Abstract
Muscle wasting occurs in a variety of clinical situations, including denervation. There is no effective pharmacological treatment for muscle wasting. In this study, we used a tibial nerve denervation model to test acupuncture plus low-frequency electric stimulation (Acu-LFES) as a therapeutic strategy for muscle atrophy. Acupuncture needles were connected to an SDZ-II electronic acupuncture device delivering pulses at 20 Hz and 1 mA; the treatment was 15 min daily for 2 wk. Acu-LFES prevented soleus and plantaris muscle weight loss and increased muscle cross-sectional area in denervated mice. The abundances of Pax7, MyoD, myogenin, and embryonic myosin heavy chain were significantly increased by Acu-LFES in both normal and denervated muscle. The number of central nuclei was increased in Acu-LFES-treated muscle fibers. Phosphorylation of Akt was downregulated by denervation leading to a decline in muscle mass; however, Acu-LFES prevented the denervation-induced decline largely by upregulation of the IGF-1 signaling pathway. Acu-LFES reduced the abundance of muscle catabolic proteins forkhead O transcription factor and myostatin, contributing to the attenuated muscle atrophy. Acu-LFES stimulated the expression of macrophage markers (F4/80, IL-1b, and arginase-1) and inflammatory cytokines (IL-6, IFNγ, and TNFα) in normal and denervated muscle. Acu-LFES also stimulated production of the muscle-specific microRNAs miR-1 and miR-206. We conclude that Acu-LFES is effective in counteracting denervation-induced skeletal muscle atrophy and increasing muscle regeneration. Upregulation of IGF-1, downregulation of myostatin, and alteration of microRNAs contribute to the attenuation of muscle atrophy in denervated mice.
Keywords: IGF-1, myostatin, macrophage, Akt, FoxO, cytokines
when skeletal muscles are denervated, they undergo atrophy. This occurs in a variety of clinical situations including trauma, motor neuron disease (amyotrophic lateral sclerosis), systemic diseases (alcoholic neuropathy or pernicious anemia), and viral infections such as polio (2, 15, 27, 39). After nerve injury, muscle fibers will progressively undergo an irreversible degeneration process if reinnervation does not occur (40). Denervation lesions result in the loss of muscle proteins, mainly reflecting increased breakdown of myofibrillar proteins, leading to muscle atrophy (45). The clinical consequences of muscle atrophy are fragility and weakness with significantly reduced quality of life. Muscle wasting is an independent index of mortality and morbidity (10, 50). There is currently no reliable pharmacological treatment to prevent muscle atrophy, so effective therapies to treat muscle wasting are needed.
Understanding the underlying mechanisms of skeletal muscle atrophy is critical for development of treatments. Skeletal muscle atrophy and hypertrophy are caused by alterations in a fine balance between protein synthesis and degradation (28). The insulin-like growth factor 1 (IGF-1)/Akt/mammalian target of rapamycin (mTOR) signaling pathway has been shown to be crucial to protein metabolism and muscle regeneration (54). Two major components in this pathway are Akt and forkhead family O (FoxO). Phosphorylation of Akt inhibits FoxO, leading to increased protein synthesis and decreased protein degradation. FoxO activation (dephosphorylation) can induce two important proteolytic pathways: the ubiquitin-proteasome pathway (UPS) and the lysosomal-autophagy pathway, leading to muscle wasting (26). In general, treatments that could upregulate IGF-1 and phosphorylate Akt would increase muscle mass and counteract muscle atrophy (45). In addition myostatin is considered a negative regulator of muscle mass (52). Therefore, inhibition of myostatin would also reduce muscle atrophy (6, 53).
Many potential therapeutic strategies to limit muscle atrophy have been proposed including the use of pharmaceuticals, antisense oligonucleotides, or gene therapy, which have been proven to be partially effective in mice (6) but have met with limited success in humans. Clinical trials for pharmacological inhibitors of myostatin are ongoing, but none have yielded a reagent for clinical use (32, 41) (https://www.clinicaltrials.gov/ct2/show/NCT01958970). The validated treatment for muscle wasting in humans is exercise, which reduces various types of muscle atrophy (1, 9). Previously we discovered that exercise has the ability to prevent a decrease of skeletal muscle mass induced by chronic kidney disease (44). Unfortunately, patients with severe diseases or those with acute illnesses are often bedridden and unable to exercise regularly. Thus there is a medical need to develop new, non-exercise-based therapies that will increase muscle mass and strength and improve patient quality of life and survival.
Acupuncture offers unique clinical advantages over pharmaceutical approaches to many disease states (8, 24). We and others have evidence that acupuncture plus low-frequency electrical stimulation (Acu-LFES) can counteract muscle atrophy (12, 31, 36, 37). Acu-LFES can suppress muscle atrophy induced by hindlimb suspension in mice (31) and increase satellite cell proliferation in skeletal muscle (37). However, the effect of Acu-LFES on muscle atrophy resulting from denervation has not been established. Various muscle and nerve stimulation paradigms have been used in the U.S. and around the world during recent years (25), including transcutaneous electrical nerve stimulation (TENS) and functional electrical stimulation (FES). In this study we will refer to Acu-LFES, which delivers electrical stimulation through needles inserted into muscle, as opposed to electrical muscle stimulation (SEMS), which delivers electrical stimulation to the skin surface by use of flat adhered electrodes. SEMS has been used as strength training in healthy people and athletes, a rehabilitation and preventive tool for disease subjects (muscle atrophy or pain release), a testing method for evaluation of neural or muscle function, and for postexercise recovery or cosmetic purposes (3, 4, 17).
The purpose of this study is to test whether Acu-LFES can be used for a therapeutic intervention for muscle atrophy induced by denervation. We investigated the impact of Acu-LFES on myogenesis, IGF-1/Akt, myostatin and FoxO signaling pathways, and microRNA expression in denervation-related muscle atrophy. We report here that Acu-LFES counteracts muscle atrophy by influencing muscle regeneration and upregulation of IGF-1 in denervated mice and could provide an additional therapeutic option for treatment of denervation muscle atrophy.
MATERIALS AND METHODS
Animals and Denervation Model
Male C57/BL6 mice (8 wk) were used for denervation models and acupuncture treatments. Mice were randomly divided into four groups: normal control, control + Acu-LFES (Acu-LFES), denervation, and denervation + Acu-LFES (denervation/Acu-LFES). Each group had four mice, and experiments were repeated three times. The denervation animal model and Acu-LFES experiments were approved by the Institutional Animal Care and Use Committee of Emory University. Denervation was induced by transection of the medial and lateral branches of the tibial nerve that innervates the plantar flexor muscles (i.e., gastrocnemius, soleus, and plantaris) close to their neuromuscular junction. The cut nerve end was sutured into the quadriceps muscle to ensure that the nerve stumps do not reinnervate.
Acu-LFES Treatment
The mice were kept in specially designed restraints so that they would remain in a recumbent position during Acu-LFES treatment. Mice were awake without any anesthesia and appeared to be comfortable throughout the treatments. Acupuncture points selected were according to the WHO Standard Acupuncture guidelines (22). The positive point (anode: Yang Ling Quan, GB34) is in the hollow of the exterior-inferior of the caput fibulae about 6 mm deep. This position is close to the superficial fibular nerve and deep fibular nerve. The negative point (cathode: Zu San Li, ST36) is 5 mm beneath the capitulum fibulae and located laterally and posterior to the knee joint about 7 mm deep and close to fibular nerve. The impulses were delivered between the two acupuncture needles. Disposable sterile needles with a diameter of 0.25 mm (Shen Li Medical & Health Material, Wujiang, China) were used. The needles were connected into SDZ-II Electronic acupuncture instrument using consistent pulse, electric frequency of 20 Hz, and electric current of 1 mA. These stimulation conditions were chosen because 1) they were effectively used in a previous study (14), 2) we have found that they are effective in treating muscle atrophy in mice with CKD (12), and 3) the mice do not appear to be uncomfortable at this level of stimulation.
Experimental Design
Denervation surgery was performed on day 1, and Acu-LFES was started on day 2. The Acu-LFES was administered for 15 min every day for 2 wk at 10 A.M. Hindlimb muscles (gastrocnemius, soleus, and plantaris) were harvested 48 h after the last Acu-LFES treatment. The control mice were placed in these specially designed restraints and remain in a recumbent position without Acu-LFES treatment for an equal amount of time and for the same number of “treatments.”
Western Blot, Muscle Histology, and Antibodies
Hindlimb muscles were homogenized in gentle lysis buffer (10 mM Tris-HCl, 10 mM NaCl, 2 mM EDTA, 0.5% NP-40, 1% glycerol, and freshly added 1 mM Na3VO4, 10 μg/ml PMSF, 5 μg/ml aprotinin, 1 μg/ml leupeptin) with phosphatase inhibitors cocktail 1 and 2 (Sigma). Proteins were subjected to Western blot analysis using previously published methods (55).
Immunohistology.
Muscles were embedded under Tissue Freezing Media (TBS; Fisher, Pittsburgh, PA) in isopentane cooled in dry ice. Cross sections (10 mm) from the midbelly of different groups of muscle were mounted on gelatin-coated slides were fixed in 4% paraformaldehyde for 10 min. Tissue was permeabilized in 0.05% Triton X-100 (in PBS) for 10 min, and quench-fixed in 50 mM NH4Cl for another 10 min. Samples were blocked with 5% bovine serum albumin for 1 h, followed by incubation overnight with first antibody. Sections were further incubated for 60 min with rhodamine red-labeled anti-rabbit IgG (diluted 1:100; Jackson Immuno Research Lab, West Grove, PA). Nuclei were stained by DAPI. Images were visualized with an Olympus 1X51 inverted fluorescence microscope and captured by DP73-1-51-17MP color camera. The positive cell number of at least 500 individual myofibers per muscle was measured using the CellSens Dimension 1.9 with count and measure full Software (Olympus, Melville, NY). Muscle fiber cross-sectional area was determined in plantaris muscles using an anti-laminin antibody (1:50 dilution; Sigma-Aldrich).
Muscle histology.
Gastrocnemius muscles were processed for cryosectioning after placing them in cryomolds containing embedding medium. The frozen sections were stained using a Masson modified IMEB trichrome stain kit according to the manufacturer's instructions (International Medical Equipment B; San Marcos, CA).
Primary antibodies.
Primary antibodies (1:1,000 dilution except indicated) that we used included Akt, p-Akt (Ser473), FoxO1, FoxO3, p-FoxO1/3 (Thr24/Thr32), mTOR, p-mTOR (Ser2448), P70S6K, p-P70S6K (Thr389), p-Smad3 (Ser423/425), p-Smad2 (Ser465/467), and Smad2/3 (D7G7) from Cell Signaling (Danvers, MA), GDF8/myostatin from Abcam (Cambridge, MA), MyoD, Pax7, Myogenin and embryonic myosin heavy chain (eMyHC) from DSHB product (University of Iowa), and GAPDH from Millipore (Burlington, MA). Protein bands were scanned and quantified using the Li-cor Odyssey infrared scanning system (Li-COR Biosciences, Lincoln, Nebraska).
Enzyme-Linked Immunosorbent Assay (ELISA)
Mouse IGF-1 in muscle lysates were quantized by IGF1 Mouse ELISA Kit (ab100695, Abcam, Cambridge, MA) and used according to manufacturer's instructions. Plasma cytokine levels were measured by Bioassay Services Team using the cytokine protein ELISA quantification assay (AssayGate, Ijamsville, MD).
Reverse Transcription and Quantitative PCR (q-PCR) for mRNA and microRNA
Total RNA was extracted using Tri-Reagent (Molecular Research, Cincinnati, OH) (13). For mRNA, reverse transcription was performed using the M-MLV reverse transcriptase (Invitrogen) and 2 μg denatured RNA according to the manufacturer's instructions. Real-time qPCR was performed with SsoAdvUniver SYBR Green PCR Reagents (Bio-Rad, Hercules, CA) using the following cycle parameters: 94°C for 2 min and 40 cycles at 94°C for 15 s, 55°C for 30 s, 72°C for 30 s with final extension at 72°C for 10 min. The quantification cycle (Cq) values was defined as the number of cycles required for the fluorescence signal to exceed the detection threshold. Individual mRNA expression was standardized to 18S rRNA and expression was calculated as the difference between the threshold values of the two genes (2-ΔCq). Melting curve analysis was routinely performed to verify the specificity of the reaction. Primers for specific genes were designed to cross intron-exon boundaries and used to generate amplicons in their linear ranges (Table 1). For microRNA, the miRCURY LNA Universal cDNA Synthesis kit (Exiqon, Woburn, MA) was used for reverse transcription of total RNA. The primers were purchased from Exiqon. The miRCURY LNA microRNA PCR SYBR Green master mix (Exiqon INC) was used for qPCR with the following cycle parameters: 95°C for 10 min and 45 cycles at 95°C for 10 s and 60°C for 60 s. Expression of individual microRNA was standardized to the mouse U6 gene.
Table 1.
Primer sequences
| Name | Sequence | Amplicon | Code | |
|---|---|---|---|---|
| IL-6 | Forward | TTCCATCCAGTTGCCTTCTTG | 101 | NM_031168 |
| Reverse | TTGGGAGTGGTATCCTCTGTGA | |||
| IFN gamma | Forward | TGCTGATGGGAGGAGATGTCT | 101 | NM_008337 |
| Reverse | TTTCTTTCAGGGACAGCCTGTT | |||
| TNF alpha | Forward | GACGTGGAACTGGCAGAAGAG | 101 | U68415.1 |
| Reverse | GCCACAAGCAGGAATGAGAAG | |||
| F4/80 | Forward | CTTTGGCTATGGGCTTCCAGTC | 165 | X93328 |
| Reverse | GCAAGGAGGACAGAGTTTATCGTG | |||
| IL-1 beta | Forward | CTTCCCCAGGGCATGTTAAG | 101 | NM_008361 |
| Reverse | ACCCTGAGCGACCTGTCTTG | |||
| IGF-1 | Forward | GACCGCACCTGCAATAAAG | 91 | NM_010512 |
| Reverse | TGTGGTGGAGCTGGTGAAG | |||
| Myostatin | Forward | GACAGTGTCAGCGTGTGGGTT | 74 | NM_001112698 |
| Reverse | CCAACACCATCACCTCCTT | |||
| Arg1 | Forward | AACACGGCAGTGGCTTTAAC | 168 | NM_007482 |
| Reverse | GAGGAGAAGGCGTTTGCTTA | |||
| 18S | Forward | CCAGAGCGAAAGCATTTGCCAAGA | 101 | X00686 |
| Reverse | TCGGCATCGTTTATGGTCGGAACT |
Statistical Analysis
Data are presented as means ± SE. To identify significant differences between two groups, comparisons were made by using the t-test. For a comparison of more than two groups, two-way ANOVA was performed with a post hoc analysis by the Student-Newman-Keuls test. A chi-square analysis was used to analyze shifts in the muscle cross-sectional area. Differences with P values < 0.05 were considered significant.
RESULTS
Acu-LFES Prevents Denervation-Induced Muscle Wasting
Mice (C57BL/6J, 8 wk) were randomly assigned into four groups: control, Acu-LFES, denervation, and denervation/Acu-LFES (n = 12/group). The ratio of individual muscle weight to body weight was measured to identify muscle wasting. The weight ratios of gastrocnemius muscle, but not soleus and plantaris muscles, in the Acu-LFES mice were increased compared with the weight ratio in control mice without Acu-LFES (Table 2). Denervation caused a reduction in relative muscle weight by 37% (soleus), 34% (plantaris), and 20% (gastrocnemius) vs. control muscle. Acu-LFES alleviated some of the muscle wasting. The average weight ratio in denervation/Acu-LFES mice was significantly higher vs. denervation mice without Acu-LFES in gastrocnemius, soleus, and plantaris muscles.
Table 2.
Muscle and body weights
| Control | Acu + LFES | Denervation | Denervation/Acu + LFES | |
|---|---|---|---|---|
| Body weight, g | 23.7 ± 1.1 | 24.5 ± 1.3 | 22.5 ± 0.9 | 23.2 ± 1.6 |
| Soleus, mg | 9.7 ± 1.0 | 10.7 ± 0.5 | 6.1 ± 0.6* | 8.5 ± 0.9*# |
| Plantaris, mg | 9.8 ± 0.3 | 10.8 ± 0.4 | 6.5 ± 0.7* | 8.7 ± 0.4*# |
| Gastrocnemius, mg | 135.9 ± 5.1 | 163.4 ± 11.1* | 109.1 ± 4.3* | 133.7 ± 4.9# |
| Soleus/body, ×105 | 40.9 ± 3.1 | 43.6 ± 4.1 | 27.1 ± 2.5* | 36.6 ± 2.4*# |
| Plantaris/body, ×105 | 41.3 ± 2.9 | 44.0 ± 3.2 | 28.9 ± 4.2* | 37.5 ± 5.1*# |
| Gastrocnemius/body, ×105 | 573.4 ± 4.1 | 666.9 ± 10.6* | 505.7 ± 7.7* | 576.3 ± 9.2# |
Data are presented as means ± SE; n = 12/group. Acu-LFES, acupuncture with low-frequency electrical stimulation. P < 0.05 is significant
vs. control,
vs. denervation.
Acu-LFES Improves Muscle Regeneration in Normal and Denervation Mice
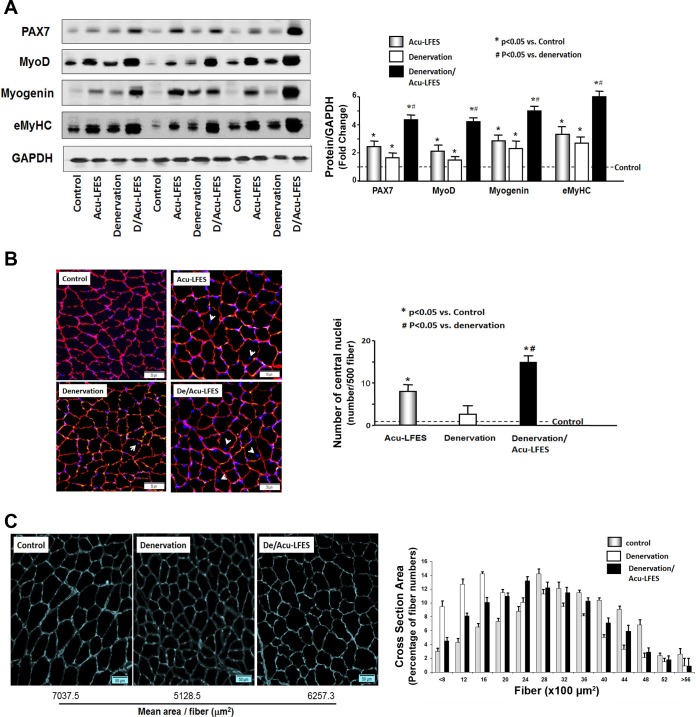
In a separate experiment, we compared control (no treatment) and manual acupuncture (insertion of the needles without electrical stimulation). We did not see a statistical difference in the myogenic protein makers (myoD, myogenin and eMyHC) or the number of central nuclei and collagen accumulation (data not shown) between the muscle from control and manual acupuncture mice. Acupuncture accompanied with electrical stimulation did show a positive effect on denervation-induced muscle atrophy as we show below. To study how Acu-LFES prevents muscle wasting in denervation mice, we first measured protein amounts of myogenesis markers which indicate the regeneration capacity of the muscles. Acu-LFES stimulates protein production under baseline conditions since in the muscle of Acu-LFES mice, the protein expressions of Pax7 (transcription factor to initial myogenesis), MyoD (proliferation marker), myogenin (differentiation marker), and eMyHC (terminal differentiation marker) were significantly increased compared with control mice (Fig. 1A). The denervation alone also increased muscle regeneration markers vs. control mice; however, Acu-LFES caused a substantial additional increase in all myogenesis markers (2.5-fold in Pax7, 2.8-fold in myoD, 2.2-fold in myogenin, and 2.5 fold in eMyHC) in denervated muscle. Interestingly, the increase in myogenesis markers by Acu-LFES on the denervation background was also significantly higher than Acu-LFES-mediated increase in control muscle without denervation. These results demonstrate that Acu-LFES counteracts denervation-induced muscle loss by activation of muscle regeneration.
Fig. 1.
Acupuncture plus low-frequency electrical stimulation (Acu-LFES) increases muscle regeneration. A: Western blot of Pax7, myoD, myogenin, and eMyHC in hindlimb (gastrocnemius, soleus, and plantaris) muscle lysates from normal control, Acu-LFES, denervation, or denervation/Acu-LFES mice. The bar graph compares the mean densities of all bands in each group expressed as a fold change from levels in normal control mice which is represented by a line at 1-fold. All band densities were normalized to the density of the GAPDH band (bars: means ± SE; n = 12/group; *P < 0.05 vs. control, #P < 0.05 vs. denervation). B: representative cross-sections from plantaris muscles after staining for laminin (red, cell perimeter) and counterstaining with DAPI (blue, nuclei) are shown in control, Acu-LFES, denervation, or denervation/Acu-LFES mice. The white arrows point to central nuclei inside of myofibers which indicates satellite cell migration. The bar graph shows the number of nuclei that have migrated to the center of the cells (central nuclei) per 500 muscle fibers (bars: means ± SE; n = 9; *P < 0.05 vs. control, #P < 0.05 vs. denervation). C: a representative cross-sectional area of plantaris muscle from control, denervation, or denervation/Acu-LFES mice are compared. Cryosections of plantaris muscle were immunostained with anti-laminin antibody. The frequency distribution of fiber cross-sectional area in control (gray bar), denervation (open bar), and denervation/Acu-LFES (black bar) mice is presented as percent cross-section area of fibers. The cross-section area is significantly different between denervation and denervation/Acu-LFES group according to a Chi-square analysis (P < 0.05, n = 6).
Central nuclei are indicators of muscle regeneration. Upon initiation of myogenesis by Acu-LFES, the central nuclei migrate into the myofibers (Fig. 1B). We found increased central nuclei in muscles of both denervation and control mice treated with Acu-LFES. However, the percentage increase in central nuclei was higher in the muscles of denervation/Acu-LFES than of control/Acu-LFES mice. We also found that Acu-LFES prevents denervation-induced decrease in the muscle fiber cross-sectional area. Muscle fiber cross-sectional area was determined in frozen sections of plantaris muscles using an anti-laminin antibody. The size of muscle fibers was significantly smaller in denervation mice without Acu-LFES than in denervation mice with Acu-LFES. Fiber area frequency distribution revealed a clear increase in the percentage of larger fibers (shift to the right) in Acu/LFES-treated denervation mice relative to the distribution curve for denervation alone (Fig. 1C). These results show that Acu-LFES effectively reduces muscle atrophy.
Acu-LFES Upregulates IGF-1 in Skeletal Muscle of Normal and Denervated Mice
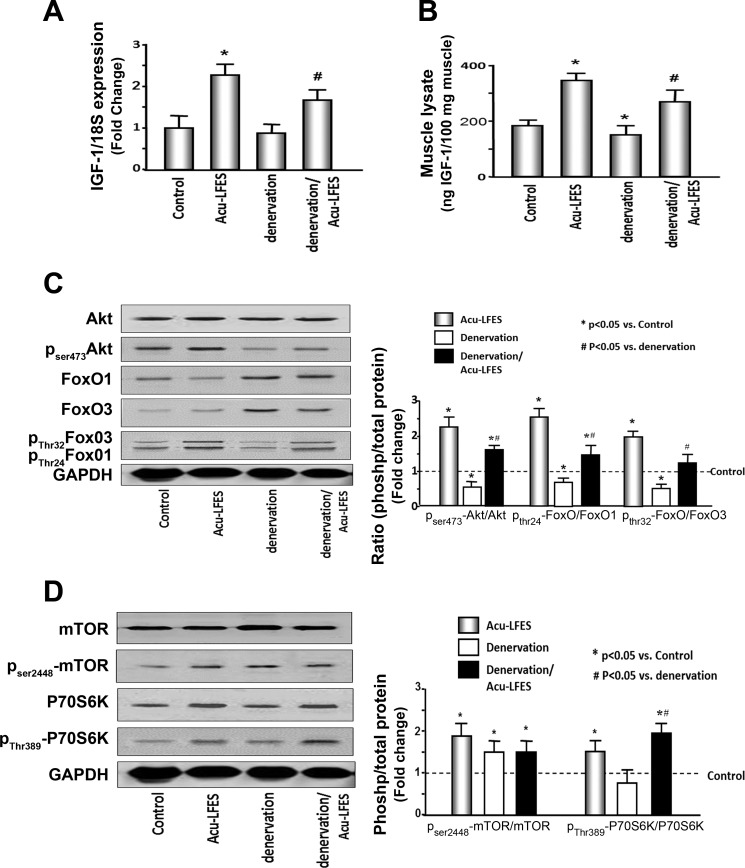
To investigate how Acu-LFES activates the muscle regeneration process, we identified mRNA expression of IGF-1 using quantitative real-time PCR (qPCR). Acu-LFES increased IGF-1 expression by 2.2-fold in normal control mice. IGF-1 expression was slightly decreased in denervated muscle at 2 wk, but was increased 1.9-fold in denervation/Acu-LFES muscle (Fig. 2A). Protein levels of IGF-1 were measured by ELISA in muscle lysates. Denervation mice showed 10% lower levels of IGF vs. control mice. Acu-LFES raised IGF-1 protein by 1.6-fold in muscle of denervation mice (Fig. 2B). These results indicate that IGF-1 is increased by Acu-LFES treatment in the muscle of normal and denervated mice, which could contribute to increased muscle regeneration.
Fig. 2.
Acu-LFES upregulates IGF-1 mRNA and protein in the muscle of denervated mice. A: total RNAs were isolated from hindlimb muscles of control, Acu-LFES, denervation, or denervation/Acu-LFES mice. The expression of IGF-1 mRNA was assayed by real-time qPCR. The bar graph shows mean mRNA from the muscles of each group of mice. Results are normalized to 18S RNA (bars: means ± SE; n = 12/group; *P < 0.05 vs. control, #P < 0.05 vs. denervation). B: IGF-1 protein levels were measured by ELISA in hindlimb muscle lysates from normal control, Acu-LFES, denervation, or denervation/Acu-LFES mice. The bar graph shows the mean of IGF-1 protein level in each group. IGF-1 levels in the muscle lysates were normalized to the total protein concentration (bars: means ± SE; n = 9; *P < 0.05 vs. control, #P < 0.05 vs. denervation). C: the protein metabolism-related proteins Akt, p-Akt, FoxO1, p-FoxO1, FoxO3, and pFoxO3 were measured by Western blotting in hindlimb muscle lysates from control, Acu-LFES, denervation, or denervation/Acu-LFES mice. Since the phosphorylation of these proteins indicates protein activation or inhibition, we expressed the data as the ratio of each phosphoprotein to total protein. The bar graph shows the fold change of the each protein band ratio compared with levels in control mice (represented by a line at 1-fold). All band densities were normalized to the density of GAPDH (bars: means ± SE; n = 12/group; *P < 0.05 vs. control, #P < 0.05 vs. denervation). D: Acu-LFES improves protein anabolic markers in skeletal muscle of denervated mice. The protein synthesis-related markers mTOR, p-mTOR, p70S6K, and p-p70S6K were measured by Western blotting in hindlimb muscle lysates from normal control, Acu-LFES, denervation, or denervation/Acu-LFES mice. The bar graph shows the fold change of the each protein band ratio compared with levels in control mice (represented by a line at 1-fold). All band densities were normalized to the density of GAPDH (bars: means ± SE; n = 12/group; *P < 0.05 vs. control, #P < 0.05 vs. denervation).
Acu-LFES Increases Akt Phosphorylation and Inhibits FoxO Activation in Denervation Mice
Activation of Akt leads to phosphorylation and inactivation of FoxO which reduces its pro-catabolic functions (46). We assayed phosphorylation of Akt, FoxO1, and FoxO3 in all groups of mice (Fig. 2C). Since phosphorylation of these proteins indicates protein activation or inhibition, we expressed the data as the ratio of phosphoprotein to total protein. Acu-LFES treatment increased the pAkt/Akt ratio 2.2-fold in normal control muscle and 2.9-fold in denervation muscle. The ratio of pFoxO1 thr24/FoxO1 increased 2.5-fold in Acu-LFES-treated normal mice and 2.1-fold in denervation muscle. The ratio of pFoxO3 thr32/FoxO3 increased 2.0-fold in Acu-LFES-treated normal mice and 2.4-fold in denervation muscle. These results demonstrate that Acu-LFES improves protein metabolism by increasing Akt and FoxO phosphorylation.
Acu-LFES Upregulates Phosphorylation of Mammalian Target of Rapamycin (mTOR) and P70S6 Kinase (P70S6K) in Normal and Denervated Mice
Phosphorylated mTOR and P70S6K are the acknowledged active forms of these proteins and upregulated during muscle protein anabolism. The ratio of phospho-mTOR to total mTOR was increased 1.8-fold by Acu-LFES in normal control muscle. Denervation alone also increased mTOR phosphorylation by 1.5-fold. Acupuncture did not promote an additional increase in phospho-mTOR above levels in the denervation muscle. The phosphorylation of the P70S6K increased 1.5-fold in Acu-LFES treated normal mice. Unlike p-mTOR, denervation did not change pP70S6K; however, pP70S6K was increased 2.5-fold by Acu-LFES treatment in the denervation mice. These results suggest that Acu-LFES increases phosphorylation of P70S6K and may lead to increased protein anabolism (Fig. 2D).
Acu-LFES Decreases Myostatin in Normal and Denervation Mice
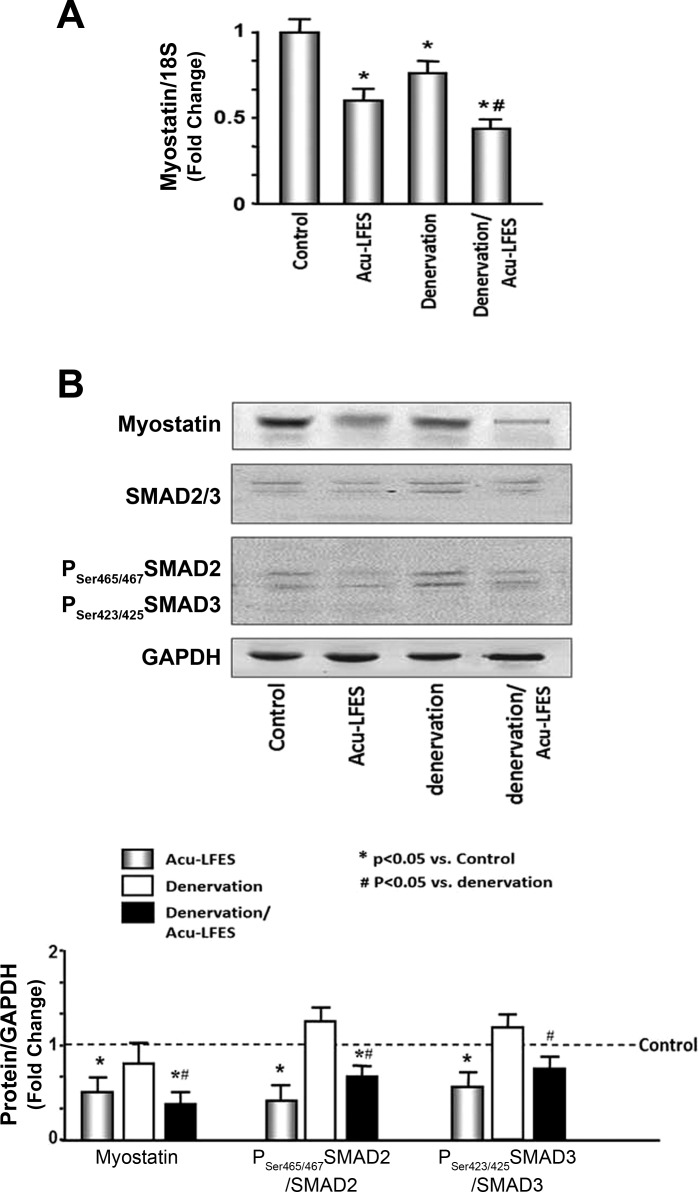
Decreased myostatin is an indicator of increasing muscle mass. To identify whether Acu-LFES changes the expression of myostatin, mRNA and protein levels of myostatin were measured in all groups of mice. Acu-LFES decreased myostatin expression by 35% in normal control mice. Denervation also decreases myostatin expression; however, Acu-LFES had an additional 23% decrease in denervation muscle (Fig. 3A). Myostatin protein was 55% decreased by Acu-LFES in normal control mouse. We did not see a significant change after 2 wk in denervation muscle; but Acu-LFES reduced the myostatin protein level by 53% in denervated muscle (Fig. 3B). Myostatin induces muscle atrophy by activation of the transcription factors SMAD2 (Mothers against decapentaplegic homolog 2) and SMAD3 (33). We found that phosphorylation (activation) of SMAD2 and SMAD3 was repressed by Acu-LFES in denervation muscle consistent with the observed decrease in myostatin. These results indicate that one of the ways that Acu-LFES ameliorates denervation-induced muscle atrophy is by suppressing myostatin, pSmad2, and pSmad3.
Fig. 3.
Acu-LFES downregulates myostatin mRNA and protein in the muscle of denervated mice. A: total RNAs were isolated from hindlimb muscles of control, Acu-LFES, denervation, or denervation/Acu-LFES mice. The expression of myostatin was assayed by real-time qPCR. The bar graph shows mRNA from the muscles of each group of mice. Results are normalized to 18S RNA (bars: means ± SE; n = 12/group; *P < 0.05 vs. control, #P < 0.05 vs. denervation). B: myostatin, SMAD 2/3, and phosphorylated SMAD 2/3 were measured by Western blotting in hindlimb muscle lysates from control, Acu-LFES, denervation, or denervation/Acu-LFES mice. The data are expressed as the ratio of each phosphoprotein to total protein. The bar graph shows the fold change of the each protein band ratio compared with levels in control mice (represented by a line at 1-fold). All band densities were normalized to the density of GAPDH (bars: means ± SE; n = 12/group; *P < 0.05 vs. control, #P < 0.05 vs. denervation).
Acu-LFES Activates Macrophages and Increases Inflammatory Cytokines in Normal and Denervated Muscle
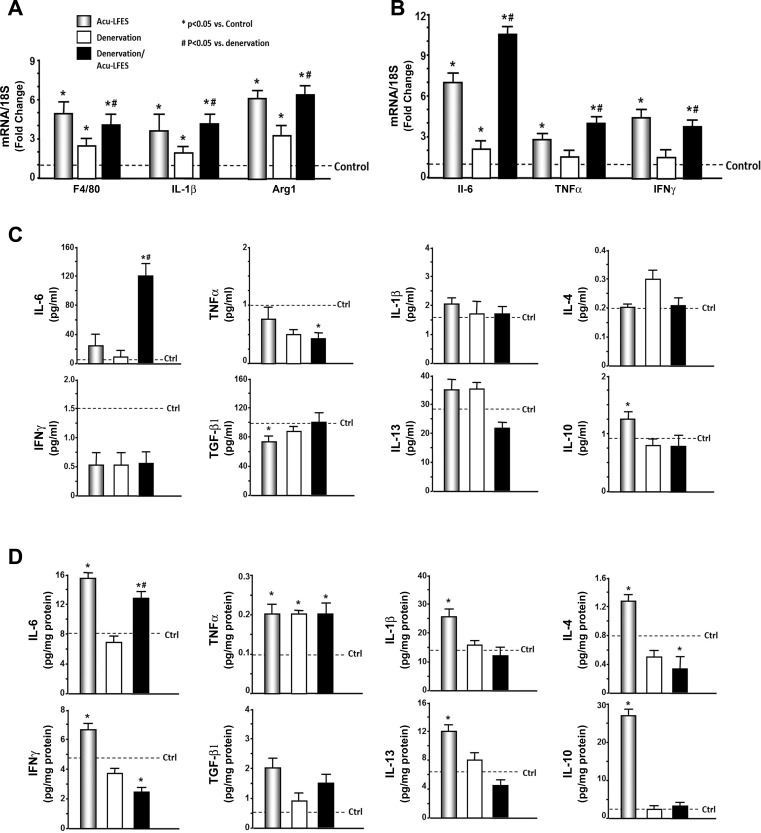
An early step in muscle regeneration is activation of macrophages and upregulation of inflammatory cytokines. We examined the markers of macrophage levels. We assayed mRNA expression of general macrophage marker F4/80, an M1 macrophage marker IL-1β, and an M2 macrophage marker arginase-1 by qPCR. F4/80 expression was elevated 5-fold in the muscle of Acu-LFES mice, 2.3-fold in the denervation muscle, and 4.4-fold in denervation/Acu-LFES mice vs. controls. IL-1β expression was elevated 3.6-fold in the muscle of Acu-LFES mice, 1.9-fold in the denervation muscle, and 4.1-fold in denervation/Acu-LFES mice vs. controls. Arginase-1 expression was increased 6.1-fold in the muscle of Acu-LFES mice, 3.2-fold in the denervation muscle, and 6.3-fold in denervation/Acu-LFES mice vs. controls (Fig. 4A). These data indicate that Acu-LFES upregulates the M1 and M2 macrophage expression after 2-wk treatments. The levels of increase were higher in M2 than M1 macrophages.
Fig. 4.
Acu-LFES increases macrophage expression and upregulates cytokines. A and B: total RNA was isolated from hindlimb muscles of control, Acu-LFES, denervation, or denervation/Acu-LFES mice. Real-time qPCR was used to assay for general macrophage marker, F4/80, M1 macrophage marker, IL-1β and M2 macrophage marker, arginase-1 (A); and inflammation cytokines IL-6, IFNγ, and TNFα (B). The bar graph shows mRNA levels in each group of muscles expressed as a fold change from the normal controls (line indicates controls set as 1-fold). Results are normalized to 18S mRNA (bars: means ± SE; n = 12/group; *P < 0.05 vs. control, #P < 0.05 vs. denervation). C: plasma was obtained from normal control, Acu-LFES, denervation, or denervation/Acu-LFES mice. Plasma cytokine levels were measured using a cytokine ELISA quantification assay. The bar graphs provide concentrations of eight cytokines compared with normal control levels (dashed lines). Bars: means ± SE; n = 9/group; *P < 0.05 vs. control and #P < 0.05 vs. denervation. D: muscle lysates were obtained from control, Acu-LFES, denervation, or denervation/Acu-LFES mice. Muscle cytokine levels were measured by cytokine ELISA and normalized by protein concentration. The bar graphs provide concentrations of eight cytokines compared with normal control levels (dashed lines). Bars: means ± SE; n = 9/group; *P < 0.05 vs. control, #P < 0.05 vs. denervation.
Since macrophages influence myogenesis through cytokines, we examined several inflammatory cytokines in the muscle of all groups of mice. The largest change was observed in IL-6 expression which was increased 7-fold in the muscle of Acu-LFES mice vs. control. Denervation also increased IL-6 expression by 2-fold and denervation/Acu-LFES has additional increase to a total of 10.5-fold compared with control mice. IFNγ expression showed a 4.5-fold increase by Acu-LFES, and no change in denervation group and 3.8-fold increase in denervation/Acu-LFES group vs. control mice. TNFα mRNAs also showed an increased expression by Acu-LFES in normal and denervation mice (Fig. 4B). To confirm that the protein levels of cytokines mirrored the altered RNA levels in response to Acu-LFES, we measured the levels of eight cytokines using ELISA assay in plasma and muscle lysate for all groups of mice. Acu-LFES did not significantly increase the plasma cytokine levels except for IL-10 (Fig. 4C), which indicates that cytokine response to Acu-LFES occurs locally. However, Acu-LFES significantly upregulated all eight cytokines in the muscle of normal mice: 2-fold in IL-6; 2-fold in TNFα; 1.4-fold in IFNγ; 4-fold in TGFβ; 1.6-fold in IL-1β; 1.6-fold in IL-4; 1.9-fold in IL-13, and 11-fold in IL-10; but Acu-LFES did not significantly increase the muscle lysates cytokine levels except for IL-6 in denervated muscle (Fig. 4D).
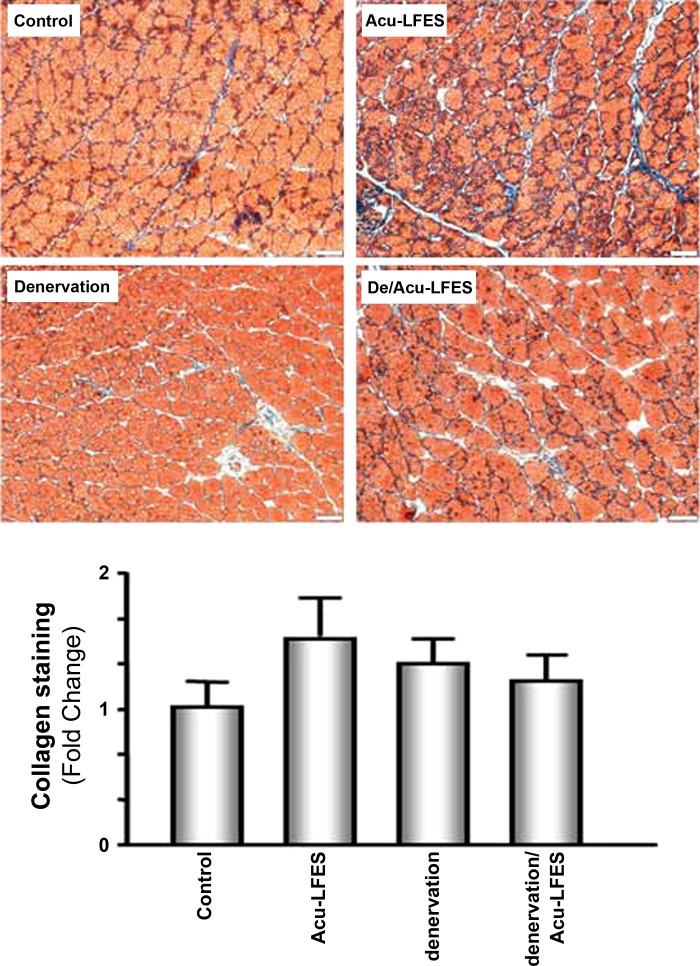
Acu-LFES Has No Significant Impact on the Abundance of Collagen in the Muscle of Normal Mice
We used Masson trichrome staining of muscle frozen sections from each condition to assess collagen content. Collagen showed a trend toward increased amount in Acu-LFES-treated normal muscle. However, using the CellSens Dimension 1.9 with Full Count & Measure Software (Olympus, Melville, NY) calculation, there were no statistic significant differences in the area of blue color staining in each group to indicate the increase in collagen accumulation (Fig. 5).
Fig. 5.
Collagen appears in the muscle of Acu-LFES mice. Muscle samples were collected 14 days after acupuncture. Muscle frozen sections were stained with Masson modified IMEB trichrome to detect collagen deposition in the gastrocnemius muscle of normal control, Acu-LFES, denervation, or denervation/Acu-LFES mice. The red color indicates muscle fiber; the blue indicates collagen; and the black indicates nuclei. The bar graph compares the area (μm2) of blue staining per 500 muscle fibers in each group expressed as a fold change from levels in control mice, which is represented by 1 (bars: means ± SE; n = 6/group; no statistically significant change).
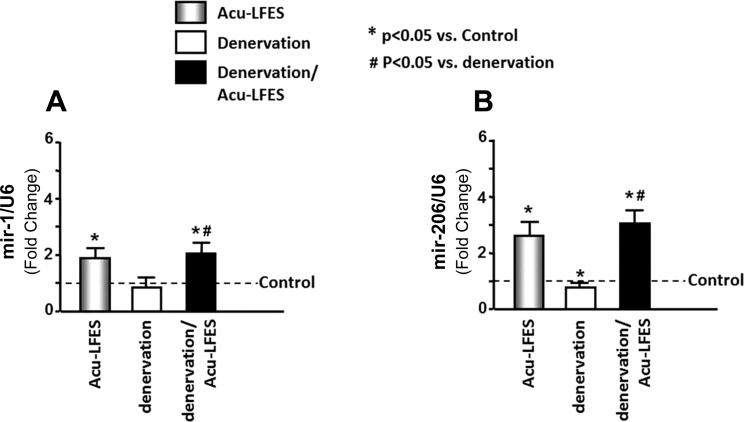
Acu-LFES Enhances the Expression of Muscle-Specific microRNA (myomiRs)
Muscle-specific microRNAs also influence muscle regeneration. To identify whether Acu-LFES stimulates the expression of muscle-specific microRNAs, we measured microRNAs by qPCR in muscle after 2 wk of Acu-LFES. The expressions of miR-1 and miR-206 were significantly increased by Acu-LFES in control mice (2.0-fold for miR-1 and 2.4-fold for miR-206). Denervation resulted in a 20% decrease in miR-1 expression but the change was not statistically significant due to the animal-to-animal variability. Acu-LFES caused increases of 2.2-fold for miR-1 expression and 2.8-fold for miR-206 expression in denervation mice (Fig. 6). The expressions of both miR-133a and miR-133b also showed a trend toward increased expression by Acu-LFES in normal and denervation muscle, but the changes were not statistically significant (data not shown). These data indicate that Acu-LFES stimulates the expressions of miR-1 and -206.
Fig. 6.
Acu-LFES increases miR-1 and miR-206 microRNA in the muscle of control and denervated mice. Total RNA was isolated from hindlimb (gastrocnemius, soleus, and plantaris) muscles of control, Acu-LFES, denervation, or denervation/Acu-LFES mice, and then assayed for specific microRNA. The expressions of miR-1 (A) and miR-206 (B) were measured using real-time qPCR with LNA-enhanced oligonucleotide primers. The bar graph shows microRNA levels in each group expressed as a fold change from levels in normal control mice, which is represented by a line at 1-fold. All band densities were normalized to the density of U6 RNA (bars: means ± SE; n = 9/group; *P < 0.05 vs. control, #P < 0.05 vs. denervation).
DISCUSSION
Numerous investigations have focused on identifying new therapeutic approaches to attenuate muscle wasting. Trials using intramuscular electrodes have been investigated to reduce denervation-induced muscle atrophy. Willand et al. showed that a 1-h/day regimen of electric stimulation using intramuscular stimulating electrodes effectively increases muscle mass and force (47, 48). However, it is not easy to translate this invasive method to clinical use. Acu-LFES is a therapy that can decrease skeletal muscle atrophy induced by hindlimb suspension in mice and has been used clinically for multiple disorders in humans (7, 31). Previously, we performed Acu-LFES on mice with chronic kidney disease-induced muscle atrophy and found that this treatment improved muscle weights and function (12). In the current study we found that Acu-LFES shows significant benefits for attenuating muscle atrophy and increasing muscle regeneration capacity. There are several potential advantages of Acu-LFES over surface electrical stimulation. First, since acupuncture needles cross the skin barrier, Acu-LFES subjects have more tolerance for higher current intensities than SEMS subjects, because skin has more sensory nerves. In addition, the electrical stimulation of Acu-LFES is delivered through metal acupuncture needles directly into the muscle, and close to the nerve. Because of the proximity, Acu-LFES yields a stronger stimulation at a given power than surface (SEMS) stimulation at the same power setting. The needle approach elicits a multiple muscle response that mimics the muscle action in treadmill training. SEMS does not elicit the same multiple muscle response. Last, muscle regeneration is an injury-regeneration process (11). In Acu-LFES the insertion of needles causes subtle myofiber injuries leading to activation of quiescent satellite cells and initiates muscle regeneration. The evidence for this injury-regeneration response is the presence of increased central nuclei and increased myogenesis markers (MyoD, myogenin, eMyHC) in the muscle of Acu-LFES-treated mice.
In general, muscle wasting is the loss of both muscle cells and protein content. Acu-LFES is a therapy that can address muscle wasting from two directions, promoting the regeneration of the muscle cells and blocking the loss of the muscle protein (44, 54). The first step of muscle regeneration is to activate myogenic stem cells, also known as satellite cells. This process involves a cascade of transcription factors that promote different steps in the regeneration process. Initiation of myogenesis is triggered by the transcription factor Pax7 (21). Proliferation and differentiation involves a complex interaction of multiple proteins of which MyoD is an essential element. Differentiation of myoblasts into myotubes requires myogenin to promote fusion of myogenic precursor cells to either new or previously existing fibers. Myosin is the main component of myofibrils (thick filaments), best known for their role in muscle contraction. Myosin heavy chain protein is a marker indicating mature muscle (38). Each of these components and markers for muscle regeneration is upregulated in the muscle of normal and denervated mouse in response to Acu-LFES.
In denervated muscle the number of satellite cells is also increased in both animals (16) and in humans (30) suggesting that, although muscle mass is reduced, muscle regeneration is stimulated. This regeneration capacity is a constant response to the denervated state since myoD, myogenin, and eMyHC are upregulated even 3 and 9 mo postdenervation (18). In the current study, we confirmed that satellite cells were activated in denervated muscle suggesting increased regenerative capacity but muscle wasting still occurred as evidenced by decreased muscle weight (Table 2) and muscle cross-sectional area (Fig. 1). Therefore, factors other than increased regeneration potential may be required to attenuate muscle atrophy in denervated muscle.
There are a few possible targets of Acu-LFES that could contribute to the attenuation of muscle atrophy. IGF-1 is a growth factor that activates satellite cells to start muscle regeneration. In addition, an increase in IGF-1 signaling will counteract muscle atrophy by promoting protein synthesis and prohibiting protein degradation induced by muscle injury and diseases (20, 42, 44, 54, 55). The expression of IGF-1 in denervated muscle changes in two phases. Expression of IGF-1 is increased at 3 days after denervation but then declines later (51). Overexpression of IGF-1 in muscle reduces the amount of myofiber atrophy by 30% in the muscle of denervated mice (35). In the current study we found that after 2 wk of Acu-LFES, IGF-1 mRNA and protein were upregulated relative to the level of IGF-1 in untreated denervated muscle. An increase in IGF-1 leads to inhibition of the pro-catabolic factor FoxO, by increasing FoxO phosphorylation, providing a means of preserving muscle mass and function.
Myostatin is a member of the TGF-β superfamily of secreted proteins and produced primarily in skeletal muscle (19). Myostatin is a potent negative regulator of muscle mass (53). In published accounts, myostatin infusion into mice led to a 33% decrease in body weight and a 35-50% decrease in muscle mass, whereas genetic or functional knockout of myostatin resulted in increased muscle mass of mice, sheep, cattle, or humans (34, 56). In denervated muscle, myostatin mRNA and protein expression gradually increased (1–14 days), and then gradually declined over a later response phase (14–56 days) (5, 23). In our 2-wk denervated mice, the levels of myostatin mRNA were decreased, but the protein amounts were not different from control levels. However, Acu-LFES reduced both myostatin mRNA expression and protein abundance in both control and denervated muscle, which could contribute to the positive effect of Acu-LFES against muscle atrophy. In agreement with our study, Takaoka et al. (37) reported that electrical acupuncture treatment suppressed myostatin expression, which led to muscle hyperplasia in mice.
MicroRNAs offer another level of control at a translational level contributing to regulation of muscle wasting (43). Direct application of miR-1, miR-133, and miR-206 to muscle by injection induced myoD, Pax7, and myogenin protein production, leading to increased muscle regeneration in a rat skeletal muscle injury model (29). Williams et al. (49) provided evidence that miR-206 is required for efficient regeneration of neuromuscular synapses after acute nerve injury. In the current study, we found that miR-206 was decreased in denervated muscle. Acu-LFES not only increased miR-1 and -206 in normal control mouse muscle, but also reversed the decrease of these microRNAs in denervated muscle.
In conclusion, Acu-LFES ameliorated denervation-induced skeletal muscle atrophy by multiple mechanisms, including improvements in muscle regeneration, upregulation of IGF-1, and downregulation of myostatin signaling pathways, and raising the expression of microRNAs. Our work contributes to a better understanding of the mechanisms involved in Acu-LFES treatment of muscle wasting.
GRANTS
Grant support for this study was by NIAMS R01-AR-060268 to X. H. Wang; and Zhejiang Provincial Natural Science Foundation of China (LY15H270017) and Zhejiang Provincial Program for the Cultivation of High-level Innovative Health Talents to Z. Su.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Z.S., L.H., J.C., F.H., M.L., H.W., and X.H.W. performed experiments; Z.S., J.C., F.H., H.C., and X.H.W. analyzed data; Z.S., F.H., H.C., and X.H.W. interpreted results of experiments; Z.S. and X.H.W. prepared figures; Z.S. and X.H.W. drafted manuscript; Z.S., J.D.K., H.C., and X.H.W. edited and revised manuscript; J.D.K. and X.H.W. approved final version of manuscript; X.H.W. conception and design of research.
REFERENCES
- 1.Adams GR, Haddad F, Bodell PW, Tran PD, Baldwin KM. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. J Appl Physiol (1985) 103: 1644–1654, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Aylward RB. Eradicating polio: today's challenges and tomorrow's legacy. Ann Tropical Med Parasitol 100: 401–413, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Babault N, Cometti C, Maffiuletti NA, Deley G. Does electrical stimulation enhance post-exercise performance recovery? Eur J Appl Physiol 111: 2501–2507, 2011. [DOI] [PubMed] [Google Scholar]
- 4.Banerjee P, Caulfield B, Crowe L, Clark A. Prolonged electrical muscle stimulation exercise improves strength and aerobic capacity in healthy sedentary adults. J Appl Physiol (1985) 99: 2307–2311, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Baumann AP, Ibebunjo C, Grasser WA, Paralkar VM. Myostatin expression in age and denervation-induced skeletal muscle atrophy. J Musculoskelet Neuronal Interact 3: 8–16, 2003. [PubMed] [Google Scholar]
- 6.Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 14: 58–74, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Consensus Conference, NIH (Anonymous). NIH Consensus Conference. Acupuncture. JAMA 280: 1518–1524, 1998. [PubMed] [Google Scholar]
- 8.Ernst G, Strzyz H, Hagmeister H. Incidence of adverse effects during acupuncture therapy-a multicentre survey. Complementary Ther Med 11: 93–97, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Gielen S, Sandri M, Kozarez I, Kratzsch J, Teupser D, Thiery J, Erbs S, Mangner N, Lenk K, Hambrecht R, Schuler G, Adams V. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: the randomized Leipzig Exercise Intervention in Chronic Heart Failure and Aging catabolism study. Circulation 125: 2716–2727, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths RD. Muscle mass, survival, and the elderly ICU patient. Nutrition 12: 456–458, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol 91: 534–551, 2001. [DOI] [PubMed] [Google Scholar]
- 12.Hu L, Klein JD, Hassounah F, Cai H, Zhang C, Xu P, Wang XH. Low-frequency electrical stimulation attenuates muscle atrophy in CKD-a potential treatment strategy. J Am Soc Nephrol 26: 626–635, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu Z, Klein JD, Mitch WE, Zhang L, Martinez I, Wang XH. MicroRNA-29 induces cellular senescence in aging muscle through multiple signaling pathways. Aging (Albany NY) 6: 160–175, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia PCR, Liu J, Tian N. Regulative effect of electro-acupuncture of different frequencies on derangement of gastric electric rhythm in rabbits. China Acupuncture 11: 801, 2006. [PubMed] [Google Scholar]
- 15.Koike H, Sobue G. Alcoholic neuropathy. Curr Opinion Neurol 19: 481–486, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Kumai Y, Ito T, Miyamaru S, Yumoto E. Modulation of MyoD- and Ki-67-positive satellite cells in the short-term denervated rat thyroarytenoid muscle. Laryngoscope 117: 2063–2067, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Lake DA. Neuromuscular electrical stimulation. An overview and its application in the treatment of sports injuries. Sports Med 13: 320–336, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Lapalombella R, Kern H, Adami N, Biral D, Zampieri S, Scordari A, di Tullio S, Marini M. Persistence of regenerative myogenesis in spite of down-regulation of activity-dependent genes in long-term denervated rat muscle. Neurol Res 30: 197–206, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol 20: 61–86, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol 15: 1537–1545, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development 138: 3639–3646, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim S. WHO Standard Acupuncture Point Locations. Evid Based Complement Alternat Med 7: 167–168, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu M, Zhang D, Shao C, Liu J, Ding F, Gu X. Expression pattern of myostatin in gastrocnemius muscle of rats after sciatic nerve crush injury. Muscle Nerve 35: 649–656, 2007. [DOI] [PubMed] [Google Scholar]
- 24.MacPherson H, Thomas K, Walters S, Fitter M. The York acupuncture safety study: prospective survey of 34,000 treatments by traditional acupuncturists. BMJ 323: 486–487, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maffiuletti NA, Minetto MA, Farina D, Bottinelli R. Electrical stimulation for neuromuscular testing and training: state-of-the-art and unresolved issues. Eur J Appl Physiol 111: 2391–2397, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 3: CD001447, 2012.22419278 [Google Scholar]
- 28.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med 335: 1897–1905, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Nakasa T, Ishikawa M, Shi M, Shibuya H, Adachi N, Ochi M. Acceleration of muscle regeneration by local injection of muscle-specific microRNAs in rat skeletal muscle injury model. J Cell Mol Med 14: 2495–2505, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olive M, Martinez-Matos JA, Pirretas P, Povedano M, Navarro C, Ferrer I. Expression of myogenic regulatory factors (MRFs) in human neuromuscular disorders. Neuropathol Appl Neurobiol 23: 475–482, 1997. [DOI] [PubMed] [Google Scholar]
- 31.Onda A, Jiao Q, Nagano Y, Akimoto T, Miyamoto T, Minamisawa S, Fukubayashi T. Acupuncture ameliorated skeletal muscle atrophy induced by hindlimb suspension in mice. Biochem Biophys Res Commun 410: 434–439, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Padhi D, Higano CS, Shore ND, Sieber P, Rasmussen E, Smith MR. Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab 99: E1967–E1975, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Sartori R, Milan G, Patron M, Mammucari C, Blaauw B, Abraham R, Sandri M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am J Physiol Cell Physiol 296: C1248–C1257, 2009. [DOI] [PubMed] [Google Scholar]
- 34.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med 350: 2682–2688, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Shavlakadze T, White JD, Davies M, Hoh JF, Grounds MD. Insulin-like growth factor I slows the rate of denervation induced skeletal muscle atrophy. Neuromuscul Disord 15: 139–146, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Su Z, Robinson A, Hu L, Klein JD, Hassounah F, Li M, Wang H, Cai H, Wang XH. Acupuncture plus low-frequency electrical stimulation (Acu-LFES) attenuates diabetic myopathy by enhancing muscle regeneration. PLoS One 10: e0134511, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takaoka Y, Ohta M, Ito A, Takamatsu K, Sugano A, Funakoshi K, Takaoka N, Sato N, Yokozaki H, Arizono N, Goto S, Maeda E. Electroacupuncture suppresses myostatin gene expression: cell proliferative reaction in mouse skeletal muscle. Physiol Genomics 30: 102–110, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol 224: 7–16, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Van Den Berg-Vos RM, Van Den Berg LH, Visser J, de Visser M, Franssen H, Wokke JH. The spectrum of lower motor neuron syndromes. J Neurol 250: 1279–1292, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Wada K, Katsuta S, Soya H. Formation process and fate of the nuclear chain after injury in regenerated myofiber. Anat Rec (Hoboken) 291: 122–128, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Wagner KR, Fleckenstein JL, Amato AA, Barohn RJ, Bushby K, Escolar DM, Flanigan KM, Pestronk A, Tawil R, Wolfe GI, Eagle M, Florence JM, King WM, Pandya S, Straub V, Juneau P, Meyers K, Csimma C, Araujo T, Allen R, Parsons SA, Wozney JM, Lavallie ER, Mendell JR. A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol 63: 561–571, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling. Endocrinology 147: 4160–4168, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Wang XH. MicroRNA in myogenesis and muscle atrophy. Curr Opin Clin Nutr Metab Care 16: 258–266, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang XH, Du J, Klein JD, Bailey JL, Mitch WE. Exercise ameliorates chronic kidney disease-induced defects in muscle protein metabolism and progenitor cell function. Kidney Int 76: 751–759, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang XH, Mitch WE. Mechanisms of muscle wasting in chronic kidney disease. Nat Rev Nephrol 10: 504–516, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang XH, Mitch WE. Muscle wasting from kidney failure—a model for catabolic conditions. Int J Biochem Cell Biol 45: 2230–2238, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willand MP, Chiang CD, Zhang JJ, Kemp SW, Borschel GH, Gordon T. Daily electrical muscle stimulation enhances functional recovery following nerve transection and repair in rats. Neurorehabil Neural Repair 29: 690–670, 2015. [DOI] [PubMed] [Google Scholar]
- 48.Willand MP, Lopez JP, de Bruin H, Fahnestock M, Holmes M, Bain JR. A new system and paradigm for chronic stimulation of denervated rat muscle. J Med Biol Eng 31: 87–92, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science 326: 1549–1554, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Windsor JA, Hill GL. Risk factors for postoperative pneumonia. The importance of protein depletion. Ann Surg 208: 209–214, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeman RJ, Zhao J, Zhang Y, Zhao W, Wen X, Wu Y, Pan J, Bauman WA, Cardozo C. Differential skeletal muscle gene expression after upper or lower motor neuron transection. Pflügers Arch 458: 525–535, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Pan J, Dong Y, Tweardy DJ, Garibotto G, Mitch WE. Stat3 activation links a C/EBPdelta to myostatin pathway to stimulate loss of muscle mass. Cell Metab 18: 368–379, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, Song Y, Min H, Wang X, Du J, Mitch WE. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J 25: 1653–1663, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang L, Wang XH, Wang H, Du J, Mitch WE. Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol 21: 419–427, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou Q, Du J, Hu Z, Walsh K, Wang XH. Evidence for adipose-muscle cross talk: opposing regulation of muscle proteolysis by adiponectin and fatty acids. Endocrinology 148: 5696–5705, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science 296: 1486–1488, 2002. [DOI] [PubMed] [Google Scholar]