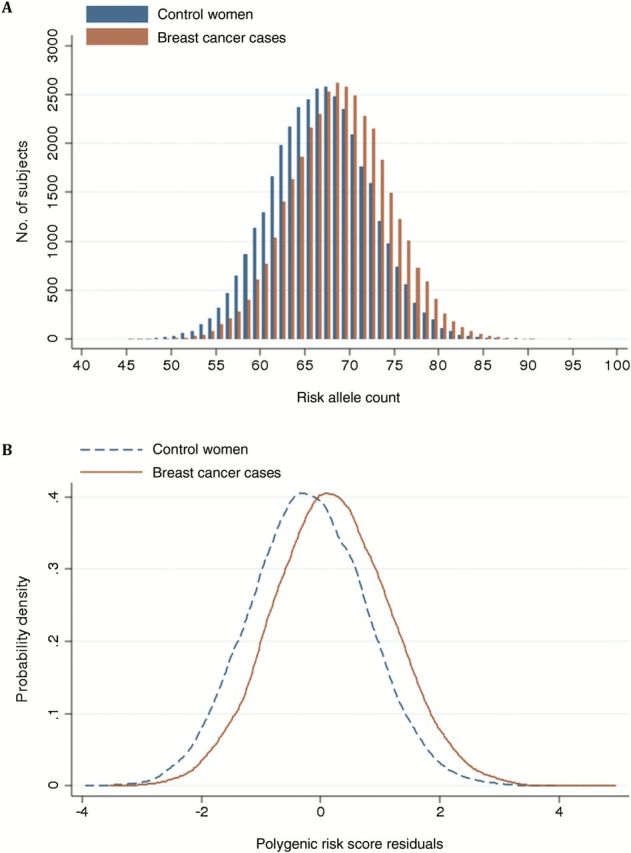
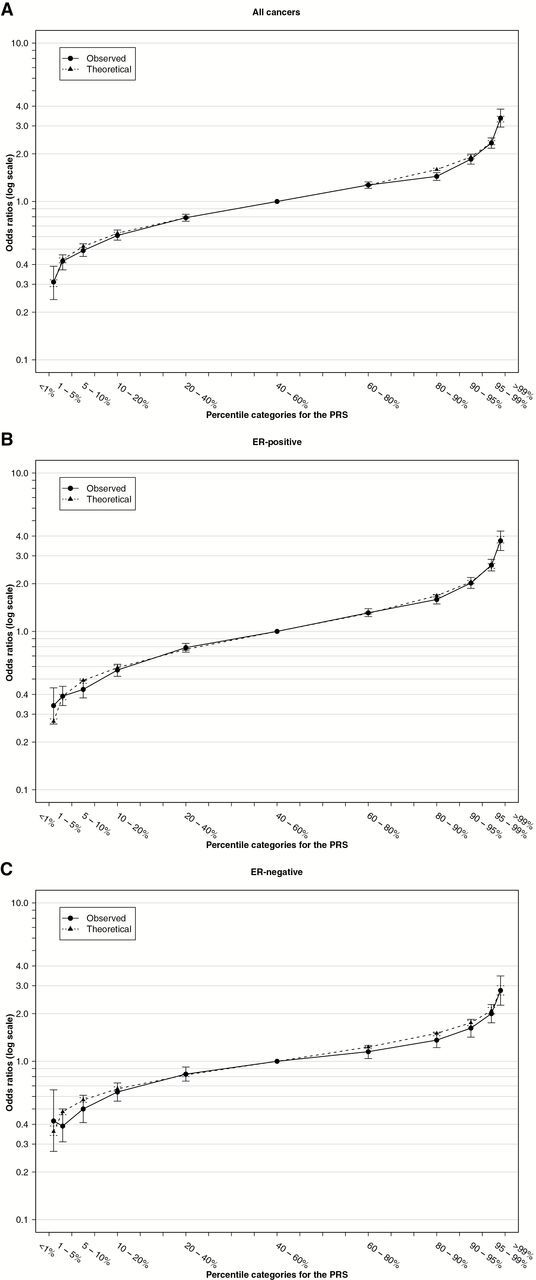
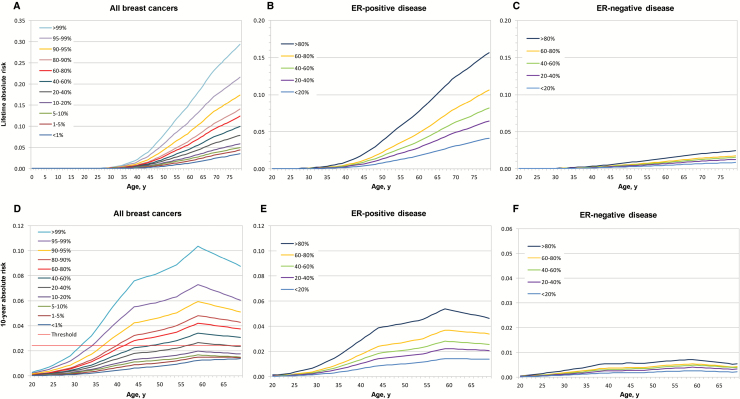
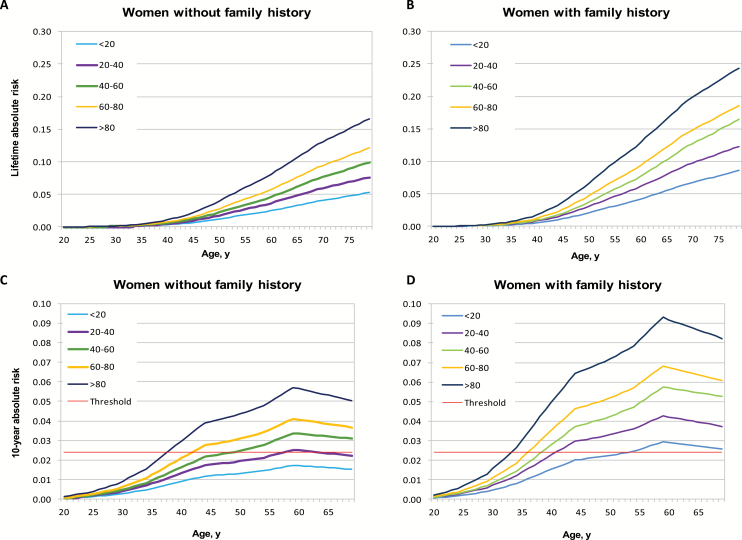
Nasim Mavaddat
Nasim Mavaddat
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,✉,
Paul D P Pharoah
Paul D P Pharoah
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Kyriaki Michailidou
Kyriaki Michailidou
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jonathan Tyrer
Jonathan Tyrer
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Mark N Brook
Mark N Brook
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Manjeet K Bolla
Manjeet K Bolla
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Qin Wang
Qin Wang
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Joe Dennis
Joe Dennis
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Alison M Dunning
Alison M Dunning
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Mitul Shah
Mitul Shah
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Robert Luben
Robert Luben
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Judith Brown
Judith Brown
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Stig E Bojesen
Stig E Bojesen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Børge G Nordestgaard
Børge G Nordestgaard
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Sune F Nielsen
Sune F Nielsen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Henrik Flyger
Henrik Flyger
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Kamila Czene
Kamila Czene
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Hatef Darabi
Hatef Darabi
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Mikael Eriksson
Mikael Eriksson
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Julian Peto
Julian Peto
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Isabel dos-Santos-Silva
Isabel dos-Santos-Silva
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Frank Dudbridge
Frank Dudbridge
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Nichola Johnson
Nichola Johnson
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Marjanka K Schmidt
Marjanka K Schmidt
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Annegien Broeks
Annegien Broeks
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Senno Verhoef
Senno Verhoef
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Emiel J Rutgers
Emiel J Rutgers
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Anthony Swerdlow
Anthony Swerdlow
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Alan Ashworth
Alan Ashworth
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Nick Orr
Nick Orr
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Minouk J Schoemaker
Minouk J Schoemaker
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jonine Figueroa
Jonine Figueroa
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Stephen J Chanock
Stephen J Chanock
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Louise Brinton
Louise Brinton
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jolanta Lissowska
Jolanta Lissowska
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Fergus J Couch
Fergus J Couch
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Janet E Olson
Janet E Olson
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Celine Vachon
Celine Vachon
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Vernon S Pankratz
Vernon S Pankratz
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Diether Lambrechts
Diether Lambrechts
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Hans Wildiers
Hans Wildiers
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Chantal Van Ongeval
Chantal Van Ongeval
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Erik van Limbergen
Erik van Limbergen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Vessela Kristensen
Vessela Kristensen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Grethe Grenaker Alnæs
Grethe Grenaker Alnæs
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Silje Nord
Silje Nord
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Anne-Lise Borresen-Dale
Anne-Lise Borresen-Dale
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Heli Nevanlinna
Heli Nevanlinna
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Taru A Muranen
Taru A Muranen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Kristiina Aittomäki
Kristiina Aittomäki
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Carl Blomqvist
Carl Blomqvist
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jenny Chang-Claude
Jenny Chang-Claude
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Anja Rudolph
Anja Rudolph
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Petra Seibold
Petra Seibold
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Dieter Flesch-Janys
Dieter Flesch-Janys
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Peter A Fasching
Peter A Fasching
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Lothar Haeberle
Lothar Haeberle
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Arif B Ekici
Arif B Ekici
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Matthias W Beckmann
Matthias W Beckmann
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Barbara Burwinkel
Barbara Burwinkel
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Frederik Marme
Frederik Marme
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Andreas Schneeweiss
Andreas Schneeweiss
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Christof Sohn
Christof Sohn
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Amy Trentham-Dietz
Amy Trentham-Dietz
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Polly Newcomb
Polly Newcomb
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Linda Titus
Linda Titus
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Kathleen M Egan
Kathleen M Egan
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
David J Hunter
David J Hunter
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Sara Lindstrom
Sara Lindstrom
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Rulla M Tamimi
Rulla M Tamimi
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Peter Kraft
Peter Kraft
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Nazneen Rahman
Nazneen Rahman
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Clare Turnbull
Clare Turnbull
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Anthony Renwick
Anthony Renwick
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Sheila Seal
Sheila Seal
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jingmei Li
Jingmei Li
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jianjun Liu
Jianjun Liu
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Keith Humphreys
Keith Humphreys
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Javier Benitez
Javier Benitez
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
M Pilar Zamora
M Pilar Zamora
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jose Ignacio Arias Perez
Jose Ignacio Arias Perez
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Primitiva Menéndez
Primitiva Menéndez
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Anna Jakubowska
Anna Jakubowska
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jan Lubinski
Jan Lubinski
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Katarzyna Jaworska-Bieniek
Katarzyna Jaworska-Bieniek
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Katarzyna Durda
Katarzyna Durda
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Natalia V Bogdanova
Natalia V Bogdanova
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Natalia N Antonenkova
Natalia N Antonenkova
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Thilo Dörk
Thilo Dörk
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Hoda Anton-Culver
Hoda Anton-Culver
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Susan L Neuhausen
Susan L Neuhausen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Argyrios Ziogas
Argyrios Ziogas
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Leslie Bernstein
Leslie Bernstein
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Peter Devilee
Peter Devilee
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Robert A E M Tollenaar
Robert A E M Tollenaar
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Caroline Seynaeve
Caroline Seynaeve
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Christi J van Asperen
Christi J van Asperen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Angela Cox
Angela Cox
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Simon S Cross
Simon S Cross
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Malcolm W R Reed
Malcolm W R Reed
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Elza Khusnutdinova
Elza Khusnutdinova
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Marina Bermisheva
Marina Bermisheva
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Darya Prokofyeva
Darya Prokofyeva
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Zalina Takhirova
Zalina Takhirova
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Alfons Meindl
Alfons Meindl
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Rita K Schmutzler
Rita K Schmutzler
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Christian Sutter
Christian Sutter
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Rongxi Yang
Rongxi Yang
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Peter Schürmann
Peter Schürmann
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Michael Bremer
Michael Bremer
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Hans Christiansen
Hans Christiansen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Tjoung-Won Park-Simon
Tjoung-Won Park-Simon
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Peter Hillemanns
Peter Hillemanns
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Pascal Guénel
Pascal Guénel
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Thérèse Truong
Thérèse Truong
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Florence Menegaux
Florence Menegaux
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Marie Sanchez
Marie Sanchez
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Paolo Radice
Paolo Radice
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Paolo Peterlongo
Paolo Peterlongo
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Siranoush Manoukian
Siranoush Manoukian
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Valeria Pensotti
Valeria Pensotti
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
John L Hopper
John L Hopper
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Helen Tsimiklis
Helen Tsimiklis
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Carmel Apicella
Carmel Apicella
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Melissa C Southey
Melissa C Southey
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Hiltrud Brauch
Hiltrud Brauch
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Thomas Brüning
Thomas Brüning
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Yon-Dschun Ko
Yon-Dschun Ko
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Alice J Sigurdson
Alice J Sigurdson
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Michele M Doody
Michele M Doody
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Ute Hamann
Ute Hamann
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Diana Torres
Diana Torres
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Hans-Ulrich Ulmer
Hans-Ulrich Ulmer
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Asta Försti
Asta Försti
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Elinor J Sawyer
Elinor J Sawyer
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Ian Tomlinson
Ian Tomlinson
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Michael J Kerin
Michael J Kerin
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Nicola Miller
Nicola Miller
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Irene L Andrulis
Irene L Andrulis
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Julia A Knight
Julia A Knight
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Gord Glendon
Gord Glendon
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Anna Marie Mulligan
Anna Marie Mulligan
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Georgia Chenevix-Trench
Georgia Chenevix-Trench
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Rosemary Balleine
Rosemary Balleine
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Graham G Giles
Graham G Giles
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Roger L Milne
Roger L Milne
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Catriona McLean
Catriona McLean
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Annika Lindblom
Annika Lindblom
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Sara Margolin
Sara Margolin
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Christopher A Haiman
Christopher A Haiman
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Brian E Henderson
Brian E Henderson
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Fredrick Schumacher
Fredrick Schumacher
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Loic Le Marchand
Loic Le Marchand
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Ursula Eilber
Ursula Eilber
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Shan Wang-Gohrke
Shan Wang-Gohrke
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Maartje J Hooning
Maartje J Hooning
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Antoinette Hollestelle
Antoinette Hollestelle
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Ans M W van den Ouweland
Ans M W van den Ouweland
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Linetta B Koppert
Linetta B Koppert
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jane Carpenter
Jane Carpenter
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Christine Clarke
Christine Clarke
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Rodney Scott
Rodney Scott
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Arto Mannermaa
Arto Mannermaa
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Vesa Kataja
Vesa Kataja
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Veli-Matti Kosma
Veli-Matti Kosma
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jaana M Hartikainen
Jaana M Hartikainen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Hermann Brenner
Hermann Brenner
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Volker Arndt
Volker Arndt
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Christa Stegmaier
Christa Stegmaier
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Aida Karina Dieffenbach
Aida Karina Dieffenbach
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Robert Winqvist
Robert Winqvist
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Katri Pylkäs
Katri Pylkäs
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Arja Jukkola-Vuorinen
Arja Jukkola-Vuorinen
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Mervi Grip
Mervi Grip
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Kenneth Offit
Kenneth Offit
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Joseph Vijai
Joseph Vijai
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Mark Robson
Mark Robson
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Rohini Rau-Murthy
Rohini Rau-Murthy
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Miriam Dwek
Miriam Dwek
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Ruth Swann
Ruth Swann
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Katherine Annie Perkins
Katherine Annie Perkins
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Mark S Goldberg
Mark S Goldberg
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
France Labrèche
France Labrèche
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Martine Dumont
Martine Dumont
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Diana M Eccles
Diana M Eccles
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
William J Tapper
William J Tapper
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Sajjad Rafiq
Sajjad Rafiq
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Esther M John
Esther M John
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Alice S Whittemore
Alice S Whittemore
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Susan Slager
Susan Slager
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Drakoulis Yannoukakos
Drakoulis Yannoukakos
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Amanda E Toland
Amanda E Toland
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Song Yao
Song Yao
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Wei Zheng
Wei Zheng
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Sandra L Halverson
Sandra L Halverson
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Anna González-Neira
Anna González-Neira
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Guillermo Pita
Guillermo Pita
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
M Rosario Alonso
M Rosario Alonso
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Nuria Álvarez
Nuria Álvarez
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Daniel Herrero
Daniel Herrero
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Daniel C Tessier
Daniel C Tessier
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,*,
Daniel Vincent
Daniel Vincent
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Francois Bacot
Francois Bacot
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Craig Luccarini
Craig Luccarini
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Caroline Baynes
Caroline Baynes
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Shahana Ahmed
Shahana Ahmed
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Mel Maranian
Mel Maranian
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Catherine S Healey
Catherine S Healey
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Jacques Simard
Jacques Simard
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Per Hall
Per Hall
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,
Douglas F Easton
Douglas F Easton
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,*,
Montserrat Garcia-Closas
Montserrat Garcia-Closas
1
Affiliations of authors:
Centre for Cancer Genetic Epidemiology, Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK (NM, PDPP, KM, MKB, QW, JD, RL, JBr, DFE); Centre for Cancer Genetic Epidemiology, Department of Oncology, University of Cambridge, Cambridge, UK (PDPP, JT, AMD, MS, CL, CB, SA, MM, CSH, DFE); Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK (MNB, ASw, MJS); Copenhagen General Population Study, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Denmark (SEB, BGN, SFN); Department of Clinical Biochemistry, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN, SFN); Faculty of Health and Medical Sciences, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (SEB, BGN); Department of Breast Surgery, Herlev Hospital, Copenhagen University Hospital, Copenhagen, Herlev, Denmark (HF); Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden (KC, HD, ME, KH, PHa); Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, UK (JP, IdSS, FD); Breakthrough Breast Cancer Research Centre, The Institute of Cancer Research, London, UK (NJ, AA, NO, MGC); Netherlands Cancer Institute, Antoni van Leeuwenhoek hospital, Amsterdam, the Netherlands (MKS, AB, SV, EJR); Division of Breast Cancer Research, Institute of Cancer Research, London, UK (ASw); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD (JF, SJC, LB, ASi, MD); Department of Cancer Epidemiology and Prevention, M. Sklodowska-Curie Memorial Cancer Center and Institute of Oncology, Warsaw, Poland (JLis); Department of Laboratory Medicine and Pathology, Mayo Clinic, Rochester, MN (FJC); Department of Health Sciences Research, Mayo Clinic, Rochester, MN (JEO, CV, VSP, SS); Vesalius Research Center, VIB, Leuven, Belgium (DL); Laboratory for Translational Genetics, Department of Oncology, University of Leuven, Leuven, Belgium (DL); Department of General Medical Oncology, University Hospitals Leuven, and Department of Oncology, KU Leuven, Leuven, Belgium (HW); Department of Radiation Oncology, University Hospital Gasthuisberg, Leuven, Belgium (EVL); Department of Radiology, University Hospital Gasthuisberg, Leuven, Belgium (CVO); Department of Genetics, Institute for Cancer Research, Oslo University Hospital, Radiumhospitalet, Oslo, Norway (VK, GGA, SN, ALBD); Institute of Clinical Medicine, University of Oslo, Oslo, Norway (VK, ALBD); Department of Clinical Molecular Biology, University of Oslo, Oslo, Norway (VK); Department of Obstetrics and Gynecology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (HN, TAM); Department of Clinical Genetics, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (KA); Department of Oncology, University of Helsinki and Helsinki University Central Hospital, Helsinki, HUS, Finland (CB); Division of Cancer Epidemiology, German Cancer Research Center, Heidelberg, Germany (JCC, AR, PS, UE); Department of Cancer Epidemiology/Clinical Cancer Registry, University Clinic Hamburg-Eppendorf, Hamburg, Germany (DFJ); University Breast Center Franconia, Department of Gynecology and Obstetrics, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany (PAF, LH, MWB); David Geffen School of Medicine, Department of Medicine Division of Hematology and Oncology, University of California at Los Angeles, CA (PAF); Institute of Human Genetics, University Hospital Erlangen, Friedrich Alexander University Erlangen-Nuremberg, Erlangen, Germany (ABE); Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany (BB, FM, ASc, CSohn, RY); Molecular Epidemiology Group, German Cancer Research Center, Heidelberg, Germany (BB, RY); National Center for Tumor Diseases, University of Heidelberg, Heidelberg, Germany (FM, ASc); University of Wisconsin Carbone Cancer Center, Madison, WI (ATD, PN); Cancer Prevention Program, Fred Hutchinson Cancer Research Center, Seattle, WA (PN); Geisel School of Medicine at Dartmouth, Hanover, NH (LT); Division of Population Sciences, Moffitt Cancer Center & Research Institute, Tampa, FL (KE); Program in Genetic Epidemiology and Statistical Genetics, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, PK); Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA (DJH, SL, RMT, PK); Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA (RMT); Section of Cancer Genetics, Institute of Cancer Research, London, UK (NR, CT, AR, SS); Human Genetics Division, Genome Institute of Singapore, Singapore (JLi, JLiu); Human Genotyping-CEGEN Unit, Human Cancer Genetics Programme, Spanish National Cancer Research Centre, Madrid, Spain (JBe, AGN, GP, MRA, NA, DH); Centro de Investigación en Red de Enfermedades Raras, Valencia, Spain (JBe); Servicio de Oncología Médica, Hospital Universitario La Paz, Madrid, Spain (MPZ); Servicio de Cirugía General y Especialidades, Hospital Monte Naranco, Oviedo, Spain (JIAP); Servicio de Anatomía Patológica, Hospital Monte Naranco, Oviedo, Spain (PM); Department of Genetics and Pathology, Pomeranian Medical University, Szczecin, Poland (AJ, JLu, KJB, KD); Department of Obstetrics and Gynaecology, Hannover Medical School, Hannover, Germany (NVB, TD, PS, TWPS, PHi); Department of Radiation Oncology, Hannover Medical School, Hannover, Germany (NVB, MB, HC); NN Alexandrov Research Institute of Oncology and Medical Radiology, Minsk, Belarus (NNA); Department of Epidemiology, University of California Irvine, Irvine, CA (HAC, AZ); Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA (SLN, LB); Department of Human Genetics and Department of Pathology, Leiden University Medical Center, Leiden, the Netherlands (PD); Department of Surgical Oncology, Leiden University Medical Center, Leiden, the Netherlands (RAEMT); Department of Clinical Genetics, Leiden University Medical Center, Leiden, the Netherlands (CJvA); Sheffield Cancer Research, Department of Oncology, University of Sheffield, UK (AC, MWRR); Academic Unit of Pathology, Department of Neuroscience, University of Sheffield, Sheffield, UK (SSC); Institute of Biochemistry and Genetics, Ufa Scientific Center of Russian Academy of Sciences, Ufa, Russia (EK, MB, ZT); Department of Genetics and Fundamental Medicine of Bashkir State University, Ufa, Russia (EK, DP); Division of Gynaecology and Obstetrics, Technische Universität München, Munich, Germany (AMe); Center for Hereditary Breast and Ovarian Cancer, University Hospital Cologne, Cologne, Germany (RKS); Center for Integrated Oncology, University Hospital Cologne, Cologne, Germany (RKS); Center for Molecular Medicine Cologne, University of Cologne, Cologne, Germany (RKS); Institute of Human Genetics, University Heidelberg, Heidelberg, Germany (CSutter); National Institute of Health and Medical Research, Center for Research in Epidemiology and Population Health, U1018, Environmental Epidemiology of Cancer, Villejuif, France (PG, TT, FM, MS); University Paris-Sud, Villejuif, France (PG, TT, FM, MS); Unit of Molecular Bases of Genetic Risk and Genetic Testing, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (PR); IFOM, Fondazione Istituto FIRC di Oncologia Molecolare, Milan, Italy (PP, VP); Unit of Medical Genetics, Department of Preventive and Predictive Medicine, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy (SM); Cogentech Cancer Genetic Test Laboratory, Milan, Italy (VP); Centre for Epidemiology & Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, Victoria, Australia (JLH, CA, GGG, RLM); Department of Pathology, University of Melbourne, Melbourne, Victoria, Australia (HT, MCS); Dr. Margarete Fischer-Bosch-Institute of Clinical Pharmacology, Stuttgart, Germany, for the GENICA Network (HB); University of Tübingen, Tübingen, Germany, for the GENICA Network (HB); German Cancer Consortium, German Cancer Research Center (DKFZ), Heidelberg, Germany (HBra, HBre, AKD); Institute for Prevention and Occupational Medicine of the German Social Accident Insurance, Institute of the Ruhr-Universität Bochum (IPA), Germany, for the GENICA Network (TB); Department of Internal Medicine, Evangelische Kliniken Bonn gGmbH, Johanniter Krankenhaus, Bonn, Germany, for the GENICA Network (YDK); Molecular Genetics of Breast Cancer, German Cancer Research Center, Heidelberg, Germany, for the GENICA Network (UH); Institute of Human Genetics, Pontificia Universidad Javeriana, Bogota, Colombia (DT); Frauenklinik der Stadtklinik Baden-Baden, Baden-Baden, Germany (HUU); Division of Molecular Genetic Epidemiology, German Cancer Research Center, Heidelberg, Germany (AF); Center for Primary Health Care Research, University of Lund, Malmö, Sweden (AF); Division of Cancer Studies, Kings College London, Guy’s Hospital, London, UK (EJS); Wellcome Trust Centre for Human Genetics and Oxford Biomedical Research Centre, University of Oxford, Oxford, UK (IT); Clinical Science Institute, University Hospital Galway, Galway, Ireland (MJK, NM); Ontario Cancer Genetics Network, Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, Ontario, Canada (ILA, GG); Department of Molecular Genetics, University of Toronto, Toronto, Ontario, Canada (ILA); Prosserman Centre for Health Research, Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada (JAK); Division of Epidemiology, Dalla Lana School of Public Health, University of Toronto, Toronto, Ontario, Canada (JAK); Department of Laboratory Medicine and Pathobiology, University of Toronto, Toronto, Ontario, Canada (AMM); Laboratory Medicine Program, University Health Network, Toronto, Ontario, Canada (AMM); Department of Genetics, QIMR Berghofer Medical Research Institute, Brisbane, Australia, for the Australian Ovarian Cancer Study Group (GCT); Peter MacCallum Cancer Center, Melbourne, Victoria, Australia, for kConFab Investigators and the Australian Ovarian Cancer Study Group; Westmead Millenium Institute for Medical Research, University of Sydney, Sydney, NSW, Australia (RB, CC); Western Sydney and Nepean Blue Mountains Local Health Districts, Sydney, Australia (RB); Cancer Epidemiology Centre, Cancer Council Victoria, Melbourne, Victoria, Australia (GGG, RLM); Anatomical Pathology, The Alfred Hospital, Melbourne, Victoria, Australia (CM); Department of Molecular Medicine and Surgery, Karolinska Institutet, Stockholm, Sweden (AL); Department of Oncology - Pathology, Karolinska Institutet, Stockholm, Sweden (SM); Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA (CAH, BEH, FS); Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI (LLM); Department of Obstetrics and Gynecology, University of Ulm, Ulm, Germany (SWG); Department of Medical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (MJH, AH, CS); Department of Clinical Genetics, Erasmus University Medical Center, Rotterdam, the Netherlands (AMWvdO); Department of Surgical Oncology, Family Cancer Clinic, Erasmus MC Cancer Institute, Rotterdam, the Netherlands (LBK); Australian Breast Cancer Tissue Bank, Westmead Millennium Institute, University of Sydney, Sydney, NSW, Australia, for the ABCTB Investigators (JC); Division of Genetics, Hunter Area Pathology Service and University of Newcastle, Callaghan, NSW, Australia (RSc); School of Medicine, Institute of Clinical Medicine, Pathology and Forensic Medicine (AMa, VMK, JMH); Cancer Center of Eastern Finland, University of Eastern Finland, Kuopio, Finland (AMa, VMK, JMH); Imaging Center, Department of Clinical Pathology, Kuopio University Hospital, Kuopio, Finland (AMa, VMK, JMH); Cancer Center, Kuopio University Hospital, Kuopio, Finland, and Jyvaskyla Central Hospital, Jyvaskyla, Finland (VK); Division of Clinical Epidemiology and Aging Research, German Cancer Research Center, Heidelberg, Germany (HB, VA, AKD); Saarland Cancer Registry, Saarbrücken, Germany (CStegmaier); Laboratory of Cancer Genetics and Tumor Biology, Department of Clinical Chemistry and Biocenter Oulu, University of Oulu, Northern Finland Laboratory Centre NordLab, Oulu, Finland (RW, KP); Department of Oncology, Oulu University Hospital, University of Oulu, Oulu, Finland (AJV); Department of Surgery, Oulu University Hospital, University of Oulu, Oulu, Finland (MG); Clinical Genetics Service, Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV, MR, RRM); Clinical Genetics Research Lab, Department of Cancer Biology and Genetics, Memorial Sloan-Kettering Cancer Center, New York, NY (KO, JV); Department of Molecular and Applied Biosciences, Faculty of Science and Technology, University of Westminster, London, UK (MDw, RSw, KAP); Department of Medicine, McGill University, Montreal, Quebec, Canada (MSG); Division of Clinical Epidemiology, McGill University Health Centre, Royal Victoria Hospital, Montreal, Quebec, Canada (MSG); Département de médecine sociale et préventive, Département de santé environnementale et santé au travail, Université de Montréal, Montreal, Quebec, Canada (FL); Cancer Genomics Laboratory for Genomics Centre, Centre Hospitalier Universitaire de Québec Research Centre and Laval University, Québec City, Quebec, Canada (MDu, JS); Faculty of Medicine, University of Southampton, UK (DME, WJT, SR); Cancer Prevention Institute of California, Fremont, CA (EMJ); Department of Health Research and Policy Stanford University School of Medicine Stanford CA (EMJ, ASW); Molecular Diagnostics Laboratory, IRRP, National Centre for Scientific Research “Demokritos”, Aghia Paraskevi Attikis, Athens, Greece (DY); Department of Molecular Virology, Immunology and Medical Genetics, Comprehensive Cancer Center, The Ohio State University, Columbus, OH (AET); Department of Cancer Prevention and Control, Roswell Park Cancer Institute, Buffalo, NY (SY); Division of Epidemiology, Department of Medicine, Vanderbilt Epidemiology Center, Vanderbilt-Ingram Cancer Center, Vanderbilt University School of Medicine, Nashville, TN (WZ, SLH); McGill University and Génome Québec Innovation Centre, Montréal, Québec, Canada (DCT, DV, FB).
1,*