Abstract
Inspiratory stretch by mechanical ventilation worsens lung injury. However, it is not clear whether and how the ventilator damages lungs in the absence of preexisting injury. We hypothesized that subtle loss of lung aeration during general anesthesia regionally augments ventilation and distension of ventilated air spaces. In eight supine anesthetized and intubated rats, hyperpolarized gas MRI was performed after a recruitment maneuver following 1 h of volume-controlled ventilation with zero positive end-expiratory pressure (ZEEP), FiO2 0.5, and tidal volume 10 ml/kg, and after a second recruitment maneuver. Regional fractional ventilation (FV), apparent diffusion coefficient (ADC) of 3He (a measurement of ventilated peripheral air space dimensions), and gas volume were measured in lung quadrants of ventral and dorsal regions of the lungs. In six additional rats, computed tomography (CT) images were obtained at each time point. Ventilation with ZEEP decreased total lung gas volume and increased both FV and ADC in all studied regions. Increases in FV were more evident in the dorsal slices. In each lung quadrant, higher ADC was predicted by lower gas volume and by increased mean values (and heterogeneity) of FV distribution. CT scans documented 10% loss of whole-lung aeration and increased density in the dorsal lung, but no macroscopic atelectasis. Loss of pulmonary gas at ZEEP increased fractional ventilation and inspiratory dimensions of ventilated peripheral air spaces. Such regional changes could help explain a propensity for mechanical ventilation to contribute to lung injury in previously uninjured lungs.
Keywords: alveolar recruitment, atelectasis, ventilator-induced lung injury, hyperpolarized gas MRI, computed tomography
investigators agree that ventilator-induced elevated tissue stretch (i.e., tidal deformation) can cause pulmonary damage de novo (23, 46) and worsen preexisting acute respiratory distress syndrome (ARDS) (1). Yet the mechanisms by which mechanical ventilation can generate injurious stretch in lungs without preexisting injury (39) (e.g., during general anesthesia) remain unclear. The pressure-causing tidal stretch (i.e., the “driving pressure”) (3) and peak inspiratory distension of the lungs (18) are each relatively low, although mild stress disrupts the extracellular matrix within only 4 h of ventilation and can initiate injury in healthy lungs (34, 37). Furthermore, general anesthesia causes lung deflation below the normal functional residual capacity (FRC), which promotes atelectasis (26, 42) and increases stress at reinflation (6, 40, 49). In fact, laboratory studies in isolated lungs have shown that atelectasis due to unimpeded deflation to a volume below FRC is harmful (38, 50). Although positive end-expiratory pressure (PEEP) maintains lung volumes and prevents atelectasis (26), clinical trials have produced ambiguous results on whether PEEP benefits the lungs of healthy patients undergoing surgery (41a) and those with ARDS (7).
Uncertainty about how and to what degree atelectasis contributes to stretch-related injury has been caused in part by the use of conventional imaging methodologies that may not capture the subtleties of ventilator-induced damage in some normal lungs. Computed tomography (CT) indicates a distribution of aeration between atelectatic vs. overstretched regions (24) when a substantial amount of tissue is not aerated and CT density is ≥100 Hounsfield units (HU) (43). However, ventilated subjects frequently exhibit areas of intermediate aeration (32), which may represent microscopic or partial atelectasis (25). In these regions, CT voxels of intermediate gray scale may contain a mixture of ventilated and atelectatic air spaces that cannot be separated from uniformly sized air spaces due to the necessity of signal averaging in the setting of low spatial resolution. A mixed aeration pattern and heterogeneity of localized inflation have been associated with increased mortality in injured lungs (i.e., ARDS) (13).
Our group has begun using hyperpolarized gas magnetic resonance imaging (HP-MRI) in an attempt to capture relationships between atelectasis and elevated tissue stretch that may be undetectable via CT. The apparent diffusion coefficient (ADC) derived from HP-MRI information measures diffusivity restriction within the alveoli and the alveolar ducts (58) and is proportional to their dimensions (56), although the dimensions are smaller than the voxel resolution of the scanner. This volume-weighted metric is particularly suited to studying the effects of atelectasis on residual ventilated air spaces because it is sensitive to enlarged microstructures (31). We showed that ADC is elevated in rats with atelectasis (8) or lung injury (9), suggesting overdistension. This gas distribution pattern was also observed in healthy lungs with microatelectasis due to suboptimal recruitment (10). On the basis of these results, it appears that sparse loss of aeration may increase the propensity to damage of neighboring alveolar units (57).
However, static measurements of air space dimensions may not fully describe the susceptibility to ventilator-induced lung injury, which results from excessive tidal stress in addition to overdistension (41). As such, this study sought to better understand the stretch of peripheral air spaces during suboptimal recruitment in noninjured lungs by regionally correlating air space dimensions as measured by ADC with fractional ventilation (FV; a measurement of gas turnover in peripheral airspaces) (33), gas volume, and CT tissue density. We hypothesized that lung regions with overall loss of gas due to derecruitment would be characterized by elevations in both FV and ADC, the latter due to the presence of ventilated air spaces in close proximity to collapsed alveoli within each voxel. This combination (colocalized increases in ADC and FV) could indicate fully recruited air spaces being overstretched due to nearby, persistent derecruitment. This hypothesis was tested in ventilated, healthy rats by analyzing the spatial distribution of HP-MRI parameters in dorsal and ventral quadrants of the lungs.
METHODS
Study Protocol
Animal preparation and care.
Studies were performed on 14 male Sprague-Dawley rats weighing 390 ± 48 g and with approval from the Institutional Animal Care and Use Committee of the University of Pennsylvania. Animals were anesthetized with pentobarbital (40–60 mg/kg ip initially, then 10–20 mg/kg hourly for maintenance), the trachea was intubated (14-gauge catheter; BD Biosciences, Franklin Lakes, NJ), and the glottis was sealed with putty (DAP Products, Baltimore, MD) (8). Animals were paralyzed using pancuronium bromide (1 mg/kg iv; Abbot Laboratories, North Chicago, IL) injected through a 24-gauge tail-vein catheter. Rats received hydration with normal saline (30 ml/kg) injected subcutaneously in the lateral neck after induction of anesthesia. After anesthetic induction and intubation, all animals were placed in the scanner in the supine position and ventilated using an MRI-compatible small-animal ventilator capable of delivering mixtures of helium, nitrogen, and oxygen (8). Airway pressure was continuously recorded using a fiber-optic sensor (Samba Sensors, Sweden); peak inspiratory pressure (PIP) and dynamic respiratory compliance (Cdyn) were measured. Heart rate and peripheral oxygen saturation (SpO2) levels were monitored by a veterinary pulse-oximeter (Nonin Medical, Plymouth, MN) attached to the hind foot. The animal's rectal temperature was maintained at 37°C by a heated pad (Gaymar Industries, Orchard Park, NY) placed under the body. After the last set of measurements, animals were removed from the scanner and euthanized by lethal pentobarbital injection.
Ventilation protocol.
Rats were ventilated in volume-control mode with a constant tidal volume (VT) of 10 ml/kg, respiratory rate of 60 breaths/min, and FiO2 0.5. PEEP was set at 0 cmH2O (ZEEP) except during alveolar recruitment maneuvers, which were performed by applying PEEP at 9 cmH2O for 1 min. This method of recruitment was chosen on the basis of our studies in healthy rats in which we found that transient application of PEEP 9 cmH2O was sufficient to improve lung aeration and ADC (8) while limiting time-dependent 3He depolarization and the adverse effects of high airway pressure. Baseline images (CT or MRI) were obtained 3 min after a first recruitment maneuver. Rats were then ventilated with ZEEP for 1 h to induce suboptimal recruitment, after which imaging was repeated. Finally, a second recruitment maneuver was performed, followed by another imaging series at ZEEP.
Imaging Techniques
Hyperpolarized-MRI.
In eight rats, imaging was performed with a 50-cm bore 4.7-T MRI scanner (Agilent Technologies, Santa Clara, CA). The MRI scanner was equipped with 12-cm 25 G/cm gradients and a quadrature 8-leg birdcage RF coil with an internal diameter of 7 cm (Stark Contrast, Erlangen, Germany) that was tuned to the 3He resonance frequency of 152.95 MHz. Using a commercial prototype (IGI.9600.He; GE Healthcare, Durham, NC), 3He gas was hyperpolarized to ∼30% over 14 h. Imaging was performed on 10-mm-thick dorsal (posterior) and ventral (anterior) coronal slices that were selected from a previous axial projection of the lung obtained using a single breath of 3He gas. The typical ventral-dorsal thickness of our rat lungs at ZEEP and VT10 ml/kg was approximately 18–22 mm. Therefore, the two slices covered the entire lung in most cases. A 1-mm gap was set between slices at the midpoint of the lung thickness, corresponding to the central airways. Regional fractional ventilation (FV) was measured from the hyperpolarized 3He signal buildup during 10 back-to-back hyperpolarized gas breaths (79% 3He, 21% O2), with images acquired during 500-ms end-inspiratory breath-holds using an HP-MRI methodology developed by our group (20). Analysis was performed on a voxel-by-voxel basis with a planar resolution of 0.47 × 0.47 mm2. In each voxel, we measured the growth of signal after each inspiration (due to the volume of “fresh” hyperpolarized gas) and indexed it by the total signal generated by residual (expiratory) and by fresh gas volume: Vfresh/(Vfresh + Vresidual) (15). Time evolution of signal intensity was then fitted to equations (20) yielding values of FV for each valid voxel.
Air space dimensions were estimated by measuring the apparent diffusion coefficient (ADC) for 3He, as described in our previous studies (8). Briefly, ADC mapping was performed with a planar resolution of 0.94 × 0.94 mm2 at the same slice position as in the fractional ventilation imaging using interleaved diffusion-weighted gradient echo imaging pulse sequence (9). End-inspiratory images were obtained following ventilation with a mixture of hyperpolarized 3He and oxygen (79% 3He, 21% O2). Each ADC acquisition was obtained during a single breath hold and consisted of six diffusion-weighted images with b values of 0, 5, 3, 2, 1, and 0 cm2/s, each corresponding to a different diffusion-sensitizing gradient. Images were analyzed by fitting the time evolution of each pixel's signal intensity to a standard equation (8) to yield maps of regional ADC values. A threshold of signal-to-noise ratio (SNR) >20 was used to identify the location of the lungs in the last image of each ventilation series and in the ADC image with zero b value. The average SNR in these two images was typically above 60, and very few low SNR voxels were identified in the lung parenchyma. The last 3He density image in the series of ventilation acquisitions was also used to estimate global and regional lung gas volume (V) at end-inspiration, which was quantified by counting voxels in images of both slices.
Computed tomography.
In six rats, high-resolution, end-inspiratory CT scans were acquired using an eXplore 120 micro-CT scanner (eXplore CT120 system; Northridge Tri-Modality Imaging, Chatsworth, CA). Settings used for imaging were as follows: 80 kVp, 32 mA, 16-ms exposure time, 220 projections (half-scan), and 100-μm isotropic resolutions. To avoid blurring due to respiratory motion, imaging was ventilator-gated and performed during 500-ms end-inspiratory and end-expiratory breath-holds. Images were reconstructed to three-dimensional whole-lung maps with 200-μm isotropic resolution using a proprietary program supplied by the scanner manufacturer (MicroView 2.2; GE Healthcare). For lung gas content determination, image thresholding was used to obtain images of all relevant three-dimensional regions of interest (ROI), including both lungs in their entirety. All pixels with CT density higher than −300 HU were excluded, thereby ensuring an adequate delineation of the aerated lung parenchyma from the surrounding chest wall and from other nonpulmonary tissue in healthy lungs. End-inspiratory lung gas volume (EILV) and end-expiratory lung volume (EELV) were calculated using CT density analysis methodology on each ROI (16). VT was computed as EILV − EELV. Because ventilator settings were identical and airway pressures were similar to those of rats receiving HP-MRI, we assumed that inflation volumes were comparable between animals receiving CT and HP-MRI.
Statistical Analysis
For analysis, ADC, FV, and 3He density maps of the ventral and dorsal slices were regridded to the same planar resolution of 0.94 × 0.94 mm2 and coregistered (52) to each other in the three subsequent conditions (baseline, 1 h, and recruitment). The maps were then binned to four ROIs corresponding to upper, lower, right, and left “lobes” of each slice, which resulted in 2 × 2 × 2 replications for each rat (spatial pseudoreplications). Means and standard errors in each ROI were calculated and used in the regional statistical analysis as mean and heterogeneity of that lobe, respectively. This step was performed to maintain regionality of information. A repeated-measures (nested) ANOVA was performed to test differences between the three conditions in the imaging markers (FV, ADC, and V). The lobe term was nested within the slice term, which was nested within the animal identification term. A priori power analysis was performed in G*power (21) before the experiments on the basis of observed effect size of increased ADC in rats. To achieve a minimum power of 80% for a repeated measure within-factor analysis with three measurements (three conditions in the same animal), eight rats are sufficient to detect the required effect size [Cohen's d = 0.5 from (8)]. Because we used a nested (pseudoreplication) methodology, eight spatial samples were used for each rat and a total of 64 samples guarantees the power of this study. ANOVA was performed, followed by a post hoc Tukey's test to identify the statistically significant differences between conditions. A variance component analysis was then performed, as detailed in the Appendix. A mixed-effect regression model was fit to the data to describe the mean ADC in each lobe (ADCm) by a linear model containing all the other imaging variables and their interactions at a regional level. Retained fixed-effects in the final minimal adequate model included mean FV (FVm), heterogeneity of FV (FVh), V, and a slice term. The random effects were used as explained above for the nested analysis (rat/slice/lobe). Statistical analysis was performed using R (R Foundation for Statistical Computing; Vienna Austria, http://www.r-project.org). An experiment-wide type I error level of 0.05 was used.
RESULTS
Hyperpolarized MRI
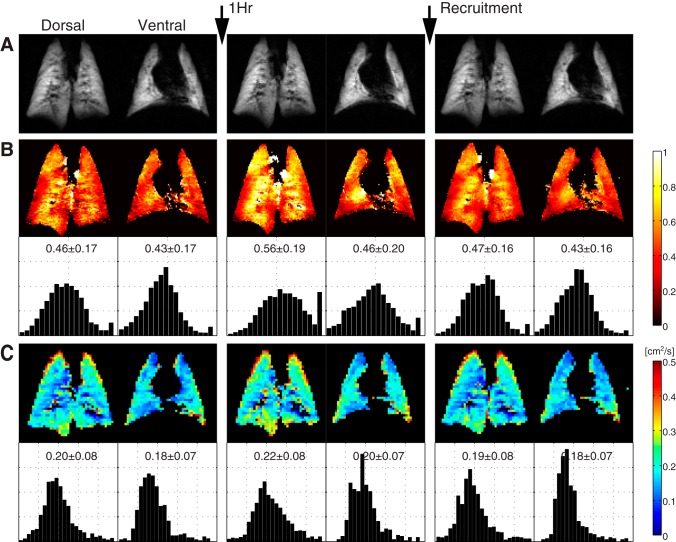
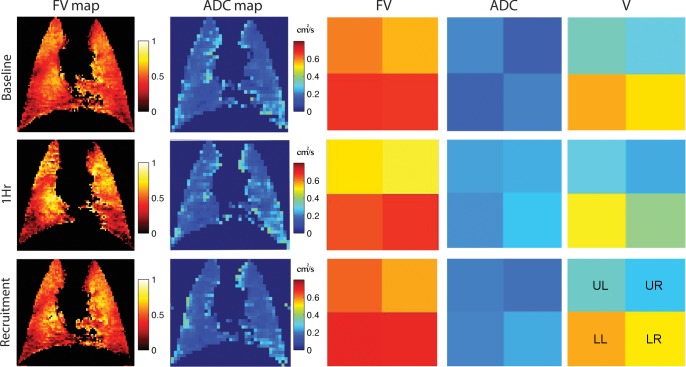
Ventilation at ZEEP for 1 h was associated with higher values for both FV and ADC, changes that are evident in HP-MRI images in the ventral and dorsal lung slices of a representative animal (Fig. 1). Frequency distributions in this individual rat suggest increased heterogeneity of FV (Fig. 1B) at 1 h. Changes in FV and ADC were mostly reversible after recruitment. Figure 2 (left) shows coregistered maps of FV and ADC in the ventral slice of a representative animal, together with mean values calculated in each of the four binned lobes (Fig. 2, right); increases in FV and ADC at 1 h were obvious in all lobes. In contrast, regional gas volume (V) declined in each lobe at 1 h but increased again after recruitment (Fig. 2).
Fig. 1.
Imaging variables obtained at baseline, after 1 h of ventilation with no positive end-expiratory pressure (PEEP), and following a recruitment maneuver in ventral and dorsal slices of a representative rat. 3He density maps (A); maps and frequency distribution histograms of regional fractional ventilation (FV) (B); and maps and frequency distributions of apparent diffusion coefficient (ADC) for 3He (C). Both FV and ADC increased at 1 h and returned to lower values after recruitment. Spreading of frequency distributions indicates higher FV heterogeneity at 1 h.
Fig. 2.
Left: coregistered maps of regional FV and ADC for 3He in the ventral slice of a representative animal. Right: mean values of FV and ADC in four regions of interest (ROI): upper left (UL), upper right (UR), lower left (LL), and lower right (LR). Gas volume (V) of each ROI (calculated from 3He density maps) is also shown. Both FV and ADC increased, whereas V decreased, in all ROIs at 1 h and returned to lower values after recruitment.
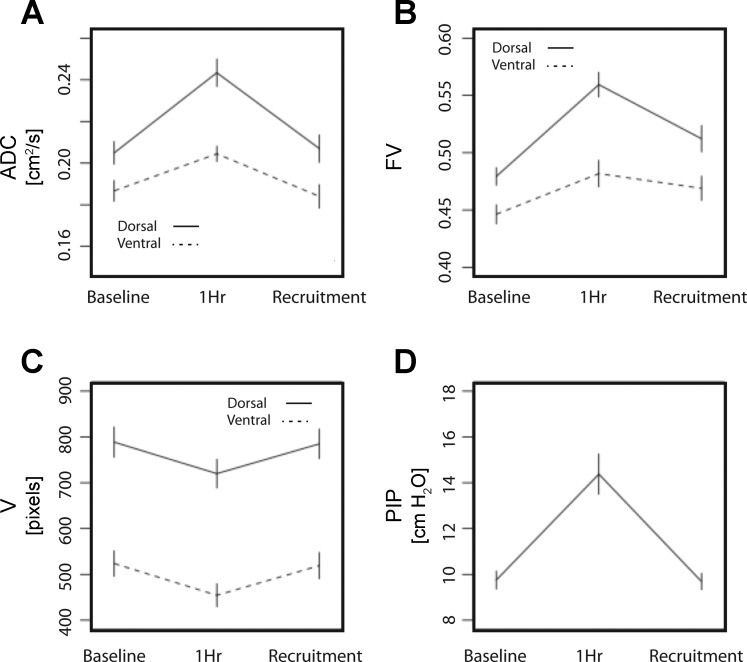
Figure 3 shows mean slice values of HP-MRI variables in the three conditions studied: the dorsal slices displayed a larger increase in ADC and FV after 1 h of ventilation than the ventral lung. The increase in PIP observed at 1 h (Fig. 3D, P < 0.01) was reversible after recruitment; dynamic compliance decreased from a baseline of 0.43 ± 0.05 to 0.29 ± 0.05 ml/cmH20 = (P < 0.01) at 1 h, then returned to 0.43 ± 0.05 ml/cmH20 after recruitment, confirming that this maneuver improved lung mechanics.
Fig. 3.
Mean values (± SD) of ADC for 3He (A); regional FV (B); inspiratory gas volume (V), quantified as pixel counts of 3He density maps (C); and peak inspiratory pressures (PIP) (D). All variables were measured in eight rats at baseline, after 1 h ventilation at zero PEEP, and after a recruitment maneuver. FV, ADC, and V were obtained from ventral (anterior) and dorsal (posterior) coronal maps. For statistical significance of MRI variables, see results of nested analysis in Table 1.
The nested ANOVA results are shown in Table 1. There were significant differences in FV, FV heterogeneity, ADC, and V among the three conditions (in all cases, P < 0.05) and between the ventral and dorsal slices (in all cases, P < 0.01) (Fig. 3, Table 1). The post hoc tests consistently confirmed significant change between 1 h and the other two conditions (in all cases, PTukey < 0.005), except for FV between 1 h and recruitment (PTukey = 0.083). Mean ADC, FV, and V values were not different between baseline and after recruitment (in all cases, PTukey > 0.1), whereas lungs still had significantly higher FV heterogeneity after recruitment (Table 1, PTukey = 0.025).
Table 1.
Repeated-measures ANOVA (nested) for differences between FV, ADC, and volume
| FVm |
FVh |
ADC |
Volume |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | df* | F | P | F | P | F | P | F | P |
| Conditions | 2 | 8.72 | 0.002 | 15.25 | <0.001 | 8.04 | 0.003 | 4.15 | 0.030 |
| Rats in conditions | 21 | 0.95 | 0.542 | 2.39 | 0.911 | 0.74 | 0.754 | 0.16 | 0.999 |
| Slices in rat | 24 | 1.97 | 0.008 | 0.62 | 0.021 | 3.82 | <0.001 | 4.52 | <0.001 |
| Lobes in slice | 144 | ||||||||
| Post hoc† | Z | PTukey | Z | PTukey | Z | PTukey | Z | PTukey | |
| Baseline − 1 h | 2 | 4.11 | <0.001 | 11.08 | <0.001 | 3.168 | 0.004 | −1.312 | <0.001 |
| 1 h − recruitment | 2 | −2.14 | 0.083 | −8.48 | <0.001 | −3.205 | 0.004 | 1.233 | <0.001 |
| Baseline − recruitment | 2 | 1.98 | 0.118 | 2.60 | 0.025 | −0.037 | 0.999 | −0.079 | 0.775 |
ADC, apparent diffusion coefficient; FV, fractional ventilation; FVm, mean fractional ventilation; FVh, heterogeneity of fractional ventilation (i.e., the standard error of FV).
Degree of freedom. †Using Tukey's test.
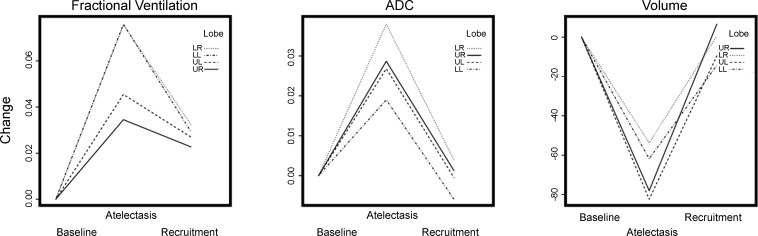
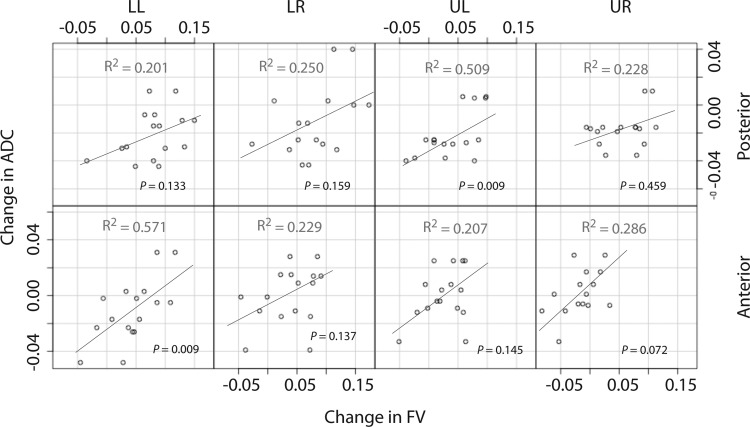
To further illustrate regional changes in FV, ADC, and V, differences in these values between each condition and the baseline are shown in each lobe after combining the ventral with the dorsal slice (Fig. 4): the increases in FV were larger in the lower lobes (Fig. 4). Figure 5 shows correlation plots between the baseline to 1-h changes in FV vs. ADC in all rats; data are grouped by lobes and slices. Increase in mean ADC was associated with increase in mean FV.
Fig. 4.
Changes from baseline in regional FV, ADC, and gas volume (from 3He density images) for each lobar region of interest (LR, LL, UR, and UL). Data in each condition were obtained after combining the ventral and dorsal slices and are expressed as the difference with the baseline values for the same lobe.
Fig. 5.
Correlations between baseline to 1-h changes in FV vs. ADC for each binned region of interest (LR, LL, UR, and UL) in ventral (anterior) and dorsal (posterior) slices.
Table 2 shows the results of the mixed-effect regression analysis. Details of the model generation are presented in the Appendix. All covariates not listed were found to be insignificant. The constant term chosen was the overall mean ADC in all the ventral ROIs for all the conditions (ADCm = 0.178). The next fixed-effect variable (slice term) showed a position-dependent gradient, with ADCm rising by ∼0.06 in the dorsal slices (ADCm = 0.235 = 0.178 + 0.057). The volume was a significant covariate with a negative strong effect on the ADCm (i.e., a 5% decrease in V increased ADCm by an average of ∼14% in ventral slices). The interaction between the remaining two effects, mean FV (FVm) and heterogeneity of FV (FVh, the standard error of FV), showed a significant positive effect on ADCm. Random effects explain the variance in the slopes of the regression terms. Approximately 24% of the variance is explained by the variability among rats and 53% by regional differences between lobes in the lung.
Table 2.
Mixed-effect model results
| Fixed effects | Estimate [× 103] | SE [× 103] | t | P |
|---|---|---|---|---|
| Constant | 178.39 | 6.343 | 28.122 | <0.001 |
| Slice, posterior | 57.46 | 8.018 | 7.167 | <0.001 |
| V | −25.09 | 3.650 | −6.874 | <0.001 |
| FVm × FVh | 4.89 | 1.738 | 2.814 | 0.0057 |
| FVm | −3.58 | 1.878 | −1.904 | 0.059 |
| FVh | 10.45 | 1.865 | 5.602 | <0.001 |
| Random effects | Estimated SD [× 103] | % Variance | ||
| Rat | 10.791 | 24.2 | ||
| Slice | 0.002 | 0.0 | ||
| Lobe | 23.584 | 52.9 | ||
| Residuals | 10.227 | 22.9 | ||
SE, standard error.
Computed Tomography
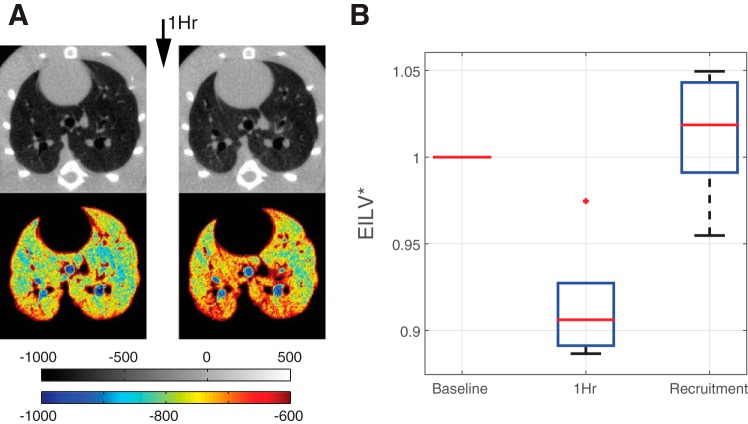
CT showed no macroscopic evidence of atelectasis after ventilation with ZEEP (Fig. 6A, top). However, a subtle increase in tissue density (indicating reduced aeration) was present at this time vs. baseline, with a gradual ventral (nondependent) to dorsal (dependent) distribution that was evident in the lung views with narrowed density window (Fig. 6A, bottom). Quantitative CT analysis showed that EILV decreased by 8.46 ± 0.03% after 1 h of ventilation at ZEEP (Fig. 6B) and was restored to near-normal values after recruitment. VT, measured by CT, was constant in all conditions (3.61 ± 0.08 ml at baseline, 3.56 ± 0.10 ml at 1 h, and 3.61 ± 0.09 ml after recruitment) and was consistent with the value set on the ventilator (3.62 ± 0.08 ml).
Fig. 6.
A: axial CT scans in a representative rat. B: trend in end inspiratory lung volume expressed as a fraction of baseline values (EILV*) (means ± SD of five rats) measured at baseline, after 1 h of ventilation at zero PEEP, and after recruitment maneuver. No macroscopically visible atelectasis was detected in the raw CT scans; however, a greater anterior-posterior density gradient was appreciated in the narrowed density windows (see color map beneath A) suggesting decreased aeration in the dorsal lung regions.
DISCUSSION
We have provided evidence that mild loss of gas content, but with no apparent macroscopic atelectasis, increases airspace dimensions, fractional ventilation, and ventilation heterogeneity in ventilated healthy rats under general anesthesia. This finding suggests that ventilator-induced injury might occur in lungs without preexisting lesions whereby suboptimal recruitment results in maldistribution of ventilation, and this in turn leads to an increase in local lung stretch (35).
The paradoxical presence of higher values for both ADC and FV in areas with modest reduction of aeration is not intuitive. On its own, elevation in FV in a region of interest after ZEEP ventilation could indicate the influx of a larger volume of “fresh” hyperpolarized gas, smaller “residual” alveolar volume in the same region, or both of these factors together, assuming that minute volume is constant. In our study, although ventilation with ZEEP caused a heterogeneous response in FV, the predominant effect was the increase of its mean values (globally and regionally) and a comparable decrease in lung gas volume. Volume loss could suggest that smaller alveoli were responsible for the higher FV because total ventilation was constant for the whole lung. However, concomitant larger mean ADC points to a prevalence of dilated rather than contracted ventilated air spaces (29, 56), because ADC is sensitive to enlargement of subacinar microstructures (31). Alveolar collapse can explain this dissociation between smaller lung volume and larger air spaces (10). Under conditions of poor recruitment, the amount of ventilated lung is smaller and inspired gas is redirected to residual open air spaces that are consequently overdistended (8). Because lung tissue is finite and total ventilation is constant, these open air spaces receive more fractional ventilation than when the lungs are optimally recruited.
Our battery of HP-MRI parameters revealed that gas volume reduction, increased air space dimensions, and FV maldistribution were colocalized after ventilation with ZEEP. Lung regions with larger loss of gas volume had higher ADC and higher and more heterogeneous FV. Using a mixed-model regression analysis (Table 2) we confirmed that mean values and heterogeneity of FV were positively correlated with ADC in each lobe. Despite a significant topographic variability in responses, the observed FV changes were more pronounced in the dependent (dorsal) lung regions. Although other researchers (5) have shown greater ventilation within poorly recruited areas of injured lungs, this is the first study to document colocalized elevations in both FV and air space dimensions in healthy lungs with suboptimal aeration. Our previous studies measured ADC as an estimate of the state of regional air space dilatation (8–10), but ADC provides a static estimate of the dimensions of ventilated peripheral air spaces at the end of inspiration; it does not quantify their dynamic behavior. Elevated air space dimensions and high FV do not necessarily coexist: the air-trapping characteristic of emphysematous lungs, for example, is associated with substantially higher ADC and lower FV (29). A means of assessing both air space dimensions and regional ventilation in mechanically ventilated subjects is extremely valuable because static deformation is less harmful than large dynamic stretch (28).
The notion that microscopic derecruitment in a given lung region causes abnormal distension and stretch of local air spaces is lent further support by our CT results. No macroscopic atelectasis was visible after ventilation at ZEEP (Fig. 6A), which is at variance with work showing regional hyperinflation secondary to discrete atelectasis after lung injury (51) or after prolonged ventilation (19) in rats. However, we observed gravity-dependent gradients of CT attenuation and reversible increases in PIP (Fig. 3) at 1 h, which suggest the presence of microatelectasis. Such sparse alveolar collapse is too small to be resolved by CT at the resolution we adopted (25). Under such conditions, subtle decruitment alters the distribution of inspired gas at the peripheral (subvoxel) level (35). The presence of such a mechanism is supported by the fact that FV was ubiquitously increased in our study, suggesting gas redistribution locally within each lobar ROI rather than between macroscopically distant regions of the lungs. Furthermore, the greater heterogeneity in FV in the form of both hypoventilated and hyperventilated air spaces suggests a maldistributive pattern of aeration, which can be generated by close proximity of collapsing and open alveoli in areas of decruitment (36). Finally, the effect of decruitment on FV and ADC was more prominent in the dorsal (dependent) regions, where density as depicted by CT was higher. Our finding of microatelectasis could be related to our use of FiO2 0.5: imaging studies have shown that higher inspired oxygen results in more substantial atelectasis (17, 43, 45), which could macroscopically redistribute (e.g., from dorsal to ventral regions) FV and ADC at ZEEP.
Although our study does not address whether marginally aerated lung tissue proceeds to ventilator-induced injury, it does present evidence of abnormal alveolar mechanics in these areas. The effect of colocalized microatelectasis and overdistension on the clinical course of ventilated patients is unknown, but recent evidence in humans suggests that heterogeneities of inflation and borderline aeration are associated with greater ARDS mortality (13), which could be due to local concentration of mechanical stress (2). Supporting this interpretation, recent studies in ventilated animals showed injury in lung tissue surrounding isolated foci of atelectasis (44) and regional aeration heterogeneities (14, 57). Maldistributions of ventilation resulting from borderline aeration are probably common during mechanical ventilation in anesthetized surgical patients: in that setting, a recent survey showed that ZEEP is used by many clinicians in combination with large VT (30). Recent studies showed that lung protective ventilation with low VT and higher PEEP improved outcomes, including rates of postoperative respiratory failure and pulmonary infections in patients undergoing high-risk abdominal surgery but with no preexisting injury (23, 46). However, studies testing higher vs. lower PEEP separately from VT size during general anesthesia had negative results (41a), clouding the analysis of the role of recruitment in surgical patients.
This study has several limitations. Most broadly, we did not measure lung stretch directly, but rather inferred the presence of abnormal inflation characteristics from the observed elevations in air space dimension and fractional ventilation after loss of gas volume at ZEEP. This approach is supported by studies that have found high correlations between local stretch, calculated as the fractional change in lung volume (22) and regional ventilation (53). In these studies, ventilation was measured as specific ventilation (sV′), defined as Vfresh/Vresidue (range 0 to infinity) (53); however, FV and sV′ are linked by a transform, and their physiological meanings (dynamic change of gas content) are superimposable. Both these metrics are related to the rapidity of gas turnover in the alveolar environment, with their largest values found in major conducting airways, and they do not represent the contributions of each individual voxel to the total ventilation of the whole lung.
Ventilation has been measured using numerous imaging techniques, and the hyperpolarized gas method we used here has its own idiosyncrasies. Compared with studies that have measured regional ventilation through the washout of inhaled tracer (11, 27, 54), the inspiratory imaging that we used is more prone to artifacts due to fresh tracer gas in the conducting airways (47). Authors using injected 13NN positron emission tomography described decreased ventilation in poorly aerated regions of lungs compared with the increased fractional ventilation reported here (55). This apparent discrepancy is likely related to the fundamental difference between the measurements. Partial or transient atelectasis may reduce overall airflow to the affected region, thus reducing ventilation while increasing the fractional replacement of the gas with each breath (e.g., during alveolar collapse). Some of the discrepancy may also be due to the differing regional sensitivities of the two methods. Washout of intravenously injected tracer has low sensitivity in poorly perfused but well-ventilated lung. Conversely, wash-in techniques such as ours are subject to low signal in air spaces with reduced gas clearance due to distal airway collapse (54). Each technique is therefore potentially subject to bias if the signal in the low-sensitivity regions is too low to quantify, but the nature and direction of bias is different.
Nevertheless, we found heterogeneity of FV to be a key characteristic of poorly recruited lung regions, suggesting that FV detected at least a portion of the alveolar units that were hypoventilated. Additionally, because rats have a pliable chest wall, our results may be hard to extrapolate to large animals and to humans in whom vertical gradients in pleural pressure are larger and chest wall recoil supports alveolar opening. However, postural changes in FV, observed by others using HP-MRI in supine rats (33), suggest vertical gradients in lung mechanics in rats as well.
ADC is weighted in favor of larger air spaces and is not affected by partial volume effects because areas of the voxels outside the lung contribute no signal. However, our HP-MRI estimates of gas volume were based on pixel counting, and as such, they were prone to overestimation where lungs were thinner than the voxels (e.g., in the lung periphery). Despite this technical limitation, changes in lung volume were similar when estimated by HP-MRI and CT in identical conditions of recruitment and derecruitment.
We performed recruitment by increasing PEEP to 9 cmH2O for a short time. Although this single recruitment maneuver was sufficient to restore baseline values of ADC and lung volume, the decrease in FV and its heterogeneity were not significant after recruitment. This was probably due to incomplete recruitment during the short duration of high PEEP, and it is possible that higher or more prolonged airway pressure elevations (48) could have restored baseline FV distributions. Inadequate recruitment of residual dorsal microatelectasis could also explain the higher baseline values of ADC in the dorsal vs. ventral slices (Fig. 3), which we did not observe in our previous study in which rats received longer recruitment maneuvers (10). We studied only one large VT value and, because of this design, we cannot assess the effects of VT selection on FV and ADC. However, lower VT in combination with better recruitment is likely to improve the maldistribution of regional ventilation in healthy lungs (54). Finally, we used a nested methodology to retain the regionality of the information (not sacrificing it to whole-lung statistics). Whereas in ANOVA tests and regression analyses a single error term is typically assumed, in our nested experiment we found that the error variance differed for each spatial and temporal scale within the same animal. In these circumstances, namely having both temporal (condition) and spatial (slices and lobes) pseudoreplications, a linear mixed-effects model may be the optimal choice for analysis (12).
Conclusions
We have provided in vivo evidence that when aeration subtly declines in previously normal lungs, fractional ventilation and air space dimensions are increased in those regions with more lung volume loss. This abnormality is undetectable by conventional monitoring or imaging and suggests the presence of elevated stretch in regions where poorly aerated lung tissue predominates. These mechanisms may have a role in the development of ventilator-induced injury.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute grants R01-HL-116342 and R01-HL-124986. Dr. Cereda is supported by a grant from the Foundation for Anesthesia Education and Research (FAER) and from the Society of Critical Care Anesthesiologists (SOCCA), and by the Transdisciplinary Awards Program in Translational Medicine and Therapeutics (Philadelphia, PA). B.P. Kavanagh is supported by operating funds from the CIHR and holds the Dr. Geoffrey Barker Chair in Critical Care Medicine.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.C., Y.X., S.K., B.P.K., and R.R.R. conception and design of research; M.C., Y.X., H.H., N.M., J.Z., and H.P. performed experiments; M.C., H.H., and N.M. analyzed data; M.C., Y.X., S.K., B.P.K., and R.R.R. interpreted results of experiments; Y.X. prepared figures; M.C. and J.C. drafted manuscript; M.C. and R.R.R. approved final version of manuscript; Y.X., J.C., S.K., B.P.K., and R.R.R. edited and revised manuscript.
ACKNOWLEDGMENTS
We acknowledge the support of Small Animal Imaging Facility at the Department of Radiology, University of Pennsylvania.
APPENDIX
Variance Component Analysis
To study the relationships between the measured regional continuous variables and factors (i.e., conditions, anatomical position), and to test the main hypothesis of our study, a variance component analysis was performed (23a). In the first step of the analysis, decision trees were used to explain variances in the data (41b). A tree-based model can be constructed by a stepwise principle. The algorithm sorts out which of the available variables explain most of the variance observed in the response variable (determined a priori), then determines a threshold value (for each identified variable) that best partitions the variance in the response. The process is repeated for values of the identified variable that are larger and smaller than the threshold until no residual explanatory variable remains.
On the basis of preliminary tree results, we chose a mixed-effect model (4) to predict lobar air space dimensions (ADCm in each ROI as response variable) from the regional stretch (FVm), aeration (V as local volume), and unevenness of ventilation in each lobar ROI (FVh). Slice term also entered the model as fixed effect because we found (in the tree analysis and from ANOVA results) that ADC was always significantly higher in the posterior slices. The random effects were chosen as in the ANOVA nested model (Rat/Slice/Lobe) to extract the variance components for different rats and anatomical positions.
This analysis was performed using the nlme package in R. The model was fit using a maximum likelihood technique and backward elimination based on Akakie information criteria (AIC) (1a). Once the significant covariates were determined, the final model was constructed using the restricted maximum likelihood technique. The first maximal model contained all the variables and all their interactions (up to four-way interactions between FVm, FVh, V, and Slice). Any factor/variable that was not significant was removed from the model; in each step the reduced model was compared with the original one with an ANOVA test and based on the change in AIC.
All the four-way and three-way interactions were nonsignificant and were removed from the model. The only two-way interaction that remained significant was between FVm and FVh. We retained all the fixed effects in the final model. The mixed-effect model results are presented in Table 2.
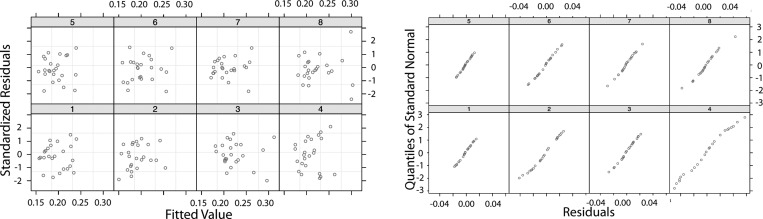
The continuous variables were scaled and centered to attain interpretable coefficients; with centering the data, the overall mean ADCm in all the anterior ROIs (for all the conditions) was selected as the constant term. Therefore, the first fixed-effect variable (slice term) yielded the overall average in posterior slice ROIs for all the conditions. The volume (V) was a significant term with a negative strong effect on the ADCm. The V term was scaled such that a 5% decrease (100 voxels in a lobar ROI with the overall average of 2,000 voxels) increased ADCm by about 14.1% (0.0251/0.178) in that same ROI in anterior slices. The interaction between the other two main effects (rm and rh) was a significant, strong positive explanatory term for ADCm: when both rm and rh increased in a lobe, ADCm also increased in that same lobe. Random effects explained the variance in the slopes of the regression terms. At the end of the analysis, 20% of the variance remained unexplained. The residuals in each rat and the resulting QQ-plot (Q stands for quantile) (54a) are shown in Fig. 7, which indicates the quality of the mixed-model regression fit. The residuals do not show any trend, and the linear QQ plots show the similarity and normality of the distributions.
Fig. 7.
Quality of the mixed-model regression fit showing residuals in each rat and resulting quantile (QQ) plots.
REFERENCES
- 1.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury, and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342: 1301–1308, 2000. [DOI] [PubMed] [Google Scholar]
- 1a.Akaike H. Likelihood of a model and information criteria. J Econometrics 16: 3–14, 1981. [Google Scholar]
- 2.Albaiceta GM, Blanch L. Beyond volutrauma in ARDS: the critical role of lung tissue deformation. Crit Care 15: 304, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372: 747–755, 2015. [DOI] [PubMed] [Google Scholar]
- 4.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Software 67: 1–48, 2015. doi: 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- 5.Bayat S, Porra L, Albu G, Suhonen H, Strengell S, Suortti P, Sovijarvi A, Petak F, Habre W. Effect of positive end-expiratory pressure on regional ventilation distribution during mechanical ventilation after surfactant depletion. Anesthesiology 119: 89–100, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Bilek AM, Dee KC, Gaver DP 3rd. Mechanisms of surface-tension-induced epithelial cell damage in a model of pulmonary airway reopening. J Appl Physiol 94: 770–783, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303: 865–873, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Cereda M, Emami K, Kadlecek S, Xin Y, Mongkolwisetwara P, Profka H, Barulic A, Pickup S, Mansson S, Wollmer P, Ishii M, Deutschman CS, Rizi RR. Quantitative imaging of alveolar recruitment with hyperpolarized gas MRI during mechanical ventilation. J Appl Physiol 110: 499–511, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cereda M, Emami K, Xin Y, Kadlecek S, Kuzma NN, Mongkolwisetwara P, Profka H, Pickup S, Ishii M, Kavanagh BP, Deutschman CS, Rizi RR. Imaging the interaction of atelectasis and overdistension in surfactant-depleted lungs. Crit Care Med 41: 527–535, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cereda M, Xin Y, Emami K, Huang J, Rajaei J, Profka H, Han B, Mongkolwisetwara P, Kadlecek S, Kuzma NN, Pickup S, Kavanagh BP, Deutschman CS, Rizi RR. Positive end-expiratory pressure increments during anesthesia in normal lung result in hysteresis and greater numbers of smaller aerated airspaces. Anesthesiology 119: 1402–1409, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chon D, Simon BA, Beck KC, Shikata H, Saba OI, Won C, Hoffman EA. Differences in regional wash-in and wash-out time constants for xenon-CT ventilation studies. Respir Physiol Neurobiol 148: 65–83, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Crawley MJ. The R book. New York: John Wiley & Sons, 2012, p. 629. [Google Scholar]
- 13.Cressoni M, Cadringher P, Chiurazzi C, Amini M, Gallazzi E, Marino A, Brioni M, Carlesso E, Chiumello D, Quintel M, Bugedo G, Gattinoni L. Lung inhomogeneity in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 189: 149–158, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Cressoni M, Chiurazzi C, Gotti M, Amini M, Brioni M, Algieri I, Cammaroto A, Rovati C, Massari D, di Castiglione CB, Nikolla K, Montaruli C, Lazzerini M, Dondossola D, Colombo A, Gatti S, Valerio V, Gagliano N, Carlesso E, Gattinoni L. Lung inhomogeneities and time course of ventilator-induced mechanical injuries. Anesthesiology 123: 618–627, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Deninger AJ, Mansson S, Petersson JS, Pettersson G, Magnusson P, Svensson J, Fridlund B, Hansson G, Erjefeldt I, Wollmer P, Golman K. Quantitative measurement of regional lung ventilation using 3He MRI. Magn Reson Med 48: 223–232, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Denison DM, Morgan MD, Millar AB. Estimation of regional gas and tissue volumes of the lung in supine man using computed tomography. Thorax 41: 620–628, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derosa S, Borges JB, Segelsjo M, Tannoia A, Pellegrini M, Larsson A, Perchiazzi G, Hedenstierna G. Reabsorption atelectasis in a porcine model of ARDS: regional and temporal effects of airway closure, oxygen, and distending pressure. J Appl Physiol 115: 1464–1473, 2013. [DOI] [PubMed] [Google Scholar]
- 18.Dreyfuss D, Soler P, Basset G, Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 137: 1159–1164, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Duggan M, McNamara PJ, Engelberts D, Pace-Asciak C, Babyn P, Post M, Kavanagh BP. Oxygen attenuates atelectasis-induced injury in the in vivo rat lung. Anesthesiology 103: 522–531, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Emami K, Kadlecek SJ, Woodburn JM, Zhu J, Yu J, Vahdat V, Pickup S, Ishii M, Rizi RR. Improved technique for measurement of regional fractional ventilation by hyperpolarized 3He MRI. Magn Reson Med 63: 137–150, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 41: 1149–1160, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Fuld MK, Easley RB, Saba OI, Chon D, Reinhardt JM, Hoffman EA, Simon BA. CT-measured regional specific volume change reflects regional ventilation in supine sheep. J Appl Physiol 104: 1177–1184, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin JE, Pereira B, Jaber S, IMPROVE Study Group . A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 369: 428–437, 2013. [DOI] [PubMed] [Google Scholar]
- 23a.Garson GD. Variance Components Analysis. Asheboro, NC: Statistical Associates Publishers, 2012. [Google Scholar]
- 24.Gattinoni L, Pesenti A, Avalli L, Rossi F, Bombino M. Pressure-volume curve of total respiratory system in acute respiratory failure. Computed tomographic scan study. Am Rev Respir Dis 136: 730–736, 1987. [DOI] [PubMed] [Google Scholar]
- 25.Gil J, Weibel ER. Morphological study of pressure-volume hysteresis in rat lungs fixed by vascular perfusion. Respir Physiol 15: 190–213, 1972. [DOI] [PubMed] [Google Scholar]
- 26.Hachenberg T, Lundquist H, Tokics L, Brismar B, Hedenstierna G. Analysis of lung density by computed tomography before and during general anaesthesia. Acta Anaesthesiol Scand 37: 549–555, 1993. [DOI] [PubMed] [Google Scholar]
- 27.Horn FC, Deppe MH, Marshall H, Parra-Robles J, Wild JM. Quantification of regional fractional ventilation in human subjects by measurement of hyperpolarized 3He washout with 2D and 3D MRI. J Appl Physiol 116: 129–139, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Hubmayr RD, Margulies SS. Regional ventilation in statically and dynamically hyperinflated dogs. J Appl Physiol 81: 1815–1821, 1996. [DOI] [PubMed] [Google Scholar]
- 29.Ishii M, Emami K, Xin Y, Barulic A, Kotzer CJ, Logan GA, Chia E, MacDuffie-Woodburn JP, Zhu J, Pickup S, Kuzma N, Kadlecek S, Podolin PL, Rizi RR. Regional function-structure relationships in lungs of an elastase murine model of emphysema. J Appl Physiol 112: 135–148, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaber S, Coisel Y, Chanques G, Futier E, Constantin JM, Michelet P, Beaussier M, Lefrant JY, Allaouchiche B, Capdevila X, Marret E. A multicentre observational study of intra-operative ventilatory management during general anaesthesia: tidal volumes and relation to body weight. Anaesthesia 67: 999–1008, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Jacob RE, Minard KR, Laicher G, Timchalk C. 3D 3He diffusion MRI as a local in vivo morphometric tool to evaluate emphysematous rat lungs. J Appl Physiol 105: 1291–1300, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malbouisson LM, Muller JC, Constantin JM, Lu Q, Puybasset L, Rouby JJ; CT Scan ARDS Study Group . Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 163: 1444–1450, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Mansson S, Deninger AJ, Magnusson P, Pettersson G, Olsson LE, Hansson G, Wollmer P, Golman K. 3He MRI-based assessment of posture-dependent regional ventilation gradients in rats. J Appl Physiol 98: 2259–2267, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Marcozzi C, Moriondo A, Solari E, Reguzzoni M, Severgnini P, Protasoni M, Passi A, Pelosi P, Negrini D. Regional lung tissue changes with mechanical ventilation and fluid load. Exp Lung Res 41: 228–240, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 28: 596–608, 1970. [DOI] [PubMed] [Google Scholar]
- 36.Mertens M, Tabuchi A, Meissner S, Krueger A, Schirrmann K, Kertzscher U, Pries AR, Slutsky AS, Koch E, Kuebler WM. Alveolar dynamics in acute lung injury: heterogeneous distension rather than cyclic opening and collapse. Crit Care Med 37: 2604–2611, 2009. [DOI] [PubMed] [Google Scholar]
- 37.Moriondo A, Pelosi P, Passi A, Viola M, Marcozzi C, Severgnini P, Ottani V, Quaranta M, Negrini D. Proteoglycan fragmentation and respiratory mechanics in mechanically ventilated healthy rats. J Appl Physiol 103: 747–756, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Muscedere JG, Mullen JB, Gan K, Slutsky AS. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149: 1327–1334, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Neto AS, Simonis FD, Barbas CS, Biehl M, Determann RM, Elmer J, Friedman G, Gajic O, Goldstein JN, Linko R, Pinheiro de Oliveira R, Sundar S, Talmor D, Wolthuis EK, Gama de Abreu M, Pelosi P, Schultz MJ; PROtective Network Investigators. Lung-protective ventilation with low tidal volumes and the occurrence of pulmonary complications in patients without acute respiratory distress syndrome: a systematic review and individual patient data analysis. Crit Care Med 43: 2155–2163, 2015. [DOI] [PubMed] [Google Scholar]
- 40.Otto CM, Markstaller K, Kajikawa O, Karmrodt J, Syring RS, Pfeiffer B, Good VP, Frevert CW, Baumgardner JE. Spatial and temporal heterogeneity of ventilator-associated lung injury after surfactant depletion. J Appl Physiol 104: 1485–1494, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Protti A, Andreis DT, Monti M, Santini A, Sparacino CC, Langer T, Votta E, Gatti S, Lombardi L, Leopardi O, Masson S, Cressoni M, Gattinoni L. Lung stress and strain during mechanical ventilation: any difference between statics and dynamics? Crit Care Med 41: 1046–1055, 2013. [DOI] [PubMed] [Google Scholar]
- 41a.PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, Pelosi P, Schultz MJ. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 384: 495–503, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41b.Quinlan JR. Simplifying decision trees. Int J Man-Machine Stud 27: 221, 1987. [Google Scholar]
- 42.Reber A, Bein T, Hogman M, Khan ZP, Nilsson S, Hedenstierna G. Lung aeration and pulmonary gas exchange during lumbar epidural anaesthesia and in the lithotomy position in elderly patients. Anaesthesia 53: 854–861, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Reber A, Engberg G, Wegenius G, Hedenstierna G. Lung aeration. The effect of pre-oxygenation and hyperoxygenation during total intravenous anaesthesia. Anaesthesia 51: 733–737, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Retamal J, Bergamini B, Carvalho AR, Bozza FA, Borzone G, Borges J, Larsson A, Hedenstierna G, Bugedo G, Bruhn A. Non-lobar atelectasis generates inflammation and structural alveolar injury in the surrounding healthy tissue during mechanical ventilation. Crit Care 18: 505, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothen HU, Sporre B, Engberg G, Wegenius G, Hogman M, Hedenstierna G. Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesia. Anesthesiology 82: 832–842, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Severgnini P, Selmo G, Lanza C, Chiesa A, Frigerio A, Bacuzzi A, Dionigi G, Novario R, Gregoretti C, de Abreu MG, Schultz MJ, Jaber S, Futier E, Chiaranda M, Pelosi P. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 118: 1307–1321, 2013. [DOI] [PubMed] [Google Scholar]
- 47.Simon BA, Kaczka DW, Bankier AA, Parraga G. What can computed tomography and magnetic resonance imaging tell us about ventilation? J Appl Physiol 113: 647–657, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steimback PW, Oliveira GP, Rzezinski AF, Silva PL, Garcia CS, Rangel G, Morales MM, Lapa ES, Capelozzi VL, Pelosi P, Rocco PR. Effects of frequency and inspiratory plateau pressure during recruitment manoeuvres on lung and distal organs in acute lung injury. Intensive Care Med 35: 1120–1128, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Steinberg JM, Schiller HJ, Halter JM, Gatto LA, Lee HM, Pavone LA, Nieman GF. Alveolar instability causes early ventilator-induced lung injury independent of neutrophils. Am J Respir Crit Care Med 169: 57–63, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS. Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99: 944–952, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsuchida S, Engelberts D, Peltekova V, Hopkins N, Frndova H, Babyn P, McKerlie C, Post M, McLoughlin P, Kavanagh BP. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med 174: 279–289, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Wang H, Dong L, O'Daniel J, Mohan R, Garden AS, Ang KK, Kuban DA, Bonnen M, Chang JY, Cheung R. Validation of an accelerated ‘demons’ algorithm for deformable image registration in radiation therapy. Phys Med Biol 50: 2887–2905, 2005. [DOI] [PubMed] [Google Scholar]
- 53.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Venegas JG, Melo MF. Measurement of regional specific lung volume change using respiratory-gated PET of inhaled 13N-nitrogen. J Nucl Med 51: 646–653, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wellman TJ, Winkler T, Costa EL, Musch G, Harris RS, Venegas JG, Vidal Melo MF. Effect of regional lung inflation on ventilation heterogeneity at different length scales during mechanical ventilation of normal sheep lungs. J Appl Physiol 113: 947–957, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54a.Wilk MB, Gnanadesikan R. Probability plotting methods for the analysis of data. Biometrika 55: 1–17, 1968. [PubMed] [Google Scholar]
- 55.Willey-Courand DB, Harris RS, Galletti GG, Hales CA, Fischman A, Venegas JG. Alterations in regional ventilation, perfusion, and shunt after smoke inhalation measured by PET. J Appl Physiol 93: 1115–1122, 2002. [DOI] [PubMed] [Google Scholar]
- 56.Woods JC, Choong CK, Yablonskiy DA, Bentley J, Wong J, Pierce JA, Cooper JD, Macklem PT, Conradi MS, Hogg JC. Hyperpolarized 3He diffusion MRI and histology in pulmonary emphysema. Magn Reson Med 56: 1293–1300, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y, Kharge AB, Perlman CE. Lung ventilation injures areas with discrete alveolar flooding, in a surface tension-dependent fashion. J Appl Physiol 117: 788–796, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yablonskiy DA, Sukstanskii AL, Leawoods JC, Gierada DS, Bretthorst GL, Lefrak SS, Cooper JD, Conradi MS. Quantitative in vivo assessment of lung microstructure at the alveolar level with hyperpolarized 3He diffusion MRI. Proc Natl Acad Sci USA 99: 3111–3116, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]