Abstract
Insufficient nitric oxide (NO) bioavailability plays an important role in endothelial dysfunction and arterial stiffening with aging. Supplementation with sodium nitrite, a precursor of NO, ameliorates age-related vascular endothelial dysfunction and arterial stiffness in mice, but effects on humans, including the metabolic pathways altered, are unknown. The purpose of this study was to determine the safety, feasibility, and efficacy of oral sodium nitrite supplementation for improving vascular function in middle-aged and older adults and to identify related circulating metabolites. Ten weeks of sodium nitrite (80 or 160 mg/day, capsules, TheraVasc; randomized, placebo control, double blind) increased plasma nitrite acutely (5- to 15-fold, P < 0.001 vs. placebo) and chronically (P < 0.10) and was well tolerated without symptomatic hypotension or clinically relevant elevations in blood methemoglobin. Endothelial function, measured by brachial artery flow-mediated dilation, increased 45-60% vs. baseline (P < 0.10) without changes in body mass or blood lipids. Measures of carotid artery elasticity (ultrasound and applanation tonometry) improved (decreased β-stiffness index, increased cross-sectional compliance, P < 0.05) without changes in brachial or carotid artery blood pressure. Aortic pulse wave velocity was unchanged. Nitrite-induced changes in vascular measures were significantly related to 11 plasma metabolites identified by untargeted analysis. Baseline abundance of multiple metabolites, including glycerophospholipids and fatty acyls, predicted vascular changes with nitrite. This study provides evidence that sodium nitrite supplementation is well tolerated, increases plasma nitrite concentrations, improves endothelial function, and lessens carotid artery stiffening in middle-aged and older adults, perhaps by altering multiple metabolic pathways, thereby warranting a larger clinical trial.
Keywords: arterial stiffness, endothelial dysfunction, metabolomics
cardiovascular diseases (CVD) remain the leading cause of mortality in modern societies, and advancing age is the primary risk factor for CVD (26). With the rapidly changing demographics of aging and record numbers of older adults, the burden of disease is projected to increase to unprecedented levels in the near future (14). As such, it is of the upmost importance to establish evidence-based interventions to prevent or delay the development and progression of CVD.
As many as 80% of all CVDs are associated with dysfunction and disorders of arteries, namely stiffening of the large elastic arteries and vascular endothelial dysfunction (24, 26). Several mechanisms are responsible for these changes, with reductions in the bioavailability of the vascular-protective molecule, nitric oxide (NO), being among the most important (7, 33, 34). The nitrite anion (inorganic nitrite), once considered an inert byproduct of NO metabolism, is now recognized as a cytoprotective molecule and the major storage form of NO in tissues. Through a one-step reduction to NO, nitrite augments circulating and tissue NO bioavailability and also acts as an independent signaling molecule, giving it substantial therapeutic potential for the prevention and treatment of arterial aging and CVD (34). Short-term supplementation with sodium nitrite reverses aortic stiffness and endothelial dysfunction in old mice (10, 35), and results of recent studies examining dietary nitrate in adults with hypertension (18) or cardiovascular risk factors (31) or sodium nitrite in patients with chronic clinical diseases (25) suggest possible benefits of these approaches in humans. However, presently there is no evidence supporting the use of chronic nitrite supplementation for improving impaired baseline arterial function in otherwise healthy middle-aged and older adults in the context of maintaining optimal vascular health and related CVD risk profiles.
Accordingly, we performed a study to determine the feasibility, safety, dosing, and efficacy of nitrite supplementation on arterial function in healthy middle-aged and older adults before conducting a larger clinical trial. We hypothesized that 10 wk of oral sodium nitrite supplementation (80 or 160 mg/day) would reduce age-related vascular endothelial dysfunction and arterial stiffness in this group. The study was designed to test key aspects necessary for conducting a future larger-scale trial, including the ability to recruit, retain, and successfully collect and analyze required data in this population; tolerability and adherence with the intervention; and efficacy and optimal dosing of nitrite supplementation for improving vascular function. This study was considered successful if >85% of randomized subjects had analyzable plasma nitrite and vascular measures at baseline and week 10 of the intervention and if no serious adverse events occurred. Finally, to gain novel molecular insight into the possible pathways by which nitrite may be acting, we also performed an unbiased analysis of the plasma metabolome to identify circulating analytes and metabolites that were related to improvements in vascular function with treatment.
MATERIALS AND METHODS
This double-blind, placebo-controlled, randomized parallel-design study was conducted at the University of Colorado Boulder Clinical Translational Research Center (CTRC), and blood was assayed at the Colorado Clinical Translational Sciences Institute CTRC Core Laboratory, Mayo Medical Laboratories, and Boulder Community Hospital.
Subjects.
Men and postmenopausal women ages 50 to 79 yr of age were recruited from Boulder and the surrounding communities. All subjects were free of cardio-metabolic diseases, including peripheral arterial disease (ankle-brachial index >0.90), as assessed by graded exercise test, blood analyses, medical history, and general physical examination by a physician. Subjects demonstrated impaired endothelial function, defined as a brachial artery flow-mediated dilation value <7%. Subjects were excluded if they had glucose-6-phosphate dehydrogenase deficiency (kinetic spectrophotometry), systolic blood pressure <100 mmHg, blood methemoglobin (MetHb) >2% (ABL825 cooximeter; Radiometer America), hypersensitivity to nitrates or nitrites, a medical history of anemia or clotting disorders, active malignancy or infection, severe obesity (body mass index >40 kg/m2), or were taking medications that interacted with nitrite or altered vascular function (i.e., phosphodiesterase-5 inhibitors, systemic β-adrenergic blockers, tricyclic antidepressants, meperidine, nitrates, anticoagulants, calcium channel blockers, hormone replacement therapy, imitrex, sumatriptan, angiotensin I-converting enzyme inhibitors, angiotensin II receptor blockers, or diuretics). No subjects took nitrate- or nitrite-related supplements. To increase the generalizability of the study, some subjects were enrolled even if they took certain medications or dietary supplements as long as they maintained their intake throughout the study and refrained from taking medications or supplements 12 h before vascular measurements. The following prescription drugs were taken by some subjects: statins (placebo n = 2, nitrite 80 mg/day n = 1), selective serotonin reuptake inhibitors (nitrite 80 mg/day n = 1, nitrite 160 mg/day n = 1), proton pump inhibitors (placebo n = 1, nitrite 80 mg/day n = 1, nitrite 160 mg/day n = 3), bupropion (placebo n = 1), finasteride (nitrite 160 mg/day n = 1), inhaled fluticasone/salmeterol (nitrite 80 mg/day n = 1), inhaled levosalbutamol (nitrite 80 mg/day n = 1), levothyroxine (placebo n = 1, nitrite 80 mg/day n = 1), lorazepam (nitrite 80 mg/day n = 1), nasal mometasone furoate monohydrate (placebo n = 2), tamsulosin (placebo n = 1), ophthalmic timolol (placebo n = 1), and zolpidem (nitrite 80 mg/day n = 1). Sixteen subjects took no medications, and 13 took no dietary supplements.
All procedures were approved by the Institutional Review Board at the University of Colorado Boulder. The nature, benefits, and risks of the study were explained to all participants, and their written informed consent was obtained before participation. This study was approved by the Food and Drug Administration (IND111401) and registered on ClinicalTrials.gov (NCT02022670).
Randomization and intervention.
Subjects were randomized using a blocked randomization scheme stratified for sex (male vs. female) and age (50–65 vs. 66–79 yr) to avoid a potential imbalance among groups for these two key baseline characteristics. Placebo or sodium nitrite capsules (40 mg or 80 mg; TheraVasc) were taken orally twice a day (morning and evening) for 10 wk. Subjects were educated by a CTRC registered dietician about foods containing moderate and high levels of nitrate or nitrite (e.g., spinach, kale, beets), and subjects were instructed to maintain a constant dietary intake of these foods and to avoid beets and beetroot juice throughout the study. Sample size was estimated using an effect size of 0.74 from published data in humans assessing the effects of oral nitrates on blood pressure (21, 36, 44), brachial artery flow-mediated dilation after forearm ischemia (44), and work from our laboratory (29).
Subject characteristics and clinical blood assays.
Body mass index and waist and hip circumferences were measured by anthropometry as previously described (42). Arterial blood pressure and heart rate were measured over the brachial artery during rest in a supine position using a semiautomated device (Dynamap XL; Johnson & Johnson).
Fasting plasma glucose was measured by enzymatic methods (Roche Diagnostic Systems) and fasting plasma insulin by radioimmunoassay (Diagnostic Systems Laboratory). Insulin resistance was estimated with the homeostasis model of insulin resistance (HOMA-IR) by the formula [fasting plasma glucose (mg/dl) × fasting plasma insulin (μU/ml)]/405 (22). The HOMA-IR has been validated against other measures of insulin sensitivity (e.g., intravenous glucose tolerance test) as a reliable estimate of insulin sensitivity (3). Fasting serum blood lipid concentrations were determined using standard assays. High-sensitivity C-reactive protein (serum, immunoturbidimetric method; Beckman Coulter; ELISA), oxidized low-density lipoprotein (EDTA-treated plasma; ALPCO Diagnostics), total antioxidant status (serum, Randox Laboratories, inhibition of the increase in absorbance by oxidized 2-acrylamideo-2-methylpropane sulfonic acid over 3 min), glutathione peroxidase (NaHep-treated whole blood, Randox Laboratories, oxidative method), norepinephrine (EGTA and glutathione-treated serum, Bio-Rad Laboratories, HPLC), renin activity (EDTA-treated plasma, DiaSorin, RIA), aldosterone (serum, Diagnostic Products, RIA), endothelin-1 (EDTA and aprotinin-treated plasma, Peninsula Laboratories, antibody method), cortisol (serum, Beckman Coulter, 1-step competitive assay), free fatty acids (serum, WaKo Chemicals USA, enzymatic method), leptin (serum, Beckman Coulter, RIA), and adiponectin (serum, Millipore, RIA) were analyzed at the Colorado Translational Sciences Institute CTRC Core Lab. Angiotensin II (EDTA- and bestastin-treated plasma, Evaluating Data, RIA) and epinephrine (EGTA and glutathione-treated serum, Bio-Rad Laboratories, HPLC) were also measured but are not reported because the majority of samples were below detection limits.
Feasibility, safety, and tolerability.
Subject adherence to the intervention was measured by pill count. Retention of subjects and adverse events (Table 4) were documented by the CTRC and reported to the Institutional Review Board and Food and Drug Administration. Tolerability and side effects of the intervention were determined by symptom questionnaire, blood pressure, and blood MetHb. A cut-off value of 12% blood MetHb or a drop of >20 mmHg systolic blood pressure were instituted as stopping criteria. Subjects ingested their first capsule in the laboratory under supervision. Because of the potential hypotensive and methemoglobinemia-inducing properties seen with higher doses of nitrite or nitrates (30), seated and postural blood pressure measures, blood sampling for MetHb levels, and a symptom questionnaire were administered before, 15 min, 30 min, 60 min, and 120 min after initial capsule ingestion. Six hours later, pulse cooximetry (Rad-57 Rainbow SET, Masimo) was performed to measure MetHb levels instead of a blood draw. Subjects were monitored for adverse events again on day 2, week 1, week 2, week 4, and week 10. These measures were performed by staff who were not involved in vascular data acquisition or analyses to ensure blinding of investigators.
Table 4.
Safety and tolerability
| Placebo | Nitrite 80 mg/day | Nitrite 160 mg/day | |
|---|---|---|---|
| Average peak blood methemoglobin, % | 0.68 ± 0.04 | 0.74 ± 0.06 | 1.10 ± 0.06 |
| Peak blood methemoglobin, % | 1.0 | 1.1 | 1.5 |
| Treatment-emergent adverse events, n | |||
| Headache | 1 | 0 | 3 |
| Nausea | 1 | 0 | 0 |
| Fatigue | 0 | 2 | 2 |
| Dizziness/lightheadedness | 1 | 1 | 2 |
| Asymptomatic orthostatic intolerance | 1 | 1 | 1 |
| Dry mouth | 1 | 0 | 0 |
| Subjects with ≥1 adverse events, n | 2 | 4 | 4 |
| Dropouts, n | 1 | 0 | 1 |
Applicable values are means ± SE.
Plasma nitrite.
To determine the acute effects of our capsules on circulating nitrite concentrations, plasma was collected before and 30 min after capsule ingestion in a subset of subjects (placebo n = 7, nitrite 80 mg/day n = 6, nitrite 160 mg/day n = 9). The timing of this blood sample was based on pharmacokinetic data from the manufacturer for capturing peak plasma concentrations (12). Plasma was also collected in all subjects at week 10 of the intervention, >12 h after capsule ingestion to determine the nonacute effects of supplementation on circulating nitrite concentrations.
Before coming into contact with blood samples or preservative solution, pipette tips and microcentrifuge tubes were washed with fresh Millipore-filtered double-distilled water (ddH2O) in triplicate to remove any nitrate or nitrite residue on labware. Whole blood was collected with syringes and tubes from the same lot (rinsing with dH2O was not possible). In a darkened room, whole blood was slowly added by syringe to prefilled tubes with freshly made (day of) N-ethylmaleimide/EDTA (10 mM/2.5 mM final concentrations, respectively), inverted several times for adequate mixing, centrifuged, plasma aliquoted, flash frozen in liquid nitrogen, and stored at −60°C until being shipped on dry ice to a laboratory for analysis. Nitrite concentrations were measured with a dedicated high-throughput HPLC system (ENO-20, EiCom) using reverse-phase chromatography and diazo-coupling for spectrophotometric detection. The system operates over a wide range of concentrations with high sensitivity (5, 6).
Vascular outcomes.
All vascular measures were performed >12 h after ingesting the last dose of sodium nitrite and 4–24 h after ingesting food, alcohol, or caffeine or performing exercise (13, 41). The same investigator (AED), who was blinded to the subject group assignment, performed all data acquisition and analyses.
Brachial artery flow-mediated dilation was assessed using high-resolution ultrasonography (Xario XG, Toshiba America Medical Systems) before and after reactive hyperemia, as described previously (9, 11). Flow-mediated dilation was measured on multiple occasions at baseline (average of 3 visits) and at 10 wk (average of 2 visits) to account for day-to-day variation in vascular function (13). Flow-mediated dilation was expressed as millimeter change (mmΔ) and percentage change (%Δ) from baseline diameter (13). Shear rate was calculated as 8 × mean velocity/occlusion diameter using pulsed Doppler signals (angle of insonation <70°) and a sample volume over the entire width of the artery during the first 10 velocity envelopes after cuff release. Arterial diameters and blood velocities were captured and analyzed by Vascular Research Tools 5 software version 5.10.9 (Medical Imaging Applications) equipped with Top Performance Analysis Integrated System with imager and frame grabber (DICOM) and vascular ECG-gating module (University of Iowa, Iowa City, IA). The coefficient of variation for baseline and peak brachial diameter in our laboratory is 0.3% and 0.6%, respectively.
Local stiffness/elasticity at the carotid artery was measured as previously described (37). Briefly, the subject's right common carotid artery maximal (i.e., end-systolic) and minimal (i.e., end-diastolic) diameters were recorded by B-mode ultrasound (Xario XG, Toshiba America Medical Systems) for >10 heart cycles (mean ± SE, 31 ± 2). Carotid blood pressure was subsequently measured via applanation tonometry of the carotid artery with a custom transducer (NonInvasive Hemodynamics Workstation, Cardiovascular Engineering) (40). Carotid artery cross-sectional compliance was calculated as [3.141592 × ((2 × carotid diastolic diameter) × (carotid systolic-diastolic diameter) + (carotid systolic-diastolic diameter)2)/4 × (carotid pulse pressure)] (41). β-Stiffness index was calculated as [Ln(carotid systolic blood pressure/carotid diastolic blood pressure)/(carotid systolic diameter-diastolic diameter)/carotid diastolic diameter] (15). β-Stiffness index is proposed to be a blood pressure-independent index of arterial stiffness (15).
Central (carotid to femoral artery) and peripheral (carotid to radial artery) pulse wave velocity and augmentation index at the carotid and radial arteries were determined by sequential applanation tonometry with ECG gating of the R wave (NonInvasive Hemodynamics Workstation, Cardiovascular Engineering) (40) at each artery (an average of 10 heart cycles) by a blinded investigator (AED). Distances between the carotid artery and suprasternal notch, radial artery and suprasternal notch, and femoral artery and suprasternal notch were measured on the body surface with a tape measure or caliper. Pulse wave velocity and augmentation index were calculated via software imbedded in the NonInvasive Hemodynamics Workstation using the equation distance/transit time and Δpressure/pulse pressure × 100, respectively (40).
Metabolomics.
EDTA-treated plasma was collected before and at week 10 of the intervention, frozen, and stored at −80°C until analysis. Compounds were extracted from samples using an established custom technique that yields molecules from four major classes (lipid, protein, carbohydrate, and nucleic acids) (23, 32, 46). Aqueous and lipid fractions were analyzed using liquid chromatography-mass spectrometry (LC-MS, 6210 ESI-TOF; Agilent Technologies), and output was analyzed using commercial and custom software (1, 46). The unbiased analysis involved assessment of metabolites from major metabolic pathways. Quality control included duplicate samples and analysis of a pooled plasma sample containing labeled or exogenous negative or positive spike-in controls, resulting in false discovery rates <3%. Spectral data were extracted and aligned based on mass and retention time using Profinder software (Agilent). Metabolites were annotated with IDBrowser in Mass Profiler Professional (MPP, Agilent) using a combination of available databases: METLIN Metabolite Database, Human Metabolome Database (HMDB), LIPID Metabolites and Pathways Strategy (LIPID MAPS) and Kyoto Encyclopedia of Genes and Genomes (KEGG). Metabolites identified as being significantly altered by treatment were confirmed using MS/MS (6520 Q-TOF, Agilent) and matched to the NIST 14 MS/MS library. Chemical formula generation was attempted for all molecules that remained unidentified in both LC-MS and LC-MS/MS analyses.
Statistical analyses.
Data are expressed as means ± SE. Statistical analyses were performed with IBM SPSS (version 22) and G*Power 3.1 software. Unless otherwise indicated, missing values or outliers (Grubb's test) were replaced with the group mean. Differences among groups in baseline subject characteristics were determined using one-way ANOVA (P < 0.05). Because this study was designed to determine dosage and sample size for a larger clinical trial, significance was set at P < 0.10 for group differences (38). Sodium nitrite treatments were compared with placebo using a mixed-model ANOVA (within factor, time; between factor, treatment group), with contrast set to changes observed in placebo controls. In the case of a significant time × treatment interaction, paired sample t-tests were performed (P < 0.05).
After metabolomics data were filtered using MPP software (Agilent), small molecules not present in at least 50% of any one group at baseline or week 10 were excluded to reduce the probability of false discovery, resulting in 3,063 detected small molecules for subsequent analyses. Molecular concentrations were transformed to a log2 scale to assess their total magnitude of change. To detect metabolites significantly altered by treatment, small molecules were required to withstand a twofold change filter and remain significant after a paired t-test (P < 0.05), while not being significantly altered (P < 0.05) in the placebo group. Significantly altered molecules were then modeled independently in linear regression models within each treatment group to assess their individual association with vascular functional outcomes.
To assess whether baseline metabolomic signatures predict responsiveness to nitrite, baseline log2-transformed concentrations of all 3,063 molecules were tested with independent linear-regression models within each dosage group. Only metabolites that were significantly associated with responsiveness to nitrite and did not show an interaction with dosage level (80 or 160 mg/day) were included. Metabolites were subsequently grouped by class using the HMDB database to determine which metabolic pathways were most involved in our analysis. A subject was designated “responsive to the intervention” if they exhibited a 1% improvement from baseline for flow-mediated dilation, an 8% improvement in carotid artery cross-sectional compliance, and a 15% improvement in β-stiffness index measures, changes shown to be predictive of mortality or CVD events (16, 43).
RESULTS
The average age of subjects was 62 ± 1 yr (range 50–77). Subjects were healthy, and there were no significant differences among groups for clinical characteristics at baseline (Tables 1 and 2). Anthropometrics, brachial artery blood pressure, fasting blood lipids, glucose, and insulin remained unchanged by the intervention. Circulating analytes, including C-reactive protein and endothelin-1, did not significantly change (Table 3).
Table 1.
Subject characteristics
| Placebo |
Nitrite 80 mg/day |
Nitrite 160 mg/day |
||||
|---|---|---|---|---|---|---|
| Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | |
| Age, yr | 62 ± 3 | — | 60 ± 2 | — | 63 ± 2 | — |
| Males/females, n | 6/4 | — | 5/5 | — | 6/5 | — |
| Body mass, kg | 75 ± 3 | 75 ± 3 | 71 ± 3 | 71 ± 3 | 70 ± 3 | 71 ± 3 |
| Body mass index, kg/m2 | 26 ± 1 | 26 ± 1 | 24 ± 1 | 25 ± 1 | 24 ± 1 | 24 ± 1 |
| Waist circumference, cm | 83 ± 3 | 83 ± 3 | 82 ± 3 | 83 ± 3 | 79 ± 3 | 79 ± 3 |
| Waist:hip ratio, U | 0.82 ± 0.03 | 0.82 ± 0.03 | 0.84 ± 0.03 | 0.85 ± 0.03 | 0.80 ± 0.03 | 0.80 ± 0.03 |
| SBP, mmHg | 121 ± 5 | 119 ± 3 | 116 ± 4 | 119 ± 4 | 122 ± 4 | 124 ± 3 |
| DBP, mmHg | 73 ± 2 | 72 ± 1 | 72 ± 2 | 74 ± 2 | 74 ± 3 | 73 ± 2 |
| Heart rate, beats/min | 57 ± 3 | 57 ± 2 | 53 ± 2 | 56 ± 2 | 59 ± 2 | 59 ± 3 |
Values are means ± SE.
SBP, systolic blood pressure; DBP, diastolic blood pressure.
Table 2.
Clinical blood characteristics
| Placebo |
Nitrite 80 mg/day |
Nitrite 160 mg/day |
||||
|---|---|---|---|---|---|---|
| Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | |
| Total cholesterol, mg/dl | 164 ± 7 | 158 ± 7 | 175 ± 8 | 174 ± 9 | 180 ± 6 | 183 ± 8 |
| HDL cholesterol, mg/dl | 59 ± 6 | 58 ± 6 | 58 ± 4 | 60 ± 4 | 57 ± 4 | 59 ± 4 |
| LDL cholesterol, mg/dl | 90 ± 5 | 87 ± 4 | 100 ± 6 | 101 ± 7 | 109 ± 6 | 108 ± 8 |
| Triglycerides, mg/dl | 79 ± 8 | 67 ± 5 | 70 ± 6 | 70 ± 13 | 75 ± 6 | 80 ± 9 |
| Glucose, mg/dl | 94 ± 1 | 91 ± 2 | 90 ± 2 | 90 ± 1 | 93 ± 1 | 91 ± 2 |
| Insulin, μU/ml | 8 ± 1 | 10 ± 2 | 9 ± 1 | 8 ± 1 | 8 ± 1 | 7 ± 1 |
| HOMA-IR, U | 1.9 ± 0.2 | 2.2 ± 0.4 | 2.0 ± 0.2 | 1.8 ± 0.3 | 1.8 ± 0.2 | 1.6 ± 0.2 |
Values are means ± SE.
HOMA-IR, homeostasis model assessment-insulin resistance.
Table 3.
Circulating factors
| Placebo |
Nitrite 80 mg/day |
Nitrite 160 mg/day |
||||
|---|---|---|---|---|---|---|
| Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | |
| C-reactive protein, mg/l | 0.59 ± 0.15 | 0.59 ± 0.09 | 0.79 ± 0.22 | 0.61 ± 0.13 | 0.50 ± 0.07 | 0.56 ± 0.08 |
| Oxidized LDL, U/l | 43 ± 3 | 42 ± 3 | 49 ± 4 | 48 ± 4 | 51 ± 3 | 50 ± 2 |
| Total antioxidant status, mmol/l | 1.37 ± 0.03 | 1.37 ± 0.04 | 1.41 ± 0.03 | 1.41 ± 0.03 | 1.36 ± 0.04 | 1.40 ± 0.04 |
| Glutathione peroxidase, U/l | 8181 ± 1054 | 8180 ± 1123 | 7109 ± 380 | 7158 ± 447 | 7392 ± 762 | 7631 ± 682 |
| Norepinephrine, pg/ml | 347 ± 38 | 342 ± 46 | 367 ± 57 | 296 ± 54 | 329 ± 42 | 255 ± 23 |
| Renin activity, ng/ml per h | 0.28 ± 0.07 | 0.26 ± 0.07 | 0.33 ± 0.05 | 0.34 ± 0.10 | 0.26 ± 0.06 | 0.24 ± 0.03 |
| Aldosterone, ng/dl | 4.7 ± 0.7 | 4.9 ± 0.9 | 4.1 ± 0.7 | 4.0 ± 0.06 | 5.3 ± 1.0 | 3.7 ± 0.5 |
| Endothelin-1, pg/ml | 6.0 ± 0.3 | 5.8 ± 0.4 | 6.4 ± 0.3 | 5.7 ± 0.3 | 5.6 ± 0.2 | 5.7 ± 0.3 |
| Cortisol, μg/ml | 9.9 ± 0.8 | 9.8 ± 1.0 | 8.3 ± 0.9 | 8.4 ± 1.2 | 9.1 ± 1.0 | 9.1 ± 1.0 |
| Free fatty acids, μmol/l | 454 ± 61 | 411 ± 60 | 497 ± 27 | 446 ± 34 | 497 ± 30 | 472 ± 51 |
| Leptin, ng/ml | 8.6 ± 2.2 | 8.0 ± 2.2 | 8.0 ± 2.6 | 7.8 ± 2.4 | 6.8 ± 2.0 | 8.7 ± 3.0 |
| Adiponectin, μg/ml | 10.8 ± 1.3 | 10.7 ± 1.4 | 10.8 ± 1.4 | 11.5 ± 1.5 | 10.5 ± 1.8 | 10.7 ± 1.5 |
Values are means ± SE.
Feasibility, safety, and tolerability.
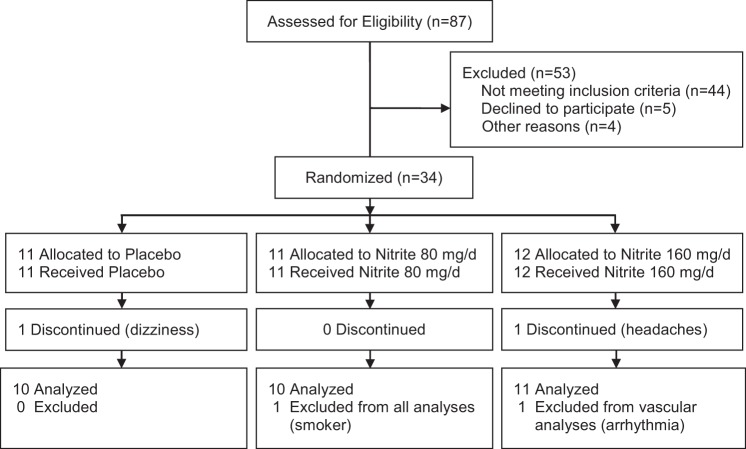
Of the 87 subjects assessed for eligibility, 34 subjects were randomized (39% enrollment rate; Fig. 1). Subjects were non-Hispanic Caucasian (84%), non-Hispanic Black/African (6%), or Hispanic (10%). With only two subjects dropping out, there was a 94% completion rate for the study. Subject adherence to the intervention was favorable, with 71% not missing taking any capsules. Of the nine subjects who missed taking a capsule, four subjects missed one, three subjects missed two and two subjects missed taking three capsules during the 10-wk intervention. Acquisition of data was successful for brachial artery flow-mediated dilation (97% analyzable), brachial artery shear rate (94% analyzable), arterial stiffness measures (94–97% analyzable), and blood analytes (71–100% analyzable).
Fig. 1.
Progress through the phases of the study.
No severe adverse events occurred (Table 4). Both doses of nitrite were well tolerated without symptomatic hypotension or clinically relevant elevations in blood methemoglobin (maximal value = 1.5%). Treatment-emergent adverse events, all of which were expected, were mild. Headache, nausea, fatigue, dizziness/lightheadedness, asymptomatic orthostatic intolerance, and dry mouth were reported. One subject, who had a history of migraines, reported headaches as her reason for dropping out of the nitrite 160 mg/day group. Another subject dropped out of the placebo group complaining of dizziness. No symptoms caused subjects to drop out of the nitrite 80 mg/day group.
Plasma nitrite.
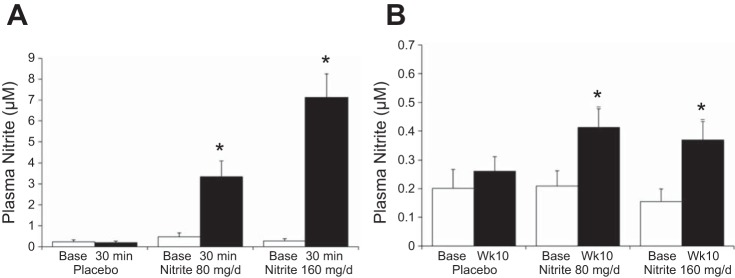
Oral sodium nitrite acutely increased plasma nitrite 5- to 15-fold at 30 min after capsule ingestion for both doses (Fig. 2A). At week 10 of the intervention, plasma nitrite remained modestly elevated in the bloodstream > 12 h after last capsule ingestion (Fig. 2B).
Fig. 2.
Changes in plasma nitrite 30 min (A) (time × treatment P < 0.001; subset of subjects: placebo n = 7, nitrite 80 mg/day n = 6, nitrite 160 mg/day n = 9) and 12–18 h (B) (time × treatment P = 0.070) after capsule ingestion at baseline (Base) and week 10 (Wk10). *P < 0.05 vs. group base.
Vascular outcomes.
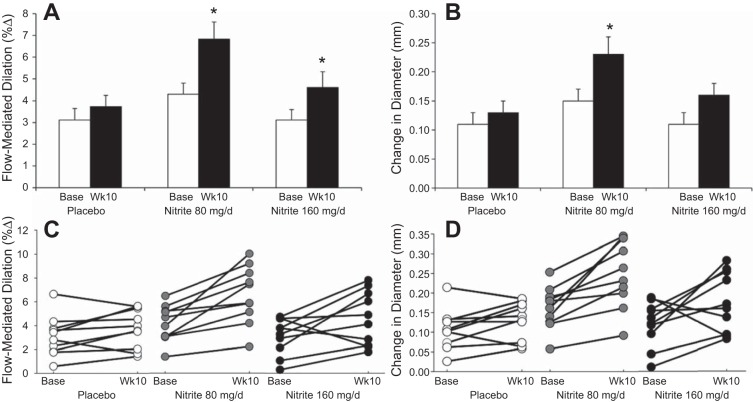
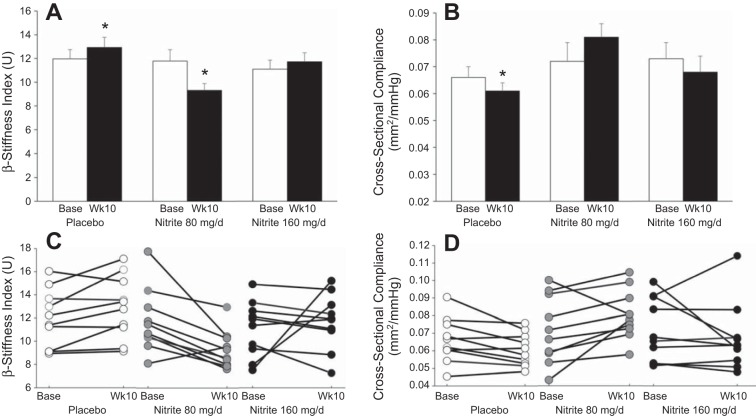
Brachial artery flow mediation dilation increased after 10 wk of nitrite supplementation for both doses (Fig. 3, A and C). Other brachial artery parameters (Table 5), including blood pressure, remained unchanged except that nitrite 80 mg/day increased the absolute change in arterial diameter (Fig. 3, B and D). Aortic stiffness, as measured by carotid to femoral pulse wave velocity, and wave reflection, as measured by augmentation index, were unchanged (Table 6). Local stiffness measures at the carotid artery had significant time × treatment interactions and, β-stiffness index was reduced from baseline in the nitrite 80 mg/day group, suggesting a possible destiffening effect of nitrite on the carotid artery (Fig. 4, A–D, Table 6).
Fig. 3.
Brachial artery flow-mediated dilation (A) (time × treatment P = 0.063) and absolute change in diameter (B) (time × treatment P = 0.099) and individual responses for flow-mediated dilation (C) and absolute change in diameter (D) at baseline and week 10. *P < 0.05 vs. group base.
Table 5.
Brachial artery parameters
| Placebo |
Nitrite 80 mg/day |
Nitrite 160 mg/day |
||||
|---|---|---|---|---|---|---|
| Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | |
| Baseline arterial diameter, mm | 3.70 ± 0.15 | 3.66 ± 0.16 | 3.59 ± 0.17 | 3.45 ± 0.12 | 3.67 ± 0.16 | 3.60 ± 0.15 |
| Peak arterial diameter, mm | 3.79 ± 0.16 | 3.81 ± 0.14 | 3.68 ± 0.12 | 3.74 ± 0.16 | 3.76 ± 0.14 | 3.78 ± 0.16 |
| Time to peak diameter, s | 52 ± 5 | 59 ± 6 | 43 ± 5 | 45 ± 3 | 47 ± 6 | 43 ± 4 |
| Shear rate, s−1* | 1743 ± 177 | 1901 ± 149 | 1872 ± 79 | 2104 ± 145 | 1832 ± 148 | 2010 ± 184 |
Values are means ± SE.
Nitrite 80 mg/day (n = 8).
Table 6.
Arterial stiffness parameters
| Placebo |
Nitrite 80 mg/day |
Nitrite 160 mg/day |
||||
|---|---|---|---|---|---|---|
| Baseline | Week 10 | Baseline | Week 10 | Baseline | Week 10 | |
| Carotid systolic pressure, mmHg | 122 ± 6 | 125 ± 4 | 121 ± 7 | 124 ± 8 | 125 ± 4 | 127 ± 7 |
| Carotid pulse pressure, mmHg | 56 ± 5 | 59 ± 4 | 57 ± 7 | 60 ± 8 | 60 ± 5 | 63 ± 6 |
| Carotid artery diameter at end-diastole, mm† | 6.55 ± 0.16 | 6.59 ± 0.13 | 6.66 ± 0.32 | 6.44 ± 0.33* | 6.58 ± 0.12 | 6.54 ± 0.12 |
| Carotid change in diameter, mm† | 0.34 ± 0.03 | 0.34 ± 0.02 | 0.37 ± 0.04 | 0.46 ± 0.06* | 0.40 ± 0.03 | 0.39 ± 0.0.03 |
| Carotid augmentation index, % | 25 ± 3 | 19 ± 5 | 20 ± 3 | 18 ± 5 | 19 ± 2 | 17 ± 2 |
| Radial augmentation index, % | −10 ± 6 | −10 ± 5 | −11 ± 5 | −11 ± 4 | −13 ± 5 | −17 ± 3 |
| Carotid to femoral PWV, cm/s‡ | 782 ± 35 | 774 ± 36 | 780 ± 55 | 845 ± 75 | 946 ± 62 | 942 ± 77 |
| Carotid to radial PWV, cm/s | 1062 ± 35 | 1040 ± 30 | 967 ± 27 | 1002 ± 39 | 1014 ± 25 | 1014 ± 34 |
Values are means ± SE.
P < 0.05 vs. group base; †P < 0.05 for time × treatment; ‡placebo (n = 9). PWV, pulse wave velocity.
Fig. 4.
Carotid artery β-stiffness index (A) (time × treatment P = 0.005) and cross-sectional compliance (B) (time × treatment P = 0.035) and individual responses for β-stiffness index (C) and cross-sectional compliance (D) at baseline and week 10. *P < 0.05 vs. group base.
Metabolomics.
To determine whether alterations in the metabolome are associated with changes in measures of vascular function, metabolites shown to change with treatment were tested for an association with functional outcomes. For subjects receiving nitrite 80 mg/day, changes in flow-mediated dilation were related to increases in two molecules, LysoPE [18:3(9Z,12Z,15Z)/0:0] and DG (28:2), and decreases in N,N-dimethyl-l-valine, 5-aminoimidazole-4-carboxamide-1-1-βd-ribofuranosyl 5′-monophosphate, and two unknown metabolites (Table 7). For subjects receiving the higher dose of nitrite, increases in l-glutamine and PC (18:1/18:1) and decreases in LysoPC (20:5) were significantly related to improvements in flow-mediated dilation. Increases in an unknown compound were significantly related to improvements in carotid artery cross-sectional compliance, whereas changes in β-stiffness index were significantly associated with increases in PE (30:0) and one unknown compound.
Table 7.
Changes in metabolites explaining variance in vascular function
| Class | β | Standard Error | P Value | |
|---|---|---|---|---|
| Flow-mediated dilation | ||||
| Nitrite 80 mg/day | ||||
| DG (28:2) | Glycerolipids | −0.646 | 0.045 | 0.044 |
| N,N-dimethyl-l-valine | Amino acids | −0.744 | 0.103 | 0.014 |
| LysoPE [18:3(9Z,12Z,15Z)/0:0] | Glycerophospholipids | 0.649 | 0.050 | 0.042 |
| C19 H50 N11 O13 P S | unknown | −0.810 | 0.238 | 0.005 |
| 5-Aminoimidazole-4-carboxamide-1-βd-ribofuranosyl 5′-monophosphate | Imidazole ribonucleosides and ribonucleotides | −0.734 | 0.361 | 0.016 |
| C35 H62 N O15 P | Unknown | −0.866 | 0.297 | 0.001 |
| Nitrite 160 mg/day | ||||
| l-glutamine | Carboxylic acid and derivatives | −0.771 | 0.071 | 0.009 |
| LysoPC (20:5) | Glycerophospholipids | −0.672 | 0.084 | 0.033 |
| PC (18:1/18:1) | Glycerophospholipids | −0.680 | 0.398 | 0.031 |
| β-Stiffness index | ||||
| Nitrite 160 mg/day | ||||
| C34 H53 N6 O2 P | Unknown | 0.785 | 0.167 | 0.007 |
| PE (30:0) | Glycerophospholipids | 0.634 | 0.592 | 0.049 |
| Cross-sectional compliance | ||||
| Nitrite 160 mg/day | ||||
| C34 H53 N6 O2 P | Unknown | −0.721 | 0.001 | 0.019 |
To determine the ability of the plasma metabolome to predict an individual's response to nitrite supplementation, baseline metabolomic profiles were compared with changes in vascular function after sodium nitrite supplementation (summary shown in Table 8). In total, 11 known metabolites populating three metabolic pathways, comprised of benzene, fatty acyl, and glycerophospholipid metabolism, were significantly associated with responsiveness to intervention for measures of flow-mediated dilation. β-Stiffness index outcomes were significantly associated with baseline concentrations of 73 metabolites from 11 metabolic pathways, including azoline, carboxylic acid, fatty acid, fatty acyl, glycerolipid, glycerophospholipid, isoprenoid, N-acrylamide, nucleoside, sphingolipid, and steroid metabolism. Lastly, an individual's responsiveness to nitrite treatment with regard to carotid artery cross-sectional compliance was significantly associated with baseline concentrations of 80 metabolites residing in 11 metabolic pathways, involving amine, carboxylic acid, fatty acid, fatty acyl, glycerolipid, glycerophospholipid, isoprenoid, prenol lipid, sphingolipid, steroid, and sterol lipid metabolites.
Table 8.
Metabolites from blood collected at baseline that predict an individual's vascular response to nitrite
| Molecular Class | Number of Metabolites Predicting Within Each Pathway |
|---|---|
| Flow-mediated dilation | |
| Benzene and substituted derivatives | 1 |
| Glycerophospholipids | 10 |
| Unknown | 15 |
| β-Stiffness index | |
| Azolines | 1 |
| Carboxylic acids and derivatives | 3 |
| Fatty acids and conjugates | 6 |
| Fatty acyls | 20 |
| Glycerolipids | 5 |
| Glycerophospholipids | 27 |
| Isoprenoids | 1 |
| N-acrylamides | 1 |
| Nucleoside and nucleotide analogues | 1 |
| Sphingolipids | 4 |
| Steroids and steroid derivatives | 4 |
| Unknown | 85 |
| Cross-sectional compliance | |
| Amines | 1 |
| Carboxylic acids and derivatives | 5 |
| Fatty acids and conjugates | 5 |
| Fatty acyls | 16 |
| Glycerolipids | 6 |
| Glycerophospholipids | 33 |
| Isoprenoids | 1 |
| Prenol lipids | 1 |
| Sphingolipids | 4 |
| Steroids and steroid derivatives | 7 |
| Sterol lipids | 1 |
| Unknown | 81 |
DISCUSSION
The primary objectives of this study were to determine our ability to recruit, retain, and successfully collect and analyze data in this population and to determine the tolerability, efficacy, and optimal dosage of chronic sodium nitrite supplementation for improving age-related impairments in endothelial function and large elastic artery stiffness. We showed that brachial artery flow-mediated dilation, a measure of endothelial function, increased with both doses of nitrite and that the elastic properties of the carotid artery, estimated by local measures of ultrasonography and tonometry, also improved. Moreover, changes in select metabolites were associated with changes in vascular function, and concentrations of several metabolites at baseline predicted responsiveness to supplementation, providing initial insight into the pathways that may be involved in the nitrite-related vascular improvements. Finally, we determined that both doses of nitrite were safe and well tolerated and that the lower dose of nitrite was sufficient to elicit these important vascular changes, solidifying the dosing protocol for a future clinical trial.
Interest in sodium nitrite as a treatment for CVD was rekindled in recent years after chronic oral supplementation was shown to protect the heart from ischemia-reperfusion injury in preclinical models (4, 8, 17) and after infusion produced transient vasodilation of conduit and resistance arteries in humans (34). Of special interest was the ability of nitrite to dilate arteries without producing tolerance (27), a phenomenon that results in a drug becoming ineffective over time, and the ability of nitrite to be stored in tissues until reduced under physiological conditions by a one-step reaction to NO in a manner independent of the enzyme endothelial nitric oxide synthase (eNOS) (5). As with CVD, advancing age is accompanied by a prooxidant milieu, in which NO bioavailability is reduced because of decreased synthesis by eNOS and/or sequestration of produced NO by the free radical superoxide (34). Therefore, oral nitrite supplementation is an attractive intervention to prevent or reduce age-related endothelial dysfunction. In the present study, we found that both doses of nitrite improved flow-mediated dilation, a measure of endothelial-dependent dilation that has been shown to predict future cardiovascular events and mortality (47, 48). To our knowledge, this is the first study to show beneficial effects of nonacute (sustained) supplementation with sodium nitrite on conduit artery health in middle-aged and older, but otherwise healthy, adults with impaired baseline endothelial function. Other recent studies have shown that 4 wk of oral nitrate, an inorganic anion that is converted by bacteria in the gut to nitrite, improves flow-mediated dilation in patients with hypertension (18) or older adults with elevated risk factors for CVD (31). In contrast, one study found that 10 wk of sodium nitrite did not alter endothelial function in patients with diabetes and peripheral arterial disease (25).
Stiffening of the large elastic arteries has been linked to CVD and is predictive of mortality and disease (24, 43). Distending pressure, NO bioavailability, and wall structure are some of the major contributors to arterial stiffness (40). In the present study, there was a significant time × treatment interaction for local arterial stiffness and compliance at the carotid artery, but aortic stiffness was unchanged by chronic nitrite supplementation. No prior studies have determined the effects of oral nitrite or nitrate on large elastic artery stiffness in healthy adults or on carotid artery elasticity in any population. Two previous studies in humans have, however, reported that 4 wk of supplementation with oral nitrate (dietary or capsules) decreases aortic stiffness in patients with elevated CVD risk factors (18, 31). Although these studies were not designed to determine the mechanisms behind these changes in stiffness, the authors speculated that decreases in blood pressure may be involved because both studies included patients with hypertension who had significant decreases in brachial artery blood pressure in response to dietary nitrate. In our study, blood pressure, measured both centrally (carotid artery) and peripherally (brachial artery), did not change. Instead, we detected a significant time × treatment effect for carotid diameter in the nitrite-supplemented groups. This, along with the increases in endothelial function in the brachial artery and increased concentrations of nitrite in the blood, suggests that NO bioavailability likely was increased by our intervention. NO lowers vascular smooth muscle tone, especially in more muscular arteries like the carotid artery (vs. the less muscular aorta) (40), possibly explaining the discrepant effects of nitrite supplementation on arterial stiffness in these two vascular beds.
Both doses of our sodium nitrite capsules increased plasma nitrite concentrations robustly at 30 min and modestly at 12 h after capsule ingestion, in agreement with their known pharmacokinetic profile (12). As for the underlying mechanisms by which physiological changes occurred, blood lipids, glucose, blood pressure, and body mass were not altered, suggesting that changes in traditional risk factors were not responsible for the improvements in vascular function with our intervention. To gain further mechanistic insight, we determined the changes in circulating metabolites with treatment. Recent studies have shown that “metabolomic signatures” can act as biomarkers and predictors of clinical disease and response to medications (2, 20, 28), making them an exciting new tool in clinical research and medicine. Our metabolomics analyses revealed combinations of specific molecular classes that were significantly associated with our vascular measures with nitrite supplementation. Of all the pathways, glycerophospholipids such as phosphatidylcholine (PC), lysoPC, phosphatidylethanolamine (PE), and lysoPE were the most frequently altered class. Decreases in lysoPC (20:5) and PC (18:1/118:1) were associated with nitrite-induced increases in brachial artery flow-mediated dilation. LysoPC (20:5) has been previously shown to be elevated in older rodents (39) and humans (19) and is implicated in atherosclerotic processes (2, 45). Furthermore, glycerophospholipids were the most abundant class of metabolites from blood samples at baseline that predicted an individual's response to nitrite for all three vascular outcome measures, suggesting that this pathway may be central to changes in physiological function with nitrite supplementation. Another class of metabolites, fatty acyls, was also predictive for carotid artery stiffness measures. These metabolites are involved in lipid oxidation and thus contribute to pathways involved in CVD (2). To our knowledge, this is the first study to determine the metabolic pathways associated with nitrite-induced changes in vascular function. The information derived from this novel technique will help to determine the metabolomic pathways of focus for future studies and may help predict individuals who will respond favorably to treatment with sodium nitrite.
Limitations of the present study include the diverse nature of the plasma metabolome, which makes it difficult to positively identify every metabolite detected, although metabolites are continually being confirmed and can potentially be validated at a later date in a larger trial. We also acknowledge that our study involved mostly Caucasians and healthy, middle-aged, and older adults. Whether sodium nitrite is well tolerated and improves vascular function in other populations, including adults with cardiovascular or kidney disease, remains to be determined.
In conclusion, we found that sodium nitrite supplementation was safe and well tolerated in healthy middle-aged and older adults with few side effects, particularly in the lower dose (nitrite 80 mg/day) group. Vascular function was improved by both doses of nitrite, especially in the nitrite 80 mg/day group, and several classes of metabolites explained the variance of vascular function with our intervention. Therefore, a large clinical trial using the lower dose of nitrite is warranted to determine the effects of sodium nitrite on age-related endothelial dysfunction and arterial stiffening. Overall, this study adds to the increasing evidence that sodium nitrite supplementation is a promising nutraceutical for the treatment of age-related processes and multiple diseases.
GRANTS
This work was supported by National Institutes of Health awards R37 AG013038 (D. Seals), T32 AG000279 (J. Justice, L. Johnson, A. DeVan), R21 HL107105 (D. Seals, A. DeVan), and CTRC Grant UL1 RR025780.
DISCLOSURES
Tony Giordano is the president and CEO of TheraVasc, and Nathan Bryan is cofounder and CSO of Neogenis Labs. Nathan Bryan also receives royalties from patents from the University of Texas Health Science Center in Houston.
AUTHOR CONTRIBUTIONS
Author contributions: A.E.D., N.S.B., M.B.M., M.B.C., A.L.S., T.G., and D.R.S. conception and design of research; A.E.D., L.C.J., F.A.B., T.D.E., C.C.-Q., N.R., N.S.B., J.R.S.-P., and C.J.B. performed experiments; A.E.D., L.C.J., F.A.B., T.D.E., J.N.J., C.C.-Q., N.R., N.S.B., M.B.M., M.B.C., C.J.B., and A.L.S. analyzed data; A.E.D., L.C.J., F.A.B., T.D.E., J.N.J., C.C.-Q., N.R., N.S.B., M.B.M., and D.R.S. interpreted results of experiments; A.E.D., L.C.J., J.N.J., and J.R.S.-P. prepared figures; A.E.D. and L.C.J. drafted manuscript; A.E.D., L.C.J., F.A.B., T.D.E., J.N.J., N.S.B., M.B.M., J.R.S.-P., M.B.C., A.L.S., T.G., and D.R.S. edited and revised manuscript; A.E.D., L.C.J., F.A.B., T.D.E., J.N.J., C.C.-Q., N.R., N.S.B., M.B.M., J.R.S.-P., M.B.C., C.J.B., A.L.S., T.G., and D.R.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Rachelle E. Kaplon, Talia Strahler, Anaheed Little, Eric Chung, Molly McNamara, Molly Nowlan, Sierra D. Hill, Rick Reisdorph, and the staff of the University of Colorado Boulder CTRC for technical assistance.
REFERENCES
- 1.Bahr TM, Hughes GJ, Armstrong M, Reisdorph R, Coldren CD, Edwards MG, Schnell C, Kedl R, LaFlamme DJ, Reisdorph N, Kechris KJ, Bowler RP. Peripheral blood mononuclear cell gene expression in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 49: 316–323, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basak T, Varshney S, Akhtar S, Sengupta S. Understanding different facets of cardiovascular diseases based on model systems to human studies: A proteomic and metabolomic perspective. J Proteomics 127: 50–60, 2015. [DOI] [PubMed] [Google Scholar]
- 3.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care 23: 57–63, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bryan NS, Calvert JW, Elrod JW, Gundewar S, Ji SY, Lefer DJ. Dietary nitrite supplementation protects against myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA 104: 19144–19149, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med 45: 468–474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med 43: 645–657, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donato AJ, Morgan RG, Walker AE, Lesniewski LA. Cellular and molecular biology of aging endothelial cells. J Mol Cell Cardiol 89: 122–135, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest 115: 1232–1240, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. J Physiol 568: 1057–1065, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fleenor BS, Sindler AL, Eng JS, Nair DP, Dodson RB, Seals DR. Sodium nitrite de-stiffening of large elastic arteries with aging: role of normalization of advanced glycation end-products. Exp Gerontol 47: 588–594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. J Appl Physiol 102: 63–71, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Greenway FL, Predmore BL, Flanagan DR, Giordano T, Qiu Y, Brandon A, Lefer DJ, Patel RP, Kevil CG. Single-dose pharmacokinetics of different oral sodium nitrite formulations in diabetes patients. Diab Technol Ther 14: 552–560, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension 55: 1075–1085, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PW, Woo YJ. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 123: 933–944, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Hirai T, Sasayama S, Kawasaki T, Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation 80: 78–86, 1989. [DOI] [PubMed] [Google Scholar]
- 16.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging 26: 631–640, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Johnson G 3rd, Tsao PS, Mulloy D, Lefer AM. Cardioprotective effects of acidified sodium nitrite in myocardial ischemia with reperfusion. J Pharmacol Exp Ther 252: 35–41, 1990. [PubMed] [Google Scholar]
- 18.Kapil V, Khambata RS, Robertson A, Caulfield MJ, Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension 65: 320–327, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JY, Kim OY, Paik JK, Kwon DY, Kim HJ, Lee JH. Association of age-related changes in circulating intermediary lipid metabolites, inflammatory and oxidative stress markers, and arterial stiffness in middle-aged men. Age (Dordr) 35: 1507–1519, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krauss RM, Zhu H, Kaddurah-Daouk R. Pharmacometabolomics of statin response. Clin Pharmacol Ther 94: 562–565, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol (Oxf) 191: 59–66, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419, 1985. [DOI] [PubMed] [Google Scholar]
- 23.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res 49: 1137–1146, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 121: 505–511, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohler ER 3rd, Hiatt WR, Gornik HL, Kevil CG, Quyyumi A, Haynes WG, Annex BH. Sodium nitrite in patients with peripheral artery disease and diabetes mellitus: safety, walking distance and endothelial function. Vasc Med 19: 9–17, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 131: e29–e322, 2015. [DOI] [PubMed] [Google Scholar]
- 27.Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res 97: 618–628, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Patel S, Ahmed S. Emerging field of metabolomics: big promise for cancer biomarker identification and drug discovery. J Pharm Biomed Anal 107: 63–74, 2015. [DOI] [PubMed] [Google Scholar]
- 29.Pierce GL, Beske SD, Lawson BR, Southall KL, Benay FJ, Donato AJ, Seals DR. Weight loss alone improves conduit and resistance artery endothelial function in young and older overweight/obese adults. Hypertension 52: 72–79, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pluta RM, Oldfield EH, Bakhtian KD, Fathi AR, Smith RK, Devroom HL, Nahavandi M, Woo S, Figg WD, Lonser RR. Safety and feasibility of long-term intravenous sodium nitrite infusion in healthy volunteers. PLoS One 6: e14504, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M, Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. J Am Coll Cardiol 63: 1584–1585, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Gutierrez V, Perez-Camino MC. Update on solid-phase extraction for the analysis of lipid classes and related compounds. J Chromatogr A 885: 321–341, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci 120: 357–375, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sindler AL, DeVan AE, Fleenor BS, Seals DR. Inorganic nitrite supplementation for healthy arterial aging. J Appl Physiol 116: 463–477, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sindler AL, Fleenor BS, Calvert JW, Marshall KD, Zigler ML, Lefer DJ, Seals DR. Nitrite supplementation reverses vascular endothelial dysfunction and large elastic artery stiffness with aging. Aging Cell 10: 429–437, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobko T, Marcus C, Govoni M, Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric Oxide 22: 136–140, 2010. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 10: 1–10, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomas-Loba A, Bernardes de Jesus B, Mato JM, Blasco MA. A metabolic signature predicts biological age in mice. Aging Cell 12: 93–101, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens 15: 445–452, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Van Pelt RE, Davy KP, Stevenson ET, Wilson TM, Jones PP, Desouza CA, Seals DR. Smaller differences in total and regional adiposity with age in women who regularly perform endurance exercise. Am J Physiol Endocrinol Metab 275: E626–E634, 1998. [DOI] [PubMed] [Google Scholar]
- 43.van Sloten TT, Schram MT, van den Hurk K, Dekker JM, Nijpels G, Henry RM, Stehouwer CD. Local stiffness of the carotid and femoral artery is associated with incident cardiovascular events and all-cause mortality: the Hoorn study. J Am Coll Cardiol 63: 1739–1747, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 51: 784–790, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu R, Huang YH, Elinder LS, Frostegard J. Lysophosphatidylcholine is involved in the antigenicity of oxidized LDL. Arterioscler Thromb Vasc Biol 18: 626–630, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Cruickshank C, Armstrong M, Mahaffey S, Reisdorph R, Reisdorph N. New sample preparation approach for mass spectrometry-based profiling of plasma results in improved coverage of metabolome. J Chromatogr A 1300C: 217–226, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation 115: 2390–2397, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]