenous CO inhibit the basophil maturation and promote its apoptosis in vitro
To determine whether HO‐1 affected basophil maturation and activation, bone marrow cells from normal mice were cultured for 10 days with 10 ng/ml IL‐3 in complete RPMI‐1640 medium and treated with different concentrations of hemin or hemin plus SnPP. The percentage of cultured basophils and their expression of Annexin V were evaluated by flow cytometry. As shown in Fig. 2, up‐regulation of HO‐1 by hemin significantly reduced the percentage of basophils (gated on FcεRIα + CD49b+ c‐kit− subset) but increased the basophil apoptosis in a dose‐dependent manner (gated on FcεRIα + CD49b+ PI− subset), whereas inhibition of HO‐1 activity by SnPP remarkably reversed the above effect (Fig. 2a right and b left and middle). Next, we investigated whether CO, a main catalytic product of HO‐1, was able to mimic the effect of HO‐1 on basophils. Basophils were treated with exogenous CO‐releasing molecule at concentrations of 5, 25 and 50 μmol/l. The results showed that exogenous CO enhanced basophil apoptosis in a dose‐dependent manner, similar to hemin treatment (Fig. 2b right), indicating that HO‐1 exerts its effect on basophils through CO.
Figure 2.
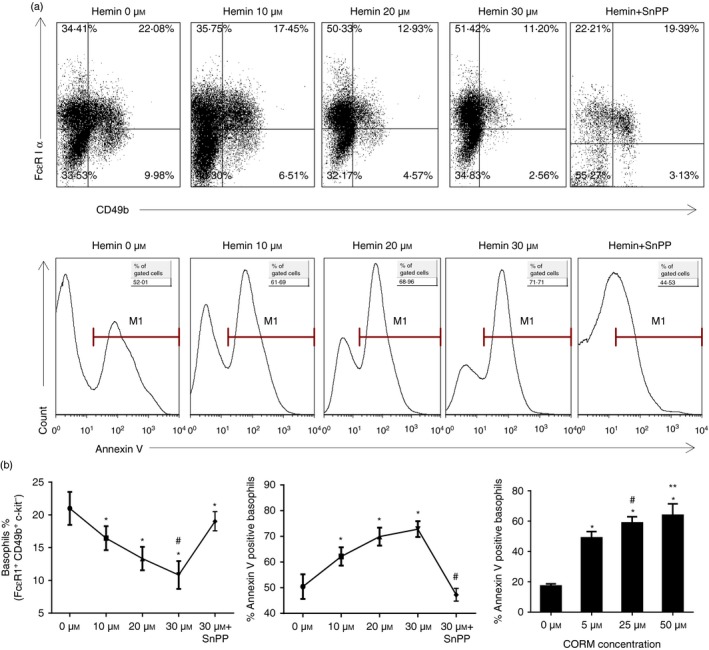
HO‐1 and exogenous CO inhibited basophil maturation and promoted their apoptosis in vitro. Bone marrow cells from normal BALB/c mice were cultured for 10 days with IL‐3. Different doses of hemin (0, 10, 20 and 30 μmol/l) or CORM (0, 5, 25, 50 μmol/l) were added. In the hemin plus SnPP group, 30 μmol/l hemin and 30 μmol/l SnPP were used respectively. Flow cytometry analysis was performed to detect the percentage and apoptosis of basophils. (a) Flow cytometeric analysis of the percentage of basophils in culture under different concentrations of hemin and hemin plus SnPP (gated on c‐kit− basophil‐enriched population; upper panel); Annexin V expression in basophils under different concentrations of hemin and hemin plus SnPP (gate on Fcε RI α + CD49b+ PI − subset; low panel). (b) Left, the percentage of basophils (gated on Fcε RI α + CD49b+ c‐kit− subset (*P < 0·05, versus 0 μmol/l group; #P < 0·05, versus hemin+SnPP group, respectively); Middle, the percentage of Annexin V expression in basophils (gate on Fcε RI α + CD49b+ PI − subset; *P < 0·05, versus 0 μmol/l group; #P < 0·05, versus 10, 20 and 30 μmol/l group, respectively); Right, the percentage of Annexin V expression in basophils (gate on Fcε RI α + CD49b+ PI − subset; (*P < 0·05, versus 0 μmol/l group, respectively; #P < 0·05, 25 μmol/l group versus 5 μmol/l group; **P < 0·05, 25 μmol/l group versus 50 μmol/l group). Data are representative of three independent experiments.
HO‐1 affects the maturation and activation of basophils in vitro
Next, we determined the effect of HO‐1 on the maturation and activation of basophils by checking the expression of basophil surface markers. Bone marrow cells from normal BALB/c mice were cultured in the same protocol. After 10 days of incubation, flow cytometry was performed to assess the expression of CD80, CD40 and MHC II and activation marker CD200R on basophils. As shown in Fig. 3, up‐regulation of HO‐1 decreased the expression of co‐stimulatory molecules (CD80, CD40 and MHC II) and activation marker CD200R on basophils to various degrees, but inhibition of HO‐1 activity by SnPP significantly reversed the effect of hemin (Fig. 3).
Figure 3.
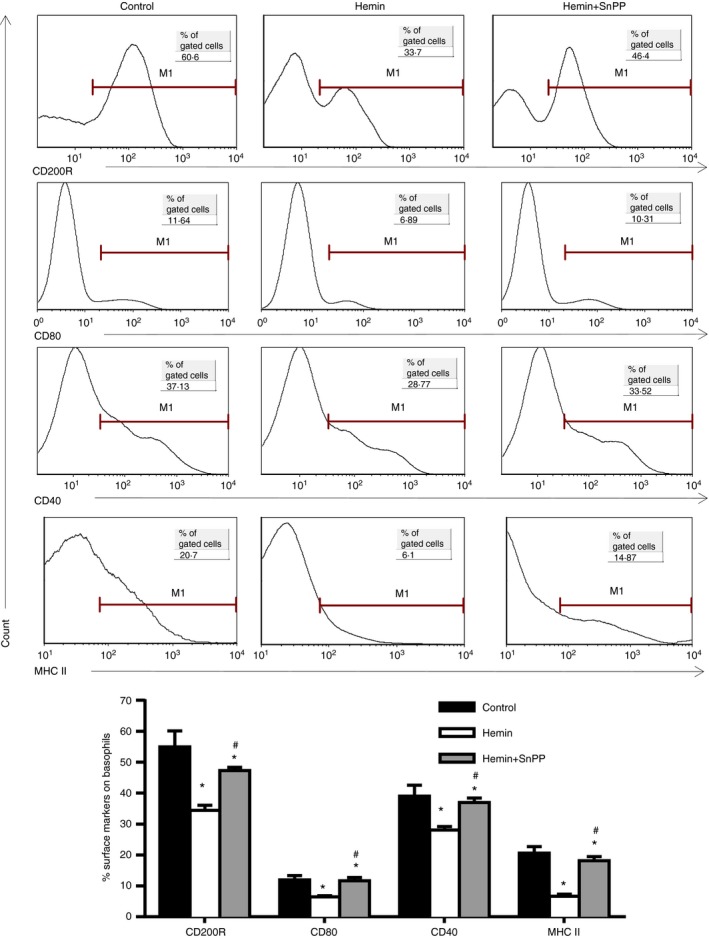
HO‐1 inhibited the expression of CD200R and co‐stimulatory molecules on basophils in vitro. Bone marrow cells from normal BALB/c mice were cultured for 10 days in complete RPMI‐1640 medium with the addition of IL‐3. Hemin and hemin+ SnPP were added at concentrations of 30 μmol/l, respectively. Expression of CD200R, CD40, CD80 and MHC II on basophils was determined by flow cytometry (gated on Fcε RI α + CD49b+ c‐kit− subset; *P < 0·05, versus control group; #P < 0·05, hemin+SnPP group versus hemin group, respectively). Data are representative of three independent experiments.
HO‐1 inhibits OVA uptake, IL‐4 release and basophil‐induced Th2 polarization
Basophils were regarded as one of the professional antigen‐presenting cells and the source of ‘early’ IL‐4. Here we investigated whether HO‐1 could affect the antigen uptake and IL‐4 releasing ability of basophils, resulting in decreased Th2 polarization. DQ‐OVA is a self‐quenched conjugate of OVA that exhibits green fluorescence on proteolytic degradation to single dye‐labelled peptides in the cells. It is regarded as a good marker of antigen processing. As shown in Fig. 4, cultured BMBs could actively take up DQ‐OVA (Fig. 4a and b left), release IL‐4 upon DNP‐OVA and anti‐DNP IgE stimulation (Fig. 4b middle) and polarize naive T cells from spleen of DO11.10 mice to the Th2 subset without exogenous IL‐4 (Fig. 4b right and c). However, up‐regulation of HO‐1 by hemin significantly inhibited the uptake by BMBs of DQ‐OVA (Fig. 4a and b left), releasing IL‐4 triggered by DNP‐OVA and anti‐DNP IgE stimulation (Fig. 4b middle), and prevented Th2 polarization (Fig. 4b right and c).
Figure 4.
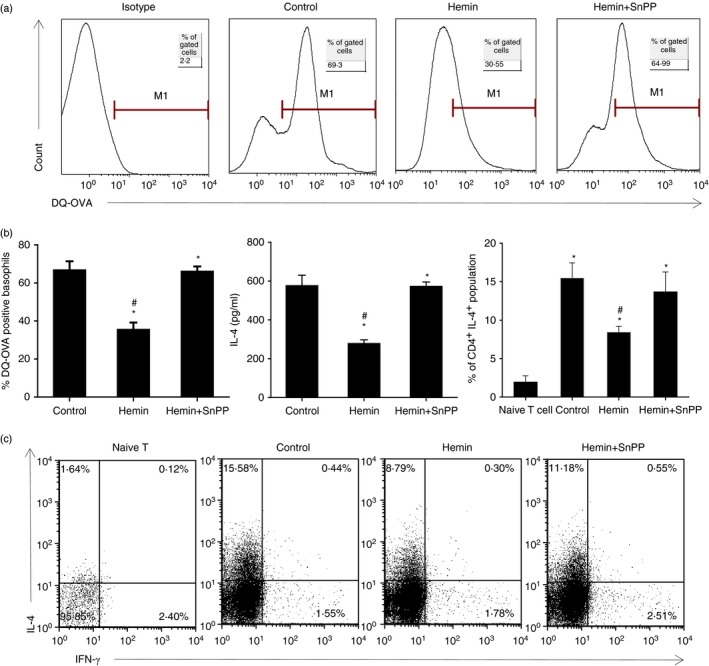
HO‐1 inhibited OVA uptake, IL‐4 releasing and basophil‐induced Th2 polarization in vitro. (a) Purified BMBs were further treated with or without hemin and hemin plus SnPP at the concentration of 30 μmol/l for 48 hr, respectively, then cultured for a further 4 hr with DQ‐OVA. DQ‐OVA taken up by basophils was examined using flow cytometry (gated on Fcε RI α + CD49b+ c‐kit− subset). (b) Left, the percentage of DNP‐OVA‐positive basophils (*P < 0·05, versus control group); Middle, IL‐4 level of sort‐purified BMBs treated with hemin or hemin plus SnPP and stimulated with anti‐DNP‐IgE in the presence of IL‐3 for 16 hr (*P < 0·05, versus control group); Right, the percentage of CD4+ IL‐4+ population (*P < 0·05, versus naive T‐cell group; #P < 0·05, versus control group without hemin or SnPP treatment). (c) Purified BMBs from BALB/c mice were treated with hemin or hemin plus SnPP and further co‐cultured with MACS‐screened splenic naive T cells (DO11.10) in the presence of DNP‐OVA, anti‐DNP IgE, IL‐3 and IL‐2 for 5 days. Intracellular staining of IL‐4 and interferon‐γ were performed and the Th2 subset (CD4+ IL‐4+) was determined by flow cytometry. Data are representative of three independent experiments.
Up‐regulation of HO‐1 attenuates Th2 immune responses and OVA‐induced allergic airway inflammation
Our previous studies proved that basophils exerted an important role in OVA‐induced allergic inflammation and HO‐1 displayed its protective function in the same animal model. So we explored whether HO‐1 can affect immune characteristics of basophils in vivo. The OVA‐induced allergic airway inflammation animal model was established as previous described. In the hemin or hemin plus SnPP groups, mice were pre‐treated with hemin or hemin plus SnPP 2 days before OVA immunization and challenge, respectively (Fig. 5a). Lung tissue derived basophils were investigated by flow cytometry. Compared with the OVA group, hemin‐treated mice displayed significantly fewer OVA‐specific Th2 subsets in MLNs (Fig. 5b and c left), decreased serum IgE level (Fig. 5c right), total cell number and eosinophil number in bronchoalveolar lavage fluid (Fig. 5d left) and IL‐4 level in lung tissue (Fig. 5d right), and attenuated allergic airway inflammation (Fig. 5e). The inhibitory role of HO‐1 can be reversed by addition of SnPP.
Figure 5.
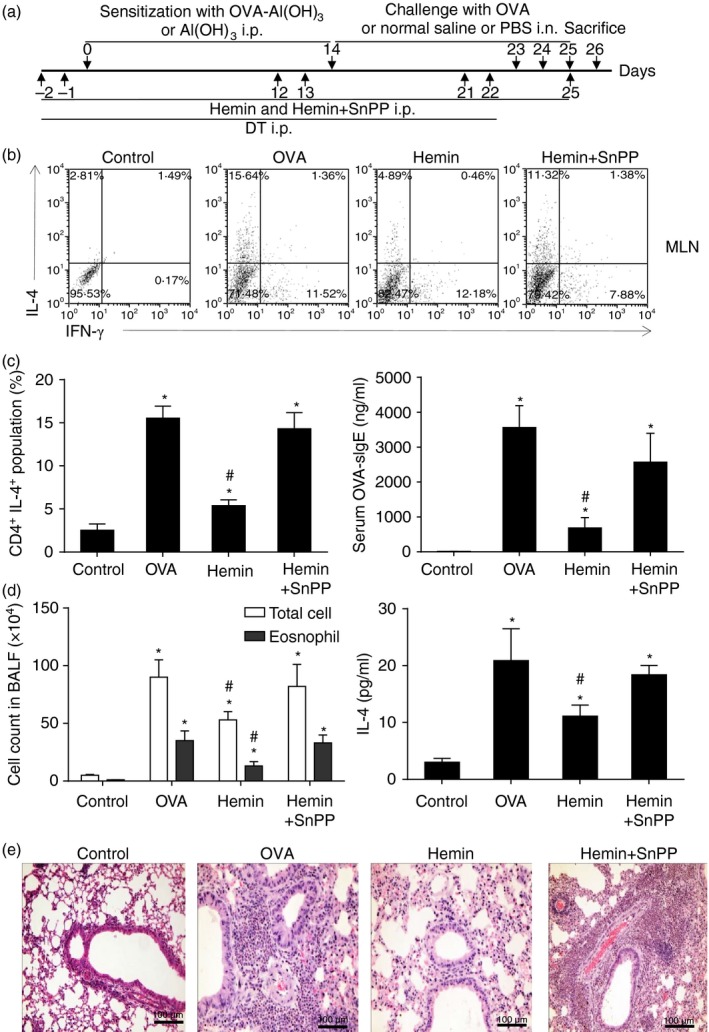
Up‐regulation of HO‐1 attenuated Th2 immune responses and OVA ‐induced allergic airway inflammation. (a) The schematic diagram of OVA‐induced asthmatic mouse model and hemin or SnPP administration. (b) Isolated cells from mediastinal lymph nodes (MLNs) were cultured with 500 μg/ml OVA for 5 days followed by intracellular staining of IL‐4 and interferon‐γ (IFN‐γ). Th1/Th2 subsets were assessed by flow cytometry (gated on CD4+ cells). (c) Left, the percentage of CD4+ IL‐4+ population from MLNs (*P < 0·05, versus control group; #P < 0·05, versus OVA group); Right, serum OVA‐sIgE level was quantified by ELISA (*P < 0·05, versus control group; #, P < 0·05, versus OVA group). (d) Left, total cell numbers count and eosinophil numbers count in bronchoalveolar lavage fluid (*P < 0·05, versus control group; #P < 0·05, versus OVA group); Right, IL‐4 level in lung tissue were quantified by ELISA (*P < 0·05, versus control group; #P < 0·05, versus OVA group). (e) Pathological change of lung tissue, scale bar, 100 μm (haematoxylin & eosin stain, 40×). Data are representative of three independent experiments.
HO‐1 reduced allergic airway inflammation invoked by adoptive transfer of basophils
To further evaluate HO‐1's protective role in basophil‐mediated OVA‐induced allergic airway inflammation, adoptive transfer of HO‐1‐modified basophils was performed as described. FACS‐purified FcεR1+ CD49b+ c‐kit− CD11c− basophils from BMBs of normal BALB/c mice were treated with 30 μmol/l hemin, SnPP or hemin plus SnPP for 48 hr and were further treated with 500 μg/ml OVA, 100 μg/ml DNP‐OVA and 10 μg/ml anti‐DNP IgE for 4 hr to activate basophils. After being washed four times, 2·5 × 105 basophils in 200 μl PBS were delivered to naive BALB/c mice by intraperitoneal injection. As shown in Fig. 6, transfusion of basophils significantly increased the proportion of Th2 cells in MLN (Fig. 6a and b left), elevated levels of pulmonary IL‐4 (Fig. 6b middle) and serum OVA‐sIgE (Fig. 6b right), invoked airway inflammation characterized by massive eosinophil infiltration in the airway compared with the control group (no basophil transfer) (Fig. 6c). Furthermore, transfer of hemin‐treated basophils showed significantly lower levels of serum OVA‐sIgE and pulmonary IL‐4, and decreased Th2 cells in MLNs compared with the basophil transfer group (OVA group). However, the effect of HO‐1 on basophil‐mediated allergic inflammation was reversed by additional SnPP treatment. These results clearly indicated that HO‐1 inhibited basophil‐mediated OVA‐induced allergic airway inflammation.
Figure 6.
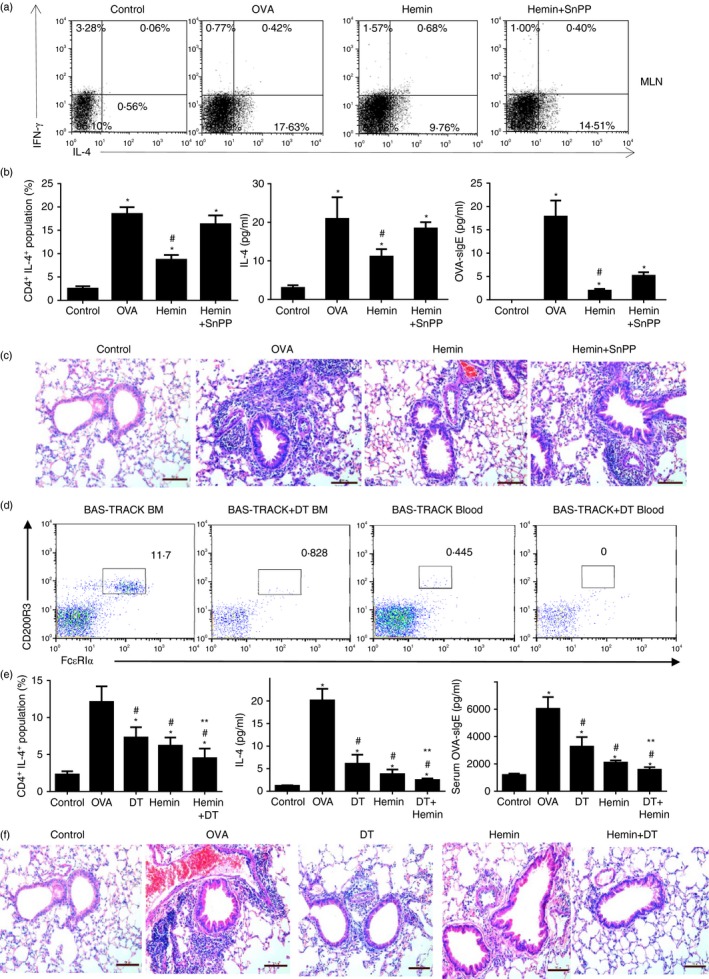
HO‐1 reduced allergic airway inflammation invoked by adoptive transfer of basophils and conditional depletion of basophils did not reverse the protective role of HO‐1. FACS purified FcεR1+ CD49b+ c‐kit− CD11c− basophils of bone marrow derived from normal BALB/c mice were treated with 30 μmol/l hemin, SnPP or hemin plus SnPP for 48 hr and were further treated with 500 μg/ml OVA, 100 μg/ml DNP‐OVA and 10 μg/ml anti‐DNP IgE for 4 hr to active basophils. A total of 2·5 × 105 basophils were delivered to naive BALB/c mice using intraperitoneal. injection at day 0 and day 14 followed by three intranasal challenges with OVA. (a) Flow cytometry assessed Th1/Th2 subsets in MLNs; gated on CD4+ cells). (b) Left, the percentage of CD4+ IL‐4+ population from MLNs (*P < 0·05, versus control group; #P < 0·05, versus OVA group); Middle, IL‐4 level in lung tissue detected by ELISA (*P < 0·05, versus control group; #P < 0·05, versus OVA group); Right, serum OVA‐sIgE level was detected by ELISA (*P < 0·05, versus control group; #P < 0·05, versus the OVA group). (c) Pathological change in lung tissue (haematoxylin & eosin stain, 40×). Diphtheria toxin (DT) ‐mediated conditional basophil‐specific depletion was performed before OVA sensitization and challenge or hemin administration. (d) DT treatment significantly decreased the percentage of basophils both in bone marrow and peripheral blood in Bas‐TRACK mice after two injections of DT (gated on CD49B+ c‐KIT − cells). (e) Left, the percentage of CD4+ IL‐4+ population from MLNs assessed by flow cytometry (gated on CD4+ cells; *P < 0·05, versus control group; #P < 0·05, versus OVA group; **P < 0·05, Hemin versus Hemin+DT group); Middle, IL‐4 level in lung tissue determined by ELISA (*P < 0·05, versus control group; #P < 0·05, versus OVA group; **P < 0·05, Hemin versus Hemin+DT group); Right, serum OVA‐sIgE level was determined by ELISA (*P < 0·05, versus control group; #P < 0·05, versus OVA group; **P < 0·05, Hemin versus Hemin+DT group). (f) Pathological change in lung tissue, scale bar, 100 μm (haematoxylin & eosin stain, 40×). Data are representative of three independent experiments.
Conditional depletion of basophil accompanying hemin administration further inhibited allergic inflammation
It is well accepted that HO‐1 exerts its protective role through regulation of multiple immune cells, we performed DT‐mediated basophil‐specific depletion to further confirm the important role of HO‐1 in OVA‐induced allergic airway inflammation. As shown in Fig. 6d, DT treatment significantly decreased the percentage of basophils both in bone marrow and peripheral blood in Bas‐TRACK mice after two injections of DT (gated on CD49B+ c‐KIT− cells). Meanwhile, DT‐mediated basophil‐specific depletion significantly reduced the OVA‐specific Th2 subset in MLN, decreased the levels of serum OVA‐sIgE and IL‐4 in lung tissue, and attenuated airway inflammation compared with the OVA group in Bas‐TRECK Tg mice after conditional depletion of basophil or hemin administration (Fig. 6e,f). Furthermore, conditional depletion of basophil accompanying hemin administration further decreased the levels of serum OVA‐sIgE, lung tissue IL‐4 and OVA‐specific Th2 subset (Fig. 6e), and attenuated allergic inflammation compared with the hemin group (Fig. 6f), which indicated that depletion of basophils did not reverse the protective role of HO‐1. This observation suggested that the HO‐1 protective role may be involved in multiple immune cells.
Up‐regulation of HO‐1 decreased pulmonary basophil number, inhibited basophil DQ‐OVA uptake, but promoted its apoptosis in vivo
Next, we investigated the effect of HO‐1 on basophils in the mouse model of OVA‐induced allergic airway inflammation. We found that OVA immunization and challenge significantly increased basophil numbers in the lung tissue, enhanced DQ‐OVA uptake by basophils and increased basophil apoptosis compared with the control group. Furthermore, induction of HO‐1 by hemin significantly decreased pulmonary basophil numbers (Fig. 7a and b left) and inhibited the ability of basophils to take up DQ‐OVA (Fig. 7b middle and c upper panel) compared with the OVA group. Meanwhile, induction of HO‐1 by hemin significantly further promoted basophil apoptosis (Fig. 7b right and c lower panel). However, inhibition of HO‐1 activity by addition of SnPP reversed the above effect of hemin (Fig. 7a–c).
Figure 7.
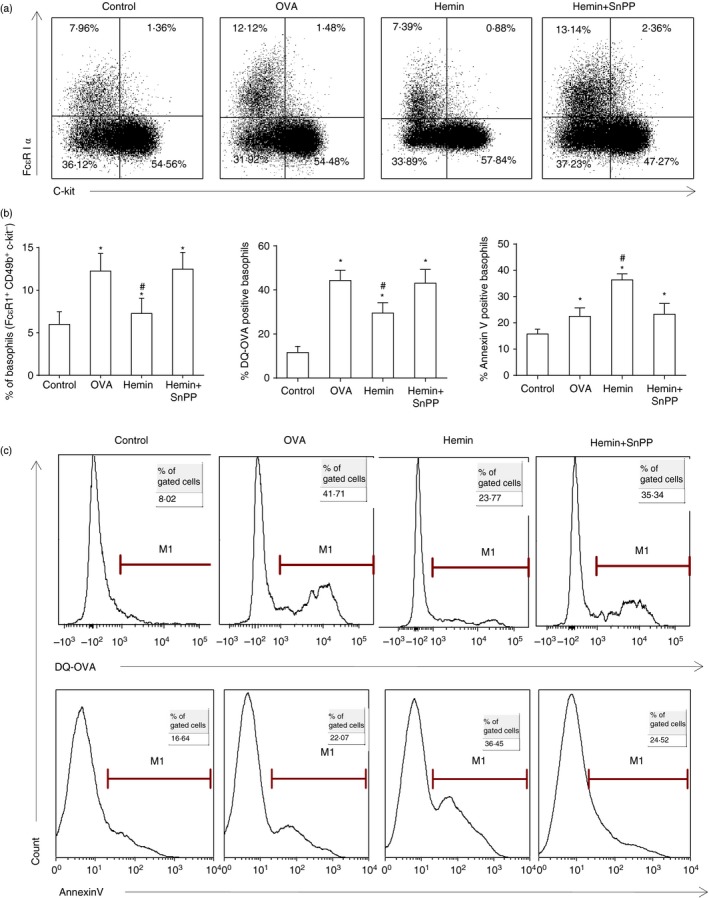
Up‐regulation of HO‐1 decreased pulmonary basophil number, inhibited basophil DQ‐OVA uptake, but promoted its apoptosis. (a) Flow cytometry analysis was performed to determine basophil amount (gate on CD49b+ basophil‐enriched population). (b) Left, the percentage of basophils (gate on CD49b+ subset; *P < 0·05, versus control group; #P < 0·05, versus OVA group); Middle, the percentage of DQ‐OVA positive basophils (gate on Fcε RI α + CD49b+ c‐kit− subset; *P < 0·05, versus control group; # P < 0·05, versus OVA group); Right, the percentage of Annexin V positive basophils (gate on Fcε RI α + CD49b+ PI + subset; *P < 0·05, versus control group; #P < 0·05, versus OVA group). (c) DQ‐OVA uptake (gate on Fcε RI α + CD49b+ c‐kit− subset) and Annexin V expression of pulmonary basophils using flow cytometry (gate on Fcε RI α + CD49b+ PI + subset). Data are representative of three independent experiments.
Up‐regulation of HO‐1 decreased co‐stimulators and activation marker CD200R expression on pulmonary basophils, and inhibited IL‐4 release from pulmonary basophils
Co‐stimulatory molecules were essential factors in the initiation of Th2 polarization. Therefore, we investigated the expression of CD80, CD40 and MHC II and its activation marker CD200R on pulmonary basophils. The results showed that OVA immunization and challenge significantly increased the levels of CD80, CD40, MHC II and activation marker CD200R. Furthermore, induction of HO‐1 treated with hemin significantly down‐regulated the expression of co‐stimulatory molecules and activation marker CD200R on pulmonary basophils (Fig. 8a and b left). Moreover, flow cytometry‐sorted pulmonary basophils from the hemin treatment group displayed attenuated ability to release IL‐4 compared with the OVA group (Fig. 8b right). However, inhibition of HO‐1 activity by SnPP reversed the above results.
Figure 8.
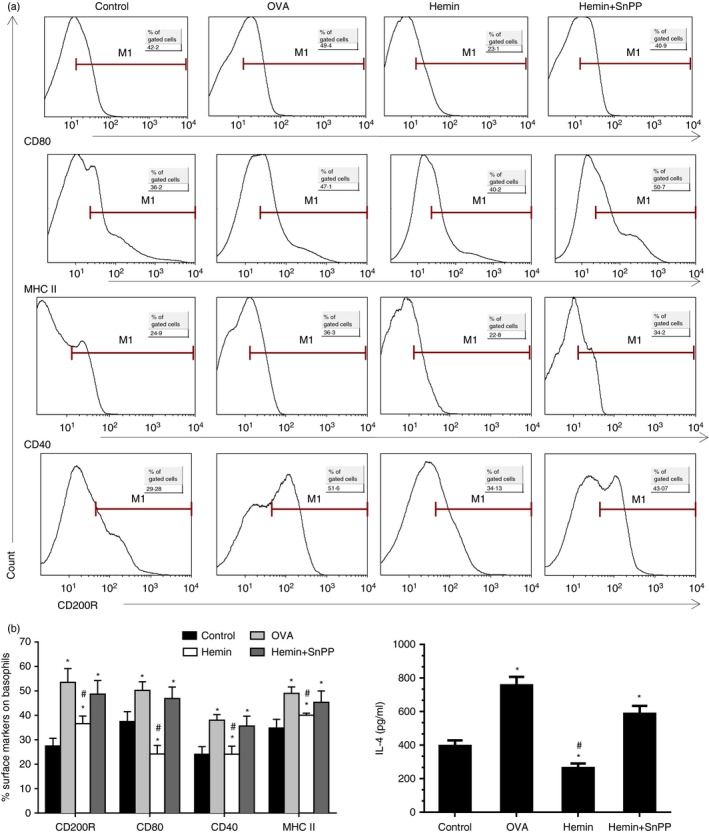
Up‐regulation of HO‐1 decreased co‐stimulators and activation marker CD200R expression on pulmonary basophils and inhibited IL‐4 release. (a) Flow cytometry analysis was performed to determine CD40, CD80, MHC II and activation marker CD200R expression on pulmonary basophils (gate on Fcε RI α + CD49b+ c‐kit− subset). (b) Left, the percentage of CD40, CD80, MHC II and CD200R positive basophils [*P < 0·05, versus control group; #P < 0·05, versus OVA group]; Right, IL‐4 level released from flow cytometry sorted pulmonary basophils upon DNP‐OVA/anti‐DNP‐IgE stimulation (*P < 0·05, versus control group; #P < 0·05, versus OVA group). Data are representative of three independent experiments.
Discussion
Heme oxygenase‐1 is known for its cytoprotective effect against oxidative injuries and differential immunological dissonance‐related diseases. Induction of HO‐1 expression has had therapeutic effects in a variety of conditions or inflammatory disorders; however, its immunological regulatory function is suspected. Studies have shown that HO‐1 affected the biological function of multiple cell types such as dendritic cells,23, 24 regulatory T cells,9, 38 mast cells27 and macrophages.2, 39 These immunocytes played a critical role in allergic diseases. Vannacci et al.36 reported that treatment with hemin significantly increased the expression of HO‐1 from partially purified human basophils, and decreased the FcεRI‐dependent release of histamine from enriched human basophils in inflammatory and allergic disease states. The present study also proved that HO‐1 was expressed in flow cytometry‐purified BMBs in vitro. Moreover, in the basophil favourite culture system, the addition of hemin to bone marrow cells significantly increased the expression of HO‐1 and its catalytic products, bilirubin and CO. It indicated that hemin was able to functionally promote HO‐1 expression and increase its activity. Furthermore, up‐regulation of HO‐1 led to a decreased percentage of basophils and promoted their apoptosis in a dose‐dependent manner. However, whether the HO‐1 induction modulates immune characteristics of basophils and suppresses the Th2 immune response in allergic airway inflammation remains to be determined.
Recent studies have demonstrated that basophils, as a new player, participate in Th2‐dominated diseases, such as allergic airway inflammation. Basophils can function as professional antigen‐presenting cells both in vivo and in vitro. They were able to take up DQ‐OVA, express MHC II and other co‐stimulatory molecules such as CD40, CD80 and CD86, release IL‐4 in the early phase, induce Th2‐cell polarization, and lead to the initiation and maintenance of a Th2 immune response.40, 41, 42, 43, 44, 45 Our previous studies further showed that in the early phase of OVA sensitization, temporary depletion of basophils with MAR‐1 or Ba103 antibody attenuated airway inflammation, represented by the significantly decreased amounts of the Th2 subset in spleen and draining lymph nodes, IL‐4 level in lung and OVA‐sIgE levels in serum. In contrast, adoptive transfer of basophils from OVA‐challenged lung tissue to naive BALB/c mice provoked the Th2 immune response.35 In this study, we demonstrated that OVA sensitization and challenge also resulted in increased numbers and elevated activation of pulmonary basophils in a murine model of allergic airway inflammation. In addition, adoptive transfer of OVA‐loaded and ‐activated BMBs to naive BALB/c mice invoked allergic airway inflammation and an OVA‐specific Th2 immune response, whereas conditional depletion of basophils by DT in Bas‐TRECK Tg mice before OVA sensitization and challenge significantly attenuated allergic airway inflammation. These data clearly demonstrated the importance of basophil in OVA‐induced allergic inflammation.
Furthermore, we found that induction of HO‐1 by hemin significantly suppressed the expression of CD200R, one of the activation markers on basophils,46 both in vivo and in vitro studies, as well as alleviating the Th2 immune response, accompanied by decreased numbers and weakened antigen uptake ability of basophils in lung tissue, lowered the expression of MHC II, CD40 and CD80, and reduced IL‐4 secretion. Adoptive transfer of hemin‐treated BMBs to naive mice also displayed less airway inflammation and Th2 immune response, which further confirmed the inhibitory role of HO‐1 on basophils. Additionally, inhibition of cell apoptosis is one of the important mechanisms that lead to the exacerbation of inflammation. Published studies show that HO‐1 is capable of anti‐apoptosis47 and pro‐apoptosis1, 48, 49 functions depending on the cell type and disease model. In the present study, we found that up‐regulation of HO‐1 can promote basophil apoptosis, which contributed to the remission of allergic inflammation. Therefore, our results demonstrated, for the first time, that HO‐1 may affect the maturation of BMBs and their recruitment to the lung tissue and promote its apoptosis.
Moreover, these effects were reversed by SnPP, which blocked HO‐1 activity, so indicating that the inhibition of basophil activation afforded by hemin was possibly the result of the increased generation of metabolites by the HO‐1 pathway, including free iron, CO, biliverdin and bilirubin. Accumulating evidence indicates that the expression of activated HO‐1 may provide an endogenous defence mechanism against pro‐inflammatory stimuli, which involved CO, biliverdin and its metabolite, bilirubin. In the current study, we used CO‐releasing molecule, an exogenous CO‐releasing molecule to mimic the role of CO. We found that exogenous CO could increase basophil apoptosis in a dose‐dependent manner, which was similar to the role of HO‐1. Conceivably, the increase of HO‐1 activity induced by hemin could serve CO‐mediated inhibitory feedback for the allergic activation of basophils, which could be relevant in the modulation of basophil‐mediated allergic reactions. Our present results demonstrated that up‐regulation of HO‐1 by hemin significantly suppressed the expression of CD200R, which was reversed by SnPP. Taken together, these results implied that HO‐1 is responsible for the inhibition of the immunological activation of basophils induced by hemin.
As mentioned above, HO‐1 exerted its protective role by affecting multiple immune cells, such as dendritic cells and regulatory T cells. So we evaluated the importance of basophils in the HO‐1‐mediated immune regulation role in OVA‐induced inflammation. We found that conditional depletion of basophil by DT in Bas‐TRECK Tg mice before OVA sensitization and challenge did not reverse the protective role of HO‐1, indicating the complicated mechanism of HO‐1.
In addition to the regulatory effect of HO‐1 on basophils, including the inhibition of the antigen‐presenting function, down‐regulation of co‐stimulatory molecules expression and reduction of IL‐4 production, we further sorted basophils from cultured cells in vitro by FACS and then co‐cultured FACS‐purified BMBs with MACS purified naive T cells from DO11.10 mice in vitro without exogenous IL‐4 to confirm the inhibitory effect of HO‐1 on basophil‐mediated Th2 polarization. As reported previously, co‐culture of naive T cells and basophils resulted in significant Th2 polarization, which was inhibited when basophils were pre‐treated with hemin. Furthermore, the inhibition of basophil‐mediated Th2 polarization by HO‐1 induction could be reversed by SnPP.
Collectively, these studies identify the previously unrecognized phenomenon that induction of HO‐1 expression can inhibit maturation and activation of basophils and promote their apoptosis to modulate their immune characteristics, including the expression of cell surface molecules and IL‐4 level. We conclude that basophils are an important target by which HO‐1 exerts protective role in Th2 cytokine‐mediated allergic airway inflammation.
Authorship contributions
Wenwei Zhong designed and performed the experiments, analysed the results, made the figures and drafted the manuscript. Caixia Di and Xiaoliang Lin participated in the experimental studies. Jiajia Lv and Yu Fan Yuan participated in the experimental studies and helped to compile the figures. Yanjie Zhang performed the statistical analysis. Jie Lv designed the experiments. Zhenwei Xia conceived of the study, designed experiments, analysed data, and helped to draft the manuscript. All authors have read and approved the final manuscript.
Disclosures
The authors declare no competing financial interests.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (91542202, 81470217, 81270084, 81070022 and 81270085) and the Shanghai Municipal Science and Technology Commission Foundation (13XD1402800, 12ZR1419100).
References
- 1. Dunn LL, Midwinter RG, Ni J, Hamid HA, Parish CR, Stocker R. New insights into intracellular locations and functions of heme oxygenase‐1. Antioxid Redox Signal 2014; 20:1723–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naito Y, Takagi T, Higashimura Y. Heme oxygenase‐1 and anti‐inflammatory M2 macrophages. Arch Biochem Biophys 2014; 564C:83–8. [DOI] [PubMed] [Google Scholar]
- 3. Gayathri G, Muthukumar S, Joseph LD, Suresh R. Immunolocalization of heme oxygenase‐1 in periodontal diseases. Indian J Dent Res 2014; 25:567–71. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Zhang L, Wu J, Di C, Xia Z. Heme oxygenase‐1 exerts a protective role in ovalbumin‐induced neutrophilic airway inflammation by inhibiting Th17 cell‐mediated immune response. J Biol Chem 2013; 288:34612–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Xia ZW, Zhong WW, Meyrowitz JS, Zhang ZL. The role of heme oxygenase‐1 in T cell‐mediated immunity: the all encompassing enzyme. Curr Pharm Des 2008; 14:454–64. [DOI] [PubMed] [Google Scholar]
- 6. Almolki A, Taille C, Martin GF, Jose PJ, Zedda C, Conti M et al Heme oxygenase attenuates allergen‐induced airway inflammation and hyperreactivity in guinea pigs. Am J Physiol Lung Cell Mol Physiol 2004; 287:L26–34. [DOI] [PubMed] [Google Scholar]
- 7. Lee MY, Seo CS, Lee JA, Lee NH, Kim JH, Ha H et al Anti‐asthmatic effects of Angelica dahurica against ovalbumin‐induced airway inflammation via upregulation of heme oxygenase‐1. Food Chem Toxicol 2011; 49:829–37. [DOI] [PubMed] [Google Scholar]
- 8. Abdureyim S, Amat N, Umar A, Upur H, Berke B, Moore N. Anti‐inflammatory, immunomodulatory, and heme oxygenase‐1 inhibitory activities of ravan napas, a formulation of uighur traditional medicine, in a rat model of allergic asthma. Evid Based Complement Alternat Med 2011; 2011:725926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xia ZW, Xu LQ, Zhong WW, Wei JJ, Li NL, Shao J et al Heme oxygenase‐1 attenuates ovalbumin‐induced airway inflammation by up‐regulation of foxp3 T‐regulatory cells, interleukin‐10, and membrane‐bound transforming growth factor‐ 1. Am J Pathol 2007; 171:1904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sheikh SZ, Hegazi RA, Kobayashi T, Onyiah JC, Russo SM, Matsuoka K et al An anti‐inflammatory role for carbon monoxide and heme oxygenase‐1 in chronic Th2‐mediated murine colitis. J Immunol 2011; 186:5506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takagi T, Naito Y, Uchiyama K, Yoshikawa T. The role of heme oxygenase and carbon monoxide in inflammatory bowel disease. Redox Rep 2010; 15:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhong W, Xia Z, Hinrichs D, Rosenbaum JT, Wegmann KW, Meyrowitz J et al Hemin exerts multiple protective mechanisms and attenuates dextran sulfate sodium‐induced colitis. J Pediatr Gastroenterol Nutr 2010; 50:132–9. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Zhang Y, Zhong W, Di C, Lin X, Xia Z. Heme oxygenase‐1 ameliorates dextran sulfate sodium‐induced acute murine colitis by regulating Th17/Treg cell balance. J Biol Chem 2014; 289:26847–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jang JU, Lee SH, Choi CU, Bahk SC, Chung HT, Yang YS. Effects of heme oxygenase‐1 inducer and inhibitor on experimental autoimmune uveoretinitis. Korean J Ophthalmol 2007; 21:238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohta K, Kikuchi T, Arai S, Yoshida N, Sato A, Yoshimura N. Protective role of heme oxygenase‐1 against endotoxin‐induced uveitis in rats. Exp Eye Res 2003; 77:665–73. [DOI] [PubMed] [Google Scholar]
- 16. Chauveau C, Remy S, Royer PJ, Hill M, Tanguy‐Royer S, Hubert FX et al Heme oxygenase‐1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL‐10 expression. Blood 2005; 106:1694–702. [DOI] [PubMed] [Google Scholar]
- 17. Al‐Huseini LM, Aw Yeang HX, Hamdam JM, Sethu S, Alhumeed N, Wong W et al Heme oxygenase‐1 regulates dendritic cell function through modulation of p38 MAPK‐CREB/ATF1 signaling. J Biol Chem 2014; 289:16442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tardif V, Riquelme SA, Remy S, Carreno LJ, Cortes CM, Simon T et al Carbon monoxide decreases endosome–lysosome fusion and inhibits soluble antigen presentation by dendritic cells to T cells. Eur J Immunol 2013; 43:2832–44. [DOI] [PubMed] [Google Scholar]
- 19. Moreau A, Hill M, Thebault P, Deschamps JY, Chiffoleau E, Chauveau C et al Tolerogenic dendritic cells actively inhibit T cells through heme oxygenase‐1 in rodents and in nonhuman primates. FASEB J 2009; 23:3070–7. [DOI] [PubMed] [Google Scholar]
- 20. Yasui Y, Nakamura M, Onda T, Uehara T, Murata S, Matsui N et al Heme oxygenase‐1 inhibits cytokine production by activated mast cells. Biochem Biophys Res Commun 2007; 354:485–90. [DOI] [PubMed] [Google Scholar]
- 21. Matsushima M, Takagi K, Ogawa M, Hirose E, Ota Y, Abe F et al Heme oxygenase‐1 mediates the anti‐allergic actions of quercetin in rodent mast cells. Inflamm Res 2009; 58:705–15. [DOI] [PubMed] [Google Scholar]
- 22. Xia ZW, Zhong WW, Xu LQ, Sun JL, Shen QX, Wang JG et al Heme oxygenase‐1‐mediated CD4+ CD25high regulatory T cells suppress allergic airway inflammation. J Immunol 2006; 177:5936–45. [DOI] [PubMed] [Google Scholar]
- 23. Gajdocsy R, Horvath I. Exhaled carbon monoxide in airway diseases: from research findings to clinical relevance. J Breath Res 2010; 4:047102. [DOI] [PubMed] [Google Scholar]
- 24. Elhini A, Abdelwahab S, Ikeda K. Heme oxygenase (HO)‐1 is upregulated in the nasal mucosa with allergic rhinitis. Laryngoscope 2006; 116:446–50. [DOI] [PubMed] [Google Scholar]
- 25. Kitada O, Kodama T, Kuribayashi K, Ihaku D, Fujita M, Matsuyama T et al Heme oxygenase‐1 (HO‐1) protein induction in a mouse model of asthma. Clin Exp Allergy 2001; 31:1470–7. [DOI] [PubMed] [Google Scholar]
- 26. Lo S, Di Palma S, Pitkin L, McCombe AW. Localisation of heme oxygenase isoforms in allergic human nasal mucosa. Eur Arch Otorhinolaryngol 2005; 262:595–8. [DOI] [PubMed] [Google Scholar]
- 27. Chakrabarty A, Emerson MR, LeVine SM. Heme oxygenase‐1 in SJL mice with experimental allergic encephalomyelitis. Mult Scler 2003; 9:372–81. [DOI] [PubMed] [Google Scholar]
- 28. Pae HOLY, Chung HT. Heme oxygenase‐1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Pat Inflamm Allergy Drug Discov 2008; 2:6. [DOI] [PubMed] [Google Scholar]
- 29. Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL‐3, but infection‐induced Th2 immunity can develop without basophil lymph node recruitment or IL‐3. J Immunol 2010; 184:1143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van Panhuys N, Prout M, Forbes E, Min B, Paul WE, Le Gros G. Basophils are the major producers of IL‐4 during primary helminth infection. J Immunol 2011; 186:2719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen‐induced T helper type 2 responses. Nat Immunol 2008; 9:310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR et al MHC class II‐dependent basophil‐CD4+ T cell interactions promote TH2 cytokine‐dependent immunity. Nat Immunol 2009; 10:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y et al Basophils contribute to TH2‐IgE responses in vivo via IL‐4 production and presentation of peptide‐MHC class II complexes to CD4+ T cells. Nat Immunol 2009; 10:706–12. [DOI] [PubMed] [Google Scholar]
- 34. Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y et al Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood 2007; 110:913–20. [DOI] [PubMed] [Google Scholar]
- 35. Zhong W, Su W, Zhang Y, Liu Q, Wu J, Di C et al Basophils as a primary inducer of the T helper type 2 immunity in ovalbumin‐induced allergic airway inflammation. Immunology 2014; 142:202–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vannacci A, Baronti R, Zagli G, Marzocca C, Pierpaoli S, Bani D et al Carbon monoxide modulates the response of human basophils to FcεRI stimulation through the heme oxygenase pathway. Eur J Pharmacol 2003; 465:289–97. [DOI] [PubMed] [Google Scholar]
- 37. Sawaguchi M1, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y et al Role of mast cells and basophils in IgE responses and in allergic airway hyperresponsiveness. J Immunol 2012; 188:1809–18. [DOI] [PubMed] [Google Scholar]
- 38. Choi BM, Pae HO, Jeong YR, Kim YM, Chung HT. Critical role of heme oxygenase‐1 in Foxp3‐mediated immune suppression. Biochem Biophys Res Commun 2005; 327:1066–71. [DOI] [PubMed] [Google Scholar]
- 39. Weis N, Weigert A, von Knethen A, Brune B. Heme oxygenase‐1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell 2009; 20:1280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen‐presenting cells for an allergen‐induced T helper type 2 response. Nat Immunol 2009; 10:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Falcone FH, Zillikens D, Gibbs BF. The 21st century renaissance of the basophil? Current insights into its role in allergic responses and innate immunity. Exp Dermatol 2006; 15:855–64. [DOI] [PubMed] [Google Scholar]
- 42. Galli SJ, Franco CB. Basophils are back!. Immunity 2008; 28:495–7. [DOI] [PubMed] [Google Scholar]
- 43. Maddur MS, Kaveri SV, Bayry J. Basophils as antigen presenting cells. Trends Immunol 2010; 31:45–8. [DOI] [PubMed] [Google Scholar]
- 44. Yoshimoto T. Basophils as Th2‐inducing antigen‐presenting cells. Int Immunol 2010; 22:543–50. [DOI] [PubMed] [Google Scholar]
- 45. Kim S, Shen T, Min B. Basophils can directly present or cross‐present antigen to CD8 lymphocytes and alter CD8 T cell differentiation into IL‐10‐producing phenotypes. J Immunol 2009; 183:3033–9. [DOI] [PubMed] [Google Scholar]
- 46. Torrero MN, Larson D, Hubner MP, Mitre E. CD200R surface expression as a marker of murine basophil activation. Clin Exp Allergy 2009; 39:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ursu ON, Sauter M, Ettischer N, Kandolf R, Klingel K. Heme oxygenase‐1 mediates oxidative stress and apoptosis in coxsackievirus B3‐induced myocarditis. Cell Physiol Biochem 2014; 33:52–66. [DOI] [PubMed] [Google Scholar]
- 48. Song R, Zhou Z, Kim PK, Shapiro RA, Liu F, Ferran C et al Carbon monoxide promotes Fas/CD95‐induced apoptosis in Jurkat cells. J Biol Chem 2004; 279:44327–34. [DOI] [PubMed] [Google Scholar]
- 49. Hill M, Pereira V, Chauveau C, Zagani R, Remy S, Tesson L et al Heme oxygenase‐1 inhibits rat and human breast cancer cell proliferation: mutual cross inhibition with indoleamine 2,3‐dioxygenase. FASEB J 2005; 19:1957–68. [DOI] [PubMed] [Google Scholar]


