A nomogram was developed and validated for disease-specific survival after resection of perihilar cholangiocarcinoma. Lymph node involvement, resection margin status, and tumor differentiation were independent prognostic factors in the derivation cohort. The proposed nomogram was better than the American Joint Committee on Cancer staging system.
Keywords: cholangiocarcinoma, survival, prognostic model, nomogram
Abstract
Background
The objective of this study was to derive and validate a prognostic nomogram to predict disease-specific survival (DSS) after a curative intent resection of perihilar cholangiocarcinoma (PHC).
Patients and methods
A nomogram was developed from 173 patients treated at Memorial Sloan Kettering Cancer Center (MSKCC), New York, USA. The nomogram was externally validated in 133 patients treated at the Academic Medical Center (AMC), Amsterdam, The Netherlands. Prognostic accuracy was assessed with concordance estimates and calibration, and compared with the American Joint Committee on Cancer (AJCC) staging system. The nomogram will be available as web-based calculator at mskcc.org/nomograms.
Results
For all 306 patients, the median overall survival (OS) was 40 months and the median DSS 41 months. Median follow-up for patients alive at last follow-up was 48 months. Lymph node involvement, resection margin status, and tumor differentiation were independent prognostic factors in the derivation cohort (MSKCC). A nomogram with these prognostic factors had a concordance index of 0.73 compared with 0.66 for the AJCC staging system. In the validation cohort (AMC), the concordance index was 0.72, compared with 0.60 for the AJCC staging system. Calibration was good in the derivation cohort; in the validation cohort patients had a better median DSS than predicted by the model.
Conclusions
The proposed nomogram to predict DSS after curative intent resection of PHC had a better prognostic accuracy than the AJCC staging system. Calibration was suboptimal because DSS differed between the two institutions. The nomogram can inform patients and physicians, guide shared decision making for adjuvant therapy, and stratify patients in future randomized, controlled trials.
introduction
Perihilar cholangiocarcinoma (PHC) is the most common malignancy of the biliary tree, with an annual incidence in the United States of one to two per 100 000 [1]. PHC arises at or near the confluence of the right and left hepatic duct. Bile duct obstruction at the confluence causes painless jaundice and intrahepatic biliary dilatation on imaging. The majority of patients are not candidates for surgical resection because of locally advanced or metastatic disease at the time of presentation [2]. Patients who can undergo a curative intent resection have an associated median overall survival (OS) that varies from 19 to 39 months [3].
Prognostic factors after resection of PHC have been identified, but prediction of long-term survival for individual patients remains inaccurate. The sixth and seventh editions of the AJCC staging systems for PHC were recently compared and found to both have modest prognostic accuracy [4]. Independent prognostic factors other than T stage, N stage, and M stage have been identified, such as a positive resection margin, moderate or poor tumor differentiation, perineural invasion, and papillary tumors [5–9]. When positive lymph nodes are not found, evaluation of less than four lymph nodes can cause understaging and is consequently an independent poor prognostic factor [10]. In patients with a negative resection margin, a close or narrow resection margin was also found to be an independent poor prognostic factor [11]. Combining these variables in a prognostic model may further improve survival predictions for individual patients after curative intent resection of PHC.
More accurate predictions for individual patients may improve identification of a high-risk group that may benefit from adjuvant treatment. Moreover, a better prognostic model will allow adequate stratification of patients in randomized, controlled trials evaluating adjuvant treatments and enable adjustment for confounding factors when comparing outcomes across centers. Such a prognostic model has been developed for most other cancers, recently including intrahepatic cholangiocarcinoma [12]. The objective of this study was to derive and validate a prognostic nomogram to predict disease-specific survival (DSS) for patients who recovered from a curative intent resection of PHC in two independent large institutional cohorts.
methods
patients
Two independent prospectively maintained databases were used, both including consecutive patients who underwent a curative intent resection for PHC. The patient series of Memorial Sloan Kettering Cancer Center (MSKCC, New York, United States) was used for development of the prognostic model; the patient series of the Academic Medical Center (AMC, Amsterdam, The Netherlands) was used for external validation. Patients were included from 1991 to 2012. All patients had a diagnosis of PHC confirmed at pathologic assessment of the resected specimen. Patients were included with tumors arising from the biliary confluence, right or left hepatic duct, or common hepatic duct. Patients with carcinoma in situ were included because death due to recurrence or long-term complications of treatment was also observed in this subgroup. Patients with 90-day postoperative mortality were excluded since the objective of the prognostic model was to inform patients and physicians about individual cancer-specific prognosis after recovery from surgery.
Patient selection for resection and perioperative management were similar between the two institutions. The main difference in treatment between centers was that all patients in AMC underwent preoperative radiation therapy with three fractions of 3.5 Gy in an effort to reduce postoperative seeding metastases [13]. Adjuvant chemotherapy was not offered in the AMC because it was not reimbursed by Dutch health insurance; in MSKCC it was discretionary and discussed with the patient. Adjuvant therapy was defined as any chemotherapy or radiotherapy that was started within 3 months after surgery. The institutional review board at both institutions approved this study.
variables
Both databases included data on patient demographics, symptoms at presentation, co-morbidities, laboratory test results, cross-sectional imaging, preoperative biliary drainage, type of surgical resection, and pathology of the resected specimen. Evaluation of less than four lymph nodes can cause understaging of lymph node-negative patients, and was previously found to be an independent poor prognostic factor [10, 14, 15]. Lymph node status was therefore divided into three groups: positive, negative with less than four lymph nodes evaluated, and negative with four or more nodes evaluated. Resection margin was also divided into three groups: a wide, narrow, or positive resection margin. A wide margin was defined as no adenocarcinoma present at both the specimen margin and any additional bile duct margins. A narrow margin was defined as the presence of adenocarcinoma at the specimen margin with a negative additional bile duct margin. A positive margin was defined as the presence of adenocarcinoma at both the specimen margin and any additional bile duct margins [11]. Preoperative tumor markers (CA 19-9 and CEA) were not considered because of missing values in the majority of patients.
Survival was measured from the date of surgery to the date of death. Patients were censored when alive at the date of last contact. The primary outcome measure was DSS.
statistical analysis
Statistical analyses were carried out using SPSS (Statistical Package for the Social Sciences), version 22, and R (a language and environment for statistical computing), version 3.0.2. Proportions were compared with Fischer's exact or χ 2 test; means were compared with t-test. Univariate analyses were conducted in the derivation cohort by Kaplan–Meier estimates of survival probabilities, partial likelihood estimation for hazard ratios and the log-rank and score tests for comparisons. A Cox proportional hazard regression model was used for multivariable modeling. Variables were a priori screened for clinical significance based on published multivariable analyses with large patient series [5–9].
A nomogram was produced to make patient-specific predictions. The predictive ability of the model was first assessed using concordance probabilities (c-statistic). The concordance probability is the chance that for any two patients, the one with the longer observed DSS also has a longer predicted DSS. Next, model calibration was checked on the derivation and external validation cohort. Patients were stratified in four quartiles based on their nomogram score. Predicted and observed (Kaplan–Meier estimated) DSS were compared for each quartile. Comparison with the AJCC staging system was carried out using concordance probabilities.
results
patients and variables
The derivation cohort (MSKCC) consisted of 173 consecutive eligible patients who had a resection of PHC. The validation cohort (AMC) consisted of 133 consecutive eligible patients. Table 1 compares patient characteristics and treatments of the two cohorts. Patients in the derivation cohort were on average 3 years older, were more frequently operated on in the 1991–2001 period (50% versus 33%), had a higher preoperative bilirubin (3.9 versus 1.1 mg/dl), were more likely to undergo preoperative percutaneous biliary drainage without endoscopic drainage (17% versus 4%) or an extended right hepatectomy (33% versus 24%), and less likely to undergo a caudate resection (36% versus 57%). In the derivation cohort, 24 patients (14%) received adjuvant chemotherapy and 25 (14%) adjuvant radiotherapy; none of the patients in the validation cohort received adjuvant chemotherapy or radiotherapy.
Table 1.
Patient characteristics
| MSKCC (N = 173) | AMC (N = 133) | P-value | |
|---|---|---|---|
| Female gender | 73 (42%) | 54 (41%) | 0.82 |
| Age (median, range) | 65 (34–89) | 62 (30–82) | 0.01 |
| Bilirubin—preoperative (mean, SEM) | 3.9 (0.45) | 1.1 (0.11) | <0.001 |
| Lobar atrophy on imaging | 0.13 | ||
| None | 108 (62%) | 81 (72%) | |
| Left | 41 (24%) | 15 (13%) | |
| Right | 24 (14%) | 16 (14%) | |
| Portal vein involvement on imaging | 0.85 | ||
| None | 108 (62%) | 68 (64%) | |
| Main/bifurcation/bilateral | 4 (2%) | 4 (4%) | |
| Left | 36 (21%) | 21 (20%) | |
| Right | 25 (14%) | 13 (12%) | |
| Bismuth classification | 0.59 | ||
| Left or right duct only | 11 (6%) | 13 (10%) | |
| 1 | 37 (21%) | 20 (15%) | |
| 2 | 22 (13%) | 17 (13%) | |
| 3A, right | 40 (23%) | 39 (29%) | |
| 3B, left | 38 (22%) | 26 (20%) | |
| 4 | 25 (14%) | 18 (14%) | |
| Blumgart classification [2] | 0.07 | ||
| T1 | 81 (47%) | 63 (56%) | |
| T2 | 66 (38%) | 28 (25%) | |
| T3 | 26 (15%) | 21 (19%) | |
| Tumor diameter on imaging | 0.78 | ||
| >3 cm | 33 (29%) | 48 (30%) | |
| Mean (SEM) | 2.34 (0.16) | 2.33 (0.16) | |
| Drainage—preoperative | <0.001 | ||
| None | 44 (25%) | 16 (12%) | |
| Percutaneous | 29 (17%) | 5 (4%) | |
| Endoscopic | 73 (42%) | 85 (64%) | |
| Both | 27 (16%) | 27 (20%) | |
| Type of resection | 0.007 | ||
| Bile duct resection alone | 32 (18%) | 21 (16%) | |
| Right hemihepatectomy | 17 (10%) | 8 (6%) | |
| Left hemihepatectomy | 49 (28%) | 37 (28%) | |
| Extended right hepatectomy | 57 (33%) | 32 (24%) | |
| Extended left hepatectomy | 12 (7%) | 15 (11%) | |
| Segment 4b/5 resection | 6 (3%) | 2 (2%) | |
| Caudate resectiona | 63 (36%) | 74 (57%) | <0.001 |
| Resection margin | 0.76 | ||
| Positive | 45 (26%) | 39 (29%) | |
| Narrow | 30 (17%) | 24 (18%) | |
| Wide | 98 (57%) | 70 (53%) | |
| Lymph node involvement | 0.06 | ||
| Yes | 51 (29%) | 28 (21%) | |
| No, <4 nodes | 72 (42%) | 52 (39%) | |
| No, at least 4 nodes | 50 (29%) | 53 (40%) | |
| Well-differentiated | 40 (24%) | 28 (22%) | 0.78 |
| Perineural invasion | 120 (69%) | 91 (68%) | 0.90 |
| Papillary tumor | 39 (23%) | 18 (14%) | 0.05 |
| T stage 3 or 4, 7th edition | 35 (26%) | 39 (23%) | 0.50 |
| AJCC stage, 7th edition | 0.84 | ||
| 0 | 8 (5%) | 3 (2%) | |
| 1 | 20 (12%) | 15 (11%) | |
| 2 | 73 (42%) | 60 (45%) | |
| 3 | 45 (26%) | 33 (25%) | |
| 4 | 27 (16%) | 22 (17%) | |
| Year of surgery, 1991–2001 | 87 (50%) | 44 (33%) | 0.003 |
| Adjuvant chemotherapy | 24 (14%) | 0 | <0.001 |
| Adjuvant radiotherapy | 25 (14%) | 0 | <0.001 |
Cross-sectional imaging and/or imaging reports were missing for some patients in the validation cohort, resulting in missing values for lobar atrophy on imaging (n = 21), portal vein involvement on imaging (n = 27), and Blumgart classification (n = 21).
aThe percentage of caudate resection is relatively low because of a large proportion of Bismuth 1 patients who had a bile duct resection without liver resection, and patients operated on in the 1990s when caudate resections were less commonly carried out.
SEM, standard error of the mean.
survival outcomes
The median OS in the study cohort (n = 306) was 40 months; the median DSS was 41 months. Estimated OS at 5 years was 35% and at 10 years 17%. DSS at 5 years was 38% and at 10 years 21%. At last follow-up, 213 patients (70%) had died; only 18 patients (8% of all deaths) had died from causes other than PHC or complications of its management. Median follow-up for patients alive at last follow-up was 48 months. The median DSS differed between the cohorts: 38 months in the derivation and 49 months in the validation cohort (P = 0.02).
univariate and multivariable analysis
Table 2 presents the results of univariate and multivariable analysis in the derivation cohort (MSKCC, n = 173). Lymph node involvement, resection margin status, and tumor differentiation were the only statistically significant independent prognostic factors in the derivation cohort. Adjuvant chemotherapy and radiotherapy were strong poor prognostic factors; however, this association disappeared after adjusting for margin status and lymph node status. None of the covariates in Table 1 that differed between the cohorts were a statistically significant independent prognostic factor. Supplementary Table S1, available at Annals of Oncology online, presents uni- and multivariable analyses for the validation cohort, of which the results are similar to the derivation cohort.
Table 2.
Univariate and multivariable analysis for DSS in the derivation cohort (MSKCC, 173 patients)
| Covariate | Univariate P-value |
Multivariable P-value* |
Multivariable HR |
Multivariable HR 95% CI |
|---|---|---|---|---|
| Lymph node involvement | <0.001 | <0.001 | ||
| Yes | 3.11 | 1.88–5.12 | ||
| No, <4 nodes evaluated | 1.43 | 0.87–2.37 | ||
| No, at least 4 nodes evaluated | Reference | – | ||
| Moderate/poor differentiation | <0.001 | <0.001 | 2.48 | 1.46–4.21 |
| Resection margin | <0.001 | <0.001 | ||
| Positive | 2.45 | 1.58–3.80 | ||
| Narrow | 1.65 | 1.01–2.72 | ||
| Wide | Reference | – | ||
| Perineural invasion | <0.001 | 0.13 | ||
| Papillary tumor | <0.001 | 0.36 | ||
| AJCC stage, 7th edition | <0.001 | 0.85 | ||
| Preoperative bilirubin | 0.004 | 0.10 | ||
| Drainage—preoperative | 0.011 | 0.20 | ||
| Lobar atrophy | 0.02 | 0.06 | ||
| Bismuth classification | 0.04 | 0.26 | ||
| T stage 3 or 4, 7th edition | 0.005 | 0.69 | ||
| Adjuvant chemotherapy | 0.02 | 0.89 | ||
| Adjuvant radiotherapy | 0.002 | 0.75 | ||
| Gender | 0.08 | |||
| Portal vein involvement | 0.79 | |||
| Tumor diameter >3 cm | 0.46 | |||
| Age (<65 versus >65 years) | 0.95 | |||
| Type of liver resection | 0.18 | |||
| Caudate resection | 0.39 | |||
| Year of surgery (1991–2001 versus 2002–2012) | 0.22 |
*Multivariable P-values when covariates were added (one at a time) to a model with lymph node involvement, tumor differentiation, and resection margin.
HR, hazard ratio; CI, confidence interval.
prognostic models
Figure 1 is a nomogram representing the prognostic model derived from the derivation cohort (MSKCC). The c-statistic of this nomogram was 0.73 compared with 0.66 for the seventh edition of the AJCC staging system. The c-statistic of the nomogram in the validation cohort (AMC) was 0.72, compared with 0.60 for the seventh edition of the AJCC staging system. Supplementary Figure S1, available at Annals of Oncology online, compares the predicted DSS at 5 years with the observed DSS in the derivation cohort (MSKCC) for four quartiles of patients stratified by nomogram score. Table 3 compares the predicted DSS at 3 and 5 years with the observed DSS in the validation cohort (AMC). A web-based calculator will be available at mskcc.org/nomograms to predict DSS at 3 and 5 years after curative intent resection of PHC.
Figure 1.
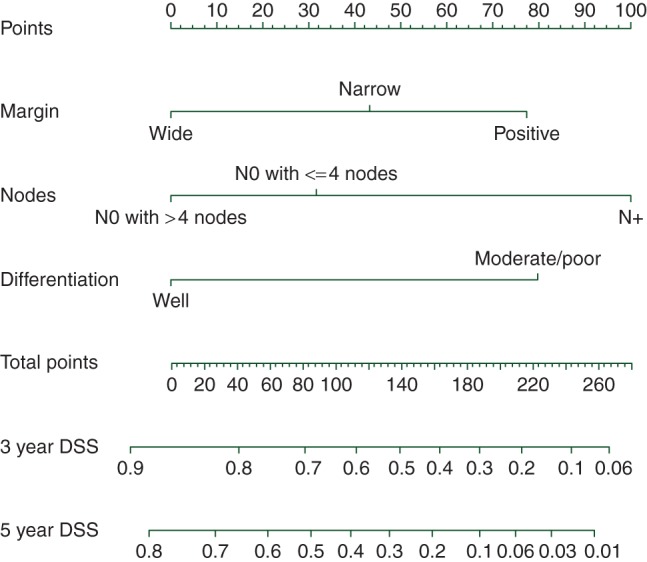
Nomogram. The predicted 3- and 5-year DSS can be read from this nomogram in two steps: (i) Draw a vertical line for each prognostic factor (margin, nodes, differentiation) from its appropriate value (e.g. for margin the values are ‘wide’, ‘narrow’, and ‘positive’) to the axis termed ‘points’ at the top of the figure. The points assigned for the value of each prognostic factor can be read where the vertical line crosses the ‘points’ axis. (ii) Add the three point scores determined at step 1 and find the sum score on the axis termed ‘total points’. Determine the predicted 3- and 5-year DSS by drawing a vertical line from the sum score on the axis termed ‘total points’ down to the 3- and 5-year DSS axes. The predicted 3-year DSS can be read where the vertical line crosses with the axis termed ‘3-year DSS’.
Table 3.
Calibration of the nomogram on the validation cohort (AMC, N = 133)
| Quartile based on nomogram score | Predicted DSS (%) | Kaplan–Meier estimated DSS (%) |
|---|---|---|
| 3-year DSS | ||
| 1 | 24 | 21 |
| 2 | 29 | 45 |
| 3 | 36 | 55 |
| 4 | 45 | 64 |
| 5-year DSS | ||
| 1 | 11 | 10 |
| 2 | 16 | 32 |
| 3 | 22 | 39 |
| 4 | 29 | 47 |
Predicted DSS at 3 and 5 years is compared with observed DSS for four quartiles of patients stratified by nomogram score.
discussion
A prognostic model was derived and externally validated to predict DSS after curative intent resection of PHC. The model was presented as a nomogram and web-based calculator based on three independent prognostic factors that are present in the pathology report of every resected PHC: lymph node involvement and count, resection margin involvement including additional margins, and tumor differentiation. The nomogram showed good discrimination with a concordance index of 0.73 in the derivation cohort and 0.72 in the validation cohort. Calibration was good in the derivation cohort; in the validation cohort patients had a better median DSS than predicted by the model. The nomogram clearly outperformed AJCC staging. The nomogram can inform patients about their prognosis, guide shared decision making for adjuvant therapy, and stratify patients in future randomized, controlled trials.
The National Comprehensive Cancer Network (NCCN) guideline recommends consideration of adjuvant treatment after resection of PHC, in particular for patients with a positive resection margin or nodal disease [16]. While the benefit of systemic chemotherapy with gemcitabine and cisplatin was demonstrated in the palliative setting, no randomized, controlled trial has been reported in the adjuvant setting [17]. The NCCN recommendation is based on a meta-analysis for adjuvant treatment of patients with any biliary cancer, including retrospective series and only one randomized, controlled trial for gallbladder cancer [18]. The greatest benefit was found for margin-positive patients with an odds ratio (OR) of 0.36 [95% confidence interval (CI) 0.14–0.92] and for node-positive patients with an OR of 0.49 (95% CI 0.30–0.80). The proposed nomogram demonstrates that margin- or node-positive patients are not the only subgroup with a poor prognosis. Margin- and node-negative patients with a moderate or poor tumor differentiation have a predicted 5-year DSS of 52%, which is similar to margin- or node-positive patients, without other poor prognostic factors. The predicted 5-year DSS drops further for patients with a moderate or poor tumor differentiation who also have a close margin (DSS 35%) or less than four lymph nodes evaluated (DSS 40%). In the absence of definitive evidence regarding the benefit of adjuvant treatment after resection of PHC, one might assume that these patients with a poor predicted 5-year DSS have a similar benefit from adjuvant chemotherapy as margin- or node-positive patients. The presented nomogram also identifies patients with a favorable predicted 5-year DSS of more than 75%, who seem less likely to benefit from adjuvant treatment. These patients have well-differentiated tumors and either no additional risk factor, a narrow resection margin, or less than four lymph nodes evaluated. They represent ∼15% of all patients with resected PHC. Better evidence about which patients benefit from adjuvant chemotherapy after resection of biliary tract cancers including PHC is anticipated from two large randomized, controlled trials (clinicaltrials.gov identifiers: NCT00363584 and NCT01313377).
The multivariable analysis in the derivation cohort of this study confirmed the prognostic factors known from the literature [7–11]. However, perineural invasion and papillary tumors were strong prognostic factors in the derivation cohort, but not independent prognostic factors due to correlation with other factors. The only published prognostic model for PHC is a risk score based on 96 patients and calculated with age, T stage, margin status, and adjuvant chemoradiation [19]. Two prognostic models have been developed for extrahepatic cholangiocarcinoma, which included both patients with perihilar and distal cholangiocarcinoma. The first model was based on the same prognostic factors as this study, although the number of evaluated lymph nodes and the difference between a wide and narrow margin were not accounted for. This model had a lower concordance index of 0.67 [20]. The second model for extrahepatic cholangiocarcinoma was based on sex, age, tumor differentiation, lymph node status, perineural invasion, and tumor location and had a considerably worse concordance index of 0.63 [21]. These three previously published prognostic models have not been externally validated. The Bismuth classification [22] and Blumgart staging [2] both aim to predict resectability rather than survival.
This study has several limitations. First, the calibration was suboptimal in the validation cohort, while the discrimination was good. A suboptimal calibration is common with external model validation due to differences in patient and tumor characteristics as well as management. In a literature review of patients undergoing curative intent surgery for PHC, the median OS ranged from 19 to 39 months across series with more than 100 patients [3]. In light of this wide range in OS, it is less surprising to find a difference of 11 months in median DSS between the derivation and validation cohort. A statistically nonsignificant difference in patients with positive lymph nodes (29% in derivation and 21% in validation cohort) may explain the difference in OS to some extent. Another explanation is that, in the validation cohort, the majority of patients were operated in the more recent period, although the year of surgery was not a prognostic factor. Finally, preoperative radiation in the validation cohort could also have contributed to a difference in OS although evidence for the benefit of radiation is lacking [13]. A second limitation involves the sample size of both cohorts. While the sample size in this study was comparable with nomograms for other less common cancers [23, 24], a larger sample size may have further improved this model. However, no other Western single-center series of resected PHC with more than 100 patients has been published [3]. Third, ∼40% of patients in both cohorts had less than four lymph nodes evaluated. These patients were potentially understaged because insufficient nodes were sampled to rule out nodal metastasis. Collecting at least four lymph nodes has been recommended, because node-negative patients had a worse prognosis if less than four lymph nodes were evaluated [14]. However, while lymphadenectomy is a standard part of the procedure, most series still have a high percentage of patients with less than four lymph nodes evaluated. This limitation has been largely resolved by including the number of evaluated lymph nodes in the nomogram. Fourth, while the tumor marker CA19-9 is a known prognostic factor for patients with PHC, it could not be evaluated in the current study because of missing values in most patients [25]. Prospective studies should evaluate CA 19-9 and specify whether it was measured before or after adequate biliary drainage. Fifth, patients were included in both cohorts over a long period spanning two decades. Although some changes in management have occurred over time, the year of resection was not a prognostic factor.
In conclusion, the proposed nomogram predicts DSS after curative intent resection of PHC based on lymph node involvement and count, resection margin involvement including additional margins, and tumor differentiation. The concordance index was good in both the derivation and the validation cohorts and clearly better than the AJCC staging. However, calibration was suboptimal because DSS differed between the two institutions. The nomogram can be used to inform patients after curative intent resection of PHC, guide adjuvant treatment decisions, and stratify patients in future adjuvant randomized, controlled trials.
funding
This work was supported by the Dutch Cancer Society (UVA 2011-4973 to BG), the Academic Medical Center Young Talent Fund (to JW), and the French Association of Hepatobiliary Surgery and Transplantation (ACHBT) (to AD). Both nonprofit organizations had no influence on the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
references
- 1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin 2014; 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 2. Jarnagin WR, Fong Y, DeMatteo RP, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg 2001; 234: 507–517; discussion 517–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Popescu I, Dumitrascu T. Curative-intent surgery for hilar cholangiocarcinoma: prognostic factors for clinical decision making. Langenbecks Arch Surg 2014; 399: 693–705. [DOI] [PubMed] [Google Scholar]
- 4. Groot Koerkamp B, Wiggers JK, Allen PJ, et al. American Joint Committee on Cancer staging for resected perihilar cholangiocarcinoma: a comparison of the 6th and 7th editions. HPB (Oxford) 2014; 16: 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nuzzo G, Giuliante F, Ardito F, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg 2012; 147: 26–34. [DOI] [PubMed] [Google Scholar]
- 6. Lee SG, Song GW, Hwang S, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Sci 2010; 17: 476–489. [DOI] [PubMed] [Google Scholar]
- 7. Furusawa N, Kobayashi A, Yokoyama T, et al. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg 2014; 38: 1164–1176. [DOI] [PubMed] [Google Scholar]
- 8. Klempnauer J, Ridder GJ, von Wasielewski R, et al. Resectional surgery of hilar cholangiocarcinoma: a multivariate analysis of prognostic factors. J Clin Oncol 1997; 15: 947–954. [DOI] [PubMed] [Google Scholar]
- 9. Song SC, Choi DW, Kow AW, et al. Surgical outcomes of 230 resected hilar cholangiocarcinoma in a single centre. ANZ J Surg 2013; 83: 268–274. [DOI] [PubMed] [Google Scholar]
- 10. Ito K, Ito H, Allen PJ, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg 2010; 251: 675–681. [DOI] [PubMed] [Google Scholar]
- 11. Endo I, House MG, Klimstra DS, et al. Clinical significance of intraoperative bile duct margin assessment for hilar cholangiocarcinoma. Ann Surg Oncol 2008; 15: 2104–2112. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 2013; 31: 1188–1195. [DOI] [PubMed] [Google Scholar]
- 13. Gerhards MF, Gonzalez DG, ten Hoopen-Neumann H, et al. Prevention of implantation metastases after resection of proximal bile duct tumours with pre-operative low dose radiation therapy. Eur J Surg Oncol 2000; 26: 480–485. [DOI] [PubMed] [Google Scholar]
- 14. Aoba T, Ebata T, Yokoyama Y, et al. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg 2013; 257: 718–725. [DOI] [PubMed] [Google Scholar]
- 15. Guglielmi A, Ruzzenente A, Campagnaro T, et al. Patterns and prognostic significance of lymph node dissection for surgical treatment of perihilar and intrahepatic cholangiocarcinoma. J Gastrointest Surg 2013; 17: 1917–1928. [DOI] [PubMed] [Google Scholar]
- 16. National Comprehensive Cancer Network. Hepatobiliary Cancers, in version 2, 2nd edition 2015. http://www.nccn.org (27 July 2015, date last accessed). [Google Scholar]
- 17. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010; 362: 1273–1281. [DOI] [PubMed] [Google Scholar]
- 18. Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol 2012; 30: 1934–1940. [DOI] [PubMed] [Google Scholar]
- 19. Kaiser GM, Paul A, Sgourakis G, et al. Novel prognostic scoring system after surgery for Klatskin tumor. Am Surg 2013; 79: 90–95. [PubMed] [Google Scholar]
- 20. van der Gaag NA, Kloek JJ, de Bakker JK, et al. Survival analysis and prognostic nomogram for patients undergoing resection of extrahepatic cholangiocarcinoma. Ann Oncol 2012; 23: 2642–2649. [DOI] [PubMed] [Google Scholar]
- 21. Song C, Kim K, Chie EK, et al. Nomogram prediction of survival and recurrence in patients with extrahepatic bile duct cancer undergoing curative resection followed by adjuvant chemoradiation therapy. Int J Radiat Oncol Biol Phys 2013; 87: 499–504. [DOI] [PubMed] [Google Scholar]
- 22. Bismuth H, Corlette MB. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 1975; 140: 170–178. [PubMed] [Google Scholar]
- 23. Gold JS, Gonen M, Gutierrez A, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 2009; 10: 1045–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang SJ, Lemieux A, Kalpathy-Cramer J, et al. Nomogram for predicting the benefit of adjuvant chemoradiotherapy for resected gallbladder cancer. J Clin Oncol 2011; 29: 4627–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kow AW, Wook CD, Song SC, et al. Role of caudate lobectomy in type III A and III B hilar cholangiocarcinoma: a 15-year experience in a tertiary institution. World J Surg 2012; 36: 1112–1121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


